Abstract
Leptospirosis is a zoonosis of global importance caused by Leptospira interrogans. The aim of this study was to compare the data between children, adolescents and adults with leptospirosis. This is a retrospective study including a total of 373 consecutive patients with leptospirosis, admitted to tertiary hospitals in Northeast of Brazil, from May 1985 to August 2010. The patients were divided into two groups (age ≤21 years and >21 years). The adults were 304 (81.5%) of the population, with a mean ge of 41 ± 13 (range 22-84) years. The pediatric group was 16 ± 3 (range 9-21) years. Signs and symptoms where similar between the groups, excepting arrhythmia, which was more frequent in adults and vomiting, more common in children (16% vs. 0%, P = 0.04 and 65% vs. 79%, P = 0.02), respectively. Adult group presented with higher serum urea (137 vs. 97 mg/dl, P = 0.002) and creatinine (4.3 vs. 3.0 mg/dl, P = 0.007). Acute kidney injury (AKI) was observed in 80%, mainly in adults (83% vs. 70% P < 0.005). Adults required renal replacement therapy more frequently than children (38% vs. 11%, P < 0.0001). Mortality was higher in adults (14.8% vs. 2.8%, P = 0.005) and in adults with AKI (93% vs. 7%, P < 0.05). There are important differences between the adults and children with leptospirosis. AKI was more frequent in adults and it was associated with increased mortality.
Keywords: Acute kidney injury, adults, children, leptospirosis, mortality
Introduction
Leptospirosis is a zoonosis of global importance caused by the pathogenic spirochetes Leptospira interrogans.[1,2] Human infection happens when there is contact with infected urine of carried mammals, especially rodents. The contact between leptospiras and humans can be directly through blood, tissue, organs or urine of carrier animals or indirectly through contaminated water or soil when there is injured skin and exposure of mucosal surfaces.[1,2,3,4] The symptomatic disease begins suddenly with headache, high-degree fever, malaise, myalgia, conjunctival suffusion and a transient rash. The severe form is characterized by jaundice, acute kidney injury (AKI) and hemorrhage, known as Weil's disease and it is mainly caused by serovars icterohaemorrhagiae, copenhageni, lai and others.[1,2] The disease is endemic in Brazil and is associated with the rainy season and floods.[5] In a recent study conducted in the State of São Paulo a total of 576 cases of lepsotpirosis were confirmed in 2008, with 73 deaths. In Sao Paulo city, with 10,990,2449 inhabitants, 172 cases with 33 deaths were recorded.[5]
There may be the difference in disease's incidence and spectrum of manifestations among pediatric and adult patients with leptospirosis and it is still not well-described. Furthermore, precise information on the frequency and types of severe manifestations in pediatric populations is limited.
The aim of this study was to compare the clinical presentation, laboratory data, morbidity and mortality between children, adolescents and adults with leptospirosis, with a focus on kidney function.
Patients and Methods
Setting and patient selection
A retrospective cohort study was conducted with a total of 373 consecutive patients with a confirmed diagnosis of leptospirosis, admitted to the São José Infectious Diseases Hospital and to the Walter Cantídio University Hospital, in the city of Fortaleza, Northeast of Brazil, from May 1985 to August 2010. The study protocol was approved by the Ethical Committee of the Institutions. All patients had confirmed diagnosis of leptospirosis, with microscopic agglutination test, with titers equal or higher than 1:800. Serology was done after at least 7 days after admission and patients with titers lower than 1:800 were excluded. Patients with negative serologies were not included, as well as those with other comorbidities, such as hypertension, diabetes, auto-immune diseases and others. All patients were from Ceará State, Northeast of Brazil.
A standardized case investigation form was used to complete demographical, epidemiological, clinical and laboratory data.
Studied parameters
Demographic characteristics such as age, gender, mean time between symptoms onset and hospital admission was analyzed, as well as duration of hospital stay, clinical manifestations, laboratory data, treatment and mortality. The clinical investigation included a record of all clinical signs and symptoms presented at hospital admission as well as arterial systolic and diastolic blood pressure, heart and respiratory rates at hospital admission and during the hospital stay. Laboratory data included an assessment of serum urea (SUr), serum creatinine (SCr), serum potassium (SK), serum sodium (SNa), total bilirubin, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase and total blood count. Hemorrhagic phenomena as gastrointestinal hemorrhage, hemoptysis and epistaxis were recorded at admission and during the hospital stay. Respiratory insufficiency was defined as the need of mechanical ventilation. The occurrence of metabolic acidosis was considered when the pH was lower than 7.35 and oliguria was defined as urine volume lower than 400 ml/day after 24 h of effective hydration. Hypotension was defined as mean arterial blood pressure (MAP) <60 mmHg and therapy with vasoactive drugs was initiated when MAP remained lower than 60 mmHg.
Dialysis was indicated in those patients that remained oliguric after effective hydration, in those cases where uremia was associated with hemorrhagic or severe respiratory failure and those with hyperkalemia or metabolic acidosis refractory to clinical treatment.
Acute kidney injury definitions
In adults, the RIFLECr criterion was used to define and stage AKI[6] while in the pediatric group, the pRIFLE definition was applied, using the Schwartz formula[7,8] to estimated creatinine clearance (eClCr).
Patients were divided into two groups according to the age (age ≤21 years vs. >21 years). A comparison of clinical and laboratory characteristics was done to investigate the differences between two groups.
Statistical analysis
The statistical analysis was performed using SPSS 20.0 for windows (SPSS Inc, Chicago, USA). Descriptive statistics are expressed as mean ± standard deviation. The primary analysis compared pediatric and adult groups. All variables were tested for normal distribution using the Kolmogorov-Smirnov test. The Student's t-test was applied to compare means of continuous variables and normal distribution data. Categorical data were tested using the Chi-square test. Values below 5% (P < 0.05) were considered to be statistically significant.
Results
Subject characteristics and clinical data
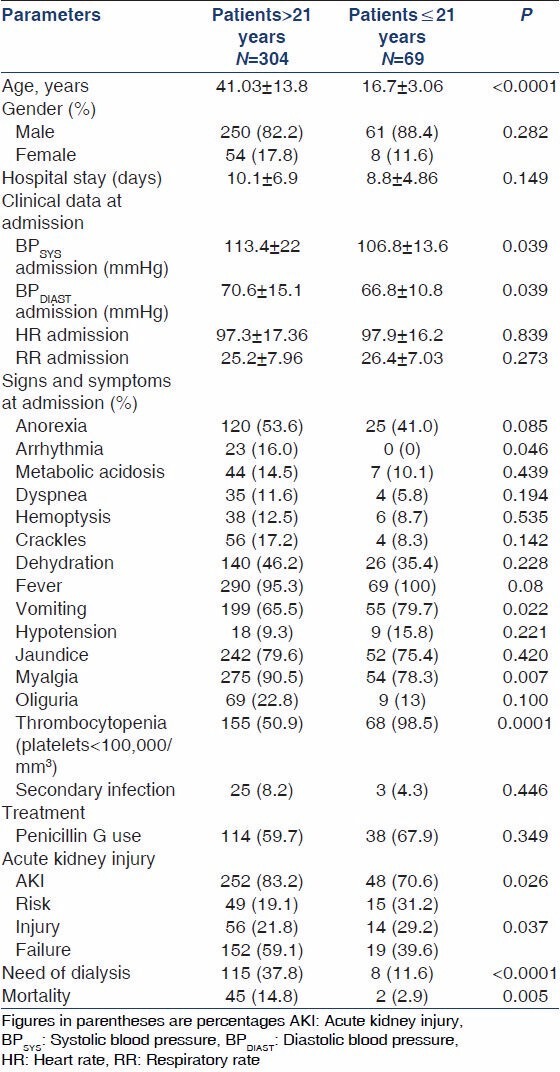
A total of 373 patients were included. The adult group represented 304 (81.5%) of the population, with a mean age of 41.03 ± 13.8 (range 22 to 84) years. The pediatric population comprised 69 (18.5%) of the total, with a mean age of 16.7 ± 3.06 years (range from 9 to 21). Regarding gender, there was no difference between the two groups (male gender, 82.2% vs. 88.4% and female, 17.8% vs. 11.6%, P = 0.282). The duration of disease was similar in both groups (10.1 ± 6.9 vs. 8.81 ± 4.8 days, P = 0.149). The most frequent signs and symptoms were the same in both groups; however, some of them were seen more frequently in adults, such as arrhythmia and myalgia. Vomiting was more frequently seen in children [Table 1].
Table 1.
Comparison of demographic and clinical manifestations between adults and children with leptospirosis
Laboratory data
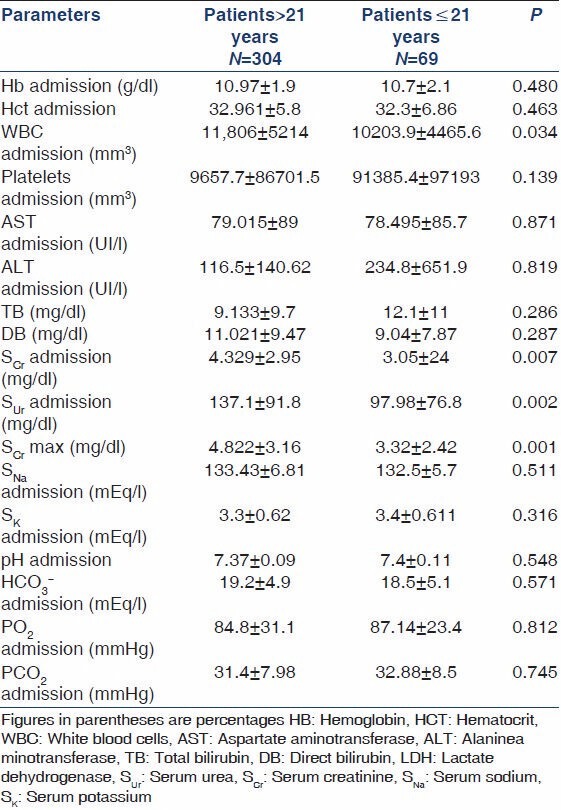
Regarding laboratory data at admission, the adult population presented with significantly higher white blood cells count (11,806 vs. 10,204 mm3, P = 0.034), while the pediatric population presented with significantly lower SUr(137.1 vs. 97.9 mg/dl, P = 0.002) and lower SCr(4.32 vs. 3.05 mg/dl, P = 0.007). Thrombocytopenia at admission was more frequent in children (98.5% vs. 50.9%, P = 0.0001). There were no significant differences between two groups regarding liver function and electrolyte balance, as can be seen in Table 2.
Table 2.
Comparison of laboratory data between adults and children with leptospirosis at admission
Renal function
AKI was observed in 300 patients (80.4%) and it was significantly more frequent in adults (83.2% vs. 70.6% P < 0.005) than in children. The distribution according to the RIFLE was as follows: Risk (19.1% vs. 31.2%), injury (21.8% vs. 29.2%), failure (59.1% vs. 39.6%) in adults and children, respectively. The adults required renal replacement therapy more frequently than children (37.8% vs. 11.6%, P < 0.0001).
Mortality
Mortality was significantly higher in adults (14.8% vs. 2.8%, P = 0.005). Adults with AKI had an even higher mortality (93.3% vs. 6.7%, P < 0.05). Mortality was also associated with AKI severity, as it was higher in the worst RIFLE stages: Risk (mortality: 6.7%), injury (11.1%) and failure (82.2%), P = 0.002. Need of dialysis (64.4% vs. 35.6%, P < 0.0001) and oliguria (54.5% vs. 45.5%, P < 0.0001) were also associated with higher mortality. No significant difference was observed in children regarding these parameters.
Discussion
Leptospirosis is a potentially fatal disease commonly observed in tropical countries.[1,2,3] The severe form (Weil disease) requires intensive care, mainly regarding renal function, including the possibility of dialysis.
Experienced clinicians in endemic areas commonly believe that leptospirosis produces milder symptoms among children.[9,10] There are several reports presenting lower rates of classic signs and symptoms of Weil's disease in children.[11,12,13] Spichler et al.,[11] in a recent study with 370 patients with severe leptospirosis, found that adults exhibited a higher frequency of jaundice and oliguria than in pediatric group. In the present study, most signs and symptoms where similar between the two groups, with the exception of arrhythmia, which was more commonly reported in adults and vomiting, which was more frequent in children. The children population in our cohort was lower than adults and this can be due to the milder course of leptospirosis in children, with the majority of cases not necessitating hospital admission.
In the present study, the adult population presented with significantly higher white blood cells count and the pediatric population presented with significantly lower SUr, SCr and higher frequency of thrombocytopenia. Spichler et al.[11] found that SCr and bilirubin were higher in adults.
In the present study, AKI was observed in a considerable number of patients (80.4%) and, unlike other infectious diseases such as visceral leishmaniasis,[14] it was significantly more common in adults. Marotto et al.[15] found AKI in 79% of 43 leptospirosis-infected children. The frequency of need of renal replacement therapy in children was similar to that in the general causes in the literature[16] and was higher in adults, evidencing a more severe kidney damage. The higher need of dialysis among adults in our cohort suggests a severe AKI in adults, i.e., children would have a milder renal disease in leptospirosis. Oliguria was also more frequent in adults than in children in a previous study.[11] The higher prevalence of AKI and oliguria in adults with leptospirosis do not appear to be related to differences in seroreactivity to leptospiral serogroups because the pattern of serogroups distribution is similar in both groups.[11] It was not possible to identify the etiologic serovar/serotype of leptospira in the present study. The serovar/serotype is an important determinant of the clinical manifestations, course and severity of the illness. The differences between the two groups presented here could have an influence of the serovar/serotype.
Renal failure is a well-known predictor of death in leptospirosis, especially in its oliguric forms.[2,17] Risk factors for death reported in the literature include advanced age, oliguria, pulmonary involvement, leukocytosis, thrombocytopenia and electrocardiographic abnormalities.[11,17,18,19] In the present study, mortality was significantly higher in adults and it was even higher in adults with AKI. This higher mortality seems to be related to a more severe AKI and more severe infection in adults.
In summary, there are important differences between adults and children with leptospirosis. AKI was more frequent in adults and it was associated with increased mortality. Pediatric patients exhibit lower mortality.
Acknowledgments
We are very grateful acknowledge to the team of physicians, residents, medical students and nurses from the Walter Cantídio University Hospital and the São José Infectious Diseases Hospital for the assistance provided to patients and for the technical support provided to the development of this research. This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (Brazilian Research Council).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–96. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–71. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–86. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 4.Vinetz JM. Leptospirosis. Curr Opin Infect Dis. 2001;14:527–38. doi: 10.1097/00001432-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Miyazato KE, Fonseca AL, Caputto LZ, Rocha KC, Azzalis LA, Junqueira VB, et al. Incidence of leptospirosis infection in the East Zone of Sao Paulo City, Brazil. Int Arch Med. 2013;6:23. doi: 10.1186/1755-7682-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure-Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 8.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 9.Rajajee S, Shankar J, Dhattatri L. Pediatric presentations of leptospirosis. Indian J Pediatr. 2002;69:851–3. doi: 10.1007/BF02723704. [DOI] [PubMed] [Google Scholar]
- 10.Silva HR, Tavares-Neto J, Bina JC, Meyer R. Leptospiral infection and subclinical presentation among children in Salvador, Bahia. Rev Soc Bras Med Trop. 2003;36:227–33. [PubMed] [Google Scholar]
- 11.Spichler A, Athanazio DA, Vilaça P, Seguro A, Vinetz J, Leake JA. Comparative analysis of severe pediatric and adult leptospirosis in Sao Paulo, Brazil. Am J Trop Med Hyg. 2012;86:306–8. doi: 10.4269/ajtmh.2012.11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz ML, Andrade J, Pereira MM. Leptospirosis in children in Rio do Janeiro. Rev Soc Bras Med Trop. 1994;27:5–9. doi: 10.1590/s0037-86821994000100002. [DOI] [PubMed] [Google Scholar]
- 13.Lopes AA, Costa E, Costa YA, Sacramento E, de Oliveira Junior AR, Lopes MB, et al. Comparative study of the in-hospital case-fatality rate of leptospirosis between pediatric and adult patients of different age groups. Rev Inst Med Trop Sao Paulo. 2004;46:19–24. doi: 10.1590/s0036-46652004000100004. [DOI] [PubMed] [Google Scholar]
- 14.Rocha NA, Oliveira MJ, Franco LF, Júnior GB, Alves MP, Sampaio AM, et al. Comparative analysis of pediatric and adult visceral leishmaniasis in Brazil. Pediatr Infect Dis J. 2013;32:e182–5. doi: 10.1097/INF.0b013e3182814eae. [DOI] [PubMed] [Google Scholar]
- 15.Marotto PC, Marotto MS, Santos DL, Souza TN, Seguro AC. Outcome of leptospirosis in children. Am J Trop Med Hyg. 1997;56:307–10. doi: 10.4269/ajtmh.1997.56.307. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurthy S, Mondal N, Narayanan P, Biswal N, Srinivasan S, Soundravally R. Incidence and etiology of acute kidney injury in southern India. Indian J Pediatr. 2013;80:183–9. doi: 10.1007/s12098-012-0791-z. [DOI] [PubMed] [Google Scholar]
- 17.Spichler AS, Vilaça PJ, Athanazio DA, Albuquerque JO, Buzzar M, Castro B, et al. Predictors of lethality in severe leptospirosis in urban Brazil. Am J Trop Med Hyg. 2008;79:911–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Tantitanawat S, Tanjatham S. Prognostic factors associated with severe leptospirosis. J Med Assoc Thai. 2003;86:925–31. [PubMed] [Google Scholar]
- 19.Esen S, Sunbul M, Leblebicioglu H, Eroglu C, Turan D. Impact of clinical and laboratory findings on prognosis in leptospirosis. Swiss Med Wkly. 2004;134:347–52. doi: 10.4414/smw.2004.10436. [DOI] [PubMed] [Google Scholar]


