Abstract
Introduction:
Naftopidil, approved initially in Japan, is an α1d-adrenergic receptor antagonist (α1-blocker) used to treat lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH). It is different from tamsulosin hydrochloride and silodosin, in that it has a higher affinity for the α1D-adrenergic receptor subtype than for the α1A subtype and has a superior efficacy to a placebo and comparable efficacy to other α1-blockers such as tamsulosin. The incidences of ejaculatory disorders and intraoperative floppy iris syndrome induced by naftopidil may also be lower than that for tamsulosin and silodosin, which have a high affinity for the α1A-adrenergic receptor subtype. However, it remains unknown if the efficacy and safety of naftopidil in Japanese men is applicable to Indian men having LUTS/BPH.
Material and Methods:
Two groups of 60 patients each, having LUTS due to BPH, were treated with tamsulosin 0.4 mg and Naftopidil 75 mg for three months. Ultrasonography (for prostate size, post-void residual volume), uroflowmetry, and the International Prostate Symptom Score (IPSS) and Quality of Life (QOL) score were recorded at the beginning of the study, and then at one and three months.
Results:
The prostate size, post-void residual volume, all the uroflowmetry variables, and the IPSS QOL scores showed a statistically significant improvement (P < 0.001) in both the groups. The improvement in the average flow rate and the QOL index was better in the naftopidil group on the intergroup comparison and was statistically significant (P < 0.001).
Conclusion:
Although the QOL life index was significantly better in the naftopidil group, overall both naftopidil and tamsulosin were found to be equally effective in the treatment of LUTS due to BPH.
Keywords: Benign prostatic hyperplasia, LUTS, naftopidil, tamsulosin
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a pathological process that contributes to, but is not the sole cause of, lower urinary tract symptoms (LUTS) in aging men. Despite intense research efforts in the past five decades to elucidate the underlying etiology of prostatic growth in older men, the cause-and-effect relationships have not been established.[1]
Lower urinary tract symptoms can be characterized as abnormal voiding sensations that occur with a frequency or severity that affects the quality of life. Common LUTS include urinary frequency, urgency, nocturia, intermittency, incomplete emptying, and a weak stream. Nocturia is the most prevalent symptom and it has been noted in one-half to two-thirds of the men studied. Other bothersome symptoms include dysuria, terminal dribbling, urinary incontinence, and genital pain.[2,3]
The development of effective therapies such as alpha-adrenergic blockers, 5-alpha-reductase inhibitors, and the possibility of their combined use, represent the most significant advance in the treatment of BPH.[4]
Currently available α-blockers have similar effects on BPH, improving symptoms by approximately 35% and the maximum urinary flow rate by 1.8 to 2.5 mL/s.[4] For the treatment of BPH/LUTS in the United States today, alfuzosin, doxazosin, terazosin, and tamsulosin are the most prescribed α1AR antagonists. Terazosin, doxazosin, and alfuzosin are non-subtype selective, in that, they block all three α1AR subtypes. In contrast, tamsulosin blocks α1a = α1dARs with 10-fold greater affnity than α1bARs, and is therefore considered to be α1AR subtype selective.[5]
Naftopidil, with three times greater affinity for α1D than for the α1A-AR subtype, is an α1-blocker that has been approved for clinical use, for LUTS/BPH, only in Japan, since 1999.[6] Different from tamsulosin and silodosin, which have a higher and extremely higher affinity for the α1A-AR subtype than for the α1D-AR subtype, respectively, naftopidil has distinct characteristics, because it has a three-fold affinity for the α1D-AR subtype than for the α1A-AR subtype. As the tissue of BPH shows nine- and three-fold increased expression of mRNA in the α1A and α1D-AR subtypes, respectively, compared to normal prostatic tissue, it has been speculated that not only α1A, but also α1D-AR, contribute to the contraction of the prostatic smooth muscle.[7]
The reports by Momose et al. and Masumori et al. have given results that tamsulosin is superior to naftopidil in the treatment of LUTS due to BPH, which are contrary to a majority of the studies published. The previous studies have concluded that naftopidil is as effective as tamsulosin. Hence, this study has been conducted to document the role of naftopidil in the treatment of LUTS due to BPH and compare its effect with tamsulosin, in the Indian population.
MATERIAL AND METHODS
This study was conducted on 120 symptomatic patients of lower urinary tract symptoms due to benign prostatic hyperplasia, attending the Outpatient Department of Surgery and Urology of Pt BDS PGIMS Rohtak, in collaboration with the Radiology Department after taking written and informed consent.
The patients were selected on the following inclusion criteria: Age more than 45 years, symptomatic benign prostatic hyperplasia for a duration of at least six months of storage symptoms (increased day time frequency, urgency and nocturia, and/or voiding symptoms (difficulty in initating micturation, feeling of incomplete voiding, impaired quality of the stream or interruption of stream), day time frequency >8 times or nocturnal frequency >2, maximum flow rate between 5 and 15 ml/second, with a voided volume of at least 150 ml, post void residual urine less than 150 ml by abdominal ultrasound, international prostate symptom score >13 points, and international prostate symptom bother score >3 points.
The patients were excluded if they had satisfied any one of the following exclusion criteria: Previous prostate surgery, severe visceral disease, postural hypotension, neurogenic bladder dysfunction, suspected prostate cancer, urethral stricture disease, history of pelvic irradiation, bladder neck disease, acute bacterial prostatitis, acute urinary tract infection, urolithiasis, history of concomitant medication that could alter the voiding pattern before inclusion (calcium antagonist monoamine oxidase inhibitors or anti cholinergic drugs), active hematuria, renal insufficiency (serum creatinine >2.0 mg/dl), severe hepatic impairment (transamimases >2 times the upper normal limit and/or total bilirubin >1.5 mg/dl), patients on antipsychotic medications, insulin-dependent diabetes mellitus, history of severe heart disease (myocardial infarction or cerebrovascular accident in the previous six months), and ascertained or suspected hypersensitivity to tamsulosin or naftopidil.
The patients were divided into two groups by a computer-generated, simple, randomized analysis.
Group 1 (tamsulosin group): Sixty patients would be administered 0.4 mg tamsulosin once daily for three months
Group 2 (naftopidil group): Sixty patients would be administered 75 mg naftopidil once daily for three months.
The IPSS self-evaluating questionnaire was filled for each one of the eligible patients. Patients in both the groups would be evaluated at the beginning of the study and then at one- and three-month intervals by means of ultrasonography, uroflowmetry, and the international prostate symptom score and quality of life index questionnaire.
The IPSS and international QOL score was calculated to assess the subjective patient response to LUTS after the start of treatment, to compare it with the uroflowmetry findings. The IPSS score contained seven symptom questions, which included, a feeling of incomplete bladder emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia, each referring to the last month, and each involving the assignment of a score from 1 to 5, for a total of a maximum of 35 points. A score of 0-7 was considered mildly symptomatic, 8-19 moderately symptomatic, and 20 to 35 severely symptomatic.
The quality of life index consists of scoring the patients on their response to the question, “How would you feel if you had to live with your urinary condition the way it is now, no better, no worse, for the rest of your life?” on a scale of 0 to 5, with 0 being most delighted and 5 being dreadful.
We performed our uroflowmetry on Laborie Urocap III uroflowmeter with Bluetooth technology.
The patients included in the study as well as the doctor conducting the study were blinded to the drug being administered and the group allocation.
OBSERVATIONS
A total of 120 patients were divided randomly into Group 1 (tamsulosin) and Group 2 (naftopidil) of 60 patients each, and were followed up for three months with no dropouts during the duration of the study.
Prostate size and post-void residual volume on ultrasonography
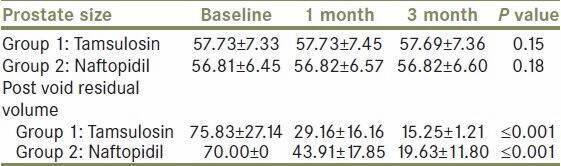
The prostate size was assessed by ultrasonography at inclusion and follow up, at one and three months. In Group 1 the mean baseline prostate size was 57.73 ± 7.33, 57.73 ± 7.45, and 57.69 ± 7.36 at inclusion, one month, and three months of follow up [Figure 1]. The reduction in size was statistically insignificant in Group 1 (P = 0.15). Similarly in Group 2 the prostate size showed a statistically insignificant reduction in size (P = 0.18), the values being 56.81 ± 6.45, 56.82 ± 6.57, and 56.82 ± 6.60, respectively [Table 1].
Figure 1.
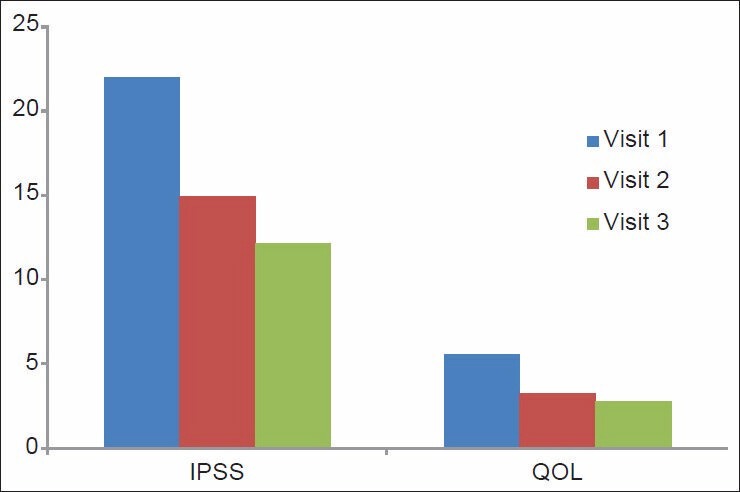
Variation in the IPSS QOL scores in the tamsulosin group
Table 1.
Variation in prostate size and post-void residual volume in group 1 and group 2
Uroflowmetry
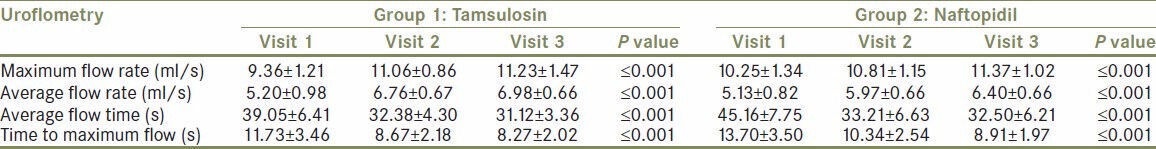
The uroflowmetry study of all the recruited patients was done before the start of treatment and on follow up at one and three months. The following parameters were measured on serial monitoring, the maximum flow rate (ml/s), average flow rate (ml/s), average flow time (s), and time to maximum flow time (s). Within each group all the parameters showed a statistically significant improvement with a P ≤ 0.001. The values obtained are shown in the Table 2.
Table 2.
Improvement in uroflometry variables on treatment with in tamsulosin and naftopidil in group 1 and group 2
International prostate symptom score and quality of life score
The IPSS scores in Group 1 were 21.95 ± 4.46, 14.91 ± 3.61, and 12.15 ± 2.65 and in Group 2 they were 21.31 ± 4.04, 13.53 ± 2.96, and 11.93 ± 1.92, respectively, at the beginning of the study, at one month, and three months. The IPSS score showed a statistically significant improvement within the groups, with a P ≤ 0.001 [Figure 1].
The QOL scores in Group 1 were 5.53 ± 0.57, 3.25 ± 0.67, and 2.78 ± 0.55 and in Group 2 they were 5.53 ± 0.57, 3.25 ± 0.67, and 2.78 ± 0.55, respectively, at the beginning of the study, at one month, and three months. The scores were statistically significant in both the groups with a P ≤ 0.001 [Figure 2].
Figure 2.
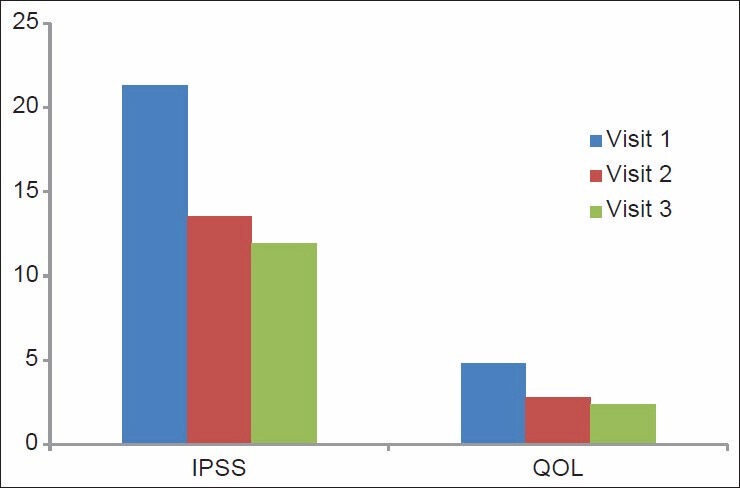
Variation in the IPSS QOL scores in the naftopidil group
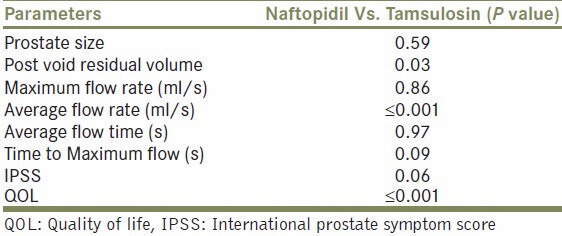
On comparing both the groups for all the parameters observed there was a statistical difference only in the average flow rate and QOL index, showing that naftopidil was better than tamsulosin. The rest of the parameters recorded did not show any statistical difference between the two drugs with respect to their effects exerted in relieving the symptoms of LUTS in patients of BPH [Table 3].
Table 3.
Inter group comparison Naftopidil Vs. Tamsulosin
In the naftopidil group, patients had complaints of dizziness, headache, and postural hypotension (6, 4, and 4%), Which were tolerable and hence Medication was not discontinued. However, in the tamsulosin group the complaints of dizziness, headache, and postural hypotension were 5, 4, and 4%. On intergroup comparison there was no statistical difference between the two drugs with respect to their side effects.
The present study had a few limitations, for example, the study did not have a placebo arm and it did not have a crossover study.
DISCUSSION
Benign enlargement of the prostate due to BPH induces bladder outlet obstruction (BOO) and results in the development of lower urinary tract symptoms (LUTS).[8] There are two mainstays of medical treatment for LUTS suggestive of BPH (LUTS/BPH): 5α-reductase inhibitors (5ARI) and α1-adrenergic receptor (AR) antagonists (α1-blockers).[9]5ARI (finasteride, dutasteride) improve BOO through reduction of the prostate volume and contributes to gradual improvement of LUTS and long-term inhibition of disease progression. On the other hand, α1-blockers improve BOO through a decrease in tone of the prostatic smooth muscle. Several α1-blockers are clinically available, including those having nonspecific affinity for α1-AR subtypes (prazosin, terazosin, doxazosin, alfuzosin) and those having specific affinity for them (tamsulosin, naftopidil, silodosin).[9]
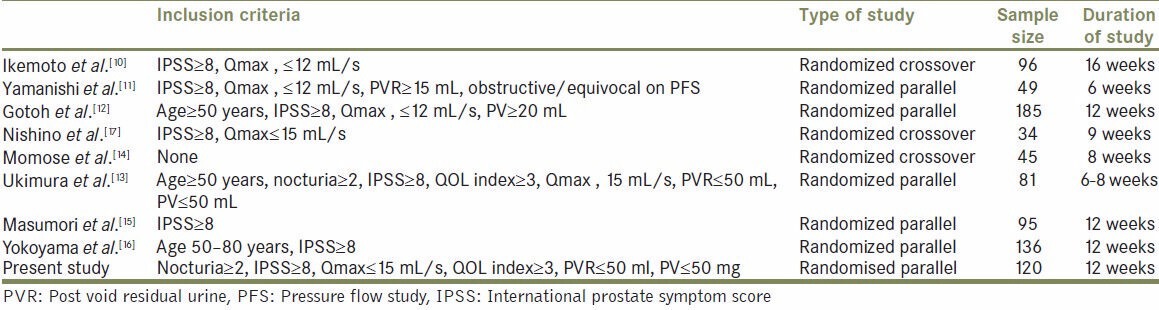
Eight randomized studies of naftopidil compared with other α1-blockers (tamsulosin in six, tamsulosin and silodosin in one, and phytotherapy in another) in contemporary English literature [Table 4], using IPSS and uroflowmetry for evaluation of LUTS, after 2003, are shown in the Table 3, which consists of various inclusion criteria, study designs, treatment durations, and sample size. A crossover design was applied to three studies (Ikemoto et al., Nishino et al., and Momose et al.). Most of the studies recruited a small number of patients, without provision of the required sample size for the hypothesis test.
Table 4.
Study Design of the various studies compared
Ikemoto et al. reported the efficacy of tamsulosin and naftopidil and showed that there was no significant difference in the incidence of adverse events between the two groups (3% versus 2%). The mean IPSS significantly decreased from 17.0 at baseline to 8.5 at 16 weeks, and 17.5 at baseline to 9.2 at 16 weeks in the N-T and the T-N groups, respectively. The QOL index and Qmax showed similar improvements in both groups.[10] Yamanishi et al. compared the clinical and urodynamic effects of naftopidil with those of phytotherapy with eviprostat. Symptomatic improvements evaluated by the IPSS and QOL index were significantly better in the naftopidil group than in the eviprostat group.[11]
Gotoh et al. compared the efficacy and safety of naftopidil and tamsulosin in a multicenter randomized trial. No intergroup differences were observed in any IPSS index after treatment. Qmax was significantly improved by 2.1 mL/second in both groups (P, 0.001 and P = 0.001) and no intergroup difference after treatment was observed (P = 0.709).[12] Ukimura et al. also directly compared the efficacy of naftopidil and tamsulosin in patients having LUTS/BPH, associated with nocturia. There were no significant differences in changes of Qmax and PVR between the two groups. The authors concluded that naftopidil provided early improvements of day frequency and nocturia compared with tamsulosin.[13]
Momose et al. also reported the results of the crossover between naftopidil and tamsulosin, in which the authors concluded that the therapeutic effects of 0.2 mg of tamsulosin on storage and voiding symptoms were superior to those of naftopidil.[14] Masumori et al. performed a head-to-head comparison of the efficacies of naftopidil and tamsulosin, in which the authors speculated that the efficacy of 0.2 mg of tamsulosin might be better than that of 50 mg of naftopidil.[15]
In the present study all the patients were followed up for the entire duration of the study with no dropouts. Our sample size and study duration were comparable to those of Ikemoto et al.,[10] Masumori et al.,[15] and Yokoyama et al.,[16] but the other studies were either too big or too small in size and duration.
On comparison of our uroflowmetry study results, we found that our study was concordant (i.e., the Maximum flow rate Qmax increased in both the groups with no intergroup difference) with all the compared studies (Ikemoto et al.,[10] Yamanishi et al.,[11] Gotoh et al.,[12] Nishino et al.,[17] Momose et al.,[14] Ukimura et al.,[13] and Yokoyama et al.),[16] except the study conducted by Masumori et al.,[15] whose results showed that Tamsulosin was better than Naftopidil in improving the Maximum flow rate Qmax.
The improvement of the IPSS and the QOL scores observed in our study (i.e. the IPSS score significantly improved in both the groups with no intergroup difference) was found to be concordant with the studies conducted by Ikemoto et al.,[10] Yamanishi et al.,[11] Gotoh et al.,[12] Nishino et al.,[17] Ukimura et al.,[13] and Yokoyama et al.,[16] but was discordant with the studies done by Masumori et al.[15] and Momose et al.,[14] who found tamsulosin to be better than naftopidil in improving the IPSS score. In our study, we found naftopidil to be better than tamsulosin in improving the overall QOL index score.
CONCLUSION
In our study, we found both naftopidil and tamsulosin to be effective in the treatment of LUTS due to BPH, in our population. Both the drugs were equally effective in improving all the subjective and objective parameters observed in the study, except for the average flow rate and the quality of life index, where naftopidil was found to superior to tamsulosin (P ≤ 0.001). Even though we were able to prove the efficacy of naftopidil in Indian patients further well-designed prospective large-scale clinical studies, having adequate statistical power, are needed.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Claus GR. Benign prostatic hyperplasia: Etiology, pathophysiology, epidemiology and natural history. In: Alan JW, editor. Campbell-Walsh Urology. 10th ed. Philadelphia: Elsevier Saunders; 2012. pp. 2570–610. [Google Scholar]
- 2.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: Results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104:352–60. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 3.Irwin DE, Milsom I, Hunsakaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur Urol. 2006;50:1306–14. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Bob D, Elisabeth E, Julia F, Geovanni E, Helen S, Roland B. Benign Prostatic Hyperplasia. Prim Care. 2010;37:583–97. [Google Scholar]
- 5.Debra AS, Claus GR. α-Adrenoceptor subtypes and lower urinary tract symptoms. Int J Urol. 2008;15:193–9. doi: 10.1111/j.1442-2042.2007.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takei R-I, Ikegaki I, Shibata K, Tsujimoto G, Asano T. Naftopidil, a novel α1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human α1-adrenoceptors. Jpn J Pharmacol. 1999;79:447–54. doi: 10.1254/jjp.79.447. [DOI] [PubMed] [Google Scholar]
- 7.Nasu K, Moriyama N, Kawabe K, Tsujimoto G, Murai M, Tanaka T, et al. Quantification and distribu-tion of α1-adrenoceptor subtype mRNAs in human prostate: Comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br J Pharmacol. 1996;119:797–803. doi: 10.1111/j.1476-5381.1996.tb15742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masumori N. Naftopidil for the treatment of urinary symptoms in patients with benign prostatic hyperplasia. Ther Clin Risk Manag. 2011;7:227–38. doi: 10.2147/TCRM.S13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapple C, Artibani W, Berges R. New medical developments in the management of LUTS in adult men. In: McConnell J, Abrams P, Denis L, Khoury S, Roehrborn C, editors. Male Lower Urinary Tract Dysfunction Evaluation and Management. 21st ed. Paris: Health Publications; 2006. pp. 143–94. [Google Scholar]
- 10.Ikemoto I, Kiyota H, Ohishi Y, Abe K, Goto H, Kishimoto K, et al. Usefulness of tamsulosin hydrochloride and naftopidil in patients with urinary disturbance caused by benign prostatic hyperplasia: A comparative, randomized, two-drug crossover study. Int J Urol. 2003;10:587–94. doi: 10.1046/j.1442-2042.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamanishi T, Yasuda K, Kamai T, Tsujii T, Sakakibara R, Uchiyama T, et al. Single-blind, randomized controlled study of the clinical and urodynamic effects of an alpha-blocker (naftopidil) and phytotherapy (eviprostat) in the treatment of benign prostatic hyperplasia. Int J Urol. 2004;11:501–9. doi: 10.1111/j.1442-2042.2004.00844.x. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh M, Kamihira O, Kinukawa T, Ono Y, Ohshima S, Origasa H. Comparison of tamsulosin and naftopidil for efficacy and safety in the treatment of benign prostatic hyperplasia: A randomized controlled trial. BJU Int. 2005;96:581–6. doi: 10.1111/j.1464-410X.2005.05688.x. [DOI] [PubMed] [Google Scholar]
- 13.Ukimura O, Kanazawa M, Fujihara A, Kamoi K, Okihara K, Miki T. Naftopidil versus tamsulosin hydrochloride for lower urinary tract symptoms associated with benign prostatic hyperplasia with special reference to the storage symptom: A prospective randomized controlled study. Int J Urol. 2008;15:1049–54. doi: 10.1111/j.1442-2042.2008.02169.x. [DOI] [PubMed] [Google Scholar]
- 14.Momose H, Hosokawa Y, Kishino T, Ono T, Oyama N. Crossover comparison study on the therapeutic effects of tamsulosin hydrochloride and naftopidil in lower urinary tract symptoms associated with benign prostatic hyperplasia. Drugs Today (Barc) 2007;43(Suppl A):1–10. [PubMed] [Google Scholar]
- 15.Masumori N, Tsukamoto T, Iwasawa A, Furuya R, Sonoda T, Mori M. Hokkaido Urological Disorders Conference Writing Group. Ejaculatory disorders caused by alpha-1 blockers for patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Comparison of naftopidil in a randomized multicenter study. Urol Int. 2009;83:49–54. doi: 10.1159/000224868. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama T, Hara R, Fukumoto K, Fujii T, Jo Y, Miyaji Y, et al. Effects of three types of alpha-1 adrenoceptor blocker on lower urinary tract symptoms and sexual function in males with benign prostatic hyperplasia. Int J Urol. 2011;18:225–30. doi: 10.1111/j.1442-2042.2010.02708.x. [DOI] [PubMed] [Google Scholar]
- 17.Nishino Y, Masue T, Miwa K, Takahashi Y, Ishihara S, Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: A randomized crossover study. BJU Int. 2006;97:747–51. doi: 10.1111/j.1464-410X.2006.06030.x. [DOI] [PubMed] [Google Scholar]


