Abstract
Flavonoids are ubiquitous in nature. They are also in food, providing an essential link between diet and prevention of chronic diseases including cancer. Anticancer effects of these polyphenols depend on several factors: Their chemical structure and concentration, and also on the type of cancer. Malignant cells from different tissues reveal somewhat different sensitivity toward flavonoids and, therefore, the preferences of the most common dietary flavonoids to various human cancer types are analyzed in this review. While luteolin and kaempferol can be considered as promising candidate agents for treatment of gastric and ovarian cancers, respectively, apigenin, chrysin, and luteolin have good perspectives as potent antitumor agents for cervical cancer; cells from main sites of flavonoid metabolism (colon and liver) reveal rather large fluctuations in anticancer activity probably due to exposure to various metabolites with different activities. Anticancer effect of flavonoids toward blood cancer cells depend on their myeloid, lymphoid, or erythroid origin; cytotoxic effects of flavonoids on breast and prostate cancer cells are highly related to the expression of hormone receptors. Different flavonoids are often preferentially present in certain food items, and knowledge about the malignant tissue-specific anticancer effects of flavonoids could be purposely applied both in chemoprevention as well as in cancer treatment.
Keywords: Diet, flavonoids, human cancers, prevention, treatment
INTRODUCTION
Numerous edible plant-derived compounds have been linked to the chemoprevention and treatment of cancer.[1,2,3,4,5,6,7,8,9] For the past decades, much research has been developed in order to discover natural compounds with potential anticancer activity[6,10,11,12] and several plant-derived agents (e.g., paclitaxel, docetaxel; vinblastine, vincristine; topotecan, irinotecan, etoposide, etc.) have been successfully used for cancer treatments.[13,14,15] Among the anticancer medications, 69% of drugs approved between 1940 and 2002 are either natural products or developed based on knowledge gained from natural products,[16,17] a rate which is much higher than in other areas of drug development.[18] Natural products offer an untold diversity of chemical structures, and it is very likely that phytochemicals will continue to be important in cancer therapeutics.[19,20,21] Application of plants in the treatment of cancer seems to be inevitable, constituting the basis for modern medical science and providing a great source for new drugs.[22,23]
Medicine and one's daily food are equally important in making a sick body well.[24] Diet is intimately linked to both the incidence and avoidance of many types of cancer[25] and dietary behavior has been identified as one of the most important modifiable determinants of cancer risk.[26] Strong and consistent epidemiological evidences suggest that a diet enriched with naturally occurring substances significantly reduces the risk for many cancers.[27,28,29,30,31] Indeed, the adoption of diets rich in vegetables and fruits, together with the maintenance of physical activity and appropriate body mass, could reduce the cancer incidence by 30-40%.[32,33,34] Moreover, several studies suggest that there is a decreased risk for different types of cancer among vegetarians.[35] Numerous classes of compounds present in fruits and vegetables are assumed to take the role of cancer-preventive agents. Among these compounds, flavonoids have been proven to be particularly important.[27,29,36]
FLAVONOIDS AS POTENT ANTICANCER AGENTS
Flavonoids are naturally occurring polyphenolic metabolites distributed throughout the plant kingdom and found in substantial amounts in fruits, vegetables, grains, nuts, seeds, tea, and traditional medicinal herbs.[37,38,39] Within individual plants, flavonoids occur in every part but are usually concentrated in the leaves and flowers.[40] Flavonoids are edible plant pigments responsible for much of the coloring in nature.[41,42]
Many of the different flavonoids are part of the regular human diet. Although they are nonessential dietary factors,[9] flavonoids are thought to be nutritionally valuable compounds,[43] being the key natural products that provide the most essential link between the diet and prevention of chronic disorders. One of the most investigated activities of flavonoids is their contribution to cancer prevention and treatment.[6]
Several thousand flavonoids are known to occur in nature, defined chemically as compounds containing a phenylchromanone structure (C6-C3-C6) with at least one hydroxyl substituent.[44,45,46,47] Flavonoids can be further divided into flavonols, flavones, flavanols, flavanones, anthocyanidins, and isoflavonoids based on the saturation level and opening of the central pyran ring[15,45,48] [Figure 1].
Figure 1.
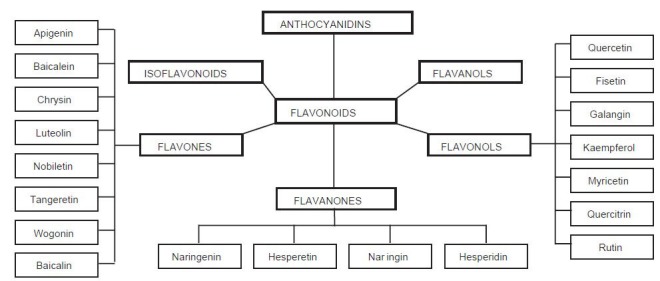
Scheme of major flavonoid aglycones and their glycosides
The daily human intake of flavonoids is quite different in amounts and classes due to various feeding habits of people from different regions and cultures.[25,49] Reports of estimated daily consumption of flavonoids range from 20 mg/day to 1 g/day.[50] As the total flavonoid intake in Western countries is estimated at 23 mg/day,[51] humans consuming high fruit and vegetable diets may ingest up to 1 g of these compounds daily.[52,53] No information is available about the content of flavonoids in the diet of vegetarians.[54,55] The main food sources of major dietary flavonoids are presented in Table 1.
Table 1.
Structures and main food sources of major flavonoid aglycones and their glycosides
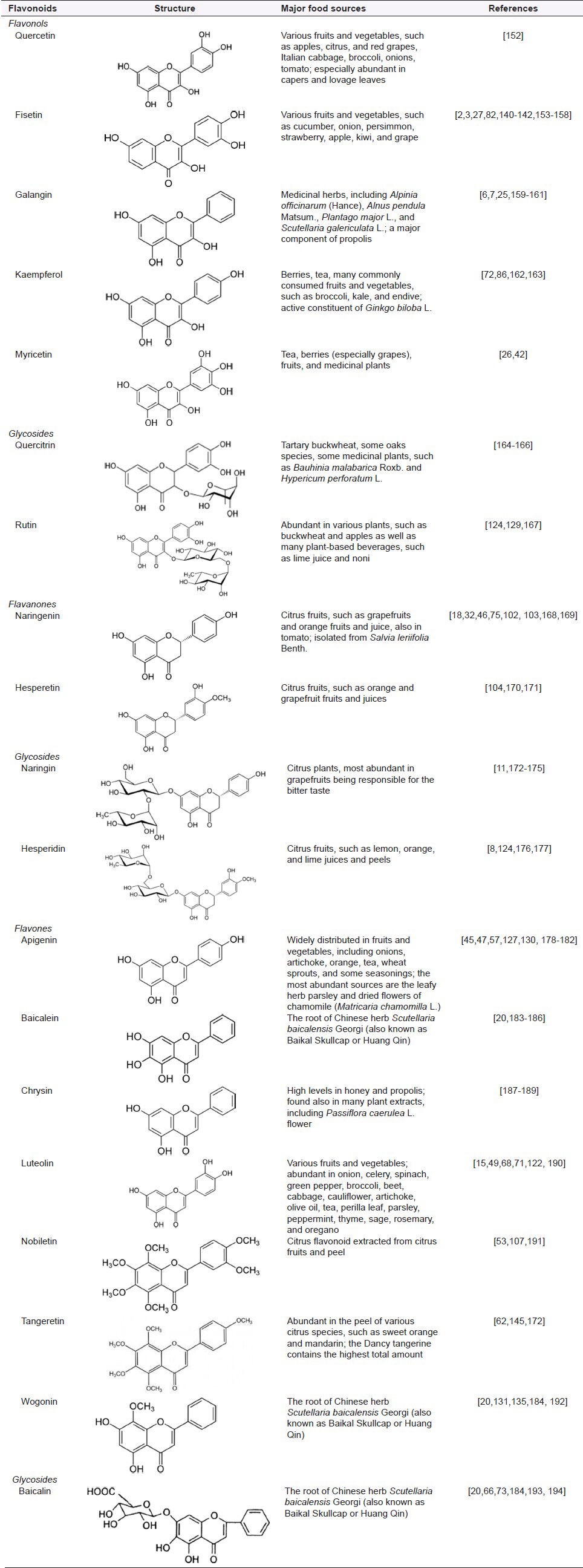
As natural products, flavonoids are regarded as safe and easily obtainable, making them ideal candidates for cancer chemoprevention or associated agents in clinical treatment.[3,43,56] Almost all artificial agents currently being used in cancer therapy are highly toxic and produce severe damage to normal cells.[57,58] The ideal anticancer agent would exert minimal adverse effects on normal tissues with maximal capacity to kill tumor cells and/or inhibit tumor growth.[12,34] The lack of substantial toxic effects for long-term therapies and inherent biological activity of flavonoids make them ideal candidates for new therapeutics.[16,59] Indeed, flavonoids have been shown to reveal cytotoxic activity toward various human cancer cells with little or no effect on normal cells, and this fact has stimulated large interest in developing of potential flavonoid-based chemotherapeutics for anticancer treatment.[60,61]
Several observations have suggested that natural flavonoids have growth inhibitory effects on various kinds of cancer cells mediated by different molecular targets and acting through diverse metabolic pathways.[62,63,64] However, the precise mechanisms responsible for the antitumor effect of flavonoids are still not thoroughly understood.[64,65] Flavonoids can easily bind to the cell membrane, penetrate in vitro cultured cells, and modulate the cellular metabolic activities.[66,67] Mitigation of oxidative damage, inactivation of carcinogen, inhibition of proliferation, promotion of differentiation, induction of cell cycle arrest and apoptosis, impairment of tumor angiogenesis, and suppression of metastasis contribute to the anticarcinogenic activities of flavonoids.[24,25,30,32,49,68] These polyphenolic compounds can interact with xenobiotics metabolizing enzymes, inhibit several kinases involved in signal transduction, interact with estrogen type II binding sites, and alter gene expression patterns.[61,69,70,71]
Normal cell growth is maintained by the balance between cell proliferation and cell death, and apoptosis is a central regulator of tissue homeostasis.[72,73] Cells from a variety of human malignancies have a decreased ability to undergo apoptosis in response to some physiological stimuli. Induction of apoptosis in malignant cells may therefore represent a promising approach to both chemoprevention and chemotherapy, and searching for agents that can specifically trigger apoptosis in tumor cells has become an attractive strategy in anticancer drug discovery.[38,74,75,76,77] The anticancer efficacy of flavonoids is due, at least in part to their ability to induce apoptosis of tumor cells.[37,78,79,80,81]
One of the most common incidents required for human cancer development known as a hallmark of malignant cells is deregulation of the cell cycle.[35,82,83] Agents that can inhibit cell-cycle progression and lead to cell-growth arrest are very important in cancer prevention and therapy studies,[35] and considerable attention has been paid to the ability of dietary flavonoids to inhibit cell-cycle progression.[82] Flavonoids have been found to arrest cell-cycle progression at either G1/S or G2/M boundaries by modulating of multiple cell cycle regulatory proteins.[69] Somewhat conflicting results have been reported with regard to the stage-specific arrest caused by one and the same compound,[59,84] and several studies have indicated the ability of flavonoids to block the cell growth at more than one stage of the cell cycle.[85]
Due to the polyphenolic structure, flavonoids have been found to possess both anti- and prooxidant action.[86] While antioxidant effect and ability to scavenge reactive oxygen species (ROS) have been shown to account for most of the reported biological effects of phenolic compounds, several recent studies have revealed that anticancer activities of flavonoids may be mediated through prooxidant action.[49,87] Cancer cells exhibit a higher and more persistent oxidative stress level compared to normal cells, rendering malignant cells more vulnerable to being killed by drugs that boost increased ROS levels, such as some flavonoids.[88,89,90,91] Whether a flavonoid acts as anti- or prooxidant depends on its dose, cell type, and also culture conditions.[37,90,92]
The specific activity of flavonoids on cell function can also depend on their chemical structure.[93,94,95] The structures of the most common dietary flavonoids are presented in Table 1. Important factors affecting cytotoxic and/or antiproliferative activities of polyphenols include the saturation of the C2-C3 bond and the position as well as the number and substitution of hydroxyl groups in the A and B rings.[69,96,97] However, even the minor modifications in the molecules can be responsible for strong variations in their activity, and flavonoids with very similar structures could not produce identical biological responses.[40,97,98,99] Indeed, some authors have suggested that the anticancer capability of flavonoids cannot be predicted based on their chemical composition and structure,[61] and it is the reason why no structure activity relationships are analyzed in the present work.
OBSCURITIES LIMITING THE USE OF FLAVONOIDS IN CANCER CHEMOPREVENTION AND TREATMENT
Flavonoids have been found to exert cytotoxic activities only at relatively high doses, within the micromolar concentration range.[26,82,100] The amount of dietary flavonoids in plasma varies according to several parameters such as functional groups and daily intake.[101] However, achieving the plasma levels sufficient to reveal antiproliferative and cytotoxic effects may not be possible via oral administration.[9,100] For example, results from human data have shown that a full glass of orange juice supplies about enough naringenin to achieve a plasma concentration of 0.5 μM;[102] a one-time consumption of approximately 550 g of grapefruit juice results in a mean peak plasma concentration of 6 μM naringenin;[103] the physiological dose of hesperetin attainable from drinking orange juice is in the range of 0.5-6 μM;[104] human plasma concentration of hesperidin reaches to 0.5 μM at 5-7 hours after ingestion of 0.5 liter of commercial orange juice providing 400 mg hesperidin;[8] typical plasma concentration of apigenin is within 10 nM range;[45] the concentration of chrysin in plasma after a single dose of 400 mg remains below 0.1 μM;[101] and maximal plasma levels of luteolin reaches to about 0.2 μM at 1-2 hours after oral administration.[31] In contrast, methoxylated flavonoids display up to 100-fold higher plasma concentrations on account of the reduced phase II conjugation reactions.[101] Higher plasma levels can be achieved through intravenous injection,[9,100] and the plasma concentration of flavonoids may also be significantly increased by regular intake for a prolonged period.[45,101,105]
Despite encouraging preclinical results, the usability of flavonoids for chemoprevention has encountered only limited success, largely because of inefficient systemic delivery and bioavailability.[106,107,108] Flavonoids are most often found in plant materials in the form of glycosides (bound to sugars), which are better soluble in water than the respective aglycones.[47,68,109] Most of the glycosides resist acid hydrolysis in the stomach[45] and are deglycosylated by β-glucosidases in the small intestine.[69] The aglycones are further glucuronidated and sulphated by the intestinal mucosa and liver before release into the blood serum.[47,68,69] It is therefore likely that phytochemicals can accumulate in the small intestine and colon at levels greater than in plasma.[110] Bioavailability of flavonoids is determined by different factors, including the sugar moiety of the polyphenolic compound and its further metabolism by the gut microflora,[11] showing that different groups of flavonoids may have different pharmacokinetic properties.[111,112] Moreover, considerable interindividual variation between humans can also influence the flavonoid metabolism, thus affecting the therapeutic action of polyphenolic compounds.[103] Furthermore, the anticancer activity would be related not only to the parent flavonoid ingested but also to its metabolites; therefore, identification and measurement of the physiological flavonoid conjugates are important to thoroughly understand the role of dietary polyphenols in human health.[31,52,69]
COMPREHENSIVE ANALYSIS OF CYTOTOXICITY OF FLAVONOIDS ON HUMAN CANCER LINES FROM DIFFERENT ORIGINS
Flavonoids have been demonstrated to suppress proliferation of various cancerous cells.[69] However, not all polyphenolic compounds share the same antiproliferative activity;[113] and depending on their structure, flavonoids display differences in the sensitivity and selectivity toward tumor cells.[26,97,114] The sensitivities of cancer cells against flavonoids can be different depending on their derived tissues,[16,115] indicating that the cytotoxicity induced by flavonoids might be related to selected cancer types.[30,116] Even in the case of flavonoids with quite similar structures, there are compound-specific effects which are relevant to modulate particular biochemical processes so that the development of certain neoplasms could be differentially influential pointing to the tissue-specific cytotoxic action.[117,118] The effectiveness of flavonoids may vary also because of the different disease etiologies.[119]
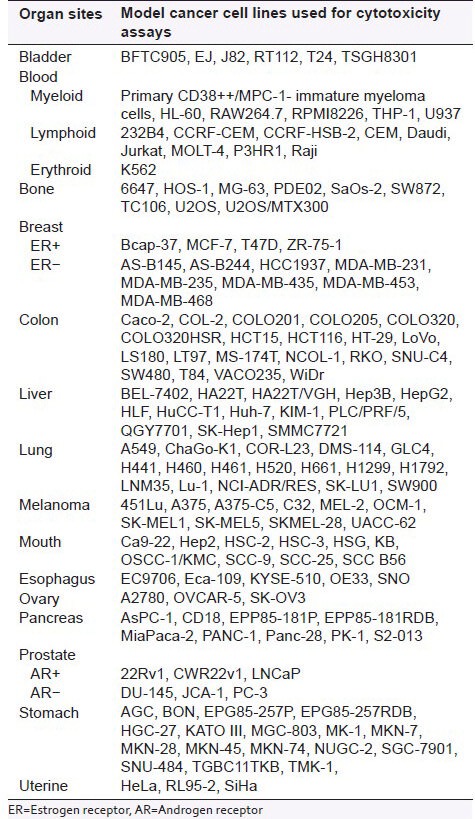
It is of interest of the current review article to determine whether the most common dietary flavonoids can exert some clear-cut preferences to certain tumor tissue types. In nature, different members of the flavonoid family are often preferentially present in some food items;[120] and knowledge about the malignant tissue-specific cytotoxic effects of flavonoids could be purposely applied both in the chemoprevention based on the genetic cancer risks and familiar anamnesis as well as in the cancer treatment. For this purpose, quantitative data characterizing the cytotoxic effects of different flavonoids on different human tumor cell lines were compiled from the literature sources, and statistical analysis to calculate the respective mean parameters was performed. IC50 values as the flavonoid concentrations required to inhibit 50% of cell growth are the most common representative indexes of the dose-response curve,[121] and these parameters were also used in the current work. The mean cytotoxic constants of the most common dietary flavonoids on cancer cell lines derived from various organ sites are presented in Table 2. Cultured human malignant cell lines used for evaluating the cytotoxicity of these compounds are listed in Table 3.
Table 2.
Cytotoxicity of flavonoids on human cancer cell lines derived from various organ sites (mean IC50±SE, μM (n)). Cell lines used for assays are presented in Table 3
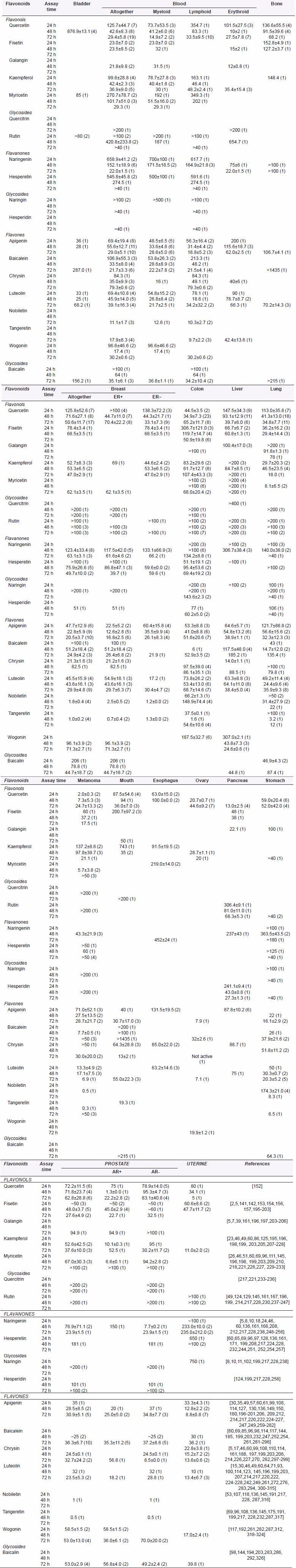
Table 3.
Human cancer cell lines used for cytotoxicity assays of flavonoids
Paucity of data complicates the analysis
Despite the extensive investigation carried out with flavonoids in the past decades, there are still quite a few parameters available, characterizing quantitatively the efficacy of polyphenolic compounds on certain cancer types. In this way, the IC50 values of flavonoids measured using the cells derived from malignant esophageal tissues are too scarce to reveal any certain specificity patterns. At the same time, data measured on bone cancer lines show only very low or even no cytotoxic activity of different flavonoids [Table 2].
Rather, few half-maximal cytotoxic parameters are available also for cell lines derived from human tumors of bladder, mouth, stomach, pancreas, and ovary. However, some activity patterns and tissue specificities of flavonoids can still be brought forth for these organ sites. In the case of bladder, cancer flavones apigenin and luteolin seem to be cytotoxically most active. Besides these two flavones, chrysin and flavonol kaempferol have also been reported to have antiproliferative activity and induce apoptosis in oral cavity cancer cells. Epithelium of the oral cavity can absorb the flavonoids directly, and should benefit for high levels of exposure to these dietary phytochemicals.[122]
Flavones apigenin, baicalein, luteolin, nobiletin, and tangeretin have shown to be the most effective flavonoids against carcinomas of stomach, whereas luteolin has even proposed to be a promising candidate agent for treatment of gastric cancer.[123]
In addition to some flavonols, such as quercetin, fisetin, and galangin, flavanone glycoside hesperidin also inhibits human pancreatic cancer cells, explaining why lime juice rich in hesperidin has been suggested to possess potential in the prevention of pancreatic cancer.[124]
The growth of human ovarian cancer cells cannot only be suppressed by several flavones including apigenin, baicalein, luteolin, and wogonin but also by flavonols quercetin and kaempferol. Kaempferol is a good candidate compound for chemoprevention of ovarian cancer; as in human studies, a significant 40% decrease in incidence of ovarian cancer was detected for individuals with the highest quintile of kaempferol consumption compared to those in the lowest quintile.[106,125] The intake of this nontoxic and inexpensive phytochemical can be easily adopted into the lifestyle of most women.[126]
Metabolic sites reveal large fluctuations toward flavonoid cytotoxicity
Present in dietary sources mostly as glycosides, flavonoids are cleaved in intestine by microbial enzymes and further metabolized in colon and liver to release into the blood as different conjugates. In this way, the epithelium of intestine is exposed to higher concentrations of flavonoids and their different metabolites than the tissues at other locations; and this would also be true for the colonic tumor cells, showing that colorectal cancer appears most relevant to dietary factors.[45,127] At the same time, the exposure to different metabolites can explain the large fluctuations in cytotoxic constants of flavonoids measured using colorectal and liver cancer cell lines. It is possible that some metabolites could be more cytotoxic than parent compounds, giving a selective anticancer activity advantage in vivo.
The other aspect important to take into consideration by analyzing the cytotoxic data of flavonoids includes their differential effect against tumors with specific mutational spectra. The differential effectiveness of inhibition of cell growth and arresting cell cycle in response to flavonoids in various colorectal cancer cell lines may be associated with the functional status of p53 and/or ras genes. While apigenin has been indicated to have stronger effect on tumors with mutations in genes which are critical to colon cancer development, thus being more effective in controlling the growth of tumors with certain mutational spectra and less effective in wild-type normal cells,[29,46,127] kaempferol and hesperetin seem to exhibit higher resistance toward mutant p53 human colon cancer cell lines.[125,128]
Some other flavonoids including quercetin and baicalein have also been shown to be useful agents for prevention and treatment of colon cancer [Table 2]. However, compared to quercetin and baicalein, their glycosides rutin and baicalin, respectively showed no growth inhibitory effects on colon cancer cells,[20,129] showing that the sugar moiety strongly affects the bioactivity of flavonoids.
Accumulated evidences have indicated that the growth of hepatocarcinoma cells can be suppressed by flavones apigenin, luteolin, wogonin, and baicalin, thus being valuable for the therapeutic intervention of human hepatomas [Table 2]. Apigenin may have some implications also in the prevention of virus infection, leading to liver cancer development;[130] wogonin possesses hepatoprotective activities against diverse pathophysiological processes associated with hepatocarcinogenesis and can be extremely competitive as anticancer drugs against malignant hepatoma.[131,132]
Blood cells are potent target sites for flavonoids
Anticancer drugs are generally more effective against leukemia than other malignancies and in this aspect flavonoids are similar to other anticancer agents.[24] The mean cytotoxic constants of various flavonoids on different blood cancer cells are depicted in the Figure 2 showing that many common dietary polyphenols exhibit growth inhibitory properties against several human hematologic malignancies.
Figure 2.
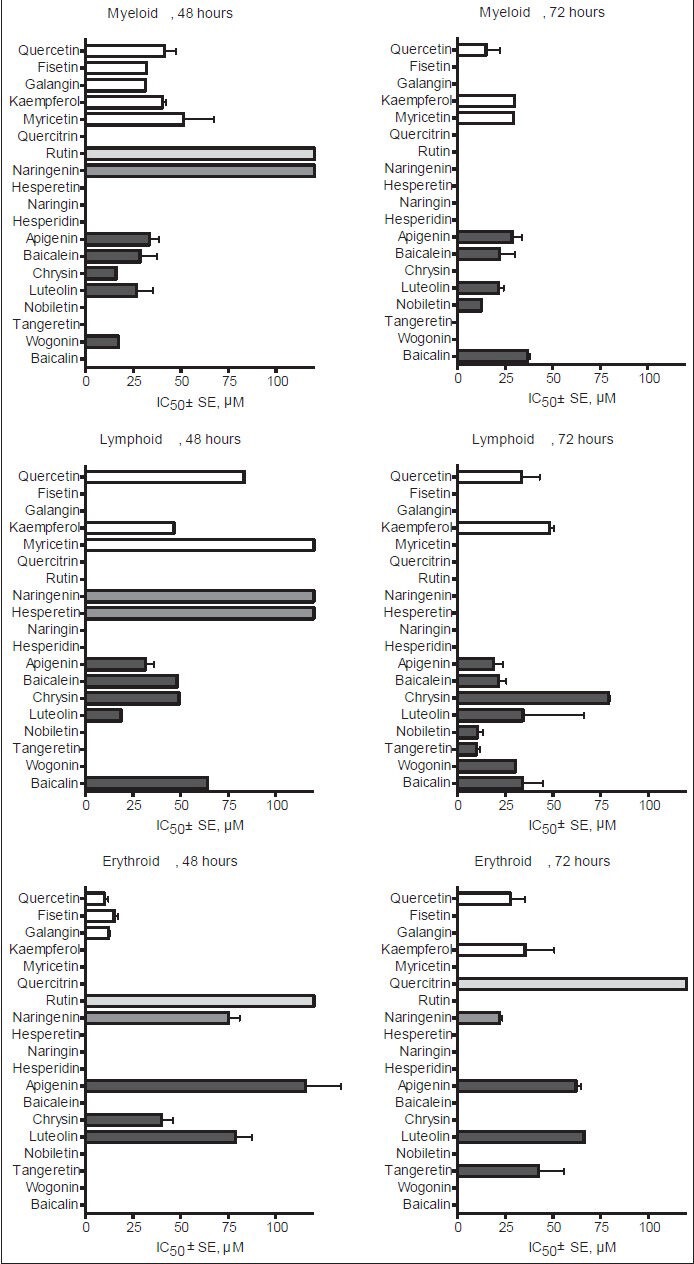
Cytotoxic effect of flavonoids on different human blood cancer cell lines
Flavonoids hold much promise for the development of new chemotherapeutics in myeloid and lymphoid leukemias.[133,134,135] In general, flavonol aglycones (quercetin, kaempferol, myricetin) seem to exhibit somewhat stronger cytotoxic activity against blood cancer cells of myeloid lineage compared to lymphocytic leukemia cell lines, whereas flavonol glycosides have no effect on the viability of different blood cancer cells. In contrast to the inactivity of flavanone naringenin in myeloid and lymphoid leukemia cell lines, this dietary polyphenol exerts cytotoxicity on erythroleukemia cells, thus revealing an opposite situation to flavones (apigenin, luteolin, tangeretin) in which cases strong anticancer activity has been measured in cell lines of myeloid and lymphoid lineages but significantly lower sensitivity is expressed toward erythroleukemia cells [Figure 2]. This knowledge could be specifically applied in chemoprevention as well as clinical trials for treatment of different hematologic malignancies.
Polyphenols affecting both hormone-dependent and -independent tumor cells
Breast and prostate cancers are hormone-dependent tumors as their development and growth can be dependent on the expression of estrogen receptors (ER) and androgen receptors (AR), respectively.
Most breast cancers are heterogeneous and consist of ER-positive and -negative cells. Therefore, agents that are able to inhibit the growth of both ER-positive and -negative tumors are of great interest.[136] Dietary flavonoids seem to display such dual activity, inhibiting both receptor-positive and -negative breast cancer cells [Table 2]. For instance, no difference in the cytotoxicity of naringenin has been found between human breast cancer cell lines expressing or not expressing ERs[24] and the regular intake of this flavanone may slow down the rate at which breast cancer cells proliferate.[103] High flavone intake has also been significantly correlated with a lower risk of breast cancer[68] and apigenin, baicalein, and luteolin may be promising candidate agents in the treatment of mammary tumors.[137,138,139] However, although apigenin can target both ER-dependent and -independent pathways, it seems to be somewhat more potent on ER-positive human breast cancer cell lines [Figure 3], thus providing more promise for the treatment of ER-positive tumors.
Figure 3.
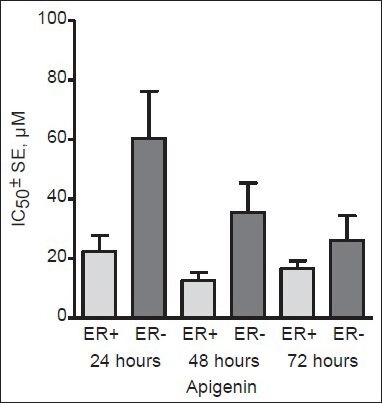
Cytotoxic effect of apigenin on human breast cancer cell lines depending on the expression of estrogen receptors
AR are the critical factors for the prostate cancer cell growth and survival and in the development of ablation-resistant prostate tumors. As presented in Table 2, flavonoids display anticancer effects both in AR-positive and -negative prostate cancer cell lines. However, flavonol aglycones (quercetin, fisetin, galangin, kaempferol, and myricetin) exert somewhat stronger cytotoxic activity on AR-dependent prostate cancer cells [Figure 4]. Indeed, quercetin has been shown to decrease the androgen receptor expression in 22rv1 human prostate cancer cells,[140] whereas fisetin can inhibit the AR signaling pathways[141,142] showing that these compounds may afford more health benefits in chemoprevention and earlier stages of prostate carcinogenesis when the tumor is still dependent on the presence of androgens. In contrast, flavanone naringenin seem to display only very low potency toward AR-positive human prostate cancer cells, suppressing at the same time the growth of androgen-independent human prostate cancer lines. Flavones like apigenin, baicalein, and baicalin express rather similar pattern of growth inhibition of both AR-positive and -negative prostate carcinoma cells, thus being independent on androgen receptor status.[66,143,144] Flavonoid treatment may offer an alternative strategy to suppress androgen-insensitive prostate tumor growth and flavonoids like naringenin, apigenin, baicalein, chrysin, and luteolin may be developed as promising chemotherapeutic agents against advanced and androgen-independent human prostate tumors.
Figure 4.
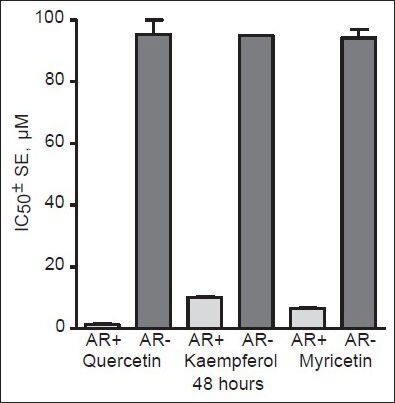
Cytotoxic effect of flavonols on human prostate cancer cell lines depending on the expression of androgen receptors
With regards to the structure of flavonoids and nature of substituents, it is especially important to point out the fact that methylation of the hydroxyl groups does not reduce the anticancer capacity but even increases it.[61,69] Therefore, polymethoxylated flavonoids, such as tangeretin and nobiletin, can be much more potent inhibitors of tumor cell growth than free hydroxylated flavonoids[69,145] [Table 2].
Lung and uterine cancer as well as melanoma cells are strongly affected by flavonoids
Cytotoxic effects of flavonoids on malignant cell lines derived from human lung and cervical cancers as well as melanoma are depicted in Figure 5. Several flavonol aglycones are able to cause decrease in cell viability with half-maximal cytotoxic doses in low micromolar range [Table 2], revealing the most potent cytotoxic activity for myricetin in lung cancer cells and quercetin in melanoma and cervical cancer cells. Flavanones display no growth inhibitory effect on lung and cervical cancer cell lines, expressing at the same time some cytotoxicity on human melanoma cells.
Figure 5.
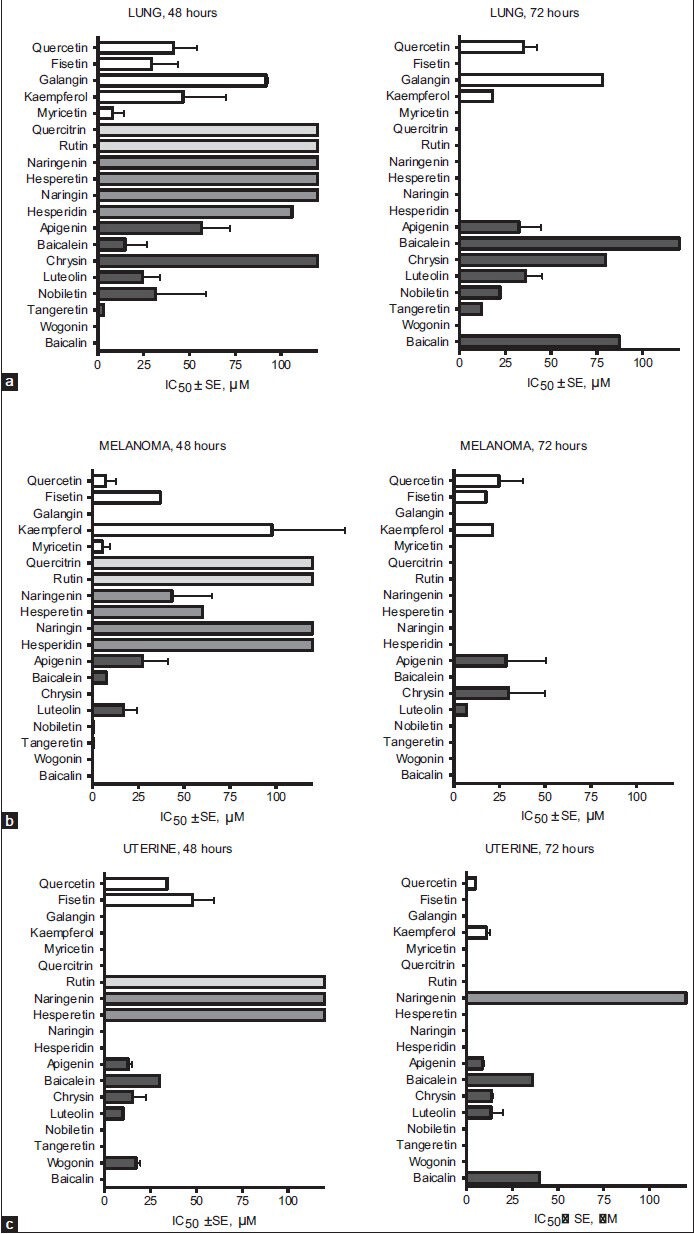
Cytotoxic effect of flavonoids on different human lung cancer cell lines (a), melanoma cell lines (b), and uterine cancer cell lines (c)
Several members of the flavone group display high cytotoxic activity against cervical cancer cells. Apigenin is probably more potent and sensitive in killing cervical cancer cells than cells of melanoma and lung cancer; the same seems to be true also for chrysin. Luteolin exerts high-level activity both in cervical cancer as well as melanoma cell lines, showing that these flavones may have good perspectives as lead compounds of potent antitumor agents for the respective target sites. On the other hand, polymethoxylated flavones nobiletin and tangeretin are among the most effective at inhibiting cancer cell growth of melanoma and lung (tangeretin) and it is also the reason why these dietary polyphenols have emerged as potential drug candidates for treatment of these malignancies [Table 2, Figure 5].
CONCLUSIONS AND FURTHER PERSPECTIVES
Flavonoids can play important beneficial roles in human nutrition and health status and chemoprevention is one of the most realistic and promising approaches for the prevention of malignant disorders.[76,118] Diet–health relationships are very complex as food items usually act through multiple pathways and each ingredient can have different molecular targets. It is also the reason why phytochemical combinations may offer greater chemoprevention than administration of single agents alone.[110] Both additive as well as synergistic interactions between several dietary flavonoids have been reported,[101,146] contributing to the health benefits of fruits and vegetables. Therefore, consumers may gain more significant health benefits from whole foods than from intake of dietary supplements.[146] However, individual phenolic compounds may also act antagonistically with other components,[147,148] and further efforts are necessary to understand their action modes as well as to provide further information for the cancer prevention in future.[149]
Flavonoids are not only promising food-derived cancer preventive compounds but could also be considered as candidates for chemotherapeutic agents, revealing potential clinical significance in the cancer treatment.[73,150] Polyphenolic compounds like quercetin, myricetin, apigenin, baicalein, chrysin, luteolin, nobiletin, and tangeretin might be valuable agents in anticancer strategies and studies of their clinical use for development of novel drugs should be continued. Beneficial effects have been described also by combining certain flavonoids with standard chemotherapeutic drugs leading to decrease in the dosage and associated toxicity while targeting specific resistance mechanisms. In this way, the genotoxic damage caused by standard chemotherapeutics to normal cells can be diminished, thereby reducing the chance of developing of secondary cancers.[45,151] Further work is certainly needed to develop and produce novel drugs from natural sources introducing structural variations into the backbone of flavonoids and modifying their structures to further improve biological activity and exhibit more potent anticancer effects.
Despite a rather short period of investigation of the anticancer action of flavonoids (for instance, apigenin was first proposed to interfere with the process of carcinogenesis only in 1980s),[45] this field has undergone an extensive development. The cytotoxic data of flavonoids compiled within the current work and relationships presented in this review article cannot only be useful in chemoprevention to choose the food items containing most active natural polyphenols on malignant cells of certain cancer types, considering the individual genetic cancer risks and familial anamnesis but also in the selection of parent compounds to design and synthesize novel chemotherapy drugs starting from the valuable material given to us by the nature.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mignet N, Seguin J, Ramos Romano M, Brulle L, Touil YS, Scherman D, et al. Development of a liposomal formulation of the natural flavonoid fisetin. Int J Pharm. 2012;423:69–76. doi: 10.1016/j.ijpharm.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 2.Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang YC, Bau DT, et al. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 2012;86:263–73. doi: 10.1007/s00204-011-0754-6. [DOI] [PubMed] [Google Scholar]
- 3.Szliszka E, Helewski KJ, Mizgala E, Krol W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int J Oncol. 2011;39:771–9. doi: 10.3892/ijo.2011.1116. [DOI] [PubMed] [Google Scholar]
- 4.Seguin J, Brullé L, Boyer R, Lu YM, Ramos Romano M, Touil YS, et al. Liposomal encapsulation of the natural flavonoid fisetin improves bioavailability and antitumor efficacy. Int J Pharm. 2013;444:146–54. doi: 10.1016/j.ijpharm.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Tolomeo M, Grimaudo S, Di Cristina A, Pipitone RM, Dusonchet L, Meli M, et al. Galangin increases the cytotoxic activity of imatinib mesylate in imatinib-sensitive and imatinib-resistant Bcr-Abl expressing leukemia cells. Cancer Lett. 2008;265:289–97. doi: 10.1016/j.canlet.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal JV, Wadegaonkar PA, Hajare SW. The bioflavonoid galangin suppresses the growth of ehrlich ascites carcinoma in Swiss Albino mice: A molecular insight. Appl Biochem Biotechnol. 2012;167:1325–39. doi: 10.1007/s12010-012-9646-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu KD, et al. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol. 2010;16:3377–84. doi: 10.3748/wjg.v16.i27.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao YC, Hsieh YS, Kuo WH, Chiou HL, Yang SF, Chiang WL, et al. The tumor-growth inhibitory activity of flavanone and 2’-OH flavanone in vitro and in vivo through induction of cell cycle arrest and suppression of cyclins and CDKs. J Biomed Sci. 2007;14:107–19. doi: 10.1007/s11373-006-9117-3. [DOI] [PubMed] [Google Scholar]
- 9.Ou YC, Kuan YH, Li JR, Raung SL, Wang CC, Hung YY, et al. Induction of apoptosis by luteolin involving akt inactivation in human 786-o renal cell carcinoma cells. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/109105. 109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen SC, Ko CH, Tseng SW, Tsai SH, Chen YC. Structurally related antitumor effects of flavanones in vitro and in vivo: Involvement of caspase 3 activtion, p21 gene expression, and reactive oxygen species production. Toxicol Appl Pharmacol. 2004;197:84–95. doi: 10.1016/j.taap.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh E, Alshatwi AA. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem Toxicol. 2013;51:97–105. doi: 10.1016/j.fct.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Shu G, Chen L, Mi X, Mei Z, Deng X. A flavonoid component from Docynia Delavayi (Franch.) Schneid represses transplanted H22 hepatoma growth and exhibits low toxic effect on tumor-bearing mice. Food Chem Toxicol. 2012;50:3166–73. doi: 10.1016/j.fct.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Jung CH, Jang BH, Go HY, Park JH, Choi YK, et al. Selective cytotoxic effects on human cancer cell lines of phenolic-rich ethyl-acetate fraction from Rhus verniciflua Stokes. Am J Chin Med. 2009;37:609–20. doi: 10.1142/S0192415X09007090. [DOI] [PubMed] [Google Scholar]
- 14.Li YL, Gan GP, Zhang HZ, Wu HZ, Li CL, Huang YP, et al. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J Ethnopharmacol. 2007;113:115–24. doi: 10.1016/j.jep.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Attoub S, Hassan AH, Vanhoecke B, Iratni R, Takahashi T, Gaben AM, et al. Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur J Pharmacol. 2011;651:18–25. doi: 10.1016/j.ejphar.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 16.Li-Weber M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Samarghandian S, Afshari JT, Davoodi S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics (Sao Paulo) 2011;66:1073–9. doi: 10.1590/S1807-59322011000600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tundis R, Loizzo MR, Menichini F, Bonesi M, Colica C, Menichini F. In vitro cytotoxic activity of extracts and isolated constituents of Salvia leriifolia Benth. against a panel of human cancer cell lines. Chem Biodivers. 2011;8:1152–62. doi: 10.1002/cbdv.201000311. [DOI] [PubMed] [Google Scholar]
- 19.Kalim MD, Bhattacharyya D, Banerjee A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern Med. 2010;10:77. doi: 10.1186/1472-6882-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CZ, Calway TD, Wen XD, Smith J, Yu C, Wang Y, et al. Hydrophobic flavonoids from Scutellaria baicalensis induce colorectal cancer cell apoptosis through a mitochondrial-mediated pathway. Int J Oncol. 2013;42:1018–26. doi: 10.3892/ijo.2013.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrahim NN, Kanthimathi MS, Abdul-Aziz A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement Altern Med. 2012;12:220. doi: 10.1186/1472-6882-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamedeyazdan S, Fathiazad F, Sharifi S, Nazemiyeh H. Antiproliferative activity of Marrubium persicum extract in the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev. 2012;13:5843–8. doi: 10.7314/apjcp.2012.13.11.5843. [DOI] [PubMed] [Google Scholar]
- 23.Suttana W, Mankhetkorn S, Poompimon W, Palagani A, Zhokhov S, Gerlo S, et al. Differential chemosensitization of P-glycoprotein overexpressing K562/Adr cells by withaferin A and Siamois polyphenols. Mol Cancer. 2010;9:99. doi: 10.1186/1476-4598-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanno S, Tomizawa A, Hiura T, Osanai Y, Shouji A, Ujibe M, et al. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol Pharm Bull. 2005;28:527–30. doi: 10.1248/bpb.28.527. [DOI] [PubMed] [Google Scholar]
- 25.Bestwick CS, Milne L. Influence of galangin on HL-60 cell proliferation and survival. Cancer Lett. 2006;243:80–9. doi: 10.1016/j.canlet.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Morales P, Haza AI. Selective apoptotic effects of piceatannol and myricetin in human cancer cells. J Appl Toxicol. 2012;32:986–93. doi: 10.1002/jat.1725. [DOI] [PubMed] [Google Scholar]
- 27.Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333:169–80. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 28.Sato F, Matsukawa Y, Matsumoto K, Nishino H, Sakai T. Apigenin induces morphological differentiation and G2-M arrest in rat neuronal cells. Biochem Biophys Res Commun. 1994;204:578–84. doi: 10.1006/bbrc.1994.2498. [DOI] [PubMed] [Google Scholar]
- 29.Chung CS, Jiang Y, Cheng D, Birt DF. Impact of adenomatous polyposis coli (APC) tumor supressor gene in human colon cancer cell lines on cell cycle arrest by apigenin. Mol Carcinog. 2007;46:773–82. doi: 10.1002/mc.20306. [DOI] [PubMed] [Google Scholar]
- 30.Kilani-Jaziri S, Frachet V, Bhouri W, Ghedira K, Chekir-Ghedira L, Ronot X. Flavones inhibit the proliferation of human tumor cancer cell lines by inducing apoptosis. Drug Chem Toxicol. 2012;35:1–10. doi: 10.3109/01480545.2011.564180. [DOI] [PubMed] [Google Scholar]
- 31.Lee EJ, Oh SY, Sung MK. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol. 2012;50:4136–43. doi: 10.1016/j.fct.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Bulzomi P, Bolli A, Galluzzo P, Acconcia F, Ascenzi P, Marino M. The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high nisphenol a background. IUBMB Life. 2012;64:690–6. doi: 10.1002/iub.1049. [DOI] [PubMed] [Google Scholar]
- 33.Dai Z, Nair V, Khan M, Ciolino HP. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol Rep. 2010;24:1087–91. [PubMed] [Google Scholar]
- 34.Morley KL, Ferguson PJ, Koropatnick J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007;251:168–78. doi: 10.1016/j.canlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–20. [PubMed] [Google Scholar]
- 36.Takagaki N, Sowa Y, Oki T, Nakanishi R, Yogosawa S, Sakai T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int J Oncol. 2005;26:185–9. [PubMed] [Google Scholar]
- 37.Pacifico S, Scognamiglio M, D’Abrosca B, Piccolella S, Tsafantakis N, Gallicchio M, et al. Spectroscopic characterization and antiproliferative activity on HepG2 human hepatoblastoma cells of flavonoid C-glycosides from Petrorhagia velutina. J Nat Prod. 2010;73:1973–8. doi: 10.1021/np100255u. [DOI] [PubMed] [Google Scholar]
- 38.Jeong JC, Kim MS, Kim TH, Kim YK. Kaempferol induces cell death through ERK and Akt-dependent down-regulation of XIAP and survivin in human glioma cells. Neurochem Res. 2009;34:991–1001. doi: 10.1007/s11064-008-9868-5. [DOI] [PubMed] [Google Scholar]
- 39.Kim DA, Jeon YK, Nam MJ. Galangin Induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food Chem Toxicol. 2012;50:684–8. doi: 10.1016/j.fct.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 40.Amado NG, Cerqueira DM, Menezes FS, da Silva JF, Neto VM, Abreu JG. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anticancer Drugs. 2009;20:543–52. doi: 10.1097/CAD.0b013e32832d1149. [DOI] [PubMed] [Google Scholar]
- 41.Lee ER, Kang YJ, Choi HY, Kang GH, Kim JH, Kim BW, et al. Induction of apoptotic cell death by synthetic naringenin derivatives in human lung epithelial carcinoma A549 cells. Biol Pharm Bull. 2007;30:2394–8. doi: 10.1248/bpb.30.2394. [DOI] [PubMed] [Google Scholar]
- 42.Shih YW, Wu PF, Lee YC, Shi MD, Chiang TA. Myricetin suppresses invasion and migration of human lung adenocarcinoma A549 cells: Possible mediation by blocking the ERK signaling pathway. J Agric Food Chem. 2009;57:3490–9. doi: 10.1021/jf900124r. [DOI] [PubMed] [Google Scholar]
- 43.Ninomiya M, Nishida K, Tanaka K, Watanabe K, Koketsu M. Structure-activity relationship studies of 5,7-dihydroxyflavones as naturally occurring inhibitors of cell proliferation in human leukemia HL-60 cells. J Nat Med. 2013;67:460–7. doi: 10.1007/s11418-012-0697-0. [DOI] [PubMed] [Google Scholar]
- 44.Wang BD, Yang ZY, Wang Q, Cai TK, Crewdson P. Synthesis, characterization, cytotoxic activities, and DNA-binding properties of the La(III) complex with Naringenin Schiff-base. Bioorg Med Chem. 2006;14:1880–8. doi: 10.1016/j.bmc.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Lefort EC, Blay J. Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res. 2013;57:126–44. doi: 10.1002/mnfr.201200424. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, VanAlstyne PC, Irons KA, Chen S, Stewart JW, Birt DF. Individual and interactive effects of apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma cell lines. Nutr Cancer. 2004;48:106–14. doi: 10.1207/s15327914nc4801_14. [DOI] [PubMed] [Google Scholar]
- 47.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise (review) Int J Oncol. 2007;30:233–45. [PubMed] [Google Scholar]
- 48.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11:2188–99. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N, Liu JH, Zhang J, Yu BY. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J Agric Food Chem. 2008;56:3876–83. doi: 10.1021/jf073520n. [DOI] [PubMed] [Google Scholar]
- 50.Guthrie N, Carroll KK. Inhibition of mammary cancer by citrus flavonoids. Adv Exp Med Biol. 1998;439:227–36. doi: 10.1007/978-1-4615-5335-9_16. [DOI] [PubMed] [Google Scholar]
- 51.Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr Cancer. 2000;38:116–22. doi: 10.1207/S15327914NC381_16. [DOI] [PubMed] [Google Scholar]
- 52.Torkin R, Lavoie JF, Kaplan DR, Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol Cancer Ther. 2005;4:1–11. [PubMed] [Google Scholar]
- 53.Yoshimizu N, Otani Y, Saikawa Y, Kubota T, Yoshida M, Furukawa T, et al. Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: Antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment Pharmacol Ther. 2004;20:95–101. doi: 10.1111/j.1365-2036.2004.02082.x. [DOI] [PubMed] [Google Scholar]
- 54.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, et al. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–21. [PubMed] [Google Scholar]
- 55.Fotsis T, Pepper MS, Montesano R, Aktas E, Breit S, Schweigerer L, et al. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin Endocrinol Metab. 1998;12:649–66. doi: 10.1016/s0950-351x(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 56.Lim do Y, Jeong Y, Tyner AL, Park JH. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G66–75. doi: 10.1152/ajpgi.00248.2006. [DOI] [PubMed] [Google Scholar]
- 57.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–20. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 58.Yuan E, Liu B, Ning Z, Chen C. Preparative separation of flavonoids in Adinandra nitida leaves by high-speed counter-current chromatography and their effects on human epidermal carcinoma cancer cells. Food Chem. 2009;115:1158–63. [Google Scholar]
- 59.Tokalov SV, Abramyuk AM, Abolmaali ND. Protection of p53 wild type cells from taxol by genistein in the combined treatment of lung cancer. Nutr Cancer. 2010;62:795–801. doi: 10.1080/01635581003605912. [DOI] [PubMed] [Google Scholar]
- 60.Plochmann K, Korte G, Koutsilieri E, Richling E, Riederer P, Rethwilm A, et al. Structure-activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch Biochem Biophys. 2007;460:1–9. doi: 10.1016/j.abb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Ben Sghaier M, Skandrani I, Nasr N, Franca MG, Chekir-Ghedira L, Ghedira K. Flavonoids and sesquiterpenes from Tecurium ramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: A structure-activity relationship study. Environ Toxicol Pharmacol. 2011;32:336–48. doi: 10.1016/j.etap.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Hirano T, Abe K, Gotoh M, Oka K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br J Cancer. 1995;72:1380–8. doi: 10.1038/bjc.1995.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu FL, Lin JK. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2008;68:61–71. doi: 10.1002/pros.20690. [DOI] [PubMed] [Google Scholar]
- 64.Galvez M, Martin-Cordero C, Lopez-Lazaro M, Cortes F, Ayuso MJ. Cytotoxic effect of Plantago spp. on cancer cell lines. J Ethnopharmacol. 2003;88:125–30. doi: 10.1016/s0378-8741(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 65.Choi EJ. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: Involvement of CDK4 and p21. Nutr Cancer. 2007;59:115–9. doi: 10.1080/01635580701419030. [DOI] [PubMed] [Google Scholar]
- 66.Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000;160:219–28. doi: 10.1016/s0304-3835(00)00591-7. [DOI] [PubMed] [Google Scholar]
- 67.Androutsopoulos VP, Ruparelia K, Arroo RR, Tsatsakis AM, Spandidos DA. CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology. 2009;264:162–70. doi: 10.1016/j.tox.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 68.Seelinger G, Merfort I, Wölfle U, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–51. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 70.Wätjen W, Weber N, Lou YJ, Wang ZQ, Chovolou Y, Kampkötter A, et al. Prenylation enhances cytotoxicity of apigenin and liquiritigenin in rat H4IIE hepatoma and C6 glioma cells. Food Chem Toxicol. 2007;45:119–24. doi: 10.1016/j.fct.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Cai X, Ye T, Liu C, Lu W, Lu M, Zhang J, et al. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol In Vitro. 2011;25:1385–91. doi: 10.1016/j.tiv.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Chen AY, Li M, Chen C, Yao Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J Surg Res. 2008;148:17–23. doi: 10.1016/j.jss.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu XF, Cai BL, Guan SM, Li Y, Wu JZ, Wang Y, et al. Baicalin induces human mucoepidermoid carcinoma Mc3 cells apoptosis in vitro and in vivo. Invest New Drugs. 2011;29:637–45. doi: 10.1007/s10637-010-9402-x. [DOI] [PubMed] [Google Scholar]
- 74.Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem Pharmacol. 2002;63:225–36. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]
- 75.Park JH, Jin CY, Lee BK, Kim GY, Choi YH, Jeong YK. Naringenin induces apoptosis through downregulation of Akt and caspase-3 activation in human leukemia THP-1 cells. Food Chem Toxicol. 2008;46:3684–90. doi: 10.1016/j.fct.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 76.Anter J, Romero-Jimenez M, Fernandez-Badmar Z, Villatoro-Pulido M, Analla M, Alonso-Moraga A, et al0. Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J Med Food. 2011;14:276–83. doi: 10.1089/jmf.2010.0139. [DOI] [PubMed] [Google Scholar]
- 77.Choi EJ, Kim GH. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J Nutr Biochem. 2009;20:285–90. doi: 10.1016/j.jnutbio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Kajimoto S, Takanashi N, Kajimoto T, Xu M, Cao J, Masuda Y, et al. Sophoranone, extracted from a traditional Chinese medicine Shan Dou Gen, induces apoptosis in human leukemia U937 cells via formation of reactive oxygen species and opening of mitochondrial permeability transition pores. Int J Cancer. 2002;99:879–90. doi: 10.1002/ijc.10414. [DOI] [PubMed] [Google Scholar]
- 79.Lin CC, Kuo CL, Lee MH, Lai KC, Lin JP, Yang JS, et al. Wogonin triggers apoptosis in human osteosarcoma U-2 OS cells through the endoplasmic reticulum stress, mitochondrial dysfunction and caspase-3-dependent signaling pathways. Int J Oncol. 2011;39:217–24. doi: 10.3892/ijo.2011.1027. [DOI] [PubMed] [Google Scholar]
- 80.Fu J, Chen D, Zhao B, Zhao Z, Zhou J, Xu J, et al. Luteolin induces carcinoma cell apoptosis through binding Hsp90 to suppress constitutive activation of STAT3. PloS One. 2012:e49194. doi: 10.1371/journal.pone.0049194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J Cell Biochem. 2009;106:73–82. doi: 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu X, Jung Ji, Cho HJ, Lim DY, Lee HS, Chun HS, et al. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135:2884–90. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- 83.Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6:1102–14. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 84.Koide T, Kamei H, Hashimoto Y, Kojima T, Terabe K, Umeda T. Influence of flavonoids on cell cycle phase as analyzed by flow-cytometry. Cancer Biother Radiopharm. 1997;12:111–5. doi: 10.1089/cbr.1997.12.111. [DOI] [PubMed] [Google Scholar]
- 85.Neves MP, Cidade H, Pinto M, Silva AM, Gales L, Damas AM, et al. Prenylated derivatives of baicalein and 3,7-dihydroxyflavone: Synthesis and study of their effects on tumor cell lines growth, cell cycle and apoptosis. Eur J Med Chem. 2011;46:2562–74. doi: 10.1016/j.ejmech.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 86.Leung HW, Lin CJ, Hour MJ, Yang WH, Wang MY, Lee HZ. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem Toxicol. 2007;45:2005–13. doi: 10.1016/j.fct.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 87.Habtemarium S, Dagne E. Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med. 2010;76:589–94. doi: 10.1055/s-0029-1240604. [DOI] [PubMed] [Google Scholar]
- 88.Valdameri G, Trombetta-Lima M, Worfel PR, Pires AR, Martinez GR, Noleto GR, et al. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem Biol Interact. 2011;193:180–9. doi: 10.1016/j.cbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Yuan L, Wang J, Xiao H, Xiao C, Wang Y, Liu X. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharmacol. 2012;265:83–92. doi: 10.1016/j.taap.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 90.Xu Y, Xin Y, Diao Y, Lu C, Fu J, Luo L, et al. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS One. 2011;6:e29169. doi: 10.1371/journal.pone.0029169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsai CF, Yeh WL, Huang SM, Tan TW, Lu DY. Wogonin induces reactive oxygen species production and cell apoptosis in human glioma cancer cells. Int J Mol Sci. 2012;13:9877–92. doi: 10.3390/ijms13089877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull. 2005;28:253–9. doi: 10.1248/bpb.28.253. [DOI] [PubMed] [Google Scholar]
- 93.Agullo G, Gamet-Payrastre L, Fernandez Y, Anciaux N, Demigne C, Rémésy C. Comparative effects of flavonoids on the growth, viability and metabolism of a colonic adenocarcinoma cell line (HT29 cells) Cancer Lett. 1996;105:61–70. doi: 10.1016/0304-3835(96)04262-0. [DOI] [PubMed] [Google Scholar]
- 94.Lee KW, Hur HJ, Lee HJ, Lee CY. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J Agric Food Chem. 2005;53:1990–5. doi: 10.1021/jf0486040. [DOI] [PubMed] [Google Scholar]
- 95.Talib WH, Zarga MH, Mahasneh AM. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from inula viscosa. Molecules. 2012;17:3291–303. doi: 10.3390/molecules17033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benavente-Garcia O, Castillo J, Lorente J, Alcaraz M, Yanez J, Martinez C, et al. Antiproliferative activity of several phenolic compounds against melanoma cell lines: Relationship between structure and activity. Agro Food Ind Hi-Tech. 2005;16:30–4. [Google Scholar]
- 97.Rodriguez J, Yanez J, Vicente V, Alcaraz M, Benavente-Garcia O, Castillo J, et al. Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: Relationship between structure and activity. Melanoma Res. 2002;12:99–107. doi: 10.1097/00008390-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Ragazzon PA, Bradshaw T, Matthews C, Iley J, Missailidis S. The characterisation of flavone-DNA isoform interactions as a basis for anticancer drug development. Anticancer Res. 2009;29:2273–83. [PubMed] [Google Scholar]
- 99.Mamadalieva NZ, Herrmann F, El-Readi MZ, Tahrani A, Hamoud R, Egamberdieva DR, et al. Flavonoids in Scutellaria immaculata and S. ramosissima (Lamiaceae) and their biological activity. J Pharm Pharmacol. 2011;63:1346–57. doi: 10.1111/j.2042-7158.2011.01336.x. [DOI] [PubMed] [Google Scholar]
- 100.Rao PS, Satelli A, Moridani M, Jenkins M, Rao US. Luteolin induces apoptosis in multidrug resistant cancer cells without affecting the drug transporter function: Involvement of cell line-specific apoptotic mechanisms. Int J Cancer. 2012;130:2703–14. doi: 10.1002/ijc.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Androutsopoulos VP, Spandidos DA. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma HepG2 cells via CYP1A-catalyzed metabolism, activation of JNK and ERK and P53/P21 up-regulation. J Nutr Biochem. 2013;24:496–504. doi: 10.1016/j.jnutbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Jin CY, Park C, Lee JH, Chung KT, Kwon TK, Kim GY, et al. Naringenin-induced apoptosis is attenuated by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human leukemia U937 cells. Toxicol In Vitro. 2009;23:259–65. doi: 10.1016/j.tiv.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 103.Harmon AW, Patel YM. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: A mechanism for impaired cellular proliferation. Breast Cancer Res Treat. 2004;85:103–10. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- 104.Yang Y, Wolfram J, Boom K, Fang X, Shen H, Ferrari M. Hesperetin impairs glucose uptake and inhibits proliferation of breast cancer cells. Cell Biochem Funct. 2013;31:374–9. doi: 10.1002/cbf.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pandey M, Kaur P, Shukla S, Abbas A, Fu P, Gupta S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol Carcinog. 2012;51:952–62. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo H, Jiang B, Li B, Li Z, Jiang BH, Chen YC. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int J Nanomedicine. 2012;7:3951–9. doi: 10.2147/IJN.S33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao H, Yang CS, Li S, Jin H, Ho CT, Patel T. Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel inhibit growth of human lung cancer cells by apoptosis. Mol Nutr Food Res. 2009;53:398–406. doi: 10.1002/mnfr.200800057. [DOI] [PubMed] [Google Scholar]
- 108.Walle T, Ta N, Kawamori T, Wen X, Tsuji PA, Walle UK. Cancer chemopreventive properties of orally bioavailable flavonoids-methylated versus unmethylated flavones. Biochem Pharmacol. 2007;73:1288–96. doi: 10.1016/j.bcp.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seibert H, Maser E, Schweda K, Seibert S, Gülden M. Cytoprotective activity against peroxide-induced oxidative damage and cytotoxicity of flavonoids in C6 rat glioma cells. Food Chem Toxicol. 2011;49:2398–407. doi: 10.1016/j.fct.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 110.Iwuchukwu OF, Tallarida RJ, Nagar S. Resveratrol in combination with other dietary polyphenols concomitantly enhances antiproliferation and UGT1A1 induction in Caco-2 cells. Life Sci. 2011;88:1047–54. doi: 10.1016/j.lfs.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun F, Zheng XY, Ye J, Wu TT, Wang JI, Chen W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr Cancer. 2012;64:599–606. doi: 10.1080/01635581.2012.665564. [DOI] [PubMed] [Google Scholar]
- 112.Rodgers EH, Grant MH. The effect of the flavonoids, quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chem Biol Interact. 1998;116:213–28. doi: 10.1016/s0009-2797(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 113.Forni C, Braglia R, Lentini A, Nuccetelli M, Provenzano B, Tabolacci C, et al. Role of transglutaminase 2 in quercetin-induced differentiation of B16-F10 murine melanoma cells. Amino Acids. 2009;36:731–8. doi: 10.1007/s00726-008-0158-y. [DOI] [PubMed] [Google Scholar]
- 114.Nagao T, Abe F, Kinjo J, Okabe H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol Pharm Bull. 2002;25:875–9. doi: 10.1248/bpb.25.875. [DOI] [PubMed] [Google Scholar]
- 115.Himeji M, Ohtsuki T, Fukazawa H, Tanaka M, Yazaki S, Ui S, et al. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007;245:269–74. doi: 10.1016/j.canlet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 116.Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate. 2001;49:285–92. doi: 10.1002/pros.10024. [DOI] [PubMed] [Google Scholar]
- 117.Bonham M, Posakony J, Coleman I, Montgomery B, Simon J, Nelson PS, et al. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin Cancer Res. 2005;11:3905–14. doi: 10.1158/1078-0432.CCR-04-1974. [DOI] [PubMed] [Google Scholar]
- 118.Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, et al. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128:539–46. doi: 10.1007/s00432-002-0373-y. [DOI] [PubMed] [Google Scholar]
- 119.Chen CH, Huang LL, Huang CC, Lin CC, Lee Y, Lu FJ. Baicalein, a novel apoptotic agent for hepatoma cell lines: A potential medicine for hepatoma. Nutr Cancer. 2000;38:287–95. doi: 10.1207/S15327914NC382_19. [DOI] [PubMed] [Google Scholar]
- 120.Kuo SM. Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells. Cancer Lett. 1996;110:41–8. doi: 10.1016/s0304-3835(96)04458-8. [DOI] [PubMed] [Google Scholar]
- 121.Hong TB, Rahumatullah A, Yogarajah T, Ahmad M, Yin KB. Potential effects of chrysin on MDA-MB-231 cells. Int J Mol Sci. 2010;11:1057–69. doi: 10.3390/ijms11031057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang SF, Yang WE, Chang HR, Chu SC, Hsieh YS. Luteolin induces apoptosis in oral squamous cancer cells. J Dent Res. 2008;87:401–6. doi: 10.1177/154405910808700413. [DOI] [PubMed] [Google Scholar]
- 123.Wu B, Zhang Q, Shen W, Zhu J. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol Cell Biochem. 2008;313:125–32. doi: 10.1007/s11010-008-9749-x. [DOI] [PubMed] [Google Scholar]
- 124.Patil JR, Chidambara Murthy KN, Jayaprakasha GK, Chetti MB, Patil BS. Bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J Agric Food Chem. 2009;57:10933–42. doi: 10.1021/jf901718u. [DOI] [PubMed] [Google Scholar]
- 125.Li W, Du B, Wang T, Wang S, Zhang J. Kaempferol induces apoptosis in human HCT116 colon cancer cells via the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement of p53 Upregulated Modulator of Apoptosis. Chem Biol Interact. 2009;177:121–7. doi: 10.1016/j.cbi.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 126.Luo H, Rankin GO, Li Z, Depriest L, Chen YC. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011;128:513–9. doi: 10.1016/j.foodchem.2011.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–10. [PubMed] [Google Scholar]
- 128.Sivagami G, Vinothkumar R, Preethy CP, Riyasdeen A, Akbarsha MA, Menon VP, et al. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line: A comparative study. Food Chem Toxicol. 2012;50:660–71. doi: 10.1016/j.fct.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 129.You HJ, Ahn HJ, Ji GE. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J Agric Food Chem. 2010;58:10886–92. doi: 10.1021/jf102871g. [DOI] [PubMed] [Google Scholar]
- 130.Cai J, Zhao XL, Liu AW, Nian H, Zhang SH. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine. 2011;18:366–73. doi: 10.1016/j.phymed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 131.Wei L, Lu N, Dai Q, Rong J, Chen Y, Li Z, et al. Different apoptotic effects of wogonin via induction of H(2)O(2) generation and Ca(2+) overload in malignant hepatoma and normal hepatic cells. J Cell Biochem. 2010;111:1629–41. doi: 10.1002/jcb.22898. [DOI] [PubMed] [Google Scholar]
- 132.Xu M, Lu N, Zhang H, Dai Q, Wei L, Li Z, et al. Wogonin induced cytotoxicity in human hepatocellular carcinoma cells by activation of unfolded protein response and inactivation of AKT. Hepatol Res. 2013;43:890–905. doi: 10.1111/hepr.12036. [DOI] [PubMed] [Google Scholar]
- 133.Romanouskaya TV, Grinev VV. Cytotoxic effect of flavonoids on leukemia cells and normal cells of human blood. Bull Exp Biol Med. 2009;148:57–9. doi: 10.1007/s10517-009-0633-9. [DOI] [PubMed] [Google Scholar]
- 134.Jang KY, Jeong SJ, Kim SH, Jung JH, Kim JH, Koh W, et al. Activation of reactive oxygen species/AMP activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer Lett. 2012;319:197–202. doi: 10.1016/j.canlet.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 135.Baumann S, Fas SC, Giaisi M, Müller WW, Merling A, Gülow K, et al. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis. Blood. 2008;111:2354–63. doi: 10.1182/blood-2007-06-096198. [DOI] [PubMed] [Google Scholar]
- 136.Guthrie N, Gapor A, Chambers AF, Carroll KK. Palm oil tocotrienols and plant flavonoids act synergistically to inhibit proliferation of estrogen receptor-negative MDA-MB-231 and -positive MCF-7 human breast cancer cells in culture. Asia Pacific J Clin Nutr. 1997;6:41–5. [PubMed] [Google Scholar]
- 137.Wang LM, Xie KP, Huo HN, Shang F, Zou W, Xie MJ. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac J Cancer Prev. 2012;13:1431–7. doi: 10.7314/apjcp.2012.13.4.1431. [DOI] [PubMed] [Google Scholar]
- 138.Kim MJ, Woo JS, Kwon CH, Kim JH, Kim YK, Kim KH. Luteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell lines. Cell Biol Int. 2012;36:339–44. doi: 10.1042/CBI20110394. [DOI] [PubMed] [Google Scholar]
- 139.Long X, Fan M, Bigsby RM, Nephew KP. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol Cancer Ther. 2008;7:2096–108. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Britton RG, Horner-Glister E, Pomenya OA, Smith EE, Denton R, Jenkins PR, et al. Synthesis and biological evaluation of novel flavonols as potential anti-prostate cancer agents. Eur J Med Chem. 2012;54:952–8. doi: 10.1016/j.ejmech.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 141.Kim JY, Jeon YK, Jeon W, Nam MJ. Fisetin induces apoptosis in Huh-7 cells via downregulation of BIRC8 and Bcl2L2. Food Chem Toxicol. 2010;48:2259–64. doi: 10.1016/j.fct.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 142.Khan N, Asim M, Afaq F, Abu Zaid M, Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–63. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seo YJ, Kim BS, Chun SY, Park YK, Kang KS, Kwon TG. Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J Korean Med Sci. 2011;26:1489–94. doi: 10.3346/jkms.2011.26.11.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen S, Ruan Q, Bedner E, Deptala A, Wang X, Hsieh TC, et al. Effects of the flavonoid baicalin and its metabolite baicalein on androgen receptor expression, cell cycle progression and apoptosis of prostate cancer cell lines. Cell Prolif. 2001;34:293–304. doi: 10.1046/j.0960-7722.2001.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677–84. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- 146.Yang J, Liu RH. Synergistic effect of apple extracts and quercetin 3-beta-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J Agric Food Chem. 2009;57:8581–6. doi: 10.1021/jf8039796. [DOI] [PubMed] [Google Scholar]
- 147.Parajuli P, Joshee N, Rimando AM, Mittal S, Yadav AK. In vitro antitumor mechanisms of various Scutellaria extracts and constituent flavonoids. Planta Med. 2009;75:41–8. doi: 10.1055/s-0028-1088364. [DOI] [PubMed] [Google Scholar]
- 148.Skupień K, Oszmiański J, Kostrzewa-Nowak D, Tarasiuk J. In vitro antileukaemic activity of extracts from berry plant leaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006;236:282–91. doi: 10.1016/j.canlet.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 149.Lu HF, Chie YJ, Yang MS, Lee CS, Fu JJ, Yang JS, et al. Apigenin induces caspase-dependent apoptosis in human lung cancer A549 cells through Bax- and Bcl-2-triggered mitochondrial pathway. Int J Oncol. 2010;36:1477–84. doi: 10.3892/ijo_00000634. [DOI] [PubMed] [Google Scholar]
- 150.Chen WY, Hsieh YA, Tsai CI, Kang YF, Chang FR, Wu YC, et al. Protoapigenone, a natural derivative of apigenin, induces mitogen-activated protein kinase-dependent apoptosis in human breast cancer cells associated with induction of oxidative stress and inhibition of glutathione S-transferase TT. Invest New Drugs. 2011;29:1347–59. doi: 10.1007/s10637-010-9497-0. [DOI] [PubMed] [Google Scholar]
- 151.Strouch MJ, Milam BM, Melstrom LG, McGill JJ, Salabat MR, Ujiki MB, et al. The flavonoid apigenin potentiates the growth inhibitory effects of gemcitabine and abrogates gemcitabine resistance in human pancreatic cancer cells. Pancreas. 2009;38:409–15. doi: 10.1097/MPA.0b013e318193a074. [DOI] [PubMed] [Google Scholar]
- 152.Sak K. Site-specific anticancer effects of dietay flavonoid quercetin. Nutr Cancer. 2014;66:177–93. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 153.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB signaling pathways. Carcinogenesis. 2009;30:300–7. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Syed DN, Afaq F, Maddodi N, Johnson JJ, Sarfaraz S, Ahmad A, et al. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/β-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011;131:1291–9. doi: 10.1038/jid.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lim do Y, Park JH. Induction of p53 contributes to apoptosis of HCT-116 human colon cancer cells induced by the diteary compound fisetin. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1060–8. doi: 10.1152/ajpgi.90490.2008. [DOI] [PubMed] [Google Scholar]
- 156.Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietay flavonoid fisetin. Int J Cancer. 2012;130:1695–705. doi: 10.1002/ijc.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–56. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haddad AQ, Fleshner N, Nelson C, Saour B, Musquera M, Venkateswaran V, et al. Antiproliferative mechanisms of the flavonoids 2,2’-dihydroxychalcone and fisetin in human prostate cancer cells. Nutr Cancer. 2010;62:668–81. doi: 10.1080/01635581003605524. [DOI] [PubMed] [Google Scholar]
- 159.Zhang W, Lan Y, Huang Q, Hua Z. Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology. 2013;65:447–55. doi: 10.1007/s10616-012-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murray TJ, Yang X, Sherr DH. Growth of a human mammary tumor cell line is blocked by galangin, a naturally occurring bioflavonoid, and is accompanied by down-regulation of cyclins D3, E, and A. Breast Cancer Res. 2006;8:R17. doi: 10.1186/bcr1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hernandez J, Goycoolea FM, Quintero J, Acosta A, Castaneda M, Dominguez Z, et al. Sonoran propolis: Chemical composition and antiproliferative activity on cancer cell lines. Planta Med. 2007;73:1469–74. doi: 10.1055/s-2007-990244. [DOI] [PubMed] [Google Scholar]
- 162.Bestwick CS, Milne L, Duthie SJ. Kaempferol induced inhibition of HL-60 cell growth results from a heterogeneous response, dominated by cell cycle alterations. Chem Biol Interact. 2007;170:76–85. doi: 10.1016/j.cbi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Ackland ML, van de Waarsenburg S, Jones R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo. 2005;19:69–76. [PubMed] [Google Scholar]
- 164.Fabjan N, Rode J, Kosir IJ, Wang Z, Zhang Z, Kreft I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a course of dietary rutin and quercitrin. J Agric Food Chem. 2003;51:6452–5. doi: 10.1021/jf034543e. [DOI] [PubMed] [Google Scholar]
- 165.Mämmelä P, Savolainen H, Lindroos L, Kangas J, Vartiainen T. Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry. J Chromatogr A. 2000;891:75–83. doi: 10.1016/s0021-9673(00)00624-5. [DOI] [PubMed] [Google Scholar]
- 166.Jiang S, Yang J, Qian D, Guo J, Shang EX, Duan JA, et al. Rapid screening and identification of metabolites of quercitrin produced by the human intestinal bacteria using ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. Arch Pharm Res. 2014;37:204–13. doi: 10.1007/s12272-013-0172-9. [DOI] [PubMed] [Google Scholar]
- 167.Thani W, Vallisuta O, Siripong P, Ruangwises N. Anti-proliferative and antioxidative activities of Thai noni/Yor (Morinda citrifolia Linn.) leaf extract. Southeast Asian J Trop Med Public Health. 2010;41:482–9. [PubMed] [Google Scholar]
- 168.Lee ER, Kang YJ, Kim HJ, Choi HY, Kang GH, Kim JH, et al. Regulation of apoptosis by modified naringenin derivatives in human colorectal carcinoma RKO cells. J Cell Biochem. 2008;104:259–73. doi: 10.1002/jcb.21622. [DOI] [PubMed] [Google Scholar]
- 169.Frydoonfar HR, McGrath DR, Spigelman AD. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid Naringenin. Colorectal Dis. 2003;5:149–52. doi: 10.1046/j.1463-1318.2003.00444.x. [DOI] [PubMed] [Google Scholar]
- 170.Alshatwi AA, Hasan TN, Shafi G, Alsaif MA, Al-Hazzani AA, Alsaif AA. A single-nucleotide polymorphism in the TP53 and MDM-2 gene modifies breast cancer risk in an ethnic Arab population. Fundam Clin Pharmacol. 2012;26:438–43. doi: 10.1111/j.1472-8206.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- 171.Zarebczan B, Pinchot SN, Kunnimalaiyaan M, Chen H. Hesperetin, a potential therapy for carcinoid cancer. Am J Surg. 2011;201:329–32. doi: 10.1016/j.amjsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quintin J, Buisson D, Thoret S, Cresteil T, Lewin G. Semisynthesis and antiproliferative evaluation of a series of 3’-aminoflavones. Bioorg Med Chem Lett. 2009;19:3502–6. doi: 10.1016/j.bmcl.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 173.Pereira RM, Andrades NE, Paulino N, Sawaya AC, Eberlin MN, Marcucci MC, et al. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules. 2007;12:1352–66. doi: 10.3390/12071352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, Abubakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wesolowska O, Wisniewski J, Sroda-Pomianek K, Bielawska-Pohl A, Paprocka M, Duś D, et al. Multidrug resistance reversal and apoptosis induction in human colon cancer cells by some flavonoids present in Citrus plants. J Nat Prod. 2012;75:1896–902. doi: 10.1021/np3003468. [DOI] [PubMed] [Google Scholar]
- 176.Nazari M, Ghorbani A, Hekmat-Doost A, Jeddi-Tehrani M, Zand H. Inactivation of nuclear factor-kB by citrus flavanone hesperidin contributes to apoptosis and chemo-sensitizing effect in Ramos cells. Eur J Pharmacol. 2011;650:526–33. doi: 10.1016/j.ejphar.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 177.Al-Ashaal HA, El-Sheltawy ST. Antioxidant capacity of hesperidin from citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm Biol. 2011;49:276–82. doi: 10.3109/13880209.2010.509734. [DOI] [PubMed] [Google Scholar]
- 178.Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2008;226:178–91. doi: 10.1016/j.taap.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 179.Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–38. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- 180.Choi SI, Jeong CS, Cho SY, Lee YS. Mechanism of apoptosis induced by apigenin in HepG2 human hepatoma cells: Involvement of reactive oxygen species generated by NADPH oxidase. Arch Pharm Res. 2007;30:1328–35. doi: 10.1007/BF02980274. [DOI] [PubMed] [Google Scholar]
- 181.Mak P, Leung YK, Tang WY, Harwood C, Ho SM. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia. 2006;8:896–904. doi: 10.1593/neo.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lu HF, Chie YJ, Yang MS, Lu KW, Fu JJ, Yang JS, et al. Apigenin induces apoptosis in human lung cancer H460 cells through caspase- and mitochondria-dependent pathways. Hum Exp Toxicol. 2011;30:1053–61. doi: 10.1177/0960327110386258. [DOI] [PubMed] [Google Scholar]
- 183.Wu B, Li J, Huang D, Wang W, Chen Y, Liao Y, et al. Baicalein mediates inhibition of migration and invasiveness of skin carcinoma through Ezrin in A431 cells. BMC Cancer. 2011;11:527. doi: 10.1186/1471-2407-11-527. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184.Zhang DY, Wu J, Ye F, Xue L, Jiang S, Yi J, et al. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003;63:4037–43. [PubMed] [Google Scholar]
- 185.Li QB, You Y, Chen ZC, Lü J, Shao J, Zou P. Role of Baicalein in the regulation of proliferation and apoptosis in human myeloma RPMI8226 cells. Chin Med J (Engl) 2006;119:948–52. [PubMed] [Google Scholar]
- 186.Chou DS, Hsiao G, Lai YA, Tsai YJ, Sheu JR. Baicalein induces proliferation inhibition in B16F10 melanoma cells by generating reactive oxygen species via 12-lipoxygenase. Free Radic Biol Med. 2009;46:1197–203. doi: 10.1016/j.freeradbiomed.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 187.Weng MS, Ho YS, Lin JK. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: Involvement of p38 mitogen-activated protein kinase. Biochem Pharmacol. 2005;69:1815–27. doi: 10.1016/j.bcp.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 188.Pichichero E, Cicconi R, Mattei M, Muzi MG, Canini A. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. Int J Oncol. 2010;37:973–81. doi: 10.3892/ijo_00000748. [DOI] [PubMed] [Google Scholar]
- 189.Ren J, Cheng H, Xin WQ, Chen X, Hu K. Induction of apoptosis by 7-piperazinethylchrysin in HCT-116 human colon cancer cells. Oncol Rep. 2012;28:1719–26. doi: 10.3892/or.2012.2016. [DOI] [PubMed] [Google Scholar]
- 190.Tsui KH, Chung LC, Feng TH, Chang PL, Juang HH. Upregulation of prostate-derived Ets factor by luteolin causes inhibition of cell proliferation and cell invasion in prostate carcinoma cells. Int J Cancer. 2012;130:2812–23. doi: 10.1002/ijc.26284. [DOI] [PubMed] [Google Scholar]
- 191.Chen KH, Weng MS, Lin JK. Tangeretin suppresses IL-1beta-induced cyclooxygenase (COX)-2 expression through inhibition of p38 MAPK, JNK, and AKT activation in human lung carcinoma cells. Biochem Pharmacol. 2007;73:215–27. doi: 10.1016/j.bcp.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 192.Zhang M, Liu LP, Chen Y, Tian XY, Qin J, Wang D, et al. Wogonin induces apoptosis in RPMI 8226, a human myeloma cell line, by downregulating phospho-Akt and overexpressing Bax. Life Sci. 2013;92:55–62. doi: 10.1016/j.lfs.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 193.Ciesielska E, Gwardys A, Metodiewa D. Anticancer, antiradical and antioxidative actions of novel Antoksyd S and its major components, baicalin and baicalein. Anticancer Res. 2002;22:2885–91. [PubMed] [Google Scholar]
- 194.Wang N, Tang LJ, Zhu GQ, Peng DY, Wang L, Sun FN, et al. Apoptosis induced by baicalin involving up-regulation of P53 and bax in MCF-7 cells. J Asian Nat Prod Res. 2008;10:1129–35. doi: 10.1080/10286020802410664. [DOI] [PubMed] [Google Scholar]
- 195.Chowdhury SA, Kishino K, Satoh R, Hashimoto K, Kikuchi H, Nishikawa H, et al. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005;25:2055–63. [PubMed] [Google Scholar]
- 196.Katalinić M, Rusak G, Domaćinović Barović J, Sinko G, Jelić D, Antolović R, et al. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur J Med Chem. 2010;45:186–92. doi: 10.1016/j.ejmech.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 197.Monasterio A, Urdaci MC, Pinchuk IV, Lopez-Moratalla N, Martinez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 198.Richter M, Ebermann R, Marian B. Quercetin-induced apoptosis in colorectal tumor cells: Possible role of EGF receptor signaling. Nutr Cancer. 1999;34:88–99. doi: 10.1207/S15327914NC340113. [DOI] [PubMed] [Google Scholar]
- 199.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–42. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 200.Lee SW, Lee JT, Lee MG, Lee HW, Ahn SJ, Lee YJ, et al. In vitro antiproliferative characteristics of flavonoids and diazepam on SNU-C4 colorectal adenocarcinoma cells. J Nat Med. 2009;63:124–9. doi: 10.1007/s11418-008-0300-x. [DOI] [PubMed] [Google Scholar]
- 201.Kim DH, Lee JT, Lee IK, Ha JH. Comparative anticancer effects of flavonoids and diazepam in cultured cancer cells. Biol Pharm Bull. 2008;31:255–9. doi: 10.1248/bpb.31.255. [DOI] [PubMed] [Google Scholar]
- 202.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465–73. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9:68–76. doi: 10.1038/sj.pcan.4500845. [DOI] [PubMed] [Google Scholar]
- 204.Ludwiczuk A, Saha A, Kuzuhara T, Asakawa Y. Bioactivity guided isolation of anticancer constituents from leaves of Alnus sieboldiana (Betulaceae) Phytomedicine. 2011;18:491–8. doi: 10.1016/j.phymed.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 205.Brown J, O’Prey J, Harrison PR. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: Involvement of the Akt and stress kinase pathways. Carcinogenesis. 2003;24:171–7. doi: 10.1093/carcin/24.2.171. [DOI] [PubMed] [Google Scholar]
- 206.Li F, Awale S, Tezuka Y, Esumi H, Kadota S. Study on the constituents of Mexican propolis and their cytotoxic activity against PANC-1 human pancreatic cancer cells. J Nat Prod. 2010;73:623–7. doi: 10.1021/np900772m. [DOI] [PubMed] [Google Scholar]
- 207.Bai N, He K, Roller M, Lai CS, Shao X, Pan MH, et al. Flavonoids and phenolic compounds from Rosmarinus officinalis. J Argic Food Chem. 2010;58:5363–7. doi: 10.1021/jf100332w. [DOI] [PubMed] [Google Scholar]
- 208.Dickancaité E, Nemeikaitè A, Kalvelytè A, Cènas N. Prooxidant character of flavonoid cytotoxicity: Structure-activity relationships. Biochem Mol Biol Int. 1998;45:923–30. [PubMed] [Google Scholar]
- 209.Lin CM, Chen CT, Lee HH, Lin JK. Prevention of cellular ROS damage by isovitexin and related flavonoids. Planta Med. 2002;68:365–7. doi: 10.1055/s-2002-26753. [DOI] [PubMed] [Google Scholar]
- 210.Rusak G, Gutzeit HO, Müller JL. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutr Res. 2005;25:143–55. [Google Scholar]
- 211.Rao YK, Geethangili M, Fang SH, Tzeng YM. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: A comparative study. Food Chem Toxicol. 2007;45:1770–6. doi: 10.1016/j.fct.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 212.Bigović D, Savikin K, Janković T, Menković N, Zdunić G, Stanojković T, et al. Antiradical and cytotoxic activity of different Helichrysum plicatum flower extracts. Nat Prod Commun. 2011;6:819–22. [PubMed] [Google Scholar]
- 213.Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF, Li KH, et al. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol Nutr Food Res. 2010;54:1585–95. doi: 10.1002/mnfr.201000005. [DOI] [PubMed] [Google Scholar]
- 214.Wang C, Kurzer MS. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr Cancer. 1997;28:236–47. doi: 10.1080/01635589709514582. [DOI] [PubMed] [Google Scholar]
- 215.Balabhadrapathruni S, Thomas TJ, Yurkow EJ, Amenta PS, Thomas T. Effects of genistein and structurally related phytoestrogens on cell cycle kinetics and apoptosis in MDA-MB-468 human breast cancer cells. Oncol Rep. 2000;7:3–12. [PubMed] [Google Scholar]
- 216.Phromnoi K, Yodkeeree S, Anuchapreeda S, Limtrakul P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol Sin. 2009;30:1169–76. doi: 10.1038/aps.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Argic Food Chem. 2002;50:5837–43. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- 218.Loizzo MR, Said A, Tundis R, Hawas UW, Rashed K, Menichini F, et al. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum Nutr. 2009;64:264–70. doi: 10.1007/s11130-009-0133-0. [DOI] [PubMed] [Google Scholar]
- 219.Kim YK, Kim YS, Choi SU, Ryu SY. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch Pharm Res. 2004;27:44–7. doi: 10.1007/BF02980044. [DOI] [PubMed] [Google Scholar]
- 220.Kwak JH, Kang MW, Roh JH, Choi SU, Zee OP. Cytotoxic phenolic compounds from Chionanthus retusus. Arch Pharm Res. 2009;32:1681–7. doi: 10.1007/s12272-009-2203-0. [DOI] [PubMed] [Google Scholar]
- 221.Loa J, Chow P, Zhang K. Studies of structure-activity relationship on plant polyphenol-induced suppression of human liver cancer cells. Cancer Chemother Pharmacol. 2009;63:1007–16. doi: 10.1007/s00280-008-0802-y. [DOI] [PubMed] [Google Scholar]
- 222.Said A, Tundis R, Hawas UW, El-Kousy SM, Rashed K, Menichini F, et al. In vitro antioxidant and antiproliferative activities of flavonoids from Ailanthus excelsa (Roxb.) (Simaroubaceae) leaves. Z Naturforsch C. 2010;65:180–6. doi: 10.1515/znc-2010-3-403. [DOI] [PubMed] [Google Scholar]
- 223.Nguyen TT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197:110–21. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 224.Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem Pharmacol. 2001;61:1205–15. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 225.O’Prey J, Brown J, Fleming J, Harrison PR. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem Pharmacol. 2003;66:2075–88. doi: 10.1016/j.bcp.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 226.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46:2042–53. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 227.Zhang Q, Zhao XH, Wang ZJ. Cytotoxicity of flavones and flavonols to a human oesophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol In Vitro. 2009;23:797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 228.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Antiproliferative activity of flavonoids on several cancer cell lines. Biosci Biotechnol Biochem. 1999;63:896–9. doi: 10.1271/bbb.63.896. [DOI] [PubMed] [Google Scholar]
- 229.Freire AC, da Silva Melo P, Aoyama H, Haun M, Durán N, Ferreira CV. Cytotoxic effect of the diterpene lactone dehydrocrotonin from Croton cajucara on human promyelocytic leukemia cells. Planta Med. 2003;69:67–9. doi: 10.1055/s-2003-37036. [DOI] [PubMed] [Google Scholar]
- 230.Aherne SA, O’Brien NM. Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and Hep G2 cells. Nutr Cancer. 1999;34:160–6. doi: 10.1207/S15327914NC3402_6. [DOI] [PubMed] [Google Scholar]
- 231.Newell AM, Yousef GG, Lila MA, Ramirez-Mares MV, de Mejia EG. Comparative in vitro bioactivities of tea extracts from six species of Ardisia and their effect on growth inhibition of HepG2 cells. J Ethnopharmacol. 2010;130:536–44. doi: 10.1016/j.jep.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 232.Yáñez J, Vicente V, Alcaraz M, Castillo J, Benavente-García O, Canteras M, et al. Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocytes cell lines: Relationship between structure and activity. Nutr Cancer. 2004;49:191–9. doi: 10.1207/s15327914nc4902_11. [DOI] [PubMed] [Google Scholar]
- 233.Xu R, Zhang Y, Ye X, Xue S, Shi J, Pan J, et al. Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro. Food Chem. 2013;138:48–53. doi: 10.1016/j.foodchem.2012.09.102. [DOI] [PubMed] [Google Scholar]
- 234.Lohezic-Le Dehevat F, Tomasi S, Fontanel D, Boustie J. Flavonols from Scurrula Ferruginea Danser (Loranthaceae) Z Naturforsch C. 2002;57:1092–5. doi: 10.1515/znc-2002-11-1224. [DOI] [PubMed] [Google Scholar]
- 235.Simirgiotis MJ, Adachi S, To S, Yang H, Reynertson KA, Basile MJ, et al. Cytotoxic chalcones and antioxidants from the fruits of a Syzygium samarangense (Wax Jambu) Food Chem. 2008;107:813–9. doi: 10.1016/j.foodchem.2007.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Browning AM, Walle UK, Walle T. Flavonoid glycosides inhibit oral cancer cell proliferation - role of cellular uptake and hydrolysis to the aglycones. J Pharm Pharmacol. 2005;57:1037–42. doi: 10.1211/0022357056514. [DOI] [PubMed] [Google Scholar]
- 237.Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6:3039–48. doi: 10.1158/1535-7163.MCT-07-0281. [DOI] [PubMed] [Google Scholar]
- 238.Ramanathan R, Tan CH, Das NP. Cytotoxic effect of plant polyphenols and fat-soluble vitamins on malignant human cultured cells. Cancer Lett. 1992;62:217–24. doi: 10.1016/0304-3835(92)90099-h. [DOI] [PubMed] [Google Scholar]
- 239.Chen YH, Chen HY, Hsu CL, Yen GC. Induction of apoptosis by the Lactuca indica L. in human leukemia cell line and its active components. J Agric Food Chem. 2007;55:1743–9. doi: 10.1021/jf063118t. [DOI] [PubMed] [Google Scholar]
- 240.Rhouma GB, Chebil L, Mustapha N, Krifa M, Ghedira K, Ghoul M, et al. Cytotoxic, genotoxic and antigenotoxic potencies of oligorutins. Hum Exp Toxicol. 2013;32:881–9. doi: 10.1177/0960327113476910. [DOI] [PubMed] [Google Scholar]
- 241.Goniotaki M, Hatziantoniou S, Dimas K, Wagner M, Demetzos C. Encapsulation of naturally occurring flavonoids into liposomes: Physicochemical properties and biological activity against human cancer cell lines. J Pharm Pharmacol. 2004;56:1217–24. doi: 10.1211/0022357044382. [DOI] [PubMed] [Google Scholar]
- 242.Li L, Henry GE, Seeram NP. Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J Agric Food Chem. 2009;57:7282–7. doi: 10.1021/jf901716j. [DOI] [PubMed] [Google Scholar]
- 243.Alía M, Mateos R, Ramos S, Lecumberri E, Bravo L, Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2) Eur J Nutr. 2006;45:19–28. doi: 10.1007/s00394-005-0558-7. [DOI] [PubMed] [Google Scholar]
- 244.Bellocco E, Barreca D, Laganà G, Leuzzi U, Tellone E, Ficarra S, et al. Influence of L-rhamnosyl-D-glycosyl derivatives on properties and biological interaction of flavonoids. Mol Cell Biochem. 2009;321:165–71. doi: 10.1007/s11010-008-9930-2. [DOI] [PubMed] [Google Scholar]
- 245.Ramos AA, Lima CF, Pereira ML, Fernandes-Ferreira M, Pereira-Wilson C. Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells: Evaluation by the comet assay. Toxicol Lett. 2008;177:66–73. doi: 10.1016/j.toxlet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 246.Tsai YC, Lin CL, Chen BH. Preparative chromatography of flavonoids and saponins in Gynostemma pentaphyllum and their antiproliferation effect on hepatoma cell. Phytomedicine. 2010;18:2–10. doi: 10.1016/j.phymed.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 247.Wei XH, Yang SJ, Liang N, Hu DY, Jin LH, Xue W, et al. Chemical constituents of Caesalpinia decapetala (Roth) Alston. Molecules. 2013;18:1325–36. doi: 10.3390/molecules18011325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fang SC, Hsu CL, Lin HT, Yen GC. Anticancer effects of flavonoid derivatives isolated from Millettia reticulata Benth in SK-Hep-1 human hepatocellular carcinoma cells. J Agric Food Chem. 2010;58:814–20. doi: 10.1021/jf903216r. [DOI] [PubMed] [Google Scholar]
- 249.Moghaddam G, Ebrahimi SA, Rahbar-Roshandel N, Foroumadi A. Antiproliferative activity of flavonoids: Influence of the sequential methoxylation state of the flavonoid structure. Phytother Res. 2012;26:1023–8. doi: 10.1002/ptr.3678. [DOI] [PubMed] [Google Scholar]
- 250.Hakimuddin F, Paliyath G, Meckling K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res Treat. 2004;85:65–79. doi: 10.1023/B:BREA.0000021048.52430.c0. [DOI] [PubMed] [Google Scholar]
- 251.Miranda CL, Stevens JF, Helmrich A, Henderson MC, Rodriguez RJ, Yang YH, et al. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem Toxicol. 1999;37:271–85. doi: 10.1016/s0278-6915(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 252.So FV, Guthrie N, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997;112:127–33. doi: 10.1016/s0304-3835(96)04557-0. [DOI] [PubMed] [Google Scholar]
- 253.Yelani T, Hussein AA, Meyer JJ. Isolation and identification of poisonous triterpenoids from Elaeodendron croceum. Nat Prod Res. 2010;24:1418–25. doi: 10.1080/14786410903052399. [DOI] [PubMed] [Google Scholar]
- 254.So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer. 1996;26:167–81. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- 255.Jin CY, Park C, Hwang HJ, Kim GY, Choi BT, Kim WJ, et al. Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells. Mol Nutr Food Res. 2011;55:300–9. doi: 10.1002/mnfr.201000024. [DOI] [PubMed] [Google Scholar]
- 256.Jeon SH, Chun W, Choi YJ, Kwon YS. Cytotoxic constituents from the bark of Salix hulteni. Arch Pharm Res. 2008;31:978–82. doi: 10.1007/s12272-001-1255-9. [DOI] [PubMed] [Google Scholar]
- 257.Alshatwi AA, Ramesh E, Periasamy VS, Subash-Babu P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam Clin Pharmacol. 2013;27:581–92. doi: 10.1111/j.1472-8206.2012.01061.x. [DOI] [PubMed] [Google Scholar]
- 258.Lee CJ, Wilson L, Jordan MA, Nguyen V, Tang J, Smiyun G. Hesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cells. Phytother Res. 2010;24(Suppl 1):S15–9. doi: 10.1002/ptr.2856. [DOI] [PubMed] [Google Scholar]
- 259.Ruela-de-Sousa RR, Fuhler GM, Blom N, Ferreira CV, Aoyama H, Peppelenbosch MP. Cytotoxicity of apigenin on leukemia cell lines: Implications for prevention and therapy. Cell Death Dis. 2010;1:e19. doi: 10.1038/cddis.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-spoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem Pharmacol. 2006;72:681–92. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 261.Sonoda M, Nishiyama T, Matsukawa Y, Moriyasu M. Cytotoxic activities of flavonoids from two Scutellaria plants in Chinese medicine. J Ethnopharmacol. 2004;91:65–8. doi: 10.1016/j.jep.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 262.Zheng PW, Chaing LC, Lin CC. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005;76:1367–79. doi: 10.1016/j.lfs.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 263.Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9:R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Choi EJ, Kim GH. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 cells. J Clin Biochem Nutr. 2009;44:260–5. doi: 10.3164/jcbn.08-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Choi EJ, Kim GH. 5-Fluorouracil combined with apigenin enhances anticancer activity through induction of apoptosis in human breast cancer MDA-MB-453 cells. Oncol Rep. 2009;22:1533–7. doi: 10.3892/or_00000598. [DOI] [PubMed] [Google Scholar]
- 266.Salama MM, Kandil ZA, Islam WT. Cytotoxic compounds from the leaves of Gaillardia aristata Pursh. growing in Egypt. Nat Prod Res. 2012;26:2057–62. doi: 10.1080/14786419.2011.606219. [DOI] [PubMed] [Google Scholar]
- 267.Forgo P, Zupkó I, Molnár J, Vasas A, Dombi G, Hohmann J. Bioactivity-guided isolation of antiproliferative compounds from Centaurea jacea L. Fitoterapia. 2012;83:921–5. doi: 10.1016/j.fitote.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 268.Hirano T, Oka K, Akiba M. Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75-1. Res Commun Chem Pathol Pharmacol. 1989;64:69–78. [PubMed] [Google Scholar]
- 269.Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr Cancer. 2001;39:139–47. doi: 10.1207/S15327914nc391_19. [DOI] [PubMed] [Google Scholar]
- 270.Cardenas M, Marder M, Blank VC, Roguin LP. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg Med Chem. 2006;14:2966–71. doi: 10.1016/j.bmc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 271.Csupor-Löffler B, Hajdú Z, Zupkó I, Molnár J, Forgo P, Vasas A, et al. Antiproliferative constituents of the roots of Conyza canadensis. Planta Med. 2011;77:1183–8. doi: 10.1055/s-0030-1270714. [DOI] [PubMed] [Google Scholar]
- 272.Csupor-Löffler B, Hajdú Z, Zupkó I, Réthy B, Falkay G, Forgo P, et al. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother Res. 2009;23:672–6. doi: 10.1002/ptr.2697. [DOI] [PubMed] [Google Scholar]
- 273.Csapi B, Hajdú Z, Zupkó I, Berényi A, Forgo P, Szabó P, et al. Bioactivity-guided isolation of antiproliferative compounds from Centaurea arenaria. Phytother Res. 2010;24:1664–9. doi: 10.1002/ptr.3187. [DOI] [PubMed] [Google Scholar]
- 274.Fullas F, Hussain RA, Chai HB, Pezzuto JM, Soejarto DD, Kinghorn AD. Cytotoxic constituents of Baccharis gaudichaudiana. J Nat Prod. 1994;57:801–7. doi: 10.1021/np50108a017. [DOI] [PubMed] [Google Scholar]
- 275.Chiang LC, Ng LT, Lin IC, Kup PL, Lin CC. Anti-proliferative effect of apigenin and its apoptotic induction in human Hep G2 cells. Cancer Lett. 2006;237:207–14. doi: 10.1016/j.canlet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 276.Yee SB, Lee JH, Chung HY, Im KS, Bae SJ, Choi JS, et al. Inhibitory effects of luteolin isolated from Ixeris sonchifolia Hance on the proliferation of HepG2 human hepatocellular carcinoma cells. Arch Pharm Res. 2003;26:151–6. doi: 10.1007/BF02976662. [DOI] [PubMed] [Google Scholar]
- 277.Das S, Sas J, Samadder A, Boujedaini N, Khuda-Bukhsh AR. Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp Biol Med (Maywood) 2012;237:1433–48. doi: 10.1258/ebm.2012.012148. [DOI] [PubMed] [Google Scholar]
- 278.King JC, Lu QY, Li G, Moro A, Takahashi H, Chen M, et al. Evidence for activation of mutated p53 by apigenin in human pancreatic cancer. Biochim Biophys Acta. 2012;1823:593–604. doi: 10.1016/j.bbamcr.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ, Golkar L, Milam B, et al. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006;5:76. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008;44:1833–45. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shenouda NS, Zhou C, Browning JD, Ansell PJ, Sakla MS, Lubahn DB, et al. Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr Cancer. 2004;49:200–8. doi: 10.1207/s15327914nc4902_12. [DOI] [PubMed] [Google Scholar]
- 282.Chen SS, Corteling R, Stevanato L, Sinden J. Polyphenols inhibit indoleamine 3,5-dioxygenase-1 enzymatic activity: A role of immunomodulation in chemoprevention. Discov Med. 2012;14:327–33. [PubMed] [Google Scholar]
- 283.Cherng JM, Shieh DE, Chiang W, Chang MY, Chiang LC. Chemopreventive effects of minor dietary constituents in common foods on human cancer cells. Biosci Biotechnol Biochem. 2007;71:1500–4. doi: 10.1271/bbb.70008. [DOI] [PubMed] [Google Scholar]
- 284.Roy MK, Nakahara K, Na TV, Trakoontivakorn G, Takenaka M, Isobe S, et al. Baicalein, a flavonoid extracted from a metanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Pharmazie. 2007;62:149–53. [PubMed] [Google Scholar]
- 285.Ma Z, Otsuyama K, Liu S, Abroun S, Ishikawa H, Tsuyama N, et al. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105:3312–8. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 286.Ciesielska E, Wolszczak M, Gulanowski B, Szulawska A, Kochman A, Metodiewa D. In vitro antileukemic, antioxidant and prooxidant activities of Antoksyd S (C/E/XXI): A comparison with baicalin and baicalein. In Vivo. 2004;18:497–503. [PubMed] [Google Scholar]
- 287.Ishii K, Tanaka S, Kagami K, Henmi K, Toyoda H, Kaise T, et al. Effects of naturally occurring polymethoxyflavonoids on cell growth, p-glycoprotein function, cell cycle, and apoptosis of daunorubicin-resistant T lymphoblastoid leukemia cells. Cancer Invest. 2010;28:220–9. doi: 10.3109/07357900902744486. [DOI] [PubMed] [Google Scholar]
- 288.Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J. 1992;282:883–9. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bednar W, Holzmann K, Marian B. Assessing 12(S)-lipoxygenase inhibitory activity using colorectal cancer cells overexpressing the enzyme. Food Chem Toxicol. 2007;45:508–14. doi: 10.1016/j.fct.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 290.Liang RR, Zhang S, Qi JA, Wang ZD, Li J, Liu PJ, et al. Preferential inhibition of hepatocellular carcinoma by the flavonoid Baicalein through blocking MEK-ERK signaling. Int J Oncol. 2012;41:969–78. doi: 10.3892/ijo.2012.1510. [DOI] [PubMed] [Google Scholar]
- 291.Matsuzaki Y, Kurokawa N, Terai S, Matsumura Y, Kobayashi N, Okita K. Cell death induced by baicalein in human hepatocellular carcinoma cell lines. Jpn J Cancer Res. 1996;87:170–7. doi: 10.1111/j.1349-7006.1996.tb03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Motoo Y, Sawaby N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994;86:91–5. doi: 10.1016/0304-3835(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 293.Leung HW, Yang WH, Lai MY, Lin CJ, Lee HZ. Inhibition of 12-lipoxygenase during baicalein-induced human lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol. 2007;45:403–11. doi: 10.1016/j.fct.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 294.Boik JC, Newman RA. A classification model to predict synergism/antagonism of cytotoxic mixtures using protein-drug docking scores. BMC Pharmacol. 2008;8:13. doi: 10.1186/1471-2210-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Cheng YH, Li LA, Lin P, Cheng LC, Hung CH, Chang NW, et al. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol Appl Pharmacol. 2012;263:360–7. doi: 10.1016/j.taap.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 296.Pidgeon GP, Kandouz M, Meram A, Honn KV. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721–7. [PubMed] [Google Scholar]
- 297.Pilátová M, Stupáková V, Varinská L, Sarisský M, Mirossay L, Mirossay A, et al. Effect of selected flavones on cancer and endothelial cells. Gen Physiol Biophys. 2010;29:134–43. doi: 10.4149/gpb_2010_02_134. [DOI] [PubMed] [Google Scholar]
- 298.Ai XH, Zheng X, Tang XQ, Sun L, Zhang YQ, Qin Y, et al. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 5,7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol. 2007;13:3824–8. doi: 10.3748/wjg.v13.i28.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem. 2004;12:6097–105. doi: 10.1016/j.bmc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 300.Lyu SY, Park WB. Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Arch Pharm Res. 2005;28:573–81. doi: 10.1007/BF02977761. [DOI] [PubMed] [Google Scholar]
- 301.Kilani-Jaziri S, Neffati A, Limem I, Boubaker J, Skandrani I, Sghair MB, et al. Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chem Biol Interact. 2009;181:85–94. doi: 10.1016/j.cbi.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 302.Chiang LC, Cianh W, Chang MY, Ng LT, Lin CC. Antileukemic activity of selected natural products in Taiwan. Am J Chin Med. 2003;31:37–46. doi: 10.1142/S0192415X03000825. [DOI] [PubMed] [Google Scholar]
- 303.Saleem A, Husheem M, Härkönen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 2002;81:327–36. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 304.Afifi FU, Abu-Dahab R. Phytochemical screening and biological activities of Eminium spiculatum (Blume) Kuntze (family Araceae) Nat Prod Res. 2012;26:878–82. doi: 10.1080/14786419.2011.565558. [DOI] [PubMed] [Google Scholar]
- 305.Hsu HF, Houng JY, Chang CL, Wu CC, Chang FR, Wu YC. Antioxidant activity, cytotoxicity, and DNA information of Glossogyne tenuifolia. J Agric Food Chem. 2005;53:6117–25. doi: 10.1021/jf050463u. [DOI] [PubMed] [Google Scholar]
- 306.Lin AS, Lin CR, Du YC, Lübken T, Chiang MY, Chen IH, et al. Acasiane A and B and farnesirane A and B, diterpene derivatives from the roots of Acacia farnesiana. Planta Med. 2009;75:256–61. doi: 10.1055/s-0028-1112201. [DOI] [PubMed] [Google Scholar]
- 307.Wu J, Yi W, Jin L, Hu D, Song B. Antiproliferative and cell apoptosis-inducing activities of compounds from Buddleja davidii in Mgc-803 cells. Cell Div. 2012;7:20. doi: 10.1186/1747-1028-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Xie YY, Yuan D, Yang JY, Wang LH, Wu CF. Cytotoxic activity of flavonoids from the flowers of Chrysanthemum morifolium on human colon cancer Colon205 cells. J Asian Nat Prod Res. 2009;11:771–8. doi: 10.1080/10286020903128470. [DOI] [PubMed] [Google Scholar]
- 309.Lee HJ, Wang CJ, Kuo HC, Chou FP, Jean LF, Tseng TH. Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicol Appl Pharmacol. 2005;203:124–31. doi: 10.1016/j.taap.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 310.Yoo DR, Jang YH, Jeon YK, Kim JY, Jeon W, Choi YJ, et al. Proteomic identification of anti-cancer proteins in luteolin-treated human hepatoma Huh-7 cells. Cancer Lett. 2009;282:48–54. doi: 10.1016/j.canlet.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 311.Chang J, Hsu Y, Kuo P, Kuo Y, Chiang L, Lin C. Increase of Bax/Bcl-XL ratio and arrest of cell cycle by luteolin in immortalized human hepatoma cell line. Life Sci. 2005;76:1883–93. doi: 10.1016/j.lfs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 312.Yu J, Liu H, Lei J, Tan W, Hu X, Zou G. Antitumor activity of chloroform fraction of Scutellaria barbata and its active constituents. Phytother Res. 2007;21:817–22. doi: 10.1002/ptr.2062. [DOI] [PubMed] [Google Scholar]
- 313.Zhao Y, Yang G, Ren D, Zhang X, Yin Q, Sun X. Luteolin suppresses growth and migration of human lung cancer cells. Mol Biol Rep. 2011;38:1115–9. doi: 10.1007/s11033-010-0208-x. [DOI] [PubMed] [Google Scholar]
- 314.Wang TT, Wang SK, Huang GL, Sun GJ. Luteolin induced-growth inhibition and apoptosis of human esophageal squamous carcinoma cell line Eca109 cells in vitro. Asian Pac J Cancer Prev. 2012;13:5455–61. doi: 10.7314/apjcp.2012.13.11.5455. [DOI] [PubMed] [Google Scholar]
- 315.Harris DM, Li L, Chen M, Lagunero FT, Go VL, Boros LG. Diverse mechanisms of growth inhibition by luteolin, resveratrol, and quercetin in MIA PaCa-2 cells: A comparative glucose tracer study with the fatty acid synthase inhibitor C75. Metabolomics. 2012;8:201–10. doi: 10.1007/s11306-011-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Luo G, Guan X, Zhou L. Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line A549 in vitro and in vivo. Cancer Biol Ther. 2008;7:966–73. doi: 10.4161/cbt.7.6.5967. [DOI] [PubMed] [Google Scholar]
- 317.Lust S, Vanhoecke B, Van Gele M, Philippé J, Bracke M, Offner F. The flavonoid tangeretin activates the unfolded protein response and synergizes with imatinib in the erythroleukemia cell line K562. Mol Nutr Food Res. 2010;54:823–32. doi: 10.1002/mnfr.200900186. [DOI] [PubMed] [Google Scholar]
- 318.Huang ST, Wang CY, Yang RC, Chu CJ, Wu HT, Pang JH. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine. 2010;17:47–54. doi: 10.1016/j.phymed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 319.Yu JS, Kim AK. Wogonin induces apoptosis by activation of ERK and p38 MAPKs signaling pathways and generation of reactive oxygen species in human breast cancer cells. Mol Cells. 2011;31:327–35. doi: 10.1007/s10059-011-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Huang KF, Zhang GD, Huang YQ, Diao Y. Wogonin induces apoptosis and down-regulates survivin in human breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int Immunopharmacol. 2012;12:334–41. doi: 10.1016/j.intimp.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 321.Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2020–33. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang W, Guo Q, You Q, Zhang K, Yang Y, Yu J, et al. Involvement of bax/bcl-2 in wogonin-induced apoptosis of human hepatoma cell line SMMC-7721. Anticancer Drugs. 2006;17:797–805. doi: 10.1097/01.cad.0000217431.64118.3f. [DOI] [PubMed] [Google Scholar]
- 323.Cao XD, Ding ZS, Jiang FS, Ding XH, Chen JZ, Chen SH, et al. Antitumor constituents from the leaves of Carya cathayensis. Nat Prod Res. 2012;26:2089–94. doi: 10.1080/14786419.2011.628174. [DOI] [PubMed] [Google Scholar]
- 324.Lee DH, Rhee JG, Lee YJ. Reactive oxygen species up-regulate p53 and Puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin. Br J Pharmacol. 2009;157:1189–202. doi: 10.1111/j.1476-5381.2009.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Shieh DE, Cheng HY, Yen MH, Chiang LC, Lin CC. Baicalin-induced apoptosis is mediated by Bcl-2-dependent, but not p53-dependent, pathway in human leukemia cell lines. Am J Chin Med. 2006;34:245–61. doi: 10.1142/S0192415X06003801. [DOI] [PubMed] [Google Scholar]
- 326.Mao W, Chen X, Yang T, Yin Y, Ge M, Luo M, et al. A rapid fluorescent screening method for cellular sensitivity to anti-cancer compound. Cytotechnology. 2012;64:451–7. doi: 10.1007/s10616-011-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


