Abstract
The incidence, risk factors and outcome of graft pyelonephritis are variably described in literature. All patients who had been transplanted at our center between January 2002 and November 2006 and had presented with acute graft dysfunction were subjected to biopsy. Those patients who had neutrophilic casts and interstitial inflammation with predominant neutrophils were included in the study. Out of the 265 patients, 110 were transplanted in the period and underwent biopsy for graft dysfunction. Out of the 110 patients, 26 had biopsy proven acute graft pyelonephritis (AGPN). Nine patients had early AGPN (within 6 months) and the other 17 had late AGPN. Nearly 19% of patients were culture negative and five patients had no clinical features of urinary tract infection; AGPN being a surprise finding on biopsy. Among the risk factors, only hepatitis C virus infection was significantly associated with AGPN. All patients received 4-6 weeks of antibiotics with at least 3 weeks of parenteral antibiotics. Majority (75%) of our patients experienced relapse of AGPN. Graft function was significantly lower 6 months after onset when compared to baseline, portending a poor outcome for these patients. Out of 26, 7 (27%) of our patients had biopsy features of concomitant acute cellular rejection. The treatment of acute rejection, however, did not improve the outcome.
Keywords: Graft dysfunction, graft pyelonephritis, kidney transplant, renal transplant, urinary tract infection
Introduction
Urinary tract infection (UTI) is the most common bacterial infection encountered in 30-60% of renal transplant recipients. However, data on acute graft pyelonephritis (AGPN) is scant with reported incidence of around 10-17%. Fiorante et al., reported 10% incidence of AGPN in the first 36 months with an incidence rate of 4.4 episodes/100 patient-years.[1,2] Variety of factors such as prolonged bladder catheterization, age, female sex, diabetes, pre-transplant UTI, underlying structural abnormalities of urinary tract, use of double J (DJ) stent, vesicoureteric reflux, episodes of acute rejection and cytomegalovirus (CMV) infection have been identified as risk factors of post-transplant UTI.[2,3] Diagnosis of AGPN is usually based on a constellation of features, e.g. fever, graft tenderness, leucocytosis, pyuria and bacteruria and biopsy is not included in the diagnostic algorithm. Clinical profile of upper and lower UTI is alike except for graft tenderness; therefore, if there is no graft tenderness, one may miss AGPN and patient shall get mistreated as a lower UTI. It is logical to presume that AGPN should result in graft dysfunction; however, literature is silent on this aspect. It may be appropriate to presume that milder forms of AGPN may not be causing graft dysfunction and in severe cases, graft dysfunction should be the rule. The association of concomitant acute rejection with AGPN has not been reported in literature since biopsies are not done for AGPN.
How AGPN impacts graft outcome has remained debatable. Pellé et al., observed poor graft outcome in patients affected by AGPN.[4] Abbott et al., observed that outcome of late AGPN is not benign while Giral et al., observed that early AGPN is a risk factor for graft loss.[5,6] However, Fiorante et al., found that AGPN has no impact on patient or graft survival.[1] Possibly because of the wide spectrum of AGPN, subjects included in different studies were different.
In view of this conflicting data on impact of AGPN on graft outcome, we planned to prospectively follow all biopsy proven cases of AGPN with graft dysfunction.
Materials and Methods
All patients who had been transplanted between January 2002 and November 2006 and presented with acute graft dysfunction were subjected to biopsy even if clinical profile suggested AGPN. The histological criteria defined as per literature and followed by us for acute (bacterial) graft pyelonephritis was presence of neutrophils in tubular lumina forming casts, between tubular epithelial cells and in the edematous interstitium.[7] Evidence of concomitant rejection was looked for and C4d staining by immunohistochemistry was performed on all biopsies. Humoral rejection was excluded by negative C4d staining. The viral etiologies were also excluded by doing BK Polyoma viral and CMV staining by immunohistochemistry. These patients with AGPN are the subjects of the study. However, in patients with features of sepsis, biopsy was delayed for 2-5 days until toxemia subsided after appropriate antibiotics.
The immunosuppressive protocol consisted of a calcineurin inhibitor (tacrolimus or cyclosporine), anti-proliferative agent (mycophenolate or azathioprine) and steroids. Induction with interleukin-2 inhibitor (basiliximab or daclizumab) or anti-thymocyte globulin (ATG) was given as per discretion of the nephrologist. As per our center protocol, high risk patients receive ATG. DJ stents were routinely placed during transplant surgery. The urinary catheter and the DJ stent were removed on the 3rd day and 14th post-operative day respectively. All patients received routine prophylactic sulfamethaxazole-trimethoprim combination for 6 months period. A bacterial growth of >105 CFU was taken as significant bacteruria. A rise in creatinine rise >25% above baseline was defined as impaired graft function.
In all patients of AGPN included in the study, baseline demographic data, history of UTIs, immunosuppressive protocol, routine blood counts, urine cultures, blood cultures, calcineurin blood levels and ultrasound findings were documented. Biopsies were performed after informed consent was obtained. The study protocol was cleared by the Ethics Committee of the Hospital.
Patient with AGPN were treated for 4-6 weeks with antibiotics that included parenteral antibiotics for the first 3 weeks. After completion of treatment, patient received 6 months of antibiotic prophylaxis. Patients with rejection were given three daily pulses of 500 mg methylprednisolone injections after completion of at least 5 days of antibiotics. Steroid pulses were given only after patient was afebrile for at least 2 days and the urine cultures had been rendered sterile. Graft pyelonephritis was classified as early if it occurred within 6 months post-transplant and late if it occurred after that. Inability of the creatinine level to settle to less than 25% over baseline, 6 months after onset of pyelonephritis was taken as adverse outcome of the graft. Any patient with relapse of pyelonephritis was again treated along the same lines as the initial episode.
Statistical analysis
Continuous and categorical variables were analyzed using the paired t-test and Fisher's exact test respectively. Correlation between continuous variables was determined using the Pearson correlation coefficient.
Ethical clearance and conflict of interest statement
The study was cleared by the Hospital Ethical Committee. The authors declare that we did not receive financial aid from any organization that sponsored the research. We declare that there was no conflict of interest in the study.
Results
A total of 265 patients transplanted in the time period January 2002-November 2006 were followed-up for a mean duration of 30.9 months (range: 1.7-58.8 months). 110 of them developed graft dysfunction and all of them underwent biopsy. The demographic data and the risk factors of patients with AGPN and the control population are represented in Table 1. Only hepatitis C virus (HCV) infection was found in our study to be a statistically significant risk factor (12/26 vs. 58/239, P = 0.016). Out of the 110 biopsies performed for evaluation of graft dysfunction, 26 had evidence of AGPN (25%). The clinical profile and laboratory findings are summarized in Table 2. Five of these patients had no clues to suggest UTI in the form of fever, dysuria, pyuria or graft tenderness. However, three of these patients were oliguric and two of them had grown Escherichia coli. The offending organism was E. coli in 73% and Klebsiella in 8%; 19% had sterile urine cultures despite showing biopsy evidence of graft pyelonephritis. None of the 26 biopsies including the five patients with no clinical features of UTI were positive for C4d staining. Seven of these 26 patients with acute graft pyelonephritis showed evidence of concomitant acute cellular rejection in the form of tubulitis [Figure 1]. Median time to AGPN after transplantation was 10.9 months (range: 0.3-56.2 months). Nine patients had early AGPN (within 6 months) and the other 17 had late AGPN.
Table 1.
Demographic data and risk factors of AGPN patients and controls
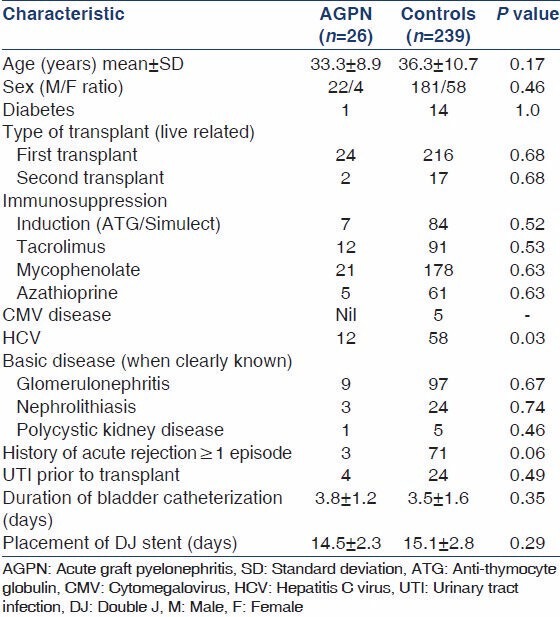
Table 2.
Summary of clinical profile and lab results in patients who had presented with graft dysfunction and had biopsy evidence of acute graft pyelonephritis
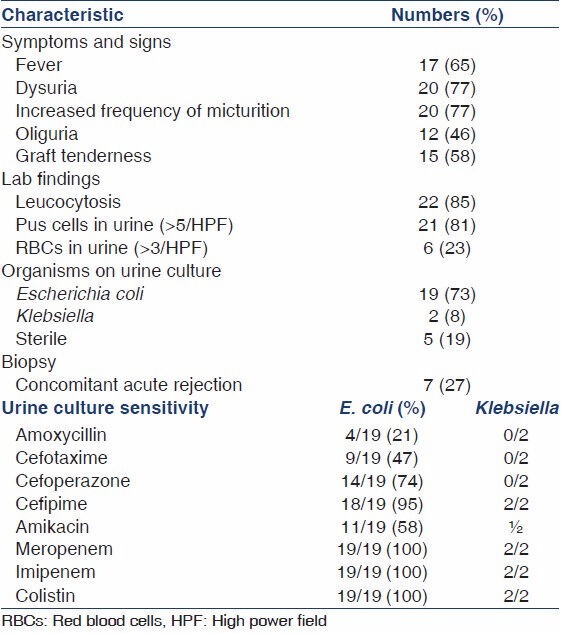
Figure 1.
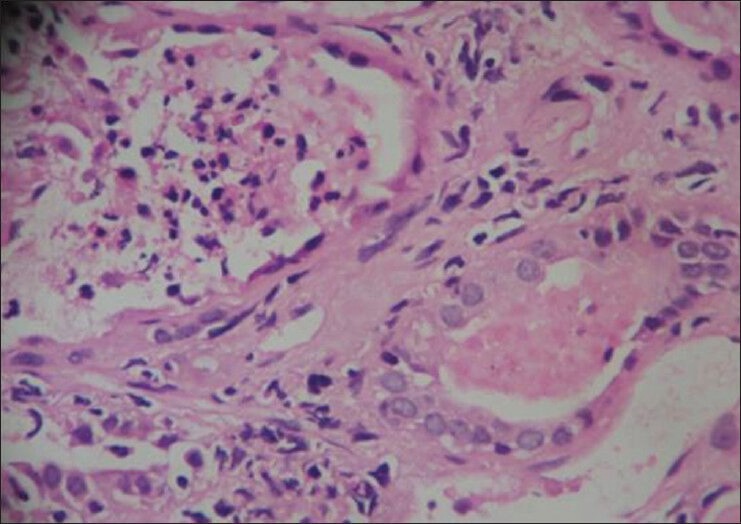
Neutrophilic casts with tubulitis (white blood cell casts to the left with lymphocytic tubulitis in the tubule to the right)
Our data showed that AGPN didn't augur well for the long-term graft outcome. Over a median follow-up of 14.6 months, patients with early as well as late AGPN showed deterioration of graft functions with most patients not returning to baseline graft function [Table 3]. Out of the seven patients with concomitant rejection and pyelonephritis, five received methylprednisolone pulses with no clear response to antirejection therapy. Outcome of patients with AGPN with or without concomitant rejection was no different (P > 0.05). 75% of AGPN patients had relapse requiring repeat courses of antibiotics.
Table 3.
Renal functions of patients with early and late AGPN and their response to treatment
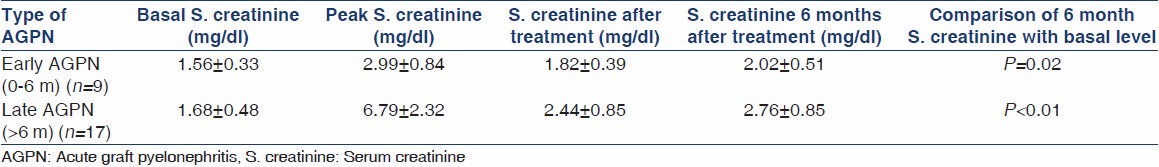
Discussion
During 3 year follow-up period, the incidence of AGPN in our center was 10% (26/265) and it was responsible for 24% (26/110) cases of graft dysfunction. The incidence of AGPN is as picked up when evaluating cases of graft dysfunction, hence if there were milder cases that did not cause graft dysfunction, they would have not got included in the present study. In a recent retrospective study, the incidence of bacteruria, irrespective of symptoms, was recorded to be as high as 59%; however, incidence of graft pyelonephritis was 10%.[1] We have documented nearly a quarter of graft dysfunction being attributable to graft pyelonephritis. This seemingly high percentage is because of the methodology used in the study where all patients of graft dysfunction irrespective of whether they had evidence of UTI or not were biopsied. Most centers exclude transplant recipients with clear evidence of UTI from biopsy; hence do not encounter graft pyelonephritis so commonly. Most studies have demonstrated the value of antibiotic prophylaxis in lowering the incidence of UTIs.[8] Despite the fact that all our patients were on cotrimoxazole prophylaxis for 6 months, 9 of the 265 patients on follow-up had developed early graft pyelonephritis.
A variety of risk factors for AGPN identified in different studies have been indwelling bladder catheters, handling and trauma to the kidney and ureter during surgery, anatomic abnormalities of the native or transplanted kidneys (such as vesicoureteric reflux, stones, or stents), neurogenic bladder especially in diabetic patients, mycophenolate, female gender, history of acute rejection, CMV infection and glomerulonephritis in native kidneys.[1,2] The use of mycophenolate, azathioprine or any specific immunosuppressive agent did not seem to predict AGPN. Our data being generated in a service hospital in South Asia, is limited by the fact that there is a bias, with younger non-diabetic adult males more likely to find a living donor; hence we refrain from commenting on age, sex and diabetes as a risk factor in our study. In our center, we do not pre-emptively follow-up CMV infection except in the immediate post-transplant period and in the developing world most transplants are D+R+; this could possibly explain our inability to find an association of CMV infection with AGPN. Unlike other studies, we did find a significant association of HCV infection with AGPN.[1,2] The high incidence of HCV infection (22.6%) in our transplant recipients could be the reason we found this association that has not been described until date. We attribute the association to the immunosuppressive effects of chronic HCV infection.
Remarkably, we found that 19% of our patients grew no organisms on urine culture. The diagnosis of graft pyelonephritis was a surprise finding on biopsy in 5/26 patients who had no symptoms of UTI. Absence of “classic” diagnostic tetrad of fever, positive urine cultures, lower urinary tract symptoms and graft dysfunction has been observed by other authors too.[9] We conclude that the gold standard of evaluation of graft dysfunction is a graft biopsy and that absence of typical symptoms of AGPN does not rule out the diagnosis.
An interesting association between acute rejection and graft pyelonephritis was discovered. It has been well-documented in anecdotal reports that graft pyelonephritis can provoke acute rejection; however, there has been no systematic study of the association.[10] Some of the authors have even refuted the claim that AGPN can provoke acute rejection.[6] This is the first study that has looked at the histopathology of graft pyelonephritis closely and demonstrated the association in 23% (6/26) of AGPN. A recent study demonstrated neutrophilic tubulitis as a marker of infection in patients with concomitant rejection.[11] We found neutrophilic casts with lymphocytic tubulitis in a quarter of our patients with AGPN. This finding could be attributed to the aggressive biopsy policy used in our study protocol. However, we could not demonstrate a response to therapy, possibly because of the nearly 1-2 weeks delay in giving the steroid pulses and the inability to use more aggressive immunosuppressive regimens in the face of severe AGPN. It is possible that an earlier use of immunosuppressives under antibiotic cover would have made a difference to the graft function, but we doubt if that policy can be adopted in the presence of active infection.
Contrary to popular belief, we found that after an episode of AGPN, the graft never recovered function to baseline levels.[1,12] There have been studies that have found AGPN is a predictor of an unfavorable graft and patient outcome.[4,5,13,14] The reason for such conflicting evidence in published literature is unclear; heterogeneous policies in defining and treating AGPN may be responsible to some extent.[6] The treatment protocols and post-transplant prophylactic strategies also are not standardized, which could be responsible for the discordant results in different studies. We attribute the poor graft outcome in our study to the severity of the AGPN with 28% of our patients having evidence of bacteremia. Our patients of graft pyelonephritis are a subset of patients who had presented with graft dysfunction in the first place; hence it is no surprise that they had a poor outcome. In addition, nearly a quarter of the patients had concomitant acute rejection that may have also contributed; although we could not prove it statistically. Giral et al., found difference in outcome with early AGPN showing greater association with bacteremia and hence, a poorer outcome.[6] In our study, both early as well as late AGPN didn't fare well; however, late AGPN seemed to have poorer outcome in terms of residual graft dysfunction. In the absence of randomized controlled trials, Kidney Disease: Improving Global Outcomes (KDIGO) has recommended initial hospitalization and parenteral antibiotics with no comment on the duration of antibiotics.[15] We used a 4-6 week antibiotic course that was successful in eradicating infection; albeit unable to reverse graft dysfunction completely. Despite the prolonged course, we had a significant percentage (75%) of relapse requiring repeat courses of antibiotics. Recurrent UTIs occur in 5.5-27% of cases and studies with longer durations of follow-up report the highest rates of recurrence.[16,17] Mortality in our data of AGPN was 15.4% and is comparable with literature.[18] AGPN is not a benign illness; not only in terms of graft outcome but also in terms of patient survival.
We conclude that patients of AGPN that present with graft dysfunction have an unfavorable long-term outcome. A quarter of our cases of acute graft dysfunction were accounted for by graft pyelonephritis. Graft pyelonephritis may occur in the absence of typical symptoms of UTI. Nearly, a fifth of our patients with graft pyelonephritis showed no growth of pathogenic bacteria on urine culture. Concomitant rejection is more common than hitherto believed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Fiorante S, Fernández-Ruiz M, López-Medrano F, Lizasoain M, Lalueza A, Morales JM, et al. Acute graft pyelonephritis in renal transplant recipients: Incidence, risk factors and long-term outcome. Nephrol Dial Transplant. 2011;26:1065–73. doi: 10.1093/ndt/gfq531. [DOI] [PubMed] [Google Scholar]
- 2.Sorto R, Irizar SS, Delgadillo G, Alberú J, Correa-Rotter R, Morales-Buenrostro LE. Risk factors for urinary tract infections during the first year after kidney transplantation. Transplant Proc. 2010;42:280–1. doi: 10.1016/j.transproceed.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Mitra S, Alangaden GJ. Recurrent urinary tract infections in kidney transplant recipients. Curr Infect Dis Rep. 2011;13:579–87. doi: 10.1007/s11908-011-0210-z. [DOI] [PubMed] [Google Scholar]
- 4.Pellé G, Vimont S, Levy PP, Hertig A, Ouali N, Chassin C, et al. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am J Transplant. 2007;7:899–907. doi: 10.1111/j.1600-6143.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 5.Abbott KC, Swanson SJ, Richter ER, Bohen EM, Agodoa LY, Peters TG, et al. Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis. 2004;44:353–62. doi: 10.1053/j.ajkd.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 6.Giral M, Pascuariello G, Karam G, Hourmant M, Cantarovich D, Dantal J, et al. Acute graft pyelonephritis and long-term kidney allograft outcome. Kidney Int. 2002;61:1880–6. doi: 10.1046/j.1523-1755.2002.00323.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiss M, Liapis H, Tomaszewski JE, Arend LJ. Hepinstall's Pathology of the Kidney. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. Pyelonephritis and other infections, reflux nephropathy, hydronephrosis, and nephrolithiasis; pp. 995–7. [Google Scholar]
- 8.Green H, Rahamimov R, Gafter U, Leibovitci L, Paul M. Antibiotic prophylaxis for urinary tract infections in renal transplant recipients: A systematic review and meta-analysis. Transpl Infect Dis. 2011;13:441–7. doi: 10.1111/j.1399-3062.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- 9.Piccoli GB, Picciotto G, Rossetti M, Burdese M, Consiglio V, Magnano A, et al. Imaging data suggesting acute pyelonephritis in the kidney graft: Report of five cases with atypical clinical presentation. Int J Antimicrob Agents. 2006;28(Suppl 1):S64–71. doi: 10.1016/j.ijantimicag.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Audard V, Amor M, Desvaux D, Pastural M, Baron C, Philippe R, et al. Acute graft pyelonephritis: A potential cause of acute rejection in renal transplant. Transplantation. 2005;80:1128–30. doi: 10.1097/01.tp.0000174343.05590.9f. [DOI] [PubMed] [Google Scholar]
- 11.Gupta G, Shapiro R, Girnita A, Batal I, McCauley J, Basu A, et al. Neutrophilic tubulitis as a marker for urinary tract infection in renal allograft biopsies with C4d deposition. Transplantation. 2009;87:1013–8. doi: 10.1097/TP.0b013e31819ca304. [DOI] [PubMed] [Google Scholar]
- 12.Kamath NS, John GT, Neelakantan N, Kirubakaran MG, Jacob CK. Acute graft pyelonephritis following renal transplantation. Transpl Infect Dis. 2006;8:140–7. doi: 10.1111/j.1399-3062.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 13.Lyerová L, Lácha J, Skibová J, Teplan V, Vítko S, Schück O. Urinary tract infection in patients with urological complications after renal transplantation with respect to long-term function and allograft survival. Ann Transplant. 2001;6:19–20. [PubMed] [Google Scholar]
- 14.Dharnidharka VR, Agodoa LY, Abbott KC. Effects of urinary tract infection on outcomes after renal transplantation in children. Clin J Am Soc Nephrol. 2007;2:100–6. doi: 10.2215/CJN.01820506. [DOI] [PubMed] [Google Scholar]
- 15.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 16.Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, et al. Infectious complications after kidney transplantation: Current epidemiology and associated risk factors. Clin Transplant. 2006;20:401–9. doi: 10.1111/j.1399-0012.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 17.Dantas SR, Kuboyama RH, Mazzali M, Moretti ML. Nosocomial infections in renal transplant patients: Risk factors and treatment implications associated with urinary tract and surgical site infections. J Hosp Infect. 2006;63:117–23. doi: 10.1016/j.jhin.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Chuang P, Parikh CR, Langone A. Urinary tract infections after renal transplantation: A retrospective review at two US transplant centers. Clin Transplant. 2005;19:230–5. doi: 10.1111/j.1399-0012.2005.00327.x. [DOI] [PubMed] [Google Scholar]


