Abstract
Context
Knowing the prevalence and risk factors of immunosuppression nonadherence after liver transplant may help guide intervention development.
Objective
To examine whether sociodemographic and psychosocial variables before liver transplant are predictive of nonadherence after liver transplant.
Design
Structured telephone interviews were used to collect self-report immunosuppression adherence and health status information. Medical record reviews were then completed to retrospectively examine the relationship between immunosuppression adherence and pretransplant variables, including sociodemographic and medical characteristics and the presence or absence of 6 hypothesized psychosocial risk factors.
Setting and Participants
A nonprobability sample of 236 adults 6 to 24 months after liver transplant at 2 centers completed structured telephone interviews.
Main Outcome Measure
Immunosuppressant medication nonadherence, categorized as missed-dose and altered-dose “adherent” or “nonadherent” during the past 6 months; immunosuppression medication holidays.
Results
Eighty-two patients (35%) were missed-dose nonadherent and 34 patients (14%) were altered-dose nonadherent. Seventy-one patients (30%) reported 1 or more 24-hour immunosuppression holidays in the past 6 months. Missed-dose nonadherence was predicted by male sex (odds ratio, 2.46; P = .01), longer time since liver transplant (odds ratio, 1.08; P = .01), pretransplant mood disorder (odds ratio, 2.52; P = .004), and pretransplant social support instability (odds ratio, 2.25; P = .03). Altered-dose nonadherence was predicted by pretransplant mood disorder (odds ratio, 2.15; P = .04) and pretransplant social support instability (odds ratio, 1.89; P = .03).
Conclusion
Rates of immunosuppressant nonadherence and drug holidays in the first 2 years after liver transplant are unacceptably high. Pretransplant mood disorder and social support instability increase the risk of nonadherence, and interventions should target these modifiable risk factors.
Liver transplant offers patients with end-stage liver disease the opportunity for enhanced quality of life and longer survival.1,2 However, these favorable outcomes are largely dependent upon a lifetime commitment to daily immunosuppression therapy. Mismanagement of immunosuppression therapy jeopardizes the technical success of solid-organ transplants and may increase the risk of graft loss, morbidity, rehospitalization, higher health service utilization and costs, retransplantation, and death.3–6 Although nonadherence to immunosuppression therapy has not yet been shown to compromise graft and patient survival rates after liver transplant specifically, liver transplant recipients typically are advised to maintain optimal adherence to the immunosuppressant medication regimen.
Nonadherence, or the extent to which patients’ medication-taking does not correspond to medical recommendations, has been the focus of increased study in solid-organ transplant for the past 2 decades. In a meta-analysis of 147 studies, Dew et al7 reported an immunosuppression nonadherence rate of 22.6 cases per 100 persons per year (PPY), among the highest nonadherence rates of all behaviors examined. Relative to kidney (36 cases per 100 PPY) or heart (14 cases per 100 PPY) transplant recipients, liver transplant recipients had significantly fewer problems taking immunosuppressant medications (7 cases per 100 PPY). However, only 7 studies examined medication adherence in liver transplant recipients (vs 32 studies in kidney transplant), and they varied considerably in the definition of nonadherence, reported prevalence of nonadherence, and methodological rigor.
As part of the patient selection process, transplant programs attempt to identify patients most at risk for nonadherence after liver transplant.8–11 Clinical assessments of nonadherence risk are based largely on factors associated with medication nonadherence in the nontransplant literature, including mood disturbances, passive coping strategies, substance abuse, prior medication nonadherence, and limited social support.12–23 Some candidates at high risk for posttransplant nonadherence are either not listed or required to participate in treatment programs designed to reduce nonadherence risk.8,24 However, whether the presence of these pretransplant factors increases the risk of immunosuppression nonadherence after transplant is not currently known. In the Dew et al7 meta-analysis, nonwhite race, inadequate social support, and poorer perceived health were the only psychosocial variables associated with immunosuppression nonadherence. However, the effect sizes were very small and highlight the need for further examination of these psychosocial factors in the liver transplant population. In the only prospective study to date, Dobbels et al25 found that pretransplant nonadherence and poor social support, but not depression, were independent predictors of immunosuppression nonadherence in transplant recipients. However, liver transplant recipients, while representing nearly half of the sample, were combined with heart and lung transplant recipients in this study.
The current study examined the prevalence of immunosuppression nonadherence in a large cohort of adult liver transplant recipients at 2 transplant centers. We also examined whether pretransplant sociodemographic and psychosocial variables were predictive of nonadherence after liver transplant. We hypothesized that immunosuppression nonadherence would be highest in patients with pretransplant mood disturbance, substance abuse, and inadequate social support.
Methods
Participants and Recruitment Procedures
Adults who underwent liver transplants at the University of Florida in Gainesville or at Beth Israel Deaconess Medical Center in Boston, Massachusetts, were invited to participate in the study. Inclusion criteria were as follows: primary liver transplant recipient, 6 to 24 months after liver transplant, age at least 18 years old, and English speaking. Patients within 6 months of liver transplant were excluded because it is common for them to experience multiple adjustments in the immunosuppression regimen in the initial months after transplant and we wanted patients to be on a stable immunosuppression regimen for several weeks before study participation. Patients more than 24 months after transplant were excluded because we were primarily interested in “early” posttransplant nonadherence. At both centers, patients were mailed a letter describing the study and then called by a research assistant who discussed the study with them. The research assistant was not affiliated with the transplant program. Verbal informed consent was obtained and documented for those who chose to take part in the study, and they were scheduled for a telephone interview.
Data Collection Procedures
A structured telephone interview was conducted by a research assistant unaffiliated with the transplant program. Patients were informed that no judgments were being made about their medication-taking behaviors, that we were interested only in understanding how we can help adults better manage their health after transplant, and that none of the information they provided as individual patients would be shared with their transplant providers. This approach was designed to facilitate candid reporting by patients. Patients were not compensated. This research was approved by the institutional review boards at both institutions.
During the interview, patients were first asked to name all of their medications. If they did not remember the medication name, the interviewer told the patient which drug(s) was part of their immunosuppression regimen. Patients were then told to answer the subsequent questions about only their immunosuppressant medication(s) and to think about only the past 6 months. To assess “missed dose” nonadherence, we asked: In the past 6 months, how often, on average, did you not take a dose of your antirejection medication? (1=never, 2=no more than a few times a month, 3=about once per week, 4=more than once per week). To assess “altered dose” nonadherence, we asked: In the past 6 months, how often, on average, did you change how you take your antirejection medication (for example, took more or less of it) without first talking to your transplant doctors, nurse, or pharmacist? (1=never, 2=no more than a few times a month, 3=about once per week, 4=more than once per week). For both questions, patients were subsequently classified as either “adherent” (response of 1 or 2) or “nonadherent” (response of 3 or 4).
Next, to assess immunosuppression holidays, we asked: In the past 6 months, have you gone more than 24 hours without taking your antirejection medication, without approval from your transplant doctors, nurse, or pharmacist? If the patients responded affirmatively, we asked how many times during the past 6 months they had gone more than 24 hours without taking immunosuppressant medication. We then asked whether any of these drug holidays lasted more than 48 hours and more than 72 hours. For the nonadherence assessment, the past 6 months was chosen to strike a balance between maximizing the sampling time frame for the patient and minimizing the likelihood of recall bias.
At the end of the interview, we asked patients to rate change in their physical health by responding to the question: Compared to the months right before your transplant, how would you rate your health in general right now? (1=much better, 2=somewhat better, 3=about the same, 4=somewhat worse, 5=much worse).
At both centers, all liver transplant patients eligible for this study previously underwent a comprehensive medical, surgical, and psychosocial evaluation to determine transplant eligibility. The psychosocial evaluations at both centers, where the first author (JRR) served as director of transplant behavioral health services, were identical and included separate clinical interviews by the transplant social worker and transplant psychologist. Psychosocial assessment findings were independently documented by both providers and consistently included summary assessments of the patient’s history of psychological and psychiatric diagnoses and treatment, medication adherence, substance abuse, primary coping strategies, and social support availability and stability. For purposes of this study, information about these psychosocial variables was abstracted from the medical record by 2 individuals at each center who were blinded to the study aims and adherence interview data. Raters independently coded each of these psychosocial variables as “present” or “absent” using criteria designed to standardize data collection, facilitate statistical analysis, and mirror the clinical presentation of this information during transplant patient selection conferences (see Table). Variables categorized as “present” were considered risk factors for nonadherence based on their association with medication nonadherence in the general medical literature. Discrepancies (85/1416, or 6%) in the ratings of the 2 coders were resolved by further review, discussion, and mutual rater agreement before statistical analysis of the data. The first author (JRR) trained the coders at both centers on how to apply the criteria (see Table). Medical record review and coding of study patients was not started until 100% agreement was achieved by the 2 coders on 5 consecutive medical records of patients who were ineligible for the study (ie, >24 months after liver transplant).
Table.
Psychosocial variables before liver transplant and their definitions
| Psychosocial variable | Risk factor present (+) | Risk factor absent (−) |
|---|---|---|
| Mood or anxiety disorder | Met DSM-IV diagnostic criteria for mood or anxiety disorder within 24 months before liver transplant | Did not meet DSM-IV diagnostic criteria for mood or anxiety disorder within 24 months before liver transplant |
| Primary coping style | Primarily passive coping style, characterized by mental or behavioral disengagement, substance use, resignation or avoidance, or denial | Primarily active coping style, characterized by planful problem-solving, seeking social support, or positive reappraisal of stressors |
| Medication adherence | One or more providers identifies problems with missing medication doses, drug holidays, or altering medications without medical consultation within 12 months before liver transplant | No providers reference problems with medication adherence behaviors |
| Substance abuse/dependence | Met DSM-IV diagnostic criteria for substance abuse or dependence before liver transplant | Did not meet DSM-IV diagnostic criteria for substance abuse or dependence before liver transplant |
| Support system availability | Patient lives alone or does not have another adult who has self-identified as a primary caregiver | Patient has an adult living with him/her who is self-identified as the patient’s primary caregiver and who intends to continue residing with patient after liver transplant |
| Support system stability | One or more providers identifies concerns about the primary caregiver’s ability to serve in such capacity, owing to physical or mental health problems, relationship instability, or substance use | No providers reference concerns about the primary caregiver’s ability to serve in such capacity |
Abbreviation: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).
The following sociodemographic data were gathered from interviews with patients or review of their medical records: current age (years), sex, race, marital status, and educational background. Also, primary diagnosis before liver transplant, laboratory Model for End-Stage Liver Disease (MELD) score at time of liver transplant, months on the waiting list, and months since liver transplant were obtained from a review of the medical records.
Statistical Analyses
Descriptive statistics were calculated to summarize the sociodemographic and medical characteristics of the sample. The proportion of patients classified as adherent or nonadherent was calculated. Frequency statistics were calculated for each of the adherence questions. Student t tests were used to examine differences between adherent and nonadherent patients on continuous variables, and χ2 or Fisher exact tests were used to identify differences between these 2 groups of patients on categorical variables. Variables with P less than .05 in the univariate analysis were included in separate stepwise logistic regression analyses to identify predictors of immunosuppression nonadherence (for missed dose and altered dose). Only those variables with an adjusted P less than .05 were included in the final model. Finally, using logistic regression analysis, we conducted a “dose-dependent” analysis26 to examine the relationship between nonadherence and different levels of psychosocial risk before liver transplant. PASW 17.0 (IBM Corporation) was used for all statistical analyses.
Results
Recipient Characteristics
Three hundred ninety-eight adult transplants were performed during the study observation period (Figure 1). One hundred twenty-eight patients (32%) did not meet study inclusion criteria. Eligible patients did not differ significantly from ineligible patients on age, sex, highest education, and primary cause of disease (all P values >.05), although ineligible patients had higher laboratory MELD scores at liver transplant than did eligible patients (P=.03). Of the 270 eligible patients, 236 (87%) completed the study and were included in the final analysis. Study participation rates and primary outcomes did not differ significantly by study site, so data from the 2 sites were combined for all analyses.
Figure 1.
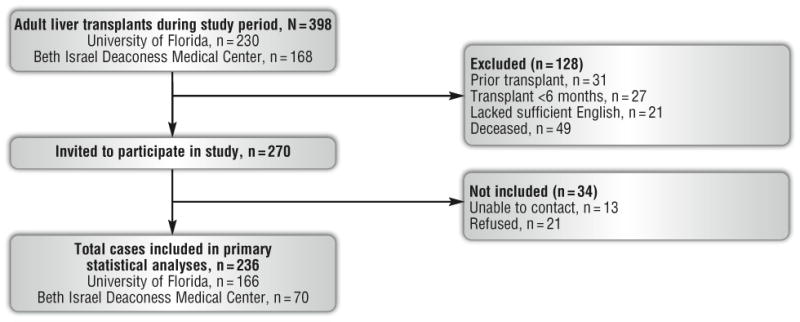
Overview of patient identification and inclusion in final data analysis.
Mean age was 53 (SD, 11) years, 143 (61%) were male, 204 (86%) were white, 160 (68%) were married, and 88 (37%) had attended college. One hundred thirteen (48%) had hepatitis C virus infection, 99 (41%) had alcohol as the primary cause of liver disease, and 48 (20%) had hepatocellular carcinoma. Mean waiting time on the transplant list was 11.1 (SD, 12) months, mean laboratory MELD score at liver transplant was 21.2 (SD, 8), and mean time from liver transplant to study participation was 14.3 (SD, 5) months.
Missed-Dose Nonadherence
Eighty-two patients (35%) were classified as missed-dose nonadherent, with 18% missing immunosuppressant medications about once per week and 17% missing immunosuppressant medications more than once per week in the past 6 months (Figure 2). Slightly more than half (52%) reported not missing any immunosuppressant medications in the past 6 months, and 14% reported missing immunosuppressant medications a few times per month or less.
Figure 2.
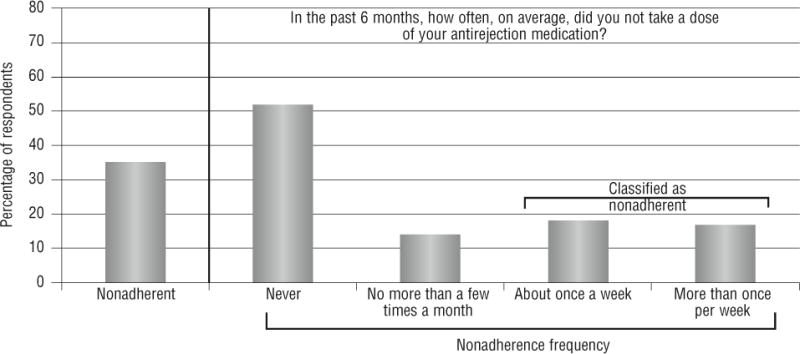
Frequency of missed-dose nonadherence.
Univariate analyses showed that, compared with adherent patients, patients with missed-dose nonadherence were more likely to have longer time since liver transplant (15.6 months vs 13.6 months, t = 2.8, P = .006), be male (70% vs 56%, P = .04), view their overall health as unchanged or worse since liver transplant (27% vs 10%, P = .003), and have the following in the 24 months before liver transplant: mood disorder (64% vs 43%, P = .002), medication nonadherence (51% vs 28%, P < .001), limited social support (47% vs 27%, P = .002), and social support instability (36% vs 18%, P = .002). In the subsequent multivariate prediction model, 4 variables were statistically significant: male sex (odds ratio [OR], 2.46; 95% CI, 1.23–4.91; P = .01), longer time since liver transplant (OR, 1.08, 95% CI, 1.02–1.14, P = .01), pretransplant mood disorder (OR, 2.52; 95% CI, 1.34–4.74; P=.004), and pretransplant social support instability (OR, 2.25, 95% CI, 1.09–4.65, P=.03). The model explained 47% of the variance in the outcome (χ2=50.87, P<.001) and correctly classified the outcome of 76% of patients.
Altered-Dose Nonadherence
Thirty-four patients (14%) were classified as altered-dose nonadherent, with 11% changing immunosuppressant medication about once per week and 3% changing immunosuppressant medication more than once per week in the past 6 months without first consulting with a transplant provider (Figure 3). Most patients (80%) reported not altering immunosuppressant medications at all.
Figure 3.
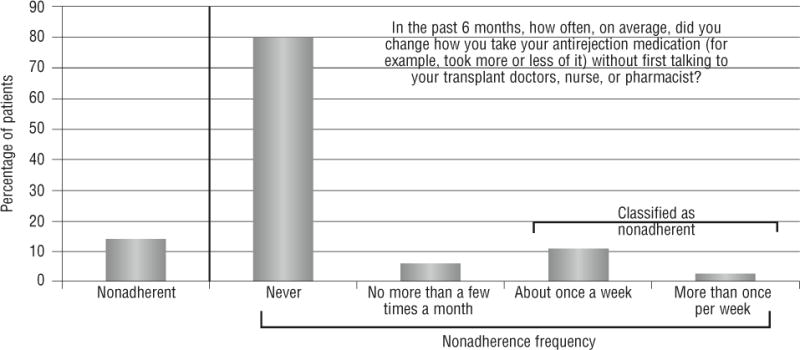
Frequency of altered-dose nonadherence.
Univariate analyses showed that, compared with adherent patients, patients with altered-dose nonadherence were more likely to have the following in the 24 months before liver transplant: mood disorder (68% vs 48%, P = .04), medication nonadherence (53% vs 31%, P = .05), limited social support (36% vs 18%, P = .02), and social support instability (41% vs 21%, P = .002). Sociodemographic characteristics, medical variables, time since liver transplant, and perceptions of overall health were not significantly associated with altered-dose nonadherence (all P values > .05). In the multivariate prediction model, pretransplant mood disorder (OR, 2.15; 95% CI, 1.13–4.45, P = .04) and pretransplant social support instability (OR, 1.89; 95% CI, 1.06–4.28, P = .03) were predictive of nonadherence. The model explained 14% of the variance in the outcome (χ2=12.00, P=.02) and correctly classified 86% of patients.
Multivariate Analyses Using a Different Definition of Nonadherence
We repeated the statistical analyses just described to ascertain whether findings could be replicated on the basis of a more restrictive definition of adherence. In this analysis, patients were classified as missed-dose adherent or altered-dose adherent if they reported never missing or altering their medication (ie, response of 1 to the 2 adherence questions) and all others were classified as nonadherent. With this classification, 48% of patients were classified as missed-dose nonadherent and and 20% as altered-dose nonadherent. Both multivariate models were significant (χ2 = 25.62, P < .001 for missed-dose nonadherence and χ2=16.42, P=.003 for altered-dose nonadherence), with pretransplant mood disorder and pretransplant social support instability retained as significant predictors of both types of nonadherence (P values < .05). Additionally, older age (P = .003) was strongly associated with missed-dose nonadherence in this multivariate analysis.
Immunosuppression Holidays
Seventy-one patients (30%) reported at least one 24-hour immunosuppression holiday in the past 6 months (Figure 4). Thirty-eight (16%) patients reported at least one 48-hour immunosuppression holiday and 23 patients (10%) reported at least one 72-hour immunosuppression holiday in the past 6 months. Compared with patients without immunosuppression holidays, those with at least one 24-hour immunosuppression holiday in the past 6 months were more likely to have a pretransplant mood disorder (65% vs 44%, P = .004), a limited social support system (48% vs 28%, P = .004), and an unstable support system (34% vs 20%, P = .02). Immunosuppression holidays were not significantly associated with sociodemographic or medical characteristics, time since liver transplant, or perceptions of current health. In the multivariate prediction model, pretransplant mood disorder (OR, 2.14, 95% CI, 1.19–3.85; P = .01) and limited social support (OR, 2.04; 95% CI, 1.09–3.82, P =.03) were predictive of nonadherence and led to correct classification of 72% of patients.
Figure 4.
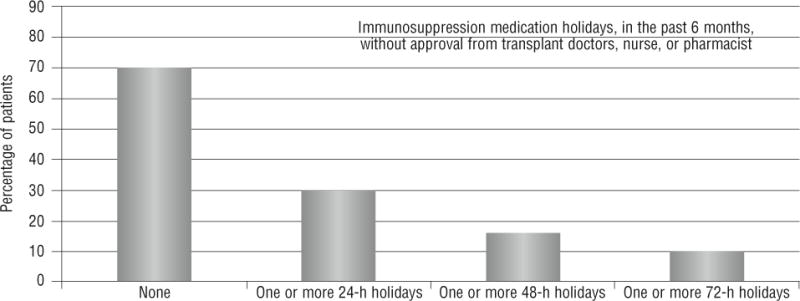
Frequency of immunosuppression holidays.
Of those patients who acknowledged taking a 24-hour immunosuppression holiday, the mean number of holidays in the past 6 months was 4.41 (SD, 4.0; range, 1–20). Men reported more immunosuppression holidays than women reported (5.25 vs 3.12, t = 2.18, P = .03), and those without a substance abuse history at time of pretransplant evaluation reported more immunosuppression holidays than those with a substance abuse history (5.88 vs 3.13, t = 3.02, P = .004).
Exploratory Dose-Dependent Analysis of Psychosocial Risk Factors
To further examine the relationship between pretransplant psychosocial risk factors and immunosuppression nonadherence, we classified patients based on how many of the 6 psychosocial risk factors they had: 0 to 1 (low risk, n = 72, 31%), 2 to 3 (moderate risk, n=106, 45%), and 4 to 6 (high risk, n=58, 25%). Figure 5 shows that a significantly higher percentage of patients (60%) in the high-risk group were classified as missed-dose nonadherent, compared with 18% and 32% in the low- and moderate-risk groups, respectively (χ2 = 24.4, P < .001).
Figure 5.
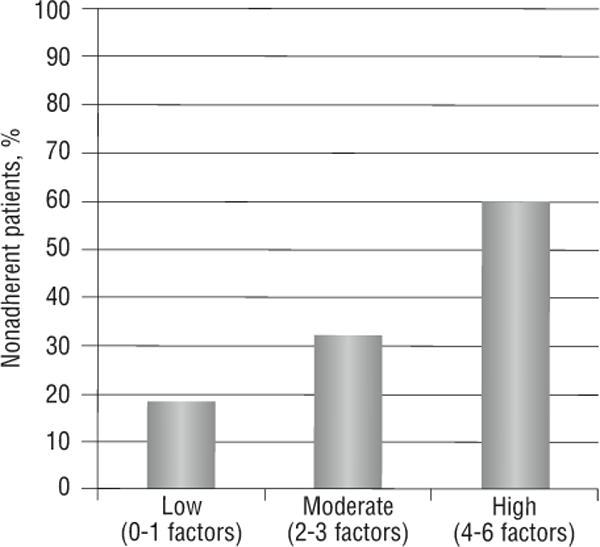
Relationship between psychosocial risk before liver transplant and missed-dose nonadherence.
Discussion
A relatively small number of studies have examined immunosuppression nonadherence in adult liver transplant recipients. Rates of immunosuppression nonadherence after liver transplant are quite variable, depending on how it is defined and what type of measurement is used, but generally range from 14% to 50%.7,27–37 The immunosuppression nonadherence rates observed in our study fall within this range. For some, immunosuppression nonadherence may be defined as taking less than the prescribed amount, considering the serious and potentially life-threatening consequences of nonadherence.28,31 By these standards, we may have underestimated immunosuppression nonadherence rates in the current study because patients who missed immunosuppression medications fewer than once per week were classified as adherent. Others have proposed that immunosuppression nonadherence be defined as a “deviation from the prescribed medication regimen sufficient to influence adversely the regimen’s intended effect.”38 However, data are insufficient to determine the level of immunosuppression nonadherence after liver transplant that adversely affects outcomes.
Nevertheless, the immunosuppression nonadherence rates observed in this study are surprising and concerning for several reasons. First, both transplant programs taking part in this study do not list patients with persistent medication nonadherence problems, so our study sample represents a select group of patients who showed evidence of good medication adherence at the time of evaluation. Second, both programs require liver transplant candidates and their caregivers to participate in a pretransplant educational program in which the importance of immunosuppression adherence is emphasized. Third, all liver transplant recipients at both programs participate in a formal predischarge medication educational intervention by the transplant pharmacist and medications are reconciled at each follow-up visit with the transplant hepatologist. Fourth, unlike many other investigations, we focused our study on those who were in the early stage of transplant survivorship (ie, ≤2 years). We expected nonadherence rates to be lower than published rates, because ample evidence shows that nonadherence to chronic medication regimens increases over time.39
Our observed drug holiday rates are lower than those reported by Drent et al,27 who found that 39% and 16% of adult liver transplant recipients reported drug holidays of 48 and 72 hours, respectively. Drent et al may have found higher drug holiday rates because they studied many patients who were several years removed from transplant. Interestingly, those with a pretransplant substance abuse history reported fewer immunosuppression holidays compared with patients without such a history. It may be that patients who have received treatment for alcohol and/or drug abuse are able to generalize relapse prevention skills to medication-taking behaviors and prevent subsequent occurrences of immunosuppression holidays. Unfortunately, we did not ask patients whether they informed their transplant providers about immunosuppression holidays. Findings of nontherapeutic immunosuppression levels or liver function abnormalities may precipitate potentially harmful immunosuppression dose increases and/or unnecessary diagnostic procedures if the transplant provider is unaware of immunosuppression nonadherence.
Male sex and longer time since liver transplant were significant predictors of immunosuppression missed-dose nonadherence. Post-hoc analysis showed that men with more limited pretransplant social support were more likely to be nonadherent than were women with similarly limited supports (61% vs 38%, P=.02). Men may have more difficulty with medication management when they lack adequate support systems, although the relationship between sex and nonadherence is equivocal and warrants further study.40–42 The finding that patients may be more nonadherent over time is not entirely unexpected. Even when faced with dire consequences of immunosuppression nonadherence, medication-taking fatigue, persistent side effects, perceptions of favorable health, and associated beliefs that immunosuppression may not be necessary, and return to normal routines all may contribute to missing medication doses. Over time, patients are likely to have fewer clinic appointments in the transplant center, which also may reduce the saliency and vigilance of medication-taking behaviors.
Mood disorder history and social support instability before transplant were strong predictors of both immunosuppression missed-dose and altered-dose nonadherence. This relationship was observed by using both our a priori definition of nonadherence and when testing a more inclusive classification of nonadherence (ie, any missed or altered immunosuppression dose in the past 6 months). Depression and anxiety are known correlates of medication nonadherence in patients with chronic illnesses.32,39,42–46 Mood disturbances can affect cognitive functioning, self-efficacy, organizational skills, and motivation, which could—individually or in combination—adversely affect a patient’s ability to attend vigilantly to a complex medication regimen. Transplant candidates and recipients should be routinely assessed and, if indicated, treated for symptoms of depression and anxiety to potentially mitigate the impact of these symptoms on behaviors related to taking immunosuppressant medications.
Our findings also corroborate previous studies showing that inadequate or unstable social support contribute to higher risk of immunosuppression nonadherence.7,26,30,47,48 Most liver transplant programs require an identified primary caregiver to provide practical assistance to the patient throughout the transplant process.8,10 However, the effectiveness and stability of the support system may be more critical to health outcomes than the mere availability of others.30 It is important that the caregiver be able to learn the posttransplant medication regimen and lifestyle changes that are necessary for optimal outcomes, as well as to facilitate immunosuppression adherence by providing cues or prompts for medication taking, positive reinforcement for healthy behaviors, and negative or corrective feedback for behaviors (eg, immunosuppressant nonadherence) that violate health expectations. The lack of a robust social network may tax a patient’s limited intrapersonal resources or coping abilities and increase stress that compromises more favorable adherence behaviors. Importantly, we did not examine the differential role of structural (eg, living arrangements) versus functional (eg, practical assistance, emotional support) social support on immunosuppression adherence, which may have different implications for medication management over time.48 Caregiver strain and burden, too, may reduce the quality and effectiveness of the support provided and, indirectly, may increase rates of medication nonadherence.49,50
Liver transplant programs carefully evaluate and identify candidates for listing who are likely to have a favorable transplant outcome.8,11 The psychosocial evaluation is an important component of this evaluation, but the utility of pretransplant psychosocial variables in predicting posttransplant adherence behaviors has infrequently been examined. We found that immunosuppression nonadherence is more likely among patients with more pretransplant psychosocial risk factors. Recipients who had 4 or more psychosocial risk factors at time of initial evaluation were significantly more likely to show signs of immunosuppression nonadherence than did those with one or fewer such risk factors. This finding further underscores the need for a comprehensive psychosocial evaluation, but perhaps more importantly emphasizes the necessity of interventions that target these symptoms to mitigate the risk of future immunosuppression nonadherence.24
Study findings should be considered within the context of several important limitations. This is a study of immunosuppression nonadherence at one point in time, and we relied entirely on patient self-report. Immunosuppression adherence patterns are known to change over time for about 25% of liver transplant patients,29 and future research should examine nonadherence prospectively and at multiple time points after transplant.25 Although we emphasized a nonjudgmental and confidential approach to data collection and employed an interviewer not involved in the patient’s clinical care, some patients may have underreported episodes of nonadherence. A multimethod approach to measuring nonadherence, including laboratory assays, is recommended for future studies.
Minority representation in this study (15%), while representative of the 2 transplant programs, is lower than the percentage of minority liver transplant recipients in the United States (29%); thus, we could not examine whether nonadherence rates and predictors differed by race or ethnicity. Although study patients did not differ significantly from nonstudy patients on sociodemographic and disease parameters, there is the possibility of selection bias that is inherent in patient-reported outcomes research. Patients with profound immunosuppression nonadherence may not have taken part in this study, either because of death, poor relationship with the transplant team, or concerns about disclosing medication-taking behaviors. Importantly, we did not assess factors (eg, cultural, cognitive, physician-patient interaction, health system variables) that may also be associated with nonadherence, and these factors could offer additional insights into the effective management of nonadherence in the transplant setting. Finally, since we focused our assessment on patients within the first 2 years after transplant, these findings should not be generalized to longer-term liver transplant survivors.
In conclusion, this is one of a few multisite studies to examine self-reported immunosuppression nonadherence in a large cohort of adult liver transplant recipients. Rates of nonadherence are moderately high and associated with some pretransplant variables that are potentially modifiable. Additional research is needed to prospectively examine predictors and patterns of nonadherence beyond the first 2 years of transplant survivorship,25 the association between subclinical levels of immunosuppression nonadherence and posttransplant clinical outcomes, and the effectiveness of behavioral health interventions to reduce the risk of posttransplant nonadherence.
Acknowledgments
The authors thank Timothy Antonellis, Kristin Gant, Ariel Hodara, Jonathan Lin, Richard McCartney, Colleen Morse, John Myers, Matthew Paek, Stacey Senat, and Hongying Tang for data collection and entry assistance.
Financial Disclosures
This study was funded, in part, by support from the Julie Henry Research Fund and the Center for Transplant Outcomes and Quality Improvement, The Transplant Institute, Beth Israel Deaconess Medical Center, Boston, MA. JRR is supported in part by research grants from the NIH/NIDDK (DK079665, DK085185) and HRSA (D71HS22061). DRN is supported in part by the NIH/NCRR Clinical and Translational Science Award to the University of Florida (UL1 RR029890).
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2010 annual data report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. Am J Transplant. 2012;12(suppl):53–74. [Google Scholar]
- 2.Butt Z, Parikh ND, Skaro AI, Ladner D, Cella D. Quality of life, risk assessment, and safety research in liver transplantation: new frontiers in health services and outcomes research. Curr Opin Organ Transplant. 2012;17(3):241–247. doi: 10.1097/MOT.0b013e32835365c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrissey PE, Flynn ML, Lin S. Medication noncompliance and its implications in transplant recipients. Drugs. 2007;67(10):1463–1481. doi: 10.2165/00003495-200767100-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kaul V, Khurana S, Munoz S. Management of medication noncompliance in solid-organ transplant recipients. BioDrugs. 2000;13(5):313–326. doi: 10.2165/00063030-200013050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9(11):2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 6.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769–776. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 7.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 8.Widows M, Rodrigue JR. Clinical practice issues in solid organ transplantation. In: Llewelyn S, Kennedy P, editors. Handbook of Clinical Health Psychology. Oxford, United Kingdom: John Wiley and Sons; 2003. pp. 277–301. [Google Scholar]
- 9.Flamme NE, Terry CL, Helft PR. The influence of psychosocial evaluation on candidacy for liver transplantation. Prog Transplant. 2008;18(2):89–96. doi: 10.1177/152692480801800205. [DOI] [PubMed] [Google Scholar]
- 10.Levenson JL, Olbrisch ME. Psychosocial evaluation of organ transplant candidates. A comparative survey of process, criteria, and outcomes in heart, liver, and kidney transplantation. Psychosomatics. 1993;34(4):314–323. doi: 10.1016/S0033-3182(93)71865-4. [DOI] [PubMed] [Google Scholar]
- 11.Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. 2002;70(3):771–783. doi: 10.1037//0022-006x.70.3.771. [DOI] [PubMed] [Google Scholar]
- 12.Sabate E. World Health Organization Report: Adherence to Long-Term Therapies Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [PubMed] [Google Scholar]
- 13.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 14.Bunzel B, Laederach-Hofmann K. Solid organ transplantation: are there predictors for posttransplant noncompliance? A literature overview. Transplantation. 2000;70(5):711–716. doi: 10.1097/00007890-200009150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dempe C, Jünger J, Hoppe S, et al. Association of anxious and depressive symptoms with medication nonadherence in patients with stable coronary artery disease. J Psychosom Res. 2013;74(2):122–127. doi: 10.1016/j.jpsychores.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Bautista LE, Vera-Cala LM, Colombo C, Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens. 2012;25(4):505–511. doi: 10.1038/ajh.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborn CY, Egede LE. The relationship between depressive symptoms and medication nonadherence in type 2 diabetes: the role of social support. Gen Hosp Psychiatry. 2012;34(3):249–253. doi: 10.1016/j.genhosppsych.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheurer D, Choudhry N, Swanton KA, Matlin O, Shrank W. Association between different types of social support and medication adherence. Am J Manag Care. 2012;18(12):e461–e467. [PubMed] [Google Scholar]
- 21.Vyavaharkar M, Moneyham L, Tavakoli A, et al. Social support, coping, and medication adherence among HIV-positive women with depression living in rural areas of the southeastern United States. AIDS Patient Care STDS. 2007;21(9):667–680. doi: 10.1089/apc.2006.0131. [DOI] [PubMed] [Google Scholar]
- 22.Grodensky CA, Golin CE, Ochtera RD, Turner BJ. Systematic review: effect of alcohol intake on adherence to outpatient medication regimens for chronic diseases. J Stud Alcohol Drugs. 2012;73(6):899–910. doi: 10.15288/jsad.2012.73.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power R, Koopman C, Volk J, et al. Social support, substance use, and denial in relationship to antiretroviral treatment adherence among HIV-infected persons. AIDS Patient Care STDS. 2003;17(5):245–252. doi: 10.1089/108729103321655890. [DOI] [PubMed] [Google Scholar]
- 24.Lisson GL, Rodrigue JR, Reed AI, Nelson DR. A brief psychological intervention to improve adherence following transplantation. Ann Transpl. 2005;10(1):52–57. [PubMed] [Google Scholar]
- 25.Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of posttransplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497–1504. doi: 10.1097/TP.0b013e3181a440ae. [DOI] [PubMed] [Google Scholar]
- 26.Dew MA, Roth LH, Thompson ME, Kormos RL, Griffith BP. Medical compliance and its predictors in the first year after heart transplantation. J Heart Lung Transplant. 1996;15(6):631–645. [PubMed] [Google Scholar]
- 27.Drent G, Haagsma EB, Geest SD, et al. Prevalence of prednisolone (non)compliance in adult liver transplant recipients. Transpl Int. 2005;18(8):960–966. doi: 10.1111/j.1432-2277.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 28.Kung M, Koschwanez HE, Painter L, Honeyman V, Broadbent E. Immunosuppressant nonadherence in heart, liver, and lung transplant patients: associations with medication beliefs and illness perceptions. Transplantation. 2012;93(9):958–963. doi: 10.1097/TP.0b013e31824b822d. [DOI] [PubMed] [Google Scholar]
- 29.De Bleser L, Dobbels F, Berben L, et al. The spectrum of nonadherence with medication in heart, liver, and lung transplant patients assessed in various ways. Transpl Int. 2011;24(9):882–891. doi: 10.1111/j.1432-2277.2011.01296.x. [DOI] [PubMed] [Google Scholar]
- 30.Scholz U, Klaghofer R, Dux R, et al. Predicting intentions and adherence behavior in the context of organ transplantation: gender differences of provided social support. J Psychosom Res. 2012;72(3):214–219. doi: 10.1016/j.jpsychores.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Stilley CS, DiMartini AF, de Vera ME, et al. Individual and environmental correlates and predictors of early adherence and outcomes after liver transplantation. Prog Transplant. 2010;20(1):58–66. doi: 10.7182/prtr.20.1.c903845857104k83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17(7):760–770. doi: 10.1002/lt.22294. [DOI] [PubMed] [Google Scholar]
- 33.Telles-Correia D, Barbosa A, Mega I, Monteiro E. Psychosocial predictors of adherence after liver transplant in a single transplant center in Portugal. Prog Transplant. 2012;22(1):91–94. doi: 10.7182/pit2012569. [DOI] [PubMed] [Google Scholar]
- 34.Morales JM, Varo E, Lázaro P. Immunosuppressant treatment adherence, barriers to adherence and quality of life in renal and liver transplant recipients in Spain. Clin Transplant. 2012;26(2):369–376. doi: 10.1111/j.1399-0012.2011.01544.x. [DOI] [PubMed] [Google Scholar]
- 35.Lamba S, Nagurka R, Desai KK, Chun SJ, Holland B, Koneru B. Self-reported non-adherence to immune-suppressant therapy in liver transplant recipients: demographic, interpersonal, and intrapersonal factors. Clin Transplant. 2012;26(2):328–335. doi: 10.1111/j.1399-0012.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 36.Schweizer RT, Rovelli M, Palmeri D, Vossler E, Hull D, Bartus S. Noncompliance in organ transplant recipients. Transplantation. 1990;49(2):374–377. doi: 10.1097/00007890-199002000-00029. [DOI] [PubMed] [Google Scholar]
- 37.O’Carroll RE, McGregor LM, Swanson V, Masterton G, Hayes PC. Adherence to medication after liver transplantation in Scotland: a pilot study. Liver Transpl. 2006;12(12):1862–1868. doi: 10.1002/lt.20828. [DOI] [PubMed] [Google Scholar]
- 38.Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant. 2009;9(1):35–41. doi: 10.1111/j.1600-6143.2008.02495.x. [DOI] [PubMed] [Google Scholar]
- 39.Meichenbaum D, Turk DC. Facilitating Treatment Adherence: A Practitioner’s Guidebook. New York, NY: Plenum Press; 1987. [Google Scholar]
- 40.Ortego C, Huedo-Medina TB, Santos P, et al. Sex differences in adherence to highly active antiretroviral therapy: a meta-analysis [Epub ahead of print] AIDS Care. 2012;24(12):1519–1534. doi: 10.1080/09540121.2012.672722. [DOI] [PubMed] [Google Scholar]
- 41.Khan NA, Yun L, Humphries K, Kapral M. Antihypertensive drug use and adherence after stroke: are there sex differences? Stroke. 2010;41(7):1445–1449. doi: 10.1161/STROKEAHA.110.579375. [DOI] [PubMed] [Google Scholar]
- 42.Nau DP, Aikens JE, Pacholski AM. Effects of gender and depression on oral medication adherence in persons with type 2 diabetes mellitus. Gend Med. 2007;4(3):205–213. doi: 10.1016/s1550-8579(07)80041-6. [DOI] [PubMed] [Google Scholar]
- 43.Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009;75(11):1223–1229. doi: 10.1038/ki.2009.51. [DOI] [PubMed] [Google Scholar]
- 44.Bautista LE, Vera-Cala LM, Colombo C, Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens. 2012;25(4):505–511. doi: 10.1038/ajh.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner GJ, Goggin K, Remien RH, et al. MACH14 Investigators. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med. 2011;42(3):352–360. doi: 10.1007/s12160-011-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chisholm-Burns MA, Spivey CA, Wilks SE. Social support and immunosuppressant therapy adherence among adult renal transplant recipients. Clin Transplant. 2010;24(3):312–320. doi: 10.1111/j.1399-0012.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 48.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23(2):207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 49.Cohen M, Katz D, Baruch Y. Stress among the family caregivers of liver transplant recipients. Prog Transplant. 2007;17(1):48–53. doi: 10.1177/152692480701700107. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigue JR, Dimitri N, Reed A, Antonellis T, Hanto DW, Curry M. Quality of life and psychosocial functioning of spouse/partner caregivers before and after liver transplantation. Clin Transplant. 2011;25(2):239–247. doi: 10.1111/j.1399-0012.2010.01224.x. [DOI] [PubMed] [Google Scholar]


