Abstract
An extensive literature documents a close association between cigarette and alcohol use. The joint pharmacological effects of alcohol and nicotine on smoking and drinking motivation may help explain this relationship. This experiment was designed to test the separate and combined pharmacological effects of nicotine and a low dose of alcohol (equivalent to 1–2 standard drinks) on substance use motivation using a double-blind and fully-crossed within-subjects design. Participants (N = 87) with a wide range of smoking and drinking patterns completed four counter-balanced experimental sessions during which they consumed an alcohol (Male: 0.3 g/kg; Female: 0.27 g/kg) or placebo beverage and smoked a nicotine (.6 mg) or placebo cigarette. Outcome measures assessed the impact of drug administration (alcohol or nicotine) on craving to smoke, craving to drink, affect, and liking of the beverage and cigarette. Results indicated that combined administration produced higher cravings to smoke for the entire sample, as well as higher cravings to drink among women and lighter drinkers. Heavier users of either alcohol or cigarettes also exhibited enhanced sensitivity to the effects of either drug in isolation. Separate, but not interactive, effects of alcohol and nicotine on mood were observed, as well as both same-drug and cross-drug effects on beverage and cigarette liking. Together, these findings support the notion that the interactive pharmacological effects of nicotine and low-doses of alcohol play an important role in motivating contemporaneous use and suggest roles for cross-reinforcement and cross-tolerance in the development and maintenance of alcohol and nicotine use and dependence.
Keywords: Alcohol, Nicotine, Smoking, Craving, Affect, Reward, Dependence, Comorbidity
There is ample evidence for a close association between alcohol and nicotine. Use of both drugs is highly prevalent within the general population and tends to covary (Falk et al., 2006). Nearly one in five individuals with nicotine dependence also meets criteria for alcohol dependence, and conversely, half of individuals with alcohol dependence are dependent on nicotine (Grant, Hasin, Chou, Stinson, & Dawson, 2004). Indeed, the strength of this relationship has led to the proposal that smoking status be used as a clinical indicator to identify those at greatest risk for alcohol misuse (McKee et al., 2007). Adolescents who initiate use of either alcohol or nicotine are significantly more likely to initiate use of the other shortly thereafter (Jackson, Sher, Cooper, & Wood, 2002). The association persists even following a period of abstinence from one substance; lapses to smoking following cessation are frequently precipitated by alcohol use (Brandon, Tiffany, Obremski, & Baker, 1990; Shiffman et al., 1996) and continued smoking may increase the risk of alcohol relapse (Gulliver, Kamholz, & Helstrom, 2006; Prochaska, Delucchi, & Hall, 2004; but see also Grant et al., 2003).
Despite robust evidence supporting the association between alcohol and nicotine use, the underlying mechanisms remain unclear. Certain traits (e.g. impulsivity) may serve as risk factors for the development of either alcohol or nicotine dependence (Jackson, Sher, & Wood, 2000). Repeated administrations of either substance over time could lead to cross-tolerance, thus mitigating the aversive effects of the other substance, or cross-reinforcement, prolonging or enhancing the pleasure derived from the other substance (Lajtha & Sershen, 2010; Pomerleau, 1995b). Once established, associative conditioning processes may maintain dual use by increasing the strength and frequency of cravings through an expanded range of relevant craving-provoking stimuli (Drobes, 2002). However, contemporaneous use of alcohol and nicotine is common across a full range of smoking and drinking patterns (Piasecki et al., 2011; Shiffman et al., 2012), suggesting that situational co-use may be motivated by the interactive pharmacological effects of the substances themselves. Further, expectancies regarding these interactive effects may play a role in driving co-use (Kahler et al., 2012; McKee, Hinson, Rounsaville, & Petrelli, 2004; Monti, Rohsenow, Colby, & Abrams, 1995). Carefully controlled laboratory-based studies may be particularly useful for elucidating the specific role that pharmacological interactions play in motivating combined use of alcohol and nicotine.
Existing laboratory studies examining the effect of alcohol on smoking have examined outcome variables such as tonic smoking urge (King & Epstein, 2005), cue-elicited smoking urge (Burton & Tiffany, 1997), and smoking behavior (Glautier, Clements, White, Taylor, & Stolerman, 1996; King, McNamara, Conrad, & Cao, 2009; McKee, Krishnan-Sarin, Shi, Mase, & O'Malley, 2006). Alcohol appears to increase motivation to smoke in both heavy (Burton & Tiffany, 1997; Sayette, Martin, Wertz, Perrott, & Peters, 2005) and light (Epstein, Sher, Young, & King, 2007; Ray et al., 2007) smokers. Furthermore, these effects appear to be dose dependent, with larger quantities of alcohol producing more robust increases in smoking motivation (King & Epstein, 2005; Mitchell, de Wit, & Zacny, 1995). However, even relatively low doses (Target BAL = 0.03 g/dl) have been shown to increase smoking behavior (McKee, et al., 2006). This line of research has focused almost exclusively on effects during the ascending limb of the blood alcohol concentration (BAC) curve, where the stimulating effects of alcohol predominate (Rueger, McNamara & King, 2009). Also noteworthy is that the majority of these studies did not examine concurrent administration of alcohol and nicotine. Given that smoking and drinking frequently occur in close temporal proximity (Shiffman & Balabanis, 1995), studies that examine the combined administration of these substances may offer insight into how their pharmacological effects impact smoking and drinking motivation. Furthermore, little consideration has been paid to how these effects may differ as a function of dependence or usage patterns.
In a parallel line of research, studies have examined the effects of nicotine on drinking alcohol. Results from these studies have been less consistent, likely in part due to the relatively small sample sizes typically employed (N’s ranging from 12 to 34). One study found that among males who were moderate users of both substances, transdermal nicotine increased craving to drink and the subjective intoxicating effects of alcohol (Kouri, McCarthy, Faust, & Lukas, 2004). Another study of both male and female light-smoking social drinkers found that a nicotine patch pretreatment increased alcohol consumption in men, but decreased it in women (Acheson, Mahler, Chi, & de Wit, 2006). However, a third study examining these effects in heavier smokers and drinkers showed transdermal nicotine reduced alcohol self-administration in both males and females (McKee, O'Malley, Shi, Mase, & Krishnan-Sarin, 2008). This latter finding runs counter to earlier work suggesting that heavier users of both alcohol and nicotine should experience greater cross-drug facilitation of use (Mello, Mendelson, & Palmieri, 1987). However, administration of nicotine via cigarettes appears to enhance alcohol consumption, at least in male, non-dependent smokers (Barrett, Tichauer, Leyton, & Pihl, 2006).
A limited number of studies have employed fully-crossed designs to examine both the separate and combined effects of alcohol and nicotine, with a mixed pattern of results. For instance, the effects of these drugs on cardiovascular response (Benowitz, Jones, & Jacob, 1986; Perkins et al., 1995), as well as on attractiveness ratings of social and environmental stimuli (Attwood, Penton-Voak, Goodwin, & Munafo, 2012), appear to be additive rather than interactive. In another study, alcohol produced a rebound in craving to smoke after it had been reduced via nicotine gum, but did not affect cravings when administered prior to the gum (Mintz et al., 1991). Still other work reveals that the sedating effects of alcohol are reversed by nicotine (Perkins, 1997; Ralevski et al., 2012), and that alcohol potentiates the rewarding effects of nicotine (Rose et al., 2004), though not all findings on this topic have been consistent (e.g. Perkins, Fonte, Blakesley-Ball, Stolinski, & Wilson, 2005).
Although research on the pharmacological effects of alcohol and nicotine has provided some insight into potential reasons for contemporaneous use of these drugs, the vast majority of studies have employed relatively small, homogenous samples. Despite concerns about individual differences in the combined effects of alcohol and nicotine (Perkins, 1997), most studies have lacked adequate power or appropriate samples for examination of these factors. In addition, procedural differences across these studies (e.g. dosages employed, route of administration, etc.) hamper cross-study comparisons and limit the conclusions that can be drawn from existing work. Thus, the present study sought to examine the effects of nicotine and a low dose of alcohol (~1–2 standard drinks) on substance use motivation when administered via traditional routes (i.e. drinking and smoking) in close temporal proximity. Alcohol doses in this range reflect an amount that even a light drinker is likely to consume during a drinking episode. It is also consistent with “priming” alcohol doses typically administered in research settings to stimulate craving to drink among heavier drinkers and dependent populations (e.g. O’Malley et al., 2002). Thus, even among heavier drinkers unlikely to consume a small dose in isolation, doses of this magnitude may reflect the early portion of a drinking episode. Indeed, responses to substances during this critical window may play a principal role in motivating further alcohol consumption. Consistent with work described above, we examined these effects during the ascending portion of BAC curve. A fully-crossed and double-blind design was used to control for the influence of expectancies. Participants with a wide range of smoking and drinking patterns were deliberately recruited to facilitate examination of usage and dependence as moderators of experimental effects. A within-subjects design was used to ensure adequate power for both experimental and moderating effects and craving to smoke and drink served as the primary outcome measures. We hypothesized that the combination of alcohol and nicotine would produce higher levels of craving for both substances when compared with either drug administered alone.
Prominent theories of addiction also emphasize the importance of affective (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004) and hedonic (Robinson & Berridge, 2003) responses to substances in motivating use. Many individuals report using drugs to increase positive mood and to experience relief from stress or other negative mood states (see Kassel, 2009). Similarly, individuals may be motivated to use drugs because they like the effects they produce (Kelley & Berridge, 2002). Importantly, evidence suggests that liking may operate relatively independently from “wanting” or an expressed desire to use the substance (Hobbs, Remington & Glautier, 2005). Accordingly, we also examined drug effects on mood and liking of the beverage and cigarette but made no explicit hypotheses regarding these secondary outcomes. Finally, given the sparse literature on individual differences in the effects of alcohol and nicotine on craving, we examined whether dependence levels, usage patterns, and gender moderated these effects. Our goal was to gain both a better understanding of how the separate and combined pharmacological effects of these drugs may serve to motivate smoking and drinking behavior, and insight into which individuals are most likely to experience these effects. Understanding the role of cross-tolerance and/or cross-reinforcement in the development and maintenance of alcohol and nicotine dependence may inform both prevention and intervention efforts.
Methods
Participants
Individuals (n = 87) between the ages of 21 and 55 who reported current use of both alcohol and cigarettes were recruited from the local community. Efforts were made to recruit a broad range of alcohol and tobacco use patterns via targeted advertisements. This included individuals who were lighter users of both substances, heavier users of both substances, or lighter users of one substance and heavier users of the other. Thus, participants were included if they reported consuming between 1 and 50 drinks per week, smoked at least one cigarette ≥ 4 days per week, smoked regularly for ≥ 2 years with a stable smoking pattern for the past year, and were not currently using any other nicotine-containing products. Exclusion criteria included: visual or hearing impairments, current pregnancy, currently seeking treatment for alcohol or tobacco use, body weight ≥ 280 lbs. (to avoid provision of excessive weight-adjusted doses of alcohol), history of medical detoxification, current use of psychotropic medications, significant medical problems that would interfere with study procedures or significant elevations on liver enzyme tests. Participants were also excluded if they met criteria for current drug dependence, depressive episode, manic episode, panic disorder, or had any history of psychosis.
Design and Procedures
During an initial baseline session to determine eligibility, participants provided informed consent, completed a battery of questionnaires, received a medical exam that included urine-based drug and pregnancy testing, provided buccal cells for genetic analysis and an expired air carbon monoxide (CO) sample, and completed a structured diagnostic interview (SCID-IV; First, Spitzer, Gibbon, & Williams, 1996). A Timeline Followback (TLFB; Sobell & Sobell, 1995) interview was conducted to determine current level of alcohol use and the previous 28 days were used to compute baseline drinking statistics.
Eligible individuals were enrolled into a fully-crossed and counter-balanced 2 (active vs. placebo beverage) x 2 (active vs. placebo cigarette) within-subjects study. Participants were instructed to smoke one of their own cigarettes one hour prior to each of the four sessions and not to smoke again thereafter. They were also instructed to abstain from alcohol the day of the appointment, as well as to abstain from caffeine for two hours and food for four hours prior to each appointment. All sessions for each participant were scheduled for the same time of day (early or mid-afternoon) to control for potential diurnal variation in outcome measures.
Each experimental session began with collection of breath samples to confirm sobriety as indicated by a breath alcohol concentration (BrAC) of zero, and provide an initial quantification of CO level. An updated TLFB was then attained for the period since the previous session followed by a brief questionnaire battery that assessed their current craving and mood (pre-drug assessment). Subsequently, participants consumed a weight- and sex-adjusted dose of alcohol (Male: 0.3 g/kg; Female: 0.27 g/kg), or an equivalent volume of placebo. Given the increased difficulty inherent to blind administration in the context of a within-subjects design, a relatively low dose of alcohol was selected to minimize the intoxicating effects thereby aiding in maintaining the blind. Due to the different pharmacokinetic profiles of alcohol and nicotine, alcohol administration always occurred first to allow additional time for absorption. Active drinks consisted of vodka (80-proof) mixed with tonic water in a 1:5 ratio. Placebo drinks consisted of de-carbonated tonic water, stored in an empty vodka bottle, and regular tonic water mixed in a 1:5 ratio, with an additional 1 ml of vodka added to the top of the glass and wiped on the rim. Both active and placebo doses were split into two separate drinks and served on ice with a small amount of lime juice added to mask the taste. Participants had 10 minutes to consume both drinks (5 minutes/drink).
Next, participants smoked one cigarette according to a fixed-pace puffing regimen, such that a puff was taken every 25 seconds in response to an auditory signal until the entire cigarette was smoked. This procedure provided some standardization of nicotine delivery without substantially altering the behavioral pattern involved in naturalistic smoking. The FTC standard nicotine yield from each cigarette was either a moderate (.6 mg; Quest 1, Vector Tobacco Inc.) or a negligible dose (.05 mg; Quest 3) and the flavor (menthol vs. non-menthol) was matched to participants’ usual brand. It should be noted that cigarettes differed solely in nicotine content and that both cigarette types also contained the myriad other constituents present in commercially available cigarettes, some of which may also have reinforcing properties (e.g. Belluzzi, Wang & Leslie, 2005). Thus, this should be considered an active placebo condition. Both the participant and primary experimenter were blind to condition (i.e. drinks and cigarettes were prepared by a second experimenter who was also responsible for obtaining breath readings). Participants were told that the beverages and cigarettes would contain different amounts of alcohol and nicotine across sessions, but were not explicitly informed of placebo conditions (Martin & Sayette, 1993). Combined with efforts to mask the taste described above, this allows for examination of pharmacological effects relative to placebo (or expectancy) effects.
Following smoking, participants completed a second brief battery of questionnaires (post-drug assessment) along with single-item ratings of how much they liked the beverage and cigarette. A second breath CO and alcohol reading was then obtained. After this, participants completed a cue reactivity task (to be described in a subsequent report). Participants were required to remain in the lab for at least one additional hour or until their BrAC level was below 0.02 (% by vol.), whichever was longer, before being transported home by a taxi or companion. All procedures were approved by the University of South Florida Institutional Review Board.
Baseline Session Measures
In addition to the baseline interviews described above, participants provided basic demographic and medical history information to confirm that eligibility criteria were met. Participants also completed the following measures to assess their substance use characteristics and symptoms of depression and anxiety. Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND; α = 0.66; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). A detailed smoking history was also obtained, including age at initiation, and number of previous quit attempts. The full 25-item version of the revised Alcohol Dependence Scale (ADS; Skinner & Allen, 1982) was administered to assess the level of alcohol dependence. However, given the original measure was developed for treatment-seeking alcoholics and the present sample included a range of drinking patterns, this measure was scored according to the reduced 9-item version developed for community samples (α = 0.65; Kahler, Strong, Stuart, Moore, & Ramsey, 2003). Finally, although participants who met formal diagnostic criteria were excluded, the Beck Anxiety Inventory (BAI; α = 0.91; Beck & Steer, 1993) and the Beck Depression Inventory - II (BDI; α = 0.90; Beck, Steer, & Brown, 1996) were used to characterize sub-threshold levels of anxiety and depression.
Experimental Session Measures
At both time points described above (pre- and post-drug), participants completed questionnaires assessing craving to smoke (Questionnaire on Smoking Urges – Brief; QSU-B; α = 0.94; Cox, Tiffany, & Christen, 2001) and craving to drink (Alcohol Urge Questionnaire; AUQ; α = 0.93; Bohn, Krahn, & Staehler, 1995). Both the QSU-B (10 items) and the AUQ (8 items) measures utilize 7-point Likert response scales. Positive (α = 0.95) and negative (α = 0.84) affect were also assessed at each time point using the Mood Form (Diener & Emmons, 1984), which consists of four positive affect and five negative affect items also rated on a 7-point response scale. Hedonic response was assessed at post-drug only using ratings of “liking” the beverage and cigarette on a 5-point Likert-type scale (ranging from “Disliked a lot” to “Liked a lot”). An additional pair of questions was included to determine if participants were able to detect differences between active and placebo drugs. Due to the within-subjects design and the fact that participants were not informed of the existence of a true placebo condition, we were unable to directly ask if they believed they received an active or placebo dose. Instead, participants provided ratings (5-point Likert scale ranging from “very different” to “very similar”) of how similar the beverage was to a drink they would normally have and how similar the cigarette was compared to their usual brand. Both the liking and similarity questions were added during the course of the study and thus were not completed by the first 13 participants. Analyses involving these measures are thus restricted to the 74 participants who completed them.
Data Analysis
The focus of the present paper is on characterizing the pharmacological effects of alcohol and nicotine on craving and other motivational variables, as well as determining if these effects vary as a function of individual differences in substance use and dependence. Analyses were conducted using a Generalized Estimating Equations (GEE; Zeger, Liang, & Albert, 1988) framework to accommodate the within-subjects design. An exchangeable working correlation matrix was specified, along with robust estimation of standard errors. Separate models were run examining each outcome measure at post-drug, controlling for pre-drug values when relevant. To prevent artifacts from arising due to the nature of the crossed design the effects of alcohol and nicotine on craving, affect, liking and perceived similarity were derived from separate models wherein the average of the two sessions in which participants received the drug of interest was contrasted with the session where both the beverages and cigarette received were placebos. This was done due to the potentially opposing effects of the drugs on subjective indices (e.g. an illusory effect created for alcohol on craving to smoke due to collapsing the double-placebo session together with nicotine-only session as the control condition). Such an approach differs from the main effect obtained by traditional ANOVA-based analyses, in which the comparison condition is produced by averaging both sessions in which the participant received a placebo for the drug of interest. These analyses were followed up with contrasts comparing results of individual sessions to the double-placebo condition. Additional models were used to test dependence, usage (i.e. cigarettes per day, drinks per week) and gender as moderators of drug effects on craving.
Results
Preliminary Analyses
Sample characteristics are presented in Table 1. Of our sample, 23% of individuals smoked fewer than 10 cigarettes per day, 44.8% smoked 10–19 cigarettes per day, and 32.2% smoked 20–40 cigarettes per day. For drinking behavior, 35.0% of the sample consumed fewer than 10 drinks per week, 38.5% consumed 10–19 drinks per week, and 35.7% consumed 20–50 drinks per week. A chi-square analysis comparing the above categories was not significant, Χ2 (4) = 1.70, p = .792, suggesting that smoking and drinking pattern varied independently. In addition, Table 2 displays Pearson correlation coefficients among continuous measures of substance use and dependence. As shown, the correlation between alcohol and nicotine dependence was not significant, nor was the correlation between cigarettes per day and drinks per week. Together, these results indicate success in recruiting a sample with a broad range of smoking and drinking patterns.
Table 1.
Sample Characteristics
| Variable | Range |
Overall (n = 87) |
Male (n = 58) |
Female (n = 29) |
|---|---|---|---|---|
| Demographic Variables | ||||
| Age | 21–54 | 29.4 (9.2) | 29.2 (8.7) | 29.9 (10.2) |
| Years of Education | 2–20 | 14.1 (3.2) | 13.8 (3.6) | 14.8 (1.9) |
| Race* | ||||
| Asian | --- | 10.3% | 13.8% | 3.4% |
| Black or African American | --- | 16.1% | 20.7% | 6.9% |
| White | --- | 73.6% | 66.7% | 89.7% |
| Ethnicity (% Hispanic) | --- | 12.6% | 10.3% | 17.2% |
| Income (% ≤ $29,999) | --- | 57.0% | 56.1% | 58.6% |
| Smoking Variables | ||||
| Fagerström Test for Nicotine Dependence | 0–9 | 3.5 (2.2) | 3.6 (2.2) | 3.2 (2.2) |
| Cigarettes Per Day | 2–40 | 14.5 (8.0) | 14.8 (7.5) | 13.9 (9.1) |
| Number of Previous Quit Attempts | 0–15 | 1.8 (2.4) | 2.1 (2.7) | 1.3 (1.6) |
| Baseline CO | 1–57 | 16.9 (11.7) | 16.5 (10.9) | 17.8 (13.4) |
| % Menthol Smokers | --- | 42.5% | 39.7% | 48.3% |
| Drinking Variables | ||||
| Alcohol Dependence Scale (9-item) | 0–9 | 4.0 (2.2) | 4.1 (2.4) | 3.9 (2.0) |
| Drinks Per Week | 1–48 | 16.8 (12.1) | 18.6 (12.7) | 13.4 (10.1) |
| Drinks per Drinking Day | 1–13.5 | 5.0 (2.5) | 5.3 (2.7) | 4.2 (1.8) |
| % Drinking Days | 7–100 | 49.0 (28.4) | 51.7 (28.8) | 43.6 (27.1) |
| Beverage Preference | ||||
| Beer | --- | 50.6% | 53.4% | 44.8% |
| Liquor | --- | 40.2% | 41.4% | 37.9% |
| Wine | --- | 9.2% | 5.2% | 17.2% |
| Affective Variables | ||||
| Beck Anxiety Inventory - Total* | 0–22 | 4.4 (4.7) | 3.5 (4.1) | 6.1 (5.5) |
| Beck Depression Inventory - Total | 0–34 | 7.6 (7.6) | 7.1 (7.8) | 8.7 (7.1) |
Note.
p < .05.
Ranges presented are for the sample. The Fagerström Test for Nicotine Dependence is scored from 0–10, whereas Alcohol Dependence Scale (9-item) is scored from 0–9. For both indices, higher scores indicate greater dependence. Variables presented as either mean (SD) or percentage. Male and female participants were compared using ANOVA for continuous variables and chi-square tests for categorical variables.
Table 2.
Associations Among Dependence and Usage
| Variable | ||||
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |
| 1. ADS-9 Item | 1.00 | --- | --- | --- |
| 2. DPW | .233* | 1.00 | --- | --- |
| 3. FTND | .020 | .148 | 1.00 | --- |
| 4. CPD | .016 | .096 | .721*** | 1.00 |
Note. ADS = Alcohol Dependence Scale (9-item version), CPD = Cigarettes Per Day, DPW = Drinks Per Week, FTND = Fagerström Test for Nicotine Dependence.
p < .05;
p < .01;
p < .001.
Although male and female participants did not differ on substance use characteristics, they did differ in both racial identity and anxiety level. However, results for analyses involving gender did not differ when these variables were included as covariates. Most outcome variables followed roughly normal distributions and thus were modeled with a linear (normal) distribution specified and an identity link function. However, both alcohol craving and negative affect exhibited markedly positive skews, so they were modeled with gamma distributions specified and log link functions. Means, standard deviations, and scale ranges for all outcome measures are presented in Table 3. Compliance with instructions to smoke within an hour of the experimental session was high. In 85% of sessions, participants reported smoking between 30 and 90 minutes of when their experimental session began, and pre-session CO levels did not differ as a function of session type. Furthermore, time since last cigarette did not moderate any of the experimental effects described below. One participant failed to complete their final (alcohol-only) session. Two additional alcohol-only sessions, and one nicotine-only session were excluded due to dosing errors. Data from remaining sessions for each of these participants was retained, as GEE allows for the use of partial data to inform parameter estimates.
Table 3.
Means (SDs) of outcome variables across time points as a function of session
| Neither Drug | Alcohol Only | Nicotine Only | Both Drugs | |||||
|---|---|---|---|---|---|---|---|---|
| Variable (Scale Range) |
Pre-Drug | Post-Drug | Pre-Drug | Post-Drug | Pre-Drug | Post-Drug | Pre-Drug | Post-Drug |
| Expired Air Carbon Monoxide |
16.8 (10.7) | 22.5 (11.3) | 16.2 (10.4) | 22.5 (10.2) | 16.4 (9.5) | 23.5 (10.6) | 15.8 (8.8) | 22.9 (9.9) |
| Breath Alcohol Content |
--- | 0.000 (0.001) | --- | 0.033 (0.012) | --- | 0.000 (0.001) | --- | 0.029 (0.012) |
| Craving to Smoke (10–70) |
35.2 (15.7) | 31.3 (15.7) | 35.9 (14.6) | 32.2 (15.3) | 37.2 (14.8) | 28.5 (14.5) | 36.1 (15.2) | 32.0 (16.3) |
| Craving to Drink (8–56) |
20.6 (11.9) | 21.2 (12.2) | 21.1 (11.5) | 21.4 (12.5) | 21.3 (12.9) | 23.2 (12.2) | 20.5 (11.7) | 23.5 (13.1) |
| Positive Mood (0–24) |
11.0 (6.4) | 10.7 (6.6) | 11.1 (6.1) | 11.1 (6.0) | 11.0 (6.5) | 11.5 (6.7) | 10.6 (6.4) | 11.6 (6.5) |
| Negative Mood (0–30) |
2.3 (4.0) | 1.8 (3.3) | 2.4 (3.4) | 1.6 (2.8) | 2.7 (4.4) | 1.4 (2.5) | 2.7 (5.1) | 1.4 (3.1) |
| Cigarette Liking (1–5) |
--- | 2.5 (1.3) | --- | 2.8 (1.3) | --- | 3.2 (1.3) | --- | 3.4 (1.3) |
| Beverage Liking (1–5) |
--- | 2.8 (1.4) | --- | 3.1 (1.3) | --- | 3.2 (1.2) | --- | 3.3 (1.3) |
| Cigarette Similarity (1–5) |
--- | 1.7 (1.0) | --- | 2.0 (1.3) | --- | 2.4 (1.5) | --- | 2.4 (1.4) |
| Beverage Similarity (1–5) |
--- | 1.9 (1.3) | --- | 2.0 (1.3) | --- | 1.8 (1.1) | --- | 2.0 (1.3) |
Biological Indices
Carbon Monoxide
Pre-drug CO did not differ across conditions. Collapsed across all sessions, CO levels increased significantly from pre-drug to post-drug, B = 6.53, 95% CI [5.76, 7.30], p < .001. Traditional main effect analyses indicated nicotine cigarettes produced larger increases in CO than placebo cigarettes, B = 1.01, 95% CI [0.36, 1.67], p = .002. Alcohol did not affect post-drug CO level, nor was there evidence of an alcohol x nicotine interaction.
Breath Alcohol Content
During alcohol sessions, participants had an average BrAC of 0.031 g/210L at the post-drug time point. Given the lack of variability in BrAC inherent to placebo beverage sessions, BrAC data from these sessions was not analyzed. However, the alcohol-only session was contrasted with the session where alcohol and nicotine were co-administered. BrAC was significantly lower in the combined drug session relative to the alcohol-only session at post-drug, B = −0.004, 95% CI [−0.006, −0.002], p < .001.
Craving, Affect, and Liking
Craving to Smoke
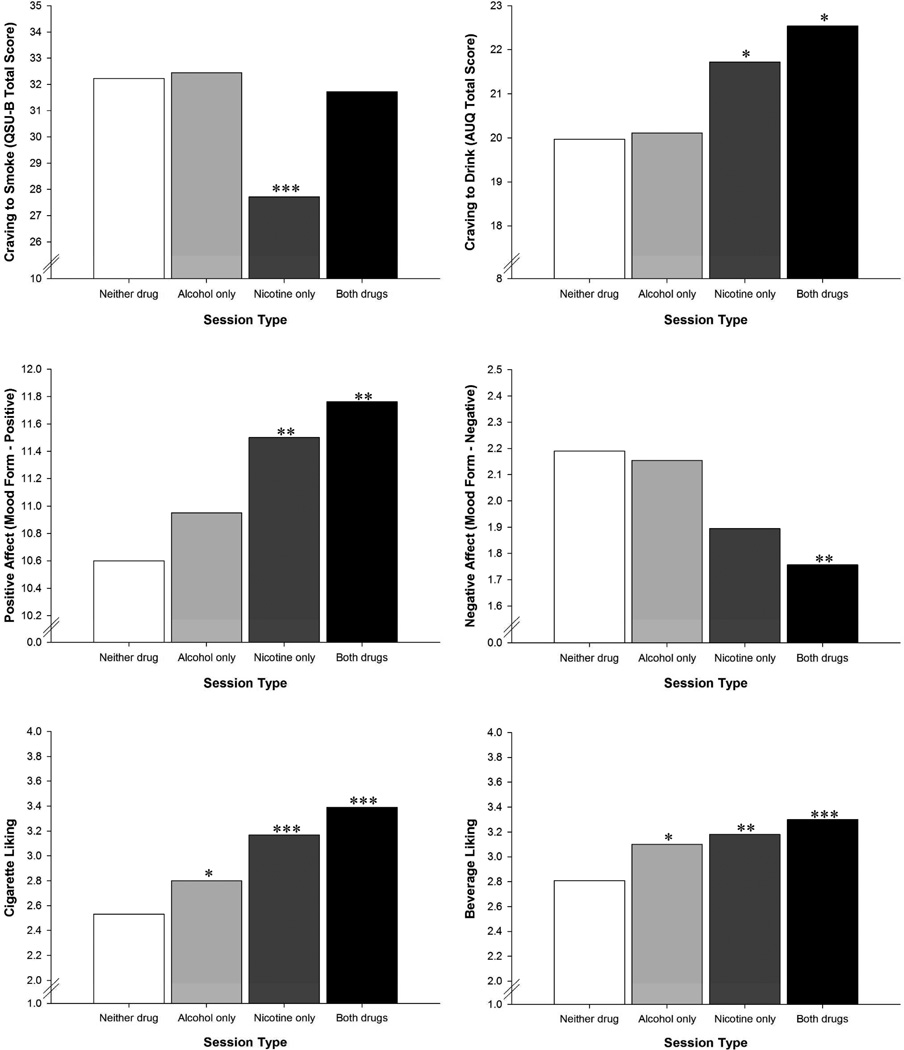
Collapsing across sessions where nicotine cigarettes were administered indicated nicotine produced a greater reduction in craving to smoke relative to the double-placebo session, B = −2.40, 95% CI [−4.71, −0.08], p = .043. However, this effect was clearly driven by the nicotine-only session, B = −4.31, 95% CI [−6.73, −1.90], p < .001 as the combined drug session did not differ from the double-placebo condition. Although there was no evidence of an alcohol effect on craving to smoke across the entire sample, there was a significant alcohol x nicotine interaction, B = 3.78, 95% CI [0.24, 7.32], p = .036, indicating that alcohol blocked the reduction in craving to smoke from nicotine administration. See Figure 1 for graphical depictions of drug effects on craving, affect and liking.
Figure 1.
Estimated marginal means for post-drug craving, affect and liking as a function of session type. Asterisks indicate results of contrasts with the double-placebo condition. * p < .05, ** p < .01, *** p < .001.
Craving to Drink
As above, collapsing across both sessions where nicotine cigarettes were administered indicated that nicotine significantly increased craving to drink relative to the double-placebo condition, B = 0.10, 95% CI [0.03, 0.18], p = .006. In contrast, when alcohol sessions were combined, there was only a trend-level difference from the double-placebo condition (B = 0.07, 95% CI [−0.01, 0.14], p = .068). This effect appeared to be driven by the combined drug session, which significantly differed from the double-placebo condition, B = 0.13, 95% CI [0.03, 0.22], p = .009. However, the alcohol x nicotine interaction term failed to reach significance. Craving to drink was significantly higher following administration of nicotine alone (B = 0.09, 95% CI [0.0, 0.17], p = .043), but not alcohol alone. Thus, the effects appear to be additive rather than interactive.
Affect
When both active conditions were compared to the double-placebo condition, results indicated that both nicotine (B = 1.04, 95% CI [0.43, 1.65], p = .001) and alcohol (B = 0.77, 95% CI [0.16, 1.38], p = .014) increased positive affect. As with craving to drink, the highest level of positive affect occurred at the combined drug session (B = 1.17, 95% CI [0.44, 1.90], p = .002), followed by the nicotine-only session (B = 0.93, 95% CI [0.26, 1.61], p = .006), with no significant effect of alcohol alone. Coupled with a non-significant interaction term, results again suggest additive rather than interactive effects.
For negative affect, collapsing both sessions where nicotine was administered indicated that nicotine produced a significant reduction in negative affect relative to the double-placebo condition (B = −0.18, 95% CI [−0.31, −0.06], p = .003). This effect was driven primarily by the combined drug session (B = −0.22, 95% CI [−0.35, −0.08], p = .001), as the nicotine-only session only differed from the double-placebo condition at the trend level (B = −0.15 95% CI [−0.31, 0.01], p = .059). Even when collapsing together combined drug session with the alcohol-only session there was no evidence of alcohol effects, nor was there an alcohol x nicotine interaction.
Beverage and Cigarette Liking
Collapsing active conditions revealed that cigarette liking was enhanced by both nicotine (B = 0.76, 95% CI [0.49, 1.03], p < .001) and alcohol (B = 0.58, 95% CI [0.36, 0.80], p < .001) relative to the double-placebo condition. The nicotine-only (B = 0.66, 95% CI [0.35, 0.97], p < .001), alcohol-only (B = 0.27, 95% CI [0.03, 0.50], p = .026) and combined drug sessions (B = 0.86, 95% CI [0.58, 1.15], p < .001) all differed significantly from the double-placebo condition. However, the interaction term was not significant, again indicating that effects of alcohol and nicotine were additive rather than interactive.
Similar results were obtained for beverage liking, which was also enhanced by both nicotine (B = 0.43, 95% CI [0.24, 0.61], p < .001) and alcohol (B = 0.39, 95% CI [0.18, 0.60], p < .001) when active conditions were collapsed and compared to the double-placebo condition. Again, the nicotine-only (B = 0.37, 95% CI [0.14, 0.61], p = .002), alcohol-only (B = 0.29, 95% CI [0.03, 0.55], p = .030) and combined drug sessions (B = 0.49, 95% CI [0.26, .71], p < .001) all differed significantly from the double-placebo condition. Once again, no interaction term was observed, indicating effects were additive.
Blind Efficacy
Traditional main effects analyses indicated perceived similarity of the beverage to one’s usual drink was not affected by alcohol, nicotine, or their interaction. Cigarettes were perceived as more similar when they contained nicotine (B = 0.71, 95% CI [0.42, 1.01], p < .001) or were accompanied by alcohol (B = 0.51, 95% CI [0.27, 0.75], p < .001). No alcohol x nicotine interaction was observed for this latter measure. Session order did not moderate any of these effects, indicating experiences in earlier sessions did not enhance participant’s ability to detect placebo conditions. When analyses examining the drug effects on craving, affect and liking reported above were repeated controlling for perceived similarity of the cigarette, most results were unaffected. The exception was the effect of nicotine on craving to smoke, which was no longer significant (p = .367). The alcohol x nicotine interaction for craving to smoke was reduced to trend-level significance (B = 3.71, 95% CI[−0.18, 7.60], p = .062).
Individual Differences in Drug Effects on Craving
All moderator analyses for both craving to smoke and craving to drink are presented in Table 4. We tested each potential moderator (gender, dependence, and usage) for interaction with the collapsed versions of the alcohol and nicotine conditions, as well as the three-way moderator x alcohol x nicotine interaction. Continuous versions of dependence and usage variables were used in all statistical analyses, but these variables were dichotomized based on median splits to illustrate effects within figures.
Table 4.
Moderators of Alcohol and Nicotine Effects on Craving to Smoke and Drink
| Craving to Smoke | Craving to Drink | ||||||
|---|---|---|---|---|---|---|---|
| B | 95% CI | p-value | B | 95% CI | p-value | ||
| Nicotine | Gender | 3.63 | [−1.00, 8.25] | .125 | 2.01 | [−14.85, 18.86] | .816 |
| FTND | [−0.28 | [−1.32, 0.75] | .591 | 5.72 | [−8.18, 19.62] | .405 | |
| ADS | [−0.10 | [−0.99, 0.80] | .832 | 2.60 | [−0.01, 5.22] | .051 | |
| CPD | [−0.31 | [−0.58, −0.03] | .028 | 0.31 | [−0.66, 1.28] | .525 | |
| DPW | 0.09 | [−0.13, 0.30] | .430 | 0.75 | [0.31, 1.19] | .001 | |
| Alcohol | Gender | 2.93 | [−1.73, 7.59] | .218 | [−1.72 | [−19.96, 16.49] | .854 |
| FTND | [−0.29 | [−1.29, 0.71] | .568 | 1.42 | [−1.26, 4.11] | .299 | |
| ADS | [−0.27 | [−1.23, 0.69] | .580 | 3.38 | [0.50, 6.26] | .021 | |
| CPD | [−0.27 | [−0.52, −0.02] | .038 | 0.51 | [−0.30, 1.33] | .217 | |
| DPW | 0.00 | [−0.23, 0.23] | .995 | 0.79 | [0.33, 1.25] | .001 | |
|
Alcohol x Nicotine |
Gender | [−1.30 | [−8.78, 6.19] | .734 | 24.34 | [6.24, 42.43] | .008 |
| FTND | [−0.30 | [−2.06, 1.50] | .738 | [−3.20 | [−8.01, 1.62] | .193 | |
| ADS | 0.46 | [−1.04, 1.97] | .547 | 1.01 | [−3.74, 5.76] | .678 | |
| CPD | [−0.10 | [−0.62, 0.43] | .720 | [−0.24 | [−1.81, 1.34] | .769 | |
| DPW | [−0.12 | [−0.40, 0.16] | .392 | [−1.27 | [−2.01, −0.54] | .001 | |
Note. Significant effects (p < .05) are bolded. ADS = Alcohol Dependence Scale (9-item version), CPD = Cigarettes Per Day, DPW = Drinks Per Week, FTND = Fagerström Test for Nicotine Dependence. Male served as the reference group for the gender parameter. Due to log scaling, parameter estimates and CIs for craving to drink have been multiplied by 100 for purposes of presentation.
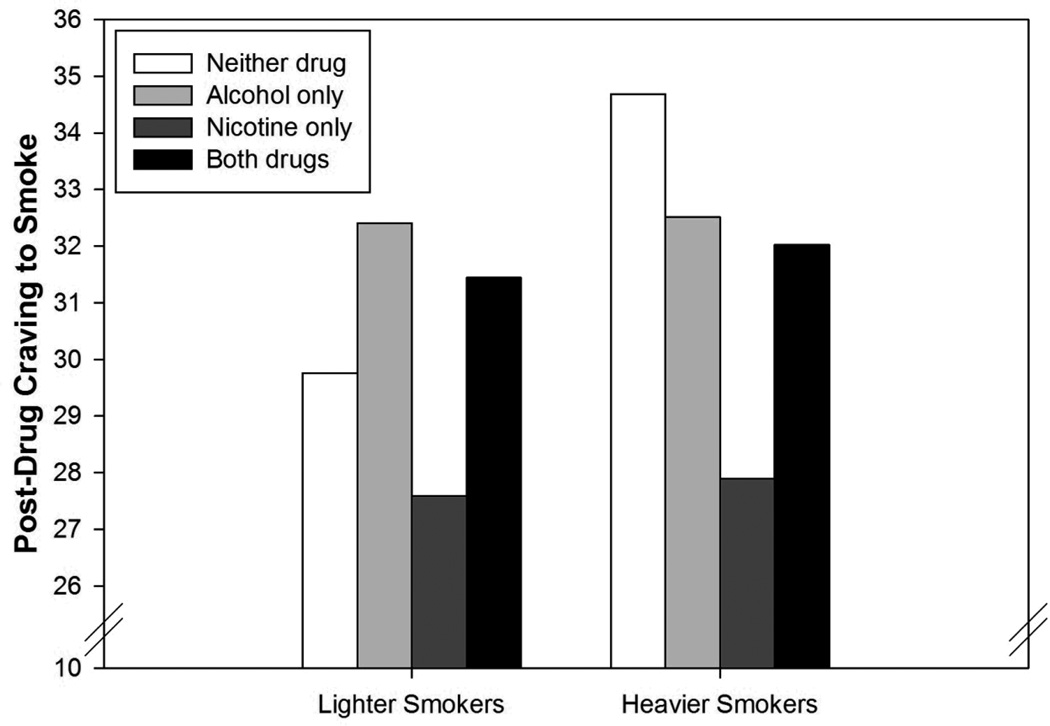
Craving to Smoke
Cigarettes per day moderated the effects of both alcohol and nicotine, but not the interaction term. As depicted in Figure 2, lighter smokers had relatively low post-drug craving to smoke (controlling for pre-drug craving) when both the beverage and cigarette were placebo, whereas heavier smokers had higher post-drug craving in this condition. Nicotine produced a greater reduction in craving to smoke among heavier smokers. However, this effect was not specific to nicotine - alcohol also reduced craving to smoke among heavier smokers.
Figure 2.
Estimated marginal means for post-drug craving to smoke as a function of session type and cigarettes per day. Cigarettes per day groups were created using median splits for the purpose of presentation (statistical analyses treated cigarettes per day as continuous). Lighter smokers consumed <15 cigarettes per day whereas heavier smokers consumed ≥ 15 cigarettes per day.
Craving to Drink
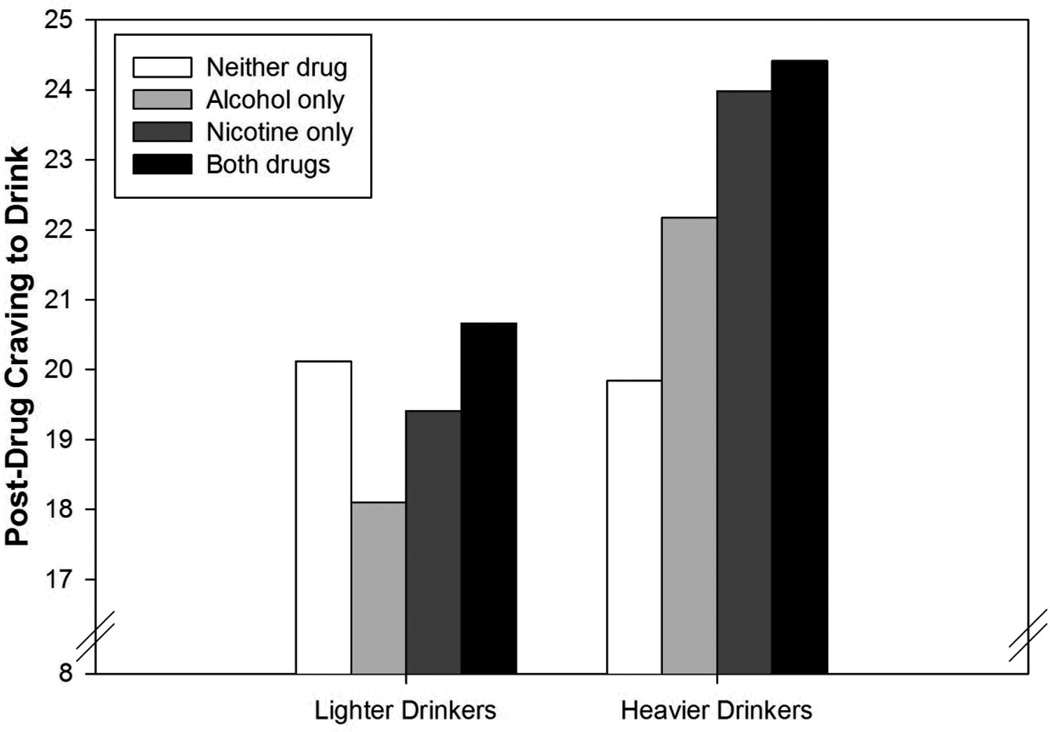
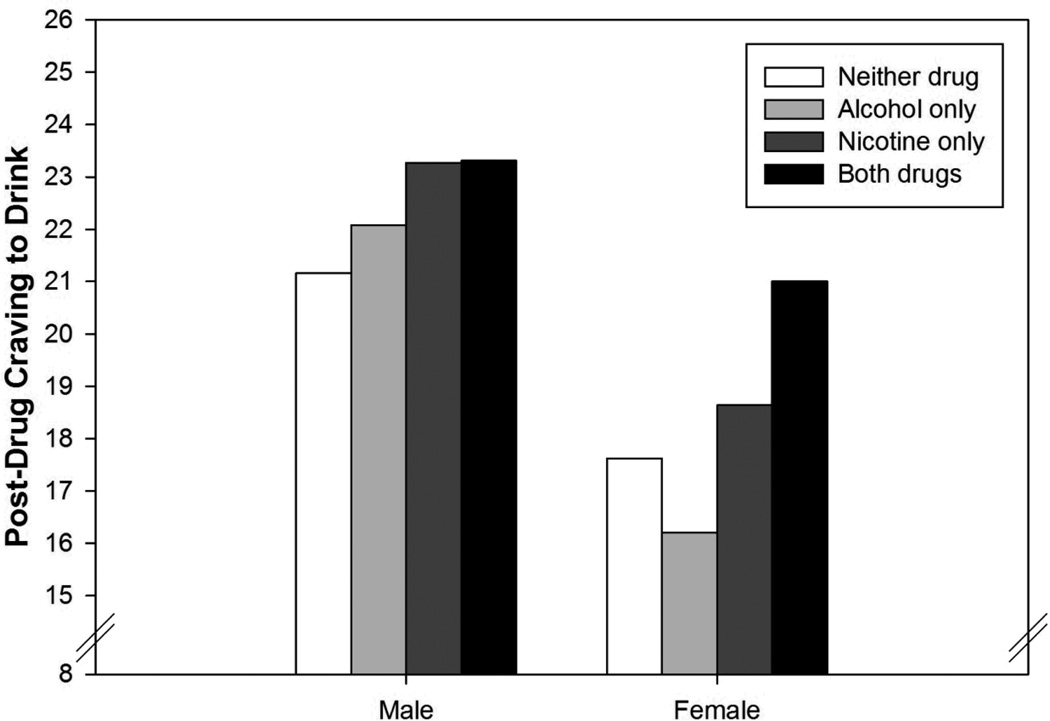
The effects of alcohol, nicotine, and co-administration on craving to drink were all moderated by drinks per week. As seen in Figure 3, lighter drinkers had lower craving to drink following administration of alcohol or nicotine relative to the session where neither drug was administered (i.e. both beverage and cigarette were placebo), whereas heavier drinkers had greater cravings following administration of either drug. Co-administration produced the highest levels of craving to drink in both groups, but the interaction term was driven by lighter drinkers, for whom co-administration reversed the reduction in craving exhibited at alcohol-only and nicotine-only sessions relative to the double-placebo condition. In contrast, heavier drinkers exhibited heightened craving during both single-drug sessions as well as co-administration. Alcohol dependence had a similar influence on the effects of alcohol and nicotine (but not their interaction). However, it did not retain significance when combined into a model with drinks per week, suggesting it did not account for any unique variance. Gender also moderated the alcohol x nicotine interaction (Figure 4). Whereas alcohol alone did not significantly impact craving to drink in either group, among women craving to drink was significantly higher when alcohol and nicotine were co-administered.
Figure 3.
Estimated marginal means for post-drug craving to drink as a function of session type and drinks per week. Drinks per week categories were created using median splits for the purpose of presentation (statistical analyses treated drinks per week as continuous). Lighter drinkers consumed ≤ 13 drinks per week whereas heavier drinkers consumed > 13 drinks per week.
Figure 4.
Estimated marginal means for post-drug craving to drink as a function of session type and gender.
Discussion
The major finding of this study is that alcohol and nicotine have interactive effects that may serve to motivate further substance use, at least during the ascending portion of the blood alcohol curve. The use of a control condition that included both placebo beverages and placebo cigarettes allows these findings to be attributed to the pharmacological properties of alcohol and nicotine rather than placebo/expectancy effects. Co-administration of a low dose of alcohol was sufficient to block the satiating effects of nicotine on craving to smoke relative to placebo controls. Importantly, this effect was not dependent on smoking rate, suggesting it may account for the high levels of tobacco use observed during drinking episodes even among comparatively lighter smokers (Shiffman, Kirchner, Ferguson, & Scharf, 2009; Shiffman & Paty, 2006). Although the impact of alcohol on the likelihood of initial smoking lapse is well-documented (e.g. Brandon, et al., 1990; Kahler, Spillane, & Metrik, 2010; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996), less consideration has been paid to how alcohol may affect the consequences of that initial lapse. If cravings to smoke are heightened rather than satiated when cigarettes are accompanied by alcohol, progression to relapse may be more likely. The effects of nicotine alone on craving to smoke were partially explained by higher perceived similarity of the nicotine cigarettes to one’s usual brand, but the interactive effects of alcohol and nicotine were relatively unaffected. The absence of a separate effect of alcohol relative to a placebo beverage may have been due to the relatively low dose of alcohol used or the relatively brief period of pre-session smoking abstinence compared to some prior research (e.g. Sayette et al., 2005), an issue that may be particularly relevant for heavier smokers.
Alcohol and nicotine also had interactive effects on craving to drink, but in contrast to results for craving to smoke, these effects differed as a function of individual difference variables (i.e. gender and drinks per week). Men were relatively insensitive to drug effects overall, perhaps due to ceiling effects. Among women, alcohol alone did not produce the expected priming effect on craving to drink, but combined alcohol and nicotine resulted in higher craving compared with all other conditions. A similar effect was observed in lighter drinkers, with co-administration resulting in greater craving to drink than administration of either substance in isolation. These effects may help explain the high rate of binge drinking among smokers (Wechsler, Dowdall, Davenport, & Castillo, 1995). Heavy drinkers are already at heightened risk for experiencing negative alcohol-related consequences (Bobak et al., 2004), but even light drinkers may experience these consequences in situations where alcohol is combined with nicotine. Similarly, research has shown that women generally experience fewer alcohol-related consequences (e.g. family or occupational problems; Wilsnack et al., 2000) than do men. Female smokers may represent a subgroup at significantly higher risk of these consequences. To our knowledge, there have been no systematic investigations of whether smoking status moderates the relationship between gender and alcohol-related consequences.
For both craving to smoke and drink, the separate effects of alcohol and nicotine were moderated by smoking rate (craving to smoke) and drinking rate (craving to drink). Possibly due to greater nicotine withdrawal, heavier smokers had significantly higher ratings of craving to smoke following administration of a placebo beverage and cigarette. However, administration of either alcohol or nicotine reduced these cravings to smoke among the heavier smokers. Similarly, heavier drinkers had higher craving to drink following administration of either alcohol or nicotine. That is, heavier users of one drug were more susceptible to the motivational influences of either drug. Consistent with a cross-tolerance/cross-sensitization framework, this finding suggests heavy use of one drug may be characterized by an increased sensitivity to multiple drugs, or perhaps a more generalized sensitivity to pharmacological influences or other interoceptive cues. Determining whether this generalized sensitivity results from heavy use or is a risk factor for developing heavy use remains an important topic for further study. Prospective studies may prove particularly informative in this regard. Given the extensive research efforts aimed at understanding individual differences in drug sensitivity and how this may relate to progression to heavy use (Pollock, 1992; Pomerleau, 1995a), it seems critical to determine if these individual differences are drug-specific or reflective of more generalized processes (e.g. propensity to experience nausea in general rather than in response to a specific drug). Such research could inform our understanding of co-occurring substance use disorders and possibly other co-morbidities in the event processes that cut across disorders are identified (e.g. susceptibility to internal physical cues could be relevant to both substance use and anxiety).
Both alcohol and nicotine independently produced elevations in positive affect, whereas only nicotine decreased negative affect. These findings are consistent with existing models of addiction that emphasize the important role that affective consequences may play in motivating drug use behavior (Cooper, Frone, Russell, & Mudar, 1995; Watkins, Koob, & Markou, 2000). The absence of an effect of alcohol on negative affect may indicate higher levels of physiological dependence on nicotine than alcohol in the present sample or be due to the relatively low dose of alcohol administered. Same-drug and cross-drug effects on liking suggests generalized enhancement of hedonic reward may also play a role in motivating dual use. For instance, both alcohol and nicotine have been shown to enhance neural reward signals (Koob et al., 1998; Rice & Cragg, 2004), which may in turn enhance responses to co-administered drugs. Perhaps surprisingly, cross-drug effects were also detected in biological indices of substance use. BrAC levels were lower when alcohol consumption was accompanied by a nicotine cigarette (relative to a placebo cigarette) despite carefully controlled dosing procedures. Prior research (Kouri et al., 2004) has provided some evidence that nicotine administration alters the pharmacokinetic profile of alcohol, but with an effect in the opposite direction of what was observed in the present study (i.e. nicotine decreased time to peak blood alcohol level, which would theoretically lead to higher BrAC when assessed during the ascending limb). In contrast, chronic smoking has been associated with lower blood alcohol levels following an alcohol-challenge task (Madden et al., 1995), though this effect was only observed during the descending limb. Evidence from animal models suggests nicotine decreases blood alcohol levels via delayed gastric emptying (Parnell, West & Chen, 2006), but to our knowledge, the present report is the first time acute administration of nicotine has been shown to produce similar effects in humans.
Study results should be interpreted in the context of several limitations. Perhaps most notably, only a single dose of alcohol and nicotine were used. The alcohol dose in particular was somewhat lower than that used in some (Burton & Tiffany, 1997; Epstein, et al., 2007), but not all (McKee, et al., 2006) previous research. Future research may wish to examine the interactive effects of nicotine with larger doses of alcohol, or across a dose-response curve. In addition, nicotine doses could not be adjusted for weight and gender, which may account for some of the observed gender differences. For instance, men might also have experienced a significant increase in craving to drink during co-administration if larger doses of nicotine had been used. Furthermore, the cigarettes used contained the wide number of constituents present in commercial cigarettes. While this enhances the external validity of the findings, little is known about the psychoactive properties of many of these other constituents and thus the conclusions should not be assumed to extend to contexts wherein alcohol and nicotine are administered in the absence of these other constituents. Alcohol administration always preceded nicotine administration in the present study and results may differ with different orders of administration. In order to allow for meaningful separation of the moderating influences of alcohol and nicotine use/dependence, we specifically recruited a subset of individuals with discordant patterns in these variables. Given a central rationale for the study is that use/dependence is correlated, recruitment of individuals with these discordant patterns of use may limit generalizability. However, we note that while correlations in patterns of alcohol/nicotine use and dependence are high, they are far from perfect. We were able to achieve this recruitment goal with only sporadic use of targeted advertisements, suggesting such individuals represent a meaningful portion of dual substance users. Only the response immediately following administration of both drugs was examined in the present report. It is critical that future research examine effects across a range of time points, with particular attention paid to the descending limb of the blood alcohol concentration curve. The effects of nicotine on cognition (see Evans & Drobes, 2009) may play a particularly critical role during the descending limb, when the sedating effects of alcohol are more prominent. Investigations that more closely examine the temporal dynamics of dual administration procedures through the use of continuous measures of smoking and drinking motivation throughout administration and/or systematic manipulation of the order of administration may also prove fruitful.
Overall, these results support the notion that alcohol and nicotine have interactive pharmacological effects that serve to motivate their combined use. Interactive effects on craving to smoke were robust across the entire sample, whereas for craving to drink these interactions were driven primarily by women and lighter drinkers. Heavier use of alcohol or nicotine appears to be characterized by enhanced sensitivity to either drug, rather than being specific to either alcohol or nicotine, consistent with a cross-tolerance/cross-reinforcement framework. Both same-drug and cross-drug effects on beverage and cigarette liking were observed, as well as separate (but not interactive) effects on mood. As research on the joint use of alcohol and tobacco continues to grow, important implications for the treatment of both disorders have begun to emerge (e.g. McKee & Weinberger, in press). A more refined understanding of the role individual differences play in the separate and combined effects of alcohol and nicotine as well as the propensity to experience cross-tolerance/cross-reinforcement may prove critical for identifying those at greatest risk of developing dual dependence and improving interventions targeting cross-drug influences that may threaten abstinence efforts within this population.
Acknowledgments
This study was funded by grant AA011157 from the National Institute on Alcohol Abuse and Alcoholism (Drobes). Additional support was provided by grant PRE14660076 from the American Heart Association (Oliver) and by NCI R25CA090314 (Blank). The authors thank Diana Diaz and Christina Reichert for their dedication to the project, as well as the Survey Methods Core of Moffitt Cancer Center for their assistance with data management.
Contributor Information
Jason A. Oliver, Department of Psychology, University of South Florida and Department of Health Outcomes and Behavior, Moffitt Cancer Center
Melissa D. Blank, Department of Health Outcomes and Behavior, Moffitt Cancer Center
Kate Janse Van Rensburg, Department of Health Outcomes and Behavior, Moffitt Cancer Center.
David A. MacQueen, Department of Psychology, University of South Florida and Department of Health Outcomes and Behavior, Moffitt Cancer Center
Thomas H. Brandon, Department of Psychology, University of South Florida and Department of Health Outcomes and Behavior, Moffitt Cancer Center
David J. Drobes, Department of Psychology, University of South Florida and Department of Health Outcomes and Behavior, Moffitt Cancer Center
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology (Berl) 2006;186(1):54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Attwood AS, Penton-Voak IS, Goodwin C, Munafo MR. Effects of acute nicotine and alcohol on the rating of attractiveness in social smokers and alcohol drinkers. Drug and Alcohol Dependence. 2012;125(1−2):43–48. doi: 10.1016/j.drugalcdep.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug and Alcohol Dependence. 2006;81(2):197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jones RT, Jacob P., 3rd Additive cardiovascular effects of nicotine and ethanol. Clinical Pharmacology & Therapeutics. 1986;40(4):420–424. doi: 10.1038/clpt.1986.200. [DOI] [PubMed] [Google Scholar]
- Bobak M, Room R, Pikhart H, Kubinova R, Malyutina S, Pajak A, Marmot M. Contribution of drinking patterns to differences in rates of alcohol related problems between three urban populations. Journal of Epidemiology & Community Health. 2004;58(3):238–242. doi: 10.1136/jech.2003.011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical & Experimental Research. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addictive Behaviors. 1990;15(2):105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92(1):15–26. [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1984;47(5):1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcoholism: Clinical & Experimental Research. 2002;26(12):1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl) 2007;190(3):321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addiction Biology. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C.: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behavioural Pharmacology. 1996;7(2):144–154. [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grant KM, Northrup JH, Agrawal S, Olsen DM, McIvor C, Romberger DJ. Smoing cessation in outpatient alcohol treatment. Addictive Disorders & Their Treatment. 2003;2:41–46. [Google Scholar]
- Gulliver SB, Kamholz BW, Helstrom AW. Smoking cessation and alcohol abstinence: what do the data tell us? Alcohol Research & Health. 2006;29(3):208–212. [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: A test of the incentive-sensitisation theory. Psychopharmacology. 2005;178:493–499. doi: 10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: onset, persistence and trajectories of use across two samples. Addiction. 2002;97(5):517–531. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Wood PK. Prospective analysis of comorbidity: tobacco and alcohol use disorders. Journal of Abnormal Psychology. 2000;109(4):679–694. doi: 10.1037//0021-843x.109.4.679. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Leventhal AM, McKee SA, Tidey JW, Rohsenow DJ. Sex differences in stimulus expectancy and pharmacologic effects of a moderate dose of alcohol on smoking lapse risk in a laboratory analogue study. Psychopharmacology (Berl) 2012;222(1):71–80. doi: 10.1007/s00213-011-2624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine & Tobacco Research. 2010;12(7):781–785. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Stuart GL, Moore TM, Ramsey SE. Item functioning of the alcohol dependence scale in a high-risk sample. Drug and Alcohol Dependence. 2003;72(2):183–192. doi: 10.1016/s0376-8716(03)00199-6. [DOI] [PubMed] [Google Scholar]
- Kassel JD, editor. Substance abuse and emotion. Washington, DC: American Psychological Association; 2009. [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drug. The Journal of Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcoholism: Clinical & Experimental Research. 2005;29(4):547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- King AC, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology (Berl) 2009;207(1):107–117. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcoholism: Clinical & Experimental Research. 1998;22(1):3–9. [pii] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug and Alcohol Dependence. 2004;75(1):55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Lajtha A, Sershen H. Nicotine: alcohol reward interactions. Neurochemical Research. 2010;35(8):1248–1258. doi: 10.1007/s11064-010-0181-8. [DOI] [PubMed] [Google Scholar]
- Madden PAF, Heath AC, Starmer GA, Whitfield JB, Martin NG. Alcohol sensitivity and smoking history in men and women. Alcoholism: Clinical and Experimental Research. 1995;19:1111–1120. doi: 10.1111/j.1530-0277.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Sayette MA. Experimental design in alcohol administration research: Limitations and alternatives in the manipulation of dosage-set. Journal of Studies on Alcohol. 1993;54:750–761. doi: 10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status as a clinical indicator for alcohol misuse in US adults. Archives of Internal Medicine. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Hinson R, Rounsaville D, Petrelli P. Survey of subjective effects of smoking while drinking among college students. Nicotine & Tobacco Research. 2004;6(1):111–117. doi: 10.1080/14622200310001656939. [DOI] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 2006;189(2):201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl) 2008;196(2):189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH. How Can We Use Our Knowledge of Alcohol-Tobacco Interactions to Reduce Alcohol Use? Annual Review of Clinical Psychology. doi: 10.1146/annurev-clinpsy-050212-185549. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: interactions with alcohol use. Psychopharmacology (Berl) 1987;93(1):8–15. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- Mintz J, Phipps CC, Arruda MJ, Glynn SM, Schneider NG, Jarvik ME. Combined use of alcohol and nicotine gum. Addictive Behaviors. 1991;16(1–2):1–10. doi: 10.1016/0306-4603(91)90034-f. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, de Wit H, Zacny JP. Effects of varying ethanol dose on cigarette consumption in healthy normal volunteers. Behavioural Pharmacology. 1995;6(4):359–365. [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Alcohol and Tobacco: From Basic Science to Clinical Practice. Bethesda, MD: U.S. Department of Health and Human Services; 1995. Smoking among alcoholics during and after treatment: Implications for models, treatment strategies, and policy. [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;189:201–210. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcoholism: Clinical & Experimental Research. 2006;30(8):1408–1413. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Combined effects of nicotine and alcohol on subjective, behavioral and physiological responses in humans. Addiction Biology. 1997;2(3):255–268. doi: 10.1080/13556219772552. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Blakesley-Ball R, Stolinski A, Wilson AS. The influence of alcohol pre-treatment on the discriminative stimulus, subjective, and relative reinforcing effects of nicotine. Behavioural Pharmacology. 2005;16(7):521–529. doi: 10.1097/01.fbp.0000175255.55774.19. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, DiMarco A, Grobe JE, Scierka A, Stiller RL. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology (Berl) 1995;119(2):205–212. doi: 10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, Sher KJ. The subjective effects of alcohol-tobacco co-use: an ecological momentary assessment investigation. Journal of Abnormal Psychology. 2011;120(3):557–571. doi: 10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. American Journal of Psychiatry. 1992;149(11):1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet. 1995a;25(2):161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Alcohol and Tobacco: From Basic Science to Clinical Practice. Bethesda, MD: U.S. Department of Health and Human Services; 1995b. Neurobiological interactions of alcohol and nicotine. [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Perry EB, Jr., D’Souza DC, Bufis V, Elander J, Limoncelli D, Petrakis I. Preliminary findings on the interactive effects of IV ethanol and IV nicotine on human behavior and cognition: a laboratory study. Nicotine & Tobacco Research. 2012;14(5):596–606. doi: 10.1093/ntr/ntr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr., Kahler CW, Leventhal AM, Monti PM, Swift R, Hutchison KE. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharmacology (Berl) 2007;193(4):449–456. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature: Neuroscience. 2004;7(6):583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine & Tobacco Research. 2004;6(1):133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Rueger SY, McNamara PJ, King AC. Expanding the Utility of the Biphasic Alcohol Effects Scale (BAES) and Initial Psychometric Support for the Brief-BAES (B-BAES) Alcoholism: Clinical & Experimental Research. 2009;33:916–924. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychology of Addictive Behaviors. 2005;19(3):263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Alcohol and Tobacco: From Basic Science to Clinical Practice. Bethesda, MD: U.S. Department of Health and Human Services; 1995. Psychosocial and Biological Mechanisms. [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. Journal of Consulting and Clinical Psychology. 1996;64(5):993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR, Ferguson SG, Scharf DM. Patterns of intermittent smoking: An analysis using Ecological Momentary Assessment. Addictive Behaviors. 2009;34(6–7):514–519. doi: 10.1016/j.addbeh.2009.01.004. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115(3):509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Experimental & Clinical Psychopharmacology. 2012;20(4):264–277. doi: 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto, CA: Addiction Research Foundation; 1995. [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine & Tobacco Research. 2000;2(1):19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Castillo S. Correlates of college student binge drinking. American Journal of Public Health. 1995;85(7):921–926. doi: 10.2105/ajph.85.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR, Ahlstrom S, Bondy S, Weiss S. Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction. 2000;95(2):251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]