Abstract
Aim:
To assess the effectiveness of laparoscopic stentless pyeloplasty for congenital ureteropelvic junction obstruction.
Materials and Methods:
This was a prospective comparative study conducted over a period of 5 years. The study included 35 cases of primary ureteropelvic junction obstruction (UPJO) with mean age of 29.5 years, divided in two groups- Group A (stent-less, 18 patients) and Group B (stented, 17 patients). Follow up ranged from one to 4years (mean 2 years). Transperitoneal laparoscopic Anderson- Hyene's pyeloplasty was standard for both the groups. Perioperative and postoperative complications were prospectively collected and analyzed by Statistical Package for Social Sciences (SPSS) 17 version using Pearson chi square test.
Results:
Both the groups were comparable with respect to preoperative differential renal function (DRF) and time required for maximum activity in minutes (tmax.min). Average post operative DRF was significantly higher than preoperative DRF in both the groups. Average tmax was significantly lower after pyeloplasty than pre operative tmax. Mean operative time, mean duration of urethral catheter, and mean duration of drain removal were comparable in both the groups. However bothersome irritative lower urinary tract symptoms (LUTS) and hematuria were significantly more in group B patients (P < 0.0001 and <0.013 respectively).
Conclusion:
In experienced hands, laparoscopic stentless pyeloplasty is as effective method for treating UPJO as its stented counterpart. It is cost effective, avoids stent-related morbidity, and could be performed without compromising the success rate. However, more randomized studies are needed to evaluate the safety of stentless pyeloplasty.
Keywords: Laparoscopic, pyeloplasty, stentless, ureteropelvic junction obstruction
INTRODUCTION
Pelvi-ureteric junction (PUJ) is the most common site of obstruction in upper urinary tract. It occurs nearly 1 in 500 to 1:1250 live births.[1,2] There are two types of ureteropelvic junction obstruction (UPJO) – more common intrinsic and the extrinsic variety.[3] The main indications for active interventions are to relieve obstruction, relieve pain, to preserve renal function and/or treat pathologies secondary to such obstruction like calculi and infections.[4] Recent improvements in pre-natal ultrasonography now allow most of the cases to be diagnosed in utero.[4] Obviously, for patients of any age, a reconstructive procedure is always indicated whenever overall renal function is compromised because of involvement in a solitary kidney or bilateral disease.[5] UPJO may not become apparent until middle age or later.[6] However, the majority of affected patients can in fact benefit from reconstructive intervention.[7] When intervention is indicated, the procedure of choice is the repair of PUJ i.e. pyeloplasty.
Surgical management of UPJO has recently been revolutionized by the introduction and widespread adoption of minimally invasive techniques as alternative to standard open reconstructive procedures in an effort to reduce the morbidity of the treatment. Initially, minimally invasive approaches included antegrade and retrograde endoscopic endopyelotomy. Although, these procedures are associated with relatively few complications, brief hospitalization and little disability, the reported success rates are lower (71_ 88%) as compared to open approach, and it has limited indications. Also these procedures have an increased risk of hemorrhage.[8]
MATERIALS AND METHODS
This prospective study was conducted in Sir Ganga Ram Hospital, New Delhi, India, in the Department of Urology from October 2007 to December 2011. Total no of patients were 35. Patients were divided in two groups as Group A (stent less, 18 patients) and Group B (stented, 17 patients). Age, gender, and preoperative differential renal function were recorded in all patients. Group A patients were explained the procedure to undergo stentless transperitoneal laparoscopic pyeloplasty and the need for stent placement, if any, in postoperative period, and the Group B patients were explained the procedure to undergo laparoscopic stented pyeloplasty. Indications for surgery were decrease in differential renal function (DRF) in presence of symptoms, decrease in DRF of more than 10% than previous reading in an asymptomatic patient, or decrease in DRF of ≥25% at any point in time, in presence or absence of symptoms, supported by diuretic renographic evidence of UPJO. The decision for not using the stent was based on patient's preference after explaining the pros and cons of the procedure, and surgeon's decision. All patients were preoperatively assessed with history, physical examination, abdominal ultrasound and X--ray kidney, ureter, and bladder (KUB), Intravenous ureterogram (I.V.U), and diuretic renal scan. All patients had radiographic evidence of UPJO and hydronephrosis on diuretic renography and I.V.U. Laboratory tests like complete blood picture, kidney function test, and urine routine and culture were done in all patients.
Exclusion criteria were patients with stone, active infection, children below 6 years of age, and renal units of PUJ obstruction with glomerular filtration rate (GFR) less than 15 ml/minute.
All patients were kept on liquid diet 1_day prior to surgery. Patients were given parentral cephalosporins and Amikacin at the time of induction of anesthesia and these antibiotics were continued postoperatively till the time of drain removal. All procedures were performed under general anesthesia. Foleys catheter was placed in the bladder in all patients before positioning. All procedures were carried out via laparoscopic transperitoneal approach and by a single surgeon. Pneumoperitoneum was created by closed technique by Veres needle and carbon dioxide gas. Three ports were placed in a standard way with variation in position as per the body habitus. Approach to the PUJ area was made by mobilizing the colon by incising the peritoneum laterally. The ureter was identified and dissected in cephalad direction to achieve mobilization of proximal ureter, ureteropelvic junction (UPJ) and renal pelvis. The pelvis was divided, diseased segment excised, watertight, spatulated uretero-pelvic anastomosis performed with a wide funnel shaped dependent new PUJ, taking care of any rotation/ twisting of the ureter. In cases of difficulty in spatulation, the ureter was brought outside body through lower working port and spatulated extra corporally (in three and two cases in Group A and B respectively). Pyeloureteral anastomosis was done with vicryl 4/0 continuous suture.
We found no difference in beginning with either posteriorly or anteriorly except that starting from anterior margin was more convenient. After completion of anterior suture line at UPJ, double J stent was indwelled in group B patients via 10 mm port, loading the stent on guide wire, and with the help of pusher. An infant feeding tube (5Fr or 6 Fr ) was placed in the ureter as a temporary splint to facilitate anastomosis. Water tight, funnel shaped, dependent anastomosis was made. A tube drain size 20 Fr was placed near the anastomosis site. Post operative complications (pain, high and or prolonged drain output, urinoma and fever), hospital stay and improvement in renal functional outcomes on technetium 99m diethylenetriaminepentaacetic acid (Tc 99 DTPA) renal scan were recorded.
Drain was removed in the post operative period when its output decreases to lessthan 50 ml in 24hr. patients with continuous drainage >600 ml on 3rd/4th pod were discharged along with drain and were explained in detail about daily recording of drain output for 24 hrs and communicated on phone. Foley's catheter was removed first followed by drain removal. In case of stented group Foley's catheter was removed after 2 days and drain was removed once output <50 ml. (after catheter removal)
Follow up
The decision for postoperative DJ stenting in stentless pyeloplasty was made if there was persistent high drain output for more than 10 days. The follow up schedule included a visit after 7 post operative day for port site clip removal and clinical examination followed by three week visit with urine analysis and detailed symptomatology and clinical examination. Diuretic renogram was done at third and or sixth month of surgery to compare with pre-operative renal scan in both Group A and Group B.
RESULTS
In our study all patients had primary UPJO. In all patients surgery was successfully completed laparoscopically. The demographic profile, pre op/ post op tmax and DRF is given in Tables 1 and 2 for group A and B patients, respectively. Mean age was 28 and 31 years in group A and B, respectively. All patients had proven significant obstruction on Tc 99 DTPA renal scan. Mean operative time was 180 mts (range155-210 mts) and mean blood loss was 50 ml (range 20-100 ml) in both the groups. In Group A patients mean duration of post operative Foley's catheter was 2 days. Drain output ranged from 500-1200 ml on first postoperative day that decreased gradually in 16 patients and drain was removed from 3rd to 5th postoperative day (mean 4 days). Two patients had persistent drainage >600 ml/24 hrs on 5th postoperative day and were discharged along with drain which was removed on 7th pod in one and 8th pod in another patient. One patient had high grade fever and vomiting on 10th pod. As the drain was removed on 3rd pod, possibly drain was blocked in immediate postoperative period. Ultrasound KUB showed features of perirenal urinoma. Subsequently DJ stent was inserted by the same surgeon with position of DJ stent checked on fluoroscopy. He was put on injectable antibiotics and hospitalized for 2-days till fever subsided. None of the patients had any signs of peritonitis or evidence of bowel injury in the post operative period.
Table 1.
Demography, tmax and DRF, both pre operatively and post operatively in Group A patients
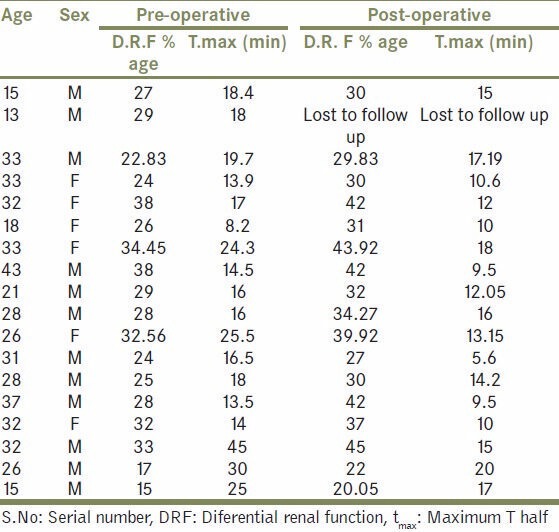
Table 2.
Demography, tmax and DRF, both pre operatively and post operatively in Group B patients
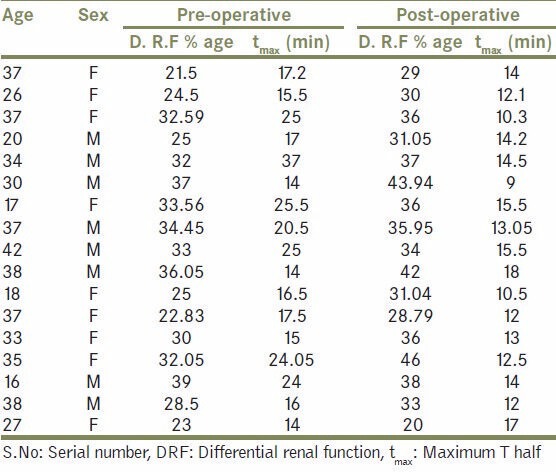
In group B patients, Foley's catheter was removed after a mean of 2 days and drain was removed on 3rd pod (when 24 hr drain output was <50 ml). On follow up these patients were noted to have significant bothersome urinary tract symptoms as compared to non stented counterpart (P value- < 0.0001) [Table 3, Figure 1]. Two patients experienced stent related pain affecting daily activities.
Table 3.
Complications in the two groups
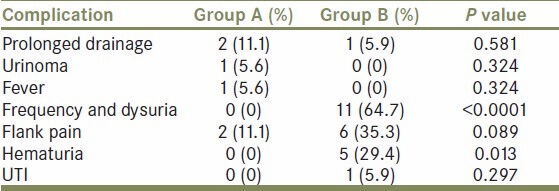
Figure 1.
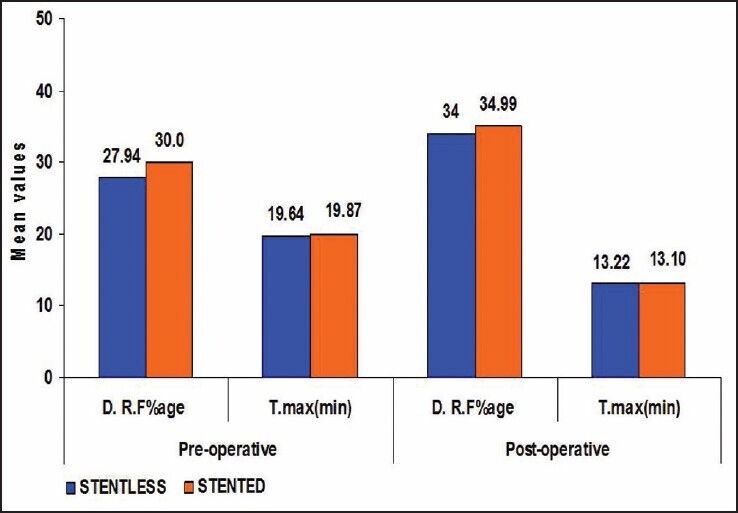
Bar diagram showing comparison between Group A and B patients with respect to their pre op/post op DRF and tmax
In stented Group 1, one patient after removal of DJ stent had no significant improvement in post operative DTPA renal scan done at 3month and on 6 month repeated DTPA renal scan showed obstruction (and deterioration of renal function). On retrograde pyelogram there was stricture distal to UPJ area. On repeat diuretic DTPA renal scan at 3months of endopyelotomy, DRF improved. In both groups the mean difference in the pre op and post op renal function is statistically significant.
Average post op DRF was significantly higher than pre op DRF in both the groups.
Likewise, average tmax was significantly lower than tmax pre operatively in both the groups. In all patients diuretic renogram at 3 month interval after surgery showed significantly improved function as compared to preoperative function (P value < 0.0001) [Table 4, Figure 1], similar to that of stented pyeloplasty. However there was no significant difference in tmax and DRF between the two groups after pyeloplasty [Table 5, Figure 2]. There was no statistically significant difference between the two groups post operatively with respect to renal function improvement, complications like urinoma formation, urinary tract infection (UTI), prolonged drainage, fever and flank pain. However bothersome irritative LUTS and hematuria were significantly more in group B patients (P < 0.0001 and <0.013 respectively [Table 3, Figure 1].
Table 4.
Comparison between pre operative and post operative DRF and tmax in both the groups
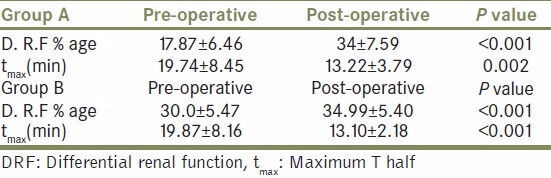
Table 5.
Pre and post operative comparison between the two groups showing that both the procedures were equally effective
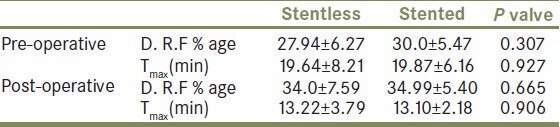
Figure 2.
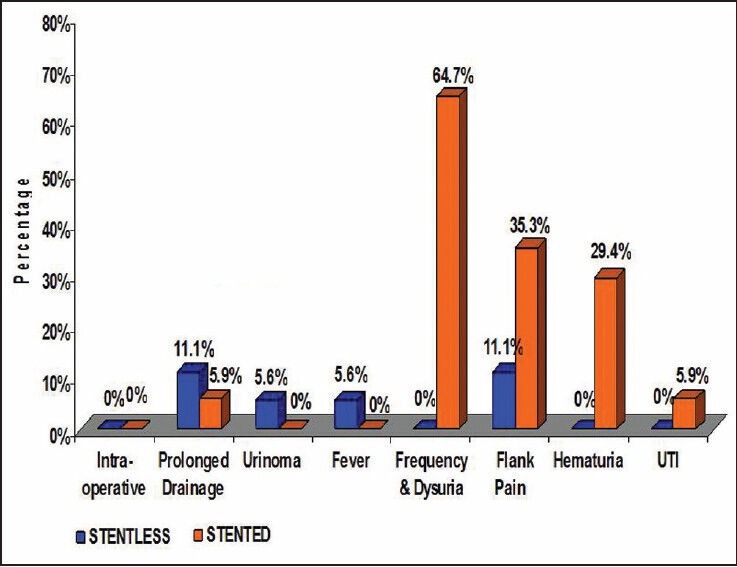
Bar diagram showing comparison of complications between the two groups
DISCUSSIONS
Laparoscopic pyeloplasty has evolved worldwide as the first minimally invasive option to match the success of open pyeloplasty, while achieving the added goals of low morbidity, improved cosmesis, short hospital stay and convalescence. Laparoscopic pyeloplasty is continuously evolving with various modifications to simplify the technique to make it a truly minimally invasive approach. There has been an ongoing debate on the merits of intubated versus non-intubated (stentless) repair of UPJO done either by laparoscopic or open technique. Despite high success rate, open pyeloplasty has the disadvantage of a loin wound and consequent increased morbidity and long convalescence.
Dismembered pyeloplasty is the popular operation when there is dyskinetic segment or proximal ureter is hooked over lower pole blood vessels.[9] The use of stenting catheters and proximal diversion at the time of pyeloplasty has been the subject of debate. Excellent results have been reported both with and without stents and diversion.[9] Stents and nephrostomy tubes, once considered integral part of pediatric reconstructive surgery are now rarely placed.[9] Most pediatric urologists now believe that routine use of stents and nephrostomy tubes is no longer indicated and is reserved for complicated cases.[10]
Laparoscopic pyeloplasty was originally developed by Schuessler et al.[11] in an attempt to duplicate the results of open pyeloplasty while simultaneously decreasing postoperative morbidity. Since then several groups have reported its successful use.[12] Although associated with greater technical complexity and a steeper learning curve, in the hands of the experienced laparoscopic surgeons it has been shown to provide lower patient morbidity, shorter hospitalization, and faster convalescence with the reported success rate matching those of open pyeloplasty (90% or higher).[12] More recently, with advancing laparoscopic skills and the introduction of robotic assisted surgery, many centers have moved to laparoscopic pyeloplasty as first-line therapy.[13] Improved suturing skills and the use of robotic assistance have greatly facilitated laparoscopic dismembered pyeloplasty for primary and secondary repairs.[13]
The use of ureteral stents following pyeloplasty ensures adequate drainage, particularly in the presence of postoperative edema.[14] The advantage of stent placement following pyeloplasty include: Lowering the risk of urinoma formation in the event of leak, thereby reducing periureteric fibrosis and restenosis,[15] and providing support and alignment of the fresh suture line.[16] The importance of the stent is highlighted when the anastomosis is not watertight, or after endopyelotomy, allowing healing of the defect while urine is diverted by the stent. However, ureteral stents are not free from risk, and potential problems include irritative urinary symptoms, flank pain and increased risk of infection,[17] migration, encrustation, retained or forgotten fragments,[18] exposure of the upper urinary tract to high pressure during urination, and need for additional procedure for removal. Although, the role of stents has been well described after endopyelotomy, its role after laparoscopic pyeloplasty, where water tight closure can be achieved remains to be evaluated.
More recently, there seems to have been a trend towards non stented repairs.[19,20] It is of interest to mention the comment from Anderson and Hynes on their technique, “We are convinced that the so called splinting of any anastomosis is not only unnecessary but it is against all the principles of plastic procedure, as it leads to fibrosis at the suture and subsequent stricture. The line of anastomosis should be wide enough and so fashioned as to render any subsequent contraction innocuous”. They did not drain the pelvis or use a trans-anastomotic tube/stent.[21] Stent has been found to be associated with stent syndrome (defined as dysuria, frequency, flank pain and hematuria commonly seen with short term placement of ureteral stents), interfere with daily activities and result in reduced quality of life.[9,22] Stents act as foreign bodies causing compromised vascularity and fibrosis at anastomotic site[9,22] Joshi et al.[17] in their study on indwelling ureteral stents reported that 78% patients had bothersome urinary symptoms that included storage symptoms, incontinence and hematuria.[12] More than 80% of patients experienced stent related pain affecting daily activities, 32% reported sexual dysfunction, and 58% reported reduced work capacity and negative economic impact. In our study in stented pyeloplasty group, 11 (65%) patients had irritative lower urinary tract symptoms needing treatment. Though stent can be removed under local anesthesia in adults, its removal requires general anesthesia in children. The benefits of stentless pyeloplasty are reduced risk of infection, avoiding the risk of developing stent syndrome, and avoiding need for cystoscopic removal after 1 month.[9,22]
The complications noted in our study in stentless pyeloplasty group were urinoma in one patient (5.5%), two patients (11%) had persistent drainage on 5th pod and were discharged along with drain, similar to the observations made by Bilen CY et al.[20] They concluded that laparoscopic stentless pyeloplasty is as feasible technique as its stented counterpart. Although, it has relatively high prolonged leakage risk, it could be performed without compromising the success rate in experienced hands.
In stented group, one patient (5.8%) developed secondary UPJO on follow up after stent removal. Retrograde endopyelotomy was done in him. It seems that stent does not replace the need of good technique of surgery and it cannot prevent re-stenosis if surgical principles are not adhered to. In our study, there was failure of laparoscopic pyeloplasty (5.8%) even when stent was placed. Smith KE et al.[15] in a study in the pediatric population concluded that stent less pyeloplasty is a safe procedure. In a study by Pasquale casale et al. (2010)[23] they concluded that stentless robotic assisted pyeloplasty is a safe and effective option for surgical treatment of UPJO. A larger prospective long-term cohort is needed to confirm the safety and efficacy of the stent less approach. In a similar study conducted by Sethi AS et al. (2010)[24] it was suggested that non stented robotic assisted pyeloplasty is a safe and feasible procedure for the treatment of ureteropelvic junction obstruction. There were no clinically significant differences between the stented and unstented groups in their study.
CONCLUSION
In experienced hand laparoscopic stentless pyeloplasty is as effective method for treating UPJO as its stented counterpart. Although, it has a relatively high initial prolonged urine leakage and prolonged hospital stay, it avoids stent related morbidity, is cost effective, and could be performed without compromising the success rate. However, since the number of patients in our study is limited, more work has to be done to evaluate the safety of stentless pyeloplasty. In our preliminary experience, we find that stent is not a mandatory requirement in pyeloplasty and there is no compromise in the outcome, while avoiding stent related problems at the same time.
Limitations of the [study]
Our study has a small size (under powered), and it is non-randomized (selection bias).
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Arger PH, Coleman BG, Mintz MC, Snyder HP, Camardese T, Arenson RL, et al. Routine fetal genitourinary tract screening. Radiology. 1985;156:485–9. doi: 10.1148/radiology.156.2.3892578. [DOI] [PubMed] [Google Scholar]
- 2.Grigon A, Filiatraut D, Homsy Y Robitaille P, Filion R, Boutin H, et al. Ureteropelvic junction stenosis: Antenatal ultrasonography diagnosis, postnatal investigation and follow-up. Radiology. 1986;160:649–51. doi: 10.1148/radiology.160.3.3526403. [DOI] [PubMed] [Google Scholar]
- 3.Joyner B. Ureteropelvic junction obstruction. In: Grosfeld JL, editor. Pediatric surgery. 6th ed. 2006. pp. 1723–1740. [Google Scholar]
- 4.Mouriquand P. Congenital Anomalies of the pyeloureteral junction. In: Grosfeld JL, Fonkalsrud EW, editors. Pediatric Surgery. 5th ed. Missouri: Mosby-Year Book; 1967. [Google Scholar]
- 5.Smith AD. Should open pyeloplasty be abandoned? J Urol. 1997;157:476–8. doi: 10.1016/s0022-5347(01)65174-7. [DOI] [PubMed] [Google Scholar]
- 6.Arun N, Kekre NS, Nath V, Gopalakrishnan G. Is open pyeloplasty still justified? Br J Urol. 1997;80:379–81. doi: 10.1046/j.1464-410x.1997.00310.x. [DOI] [PubMed] [Google Scholar]
- 7.Schoeffer AJ, Grayhack JT. In: Surgical management of ureteropelvic junction obstruction. 5th ed. Walsh PC, Gittes RF, editors. Philadelphia: WB Saunders Co; 1986. [Google Scholar]
- 8.Meretyk I, Meretyk S, Clayman RV. Endopyelotomy: Comparison of ureteroscopy, retrograde and antegrade percutaneous techniques. J Urol. 1992;148:775–82. doi: 10.1016/s0022-5347(17)36717-4. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DH, Aliabadi H, Ercole CJ, Gonzalez R. Non intubated Anderson Hynes repair of ureteropelvic junction, observation in 60 patients. J Urol. 1989;142:704–6. doi: 10.1016/s0022-5347(17)38859-6. [DOI] [PubMed] [Google Scholar]
- 10.Foley FE. New plastic operation for stricture at ureteropelvic junction. J Urol. 1937;38:643. [PubMed] [Google Scholar]
- 11.Schuessler WW, Grune MT, Tecuanhuey LV, Preminger GM. Laparoscopic dismembered pyeloplasty. J Urol. 1993;150:1795–9. doi: 10.1016/s0022-5347(17)35898-6. [DOI] [PubMed] [Google Scholar]
- 12.Adeyoju AB, Hrouda D, Gill IS. Laproscopic Pyeloplasty: The first decade. BJU Int. 2004;94:264–7. doi: 10.1111/j.1464-410X.2003.04959.x. [DOI] [PubMed] [Google Scholar]
- 13.Atug F, Burgess SV, Castle EP, Thomas R. Role of Robotics in the management of secondary ureteropelvic junction obstruction. Int J Clin Pract. 2006;60:9–11. doi: 10.1111/j.1368-5031.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerber GS, Stockton BR. Use of stents after ureteroscopic stone removal. J Endourol. 2006;20:383–5. doi: 10.1089/end.2006.20.383. [DOI] [PubMed] [Google Scholar]
- 15.Smith KE, Holmes N, lieh JI, Mandell J, Baskin LS, Kogan BA, et al. Stented versus nonstented pediatric pyeloplasty: A modern series and review of the literature. J Urol. 2002;168:1127–30. doi: 10.1016/S0022-5347(05)64607-1. [DOI] [PubMed] [Google Scholar]
- 16.Lawrentschuk N, Russell JM. Ureteric stenting 25 years on: Routine or risky. A N Z Surg. 2004;74:243–7. doi: 10.1111/j.1445-2197.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 17.Joshi HB, Stainthrope A, MacDonagh RP, Keeiey JR, Keeley JR, Timoney AG, Barry MJ. Indwelling ureteral stents: Evaluation of symptoms, quality of life and utility. J UROL. 2003;169:1065–9. doi: 10.1097/01.ju.0000048980.33855.90. [DOI] [PubMed] [Google Scholar]
- 18.Somers WJ. Management of forgotten or retained indwelling ureteral stents. Urology. 1996;47:431–5. doi: 10.1016/S0090-4295(99)80468-3. [DOI] [PubMed] [Google Scholar]
- 19.Shalhav AL, Mikhail AA, Orvieto MA, Gofrit ON, Gerber GS, Zorn KC. Adult stentless laparoscopic pyeloplasty. JSLS. 2007;11:8–13. [PMC free article] [PubMed] [Google Scholar]
- 20.Bilen CY, Bayazit Y, Güdeloðlu A, Abat D, Inci K, Doran S. Laparoscopic pyeloplasty in adults: Stented versus stentless. J Endourol. 2011;25:645–50. doi: 10.1089/end.2010.0401. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JC, Hynes W. Retrocaval ureter. A case diagnosed preoperatively and treated successfully by a plastic operation. Br J Urol. 1949;21:209–14. doi: 10.1111/j.1464-410x.1949.tb10773.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwyzer A. New pyeloureteral plastic operation for hydronephrosis. Surg Clin North Am. 1923;3:1441. [Google Scholar]
- 23.Casale P, Lembert S. J Robotic surg. 2010;3:215–27. doi: 10.1007/s11701-009-0164-4. [DOI] [PubMed] [Google Scholar]
- 24.Sethi AS, Regan SM, Sundaram CP. Robot-assisted laparoscopic pyeloplasty with and without a ureteral stent. J Endourol. 2011;2:239–43. doi: 10.1089/end.2010.0192. [DOI] [PubMed] [Google Scholar]


