Abstract
Aim:
To evaluate the expression of two different lymphatic vascular density (LVD) markers (D2-40 and LYVE-1) and a lymphangiogenic cytokine (Vascular Endothelial Growth Factor-C, [VEGF-C]) in prostate carcinoma and to investigate their relationship with the lymph node status.
Settings and Design:
Archival material study of 92 non-consecutive radical prostatectomy specimens.
Materials and Methods:
The mean LVD was assessed immunohistochemically in 24 prostate carcinoma specimens from patients with clinically localized disease, who were found to have nodal metastasis (pN1), and was compared with 68 pN0 cases. Furthermore, the mean LVD, VEGF-C expression, and lymphatic invasion were examined in relation to lymph node involvement.
Results:
Peritumoral (but not intratumoral) mean LVD assessed by D2-40 was higher in pN1 tumors (P = 0.015). LYVE-1 expression was limited and not associated with lymph node status. The VEGF-C expression was higher in the N1 cases and also correlated with the increased mean LVD in both the peri- and intratumoral compartments. Lymphatic invasion was strongly associated with nodal metastasis and higher VEGF-C expression.
Conclusions:
Our results indicate that increased peritumoral (but not intratumoral) LVD in the tumor specimen is associated with lymph node metastasis. Increased expression of VEGF-C is associated with higher LVD (in both intratumoral and peritumoral compartments) and with positive lymph node status, indicating a possible dual role in both lymphangiogenesis and lymphatic vessel invasion.
Keywords: Immunohistochemistry, lymphangiogenesis, prostate adenocarcinoma, vascular endothelial growth factor-C, vessel density
INTRODUCTION
Widespread interest in the screening and early detection of prostate cancer has led to an increase in patients diagnosed with ‘localized disease,’ who undergo radical prostatectomy.[1] Nevertheless, 5-12% of the patients who receive surgical treatment for clinically localized disease, harbor metastasis in the regional lymph nodes, suggesting that the currently available methods of clinical staging (lymphangiography, computed tomography, magnetic resonance imaging, Partin tables) are inadequate.[2,3] Recent interest in molecular staging is based on promising results of studies that employ lymphangiogenesis markers and lymphangiogenic factors in the prediction of nodal status. Studies on breast, cervical, and gastric tumors have revealed a strong association of lymphatic vessel density (LVD) with nodal metastasis and prognosis.[4,5] Detection of lymphatic vessels is reliably accomplished by immunohistochemistry using the D2-40 and LYVE-1 antibodies. However, limited studies on prostate carcinoma have given contradictory results.[6,7] The discrepancy in the previous results may in part be due to the lack of lymphatic-specific markers in the past and the heterogeneity of protein expression in lymphatic endothelial cells, making the assessment of lymphangiogenesis inaccurate.[8]
The aim of our study was to evaluate the tumoral lymphatic parameters in patients who were subjected to radical prostatectomy for clinically localized disease, but were found to have positive nodes (pN1). We hypothesized that increased lymph vessel density in the tumor specimen would correlate with positive node status. We used the lymphatic-specific markers D2-40 and LYVE-1, and compared their value as markers of LVD. Moreover, we evaluated the expression of the vascular endothelial growth factor-C (VEGF-C) in relation to the mean LVD and lymph node status.
MATERIALS AND METHODS
Patients and specimens
Pathology reports of 258 patients with prostate carcinoma, who underwent radical prostatectomy with pelvic lymph node dissection for clinically localized disease (stage T1-2, N0, M0), at the General University Hospital of Patras, between 2003 and 2009, were reviewed. Twenty-four of these men (9.3%) were pathologically assessed to have regional LN metastasis (LN+, Stage pT1-2, N1, M0). We further randomly selected 68 cases from the pN0 (LN–) cohort. After an Institutional Review Board approval, archival formalin-fixed, paraffin-embedded tissue blocks, representative of the reported adenocarcinoma, were selected for each case. The tumors were graded according to the Gleason system and staged according to the Tumor Node Metastasis (TNM) (AJCC 2009) staging system for radical prostatectomy. The cases were further divided in three groups, according to Gleason score: Grade ≤7 (3 + 4), grade = 7 (4 + 3), and grade ≥8.
Immunohistochemistry
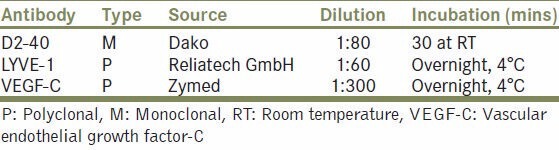
Briefly, serial 4 μm sections were deparaffinized by incubation in xylene, at 60°C, and rehydrated in a series of graded alcohol solutions, followed by washing in tris-buffered saline (TBS) (pH 7.6). Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in distilled water, followed by washing in TBS. The sections were treated in a microwave oven, in a citrate buffer (pH 6.0), for antigen retrieval. Non-specific binding was blocked by treating the slides for 12 minutes with 2% bovine serum albumin in TBS. The commercially available antibodies for podoplanin (D2-40), LYVE-1, and VEGF-C were used as described in Table 1. The bound primary antibody was detected with the Envision TM detection kit (DAKO, Hamburg, Germany) and visualized using diaminobenzidine (DAB) as the chromogen. Finally, the tissue sections were counterstained with hematoxylin and dehydrated through graded ethanols and xylene.
Table 1.
Antibody characteristics
Immunohistochemical evaluation
All slides were assessed independently by one pathologist (HP) and one investigator (IL). In cases of discrepant scoring, an agreement was reached after discussion. Intratumoral and peritumoral tissue lymphatics were evaluated separately in all specimens, as previously described.[8] The intratumoral compartment was defined as the area encompassing all the cancer glands present in the section, while the peritumoral compartment was a 1 mm wide area around the intratumoral compartment. Any discrete D2-40 or LYVE-1 positive endothelial cell or cell cluster separate from adjacent structures, regardless of the presence of lumens, was counted as one lymphatic vessel. Lymphatic vessel density, counted at ×200 magnification, was defined as the mean number of lymphatic vessels per field in four optical areas (‘hot-spots’ scanned at low power).
The VEGF-C expression by the neoplastic cells was evaluated in a semiquantitative fashion, by developing a score (Histoscore), where both intensity and distribution of the staining were taken into account. Distribution was graded on a scale of 0 to 3 based on the percentage of positive cells (0: Immunoreactivity in <20% of cells, 1: 20-45% of the cells, 2: 45-75% of the cells, 3: >75% of the cells). Intensity of staining was scored as follows: Score 0: Negative, 1: Weak, 2: Moderate, and 3: Strong staining. Negative staining corresponded to complete absence of staining, strong corresponded to staining that could be easily recognized at ×4 magnification, weak corresponded to staining that could be recognized only at ×20 magnification, and moderate was the staining with intensity values between weak and strong. The two scores were multiplied and the immunoreactivity score (values from 0-9) was determined as follows: Score 0 as negative; score 1 (values 1, 2, 3) as weakly positive; score 2 (values 4, 6) as moderately positive, and score 3 (value 9) as strongly positive.
Another histopathological parameter recorded was the presence of inflammation, assessed semiquantitatively in three levels (low, moderate, and high), in relation to the inflammatory cell (lymphocytes, macrophages, and mast cells) concentration and their overall expanse in the section. Moreover, lymphatic invasion was evaluated using both the pathology reports and the immunostained slides, and its possible correlation to the lymph node status, LVD, and VEGF-C expression was examined.
Statistical analysis
All calculations were performed using the commercially available GraphPad PRISMTM 5.0 statistical software. The unpaired t-test and analysis of variance (ANOVA) were used for mean LVD comparisons between two (nodal status, T stage) or three (Gleason score) groups, accordingly. The Mann-Whitney and Kruskal-Wallis tests were used for non-parametric data comparisons between groups. The association of the pathological parameters and the VEGF-C index with the nodal status, lymphatic invasion, and Gleason score was examined using the Fisher's exact test and the Pearson Chi-square test, respectively. A 5% significance level was used for all the tests.
RESULTS
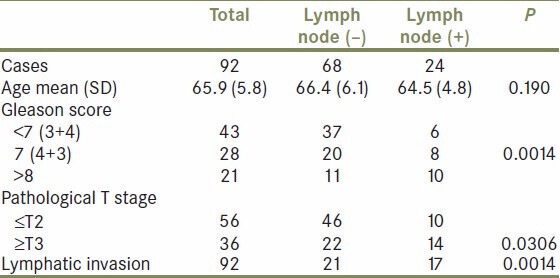
The mean (±SD) patient age at operation was 65.9 (±5.8) years. The two groups (LN- and LN+) were matched for age (t-test, P = 0.190). A higher Gleason score, T stage ≥T3, and lymphatic invasion by tumor cells were associated with positive lymph node status [Table 2].
Table 2.
Histopathological parameters and lymph node status
D2-40 and LYVE-1 positive lymph vessels in prostate adenocarcinoma
D2-40 positive vessels were present in all cases of prostate carcinoma. The immunostaining was limited to thin-walled vessels, with no intramural presence of blood cells, which was consistent with the appearance of lymphatics [Figure 1a and b]. The vessels were found both inside the tumor and in the peritumoral area. At the intratumoral compartment, the lymph vessels were mainly small and collapsed, compared to the more abundant lymphatics at the tumor periphery. In the ‘normal’ prostate gland a few lymph vessels were also present (data not shown). Occasionally, basal cells of the prostatic glandular epithelium were positive for D2-40. The mean LVD in the tumor periphery was higher than that in the intratumoral compartment (mean ± SEM: 7.833 ± 0.415 vs. 5.458 ± 0.458). When the mean LVD was compared between the lymph node positive and negative cases, a statistically significant difference was present for the peritumoral, but not the intratumoral lymphatics (unpaired t-test, P = 0.015 and P = 0.252, respectively, Figure 2a).
Figure 1.
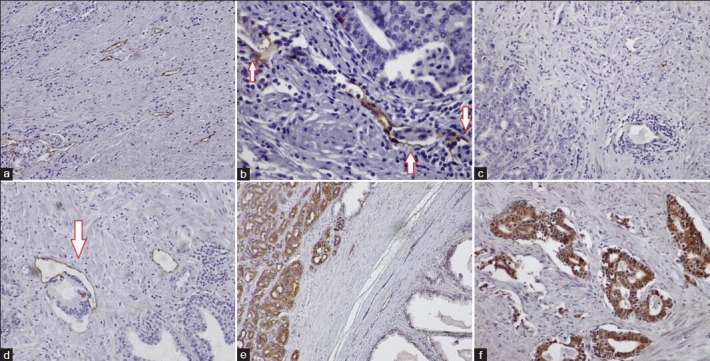
Detection of lymphatics by D2-40 immunostaining in prostate carcinoma (a-b). Note peritumoral lymphatic invasion (B, arrows). Limited LYVE-1 expression in prostate cancer (c-d). Note invasion of lymphatic vessel by tumor in D (arrow). Immunostaining for VEGF-C in prostate carcinoma (e–f). Strong cytoplasmic staining was present in the tumor cells, with extremely limited staining in the stroma and BPH cells (e). All microphotographs ×200 magnification
Figure 2.
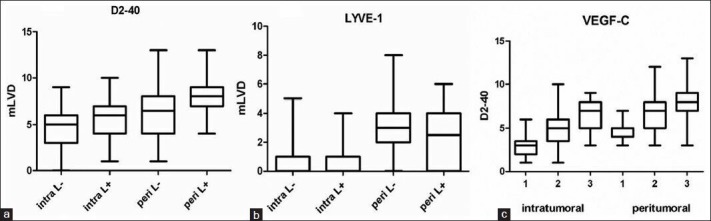
(a and b) Mean LVD in the intratumoral and peritumoral compartments of LN (-) and LN (+) tumors using D2-40 and LYVE-1, respectively. (c) Mean LVD (by D2-40) in relation to VEGF-C expression. X-axis scores: Grade groups 1, 2, and 3. All graphs: Whiskers: Minimum to maximum, line at mean
LYVE-1 overall immunostaining was quite limited compared to D2-40 (mean ± SEM: 2.333 ± 0.424 vs. 0.541 ± 0.208 in the peri- and intratumoral compartments, respectively). In the lymph node negative group, 32 cases (47%) in the intratumoral and six (9%) in the peritumoral compartment were completely negative (score 0). In the node positive group, the negative cases were 16 (66%) and nine (37%), respectively. LYVE-1 immunostaining was limited to a few thin lymphatics [Figure 1c and d]. When LVD was compared between the LN- and LN + cases no differences were present (unpaired t-test, P = 0.280 and P = 0.406, for intra- and peritumoral lymphatics, respectively, Figure 2b).
We further examined the possible relationship of the mean LVD (for both D2-40 and LYVE-1) with the Gleason grade. No statistical differences were found between grade-groups (ANOVA, P > 0.05 in all cases).
VEGF-C expression is higher in prostate adenocarcinoma with lymph node metastasis
Immunostaining for VEGF-C was limited to the glandular compartment of the tumors, with no positivity in the stromal elements. The tumor cells showed either a strong cytoplasmic or a combined membrane and cytoplasmic staining. Staining in normal prostate glands was practically negative. The VEGF-C expression was higher in patients with lymph node metastasis (Mann-Whitney test, P = 0.042). When the mean LVD was evaluated in relation to the degree of VEGF-C expression, significant differences were present for D2-40, but not for LYVE-1 staining (ANOVA, P < 0.0001 for both the intratumoral and peritumoral lymphatics, Figure 2c).
Lymphatic invasion is associated with higher LVD and VEGF-C expression
The presence of lymphatic invasion was higher (58.3%) in the LN + group compared to 30.1% in the LN- group (Fisher's test, P = 0.0014). Lymphatic invasion was also associated with a higher mean LVD for D2-40 in both the intra- and peritumoral compartments (t-test, P = 0.0034 and P = 0.0096, respectively). VEGF-C expression was also significantly higher in cases with lymphatic invasion present (Mann-Whitney, P = 0.0009).
Inflammation and LVD
Chronic inflammatory infiltration (mainly by lymphocytes, but also by macrophages and mast cells) was present in all cases. The degree of inflammation varied: Thirty-three cases (35.9%) were listed as grade I, 44 cases (47.8%) as grade II, and 15 cases (16.3%) as grade III. The mean LVD (D2-40 staining) for both intra- and peritumoral lymphatics was related to the degree of inflammation (ANOVA, P < 0.0001 in all cases). The expression of VEGF-C was also higher in relation to the inflammatory infiltrate (Kruskal-Wallis test, P < 0.0001). The degree of inflammation correlated well with the Gleason score (Pearson Chi square, P = 0.020), but not with the lymph node status (Pearson Chi square, P = 0.117).
DISCUSSION
In patients with prostate carcinoma, lymph node metastasis has been proved to be a strong predictor of poor prognosis.[9,10] Clinical staging of the lymph node status has certain difficulties. In our series, 9.3% of the patients who underwent radical prostatectomy, for organ-confined disease, were found with positive lymph nodes on histopathological examination; a percentage was comparable with the findings in other studies.[6,8] Understanding lymphangiogenesis and lymphatic architecture, therefore, seems a promising step to a more accurate molecular staging in the future.
Although discrepancies in the results of available studies still exist, it is generally agreed that the mean lymph vessel density (LVD) correlates with node metastasis in several tumors.[4,5,11] In this study we have evaluated LVD by utilizing two lymphatic markers, D2-40 and LYVE-1. The monoclonal antibody D2-40 against human podoplanin has so far given good results on the specific staining of lymphatics in several tumors, including prostate carcinoma.[11,12,13] Conversely, LYVE-1, a lymphatic vessel endothelial hyaluronan receptor has not been widely used. Our findings suggest that D2-40 immunostaining is superior to LYVE-1 for both peri- and intratumoral lymphatics. LYVE-1 staining is limited in extent (compared to D2-40) and has also failed to reveal lymphatics in many cases. Moreover, LYVE-1 immunostaining does not differ in LN + and LN- cases. On the other hand, D2-40 stained lymphatics are present in all cases and LVD assessed by D2-40 is strongly associated with lymph node metastasis. In a study by Soh et al., the LVD determined by D2-40 is 30 times higher than that determined by LYVE-1 staining.[14] The inconsistency in the above results can be explained by the heterogeneity of lymphatic endothelial cells (LEC) in relation to the protein expression pattern. It is proposed that D2-40/podoplanin can be an ubiquitous marker for lymphatics, while markers such as LYVE-1 or VEGFR-3 may be expressed only in a specific subpopulation of LEC in the prostate tissue.[8] Similar differential expression of endothelial markers has been shown in other tissues as well, possibly accounting for the inconsistent results with lymphatic markers, in different tumor types.[15] Another possible explanation may lie in the fact that practically all cases of prostate carcinoma in this study have presented a degree of inflammation. The effect that inflammation exerts on LYVE-1 consists of a loss of surface expression of the protein in the LEC, coupled with a shutdown in gene expression.[16] Therefore, our data suggest that, at least in the case of prostate carcinoma, LYVE-1 may not be an optimal lymphatic marker and D2-40 immunostaining must be preferred.
It has long been debated whether peritumoral or intratumoral lymphatics are functional and actually participate in tumor metastasis.[17] In our series the mean LVD determined by D2-40 was strongly associated with the positive lymph nodes in the case of peritumoral, but not in the case of intratumoral lymphatics. Our findings are in line with other studies, which emphasize the importance of peritumoral lymphatics.[8,13,18] It has been proposed that intratumoral lymphatics, although increased compared to normal tissue, are not functional and display abnormal function.[18] In head and neck, pancreatic, and thyroid carcinoma, the intratumoral vessels are considered to contribute to nodal metastasis by facilitating tumor cells to leave the primary site. However, in the case of prostate carcinoma intratumoral lymphatics are probably not major routes for nodal involvement.[8] Instead, peritumoral lymphatics, either pre-existing or induced, seem to suffice as conduits for the dissemination of tumor cells to the regional lymph nodes.[12] However, one must be cautious when interpreting such findings, as the difference in numbers of peri- and intratumoral lymphatics, although statistically significant, is subtle. Moreover, other factors independent of LVD (i.e., Gleason grade and T stage) may also influence the lymphatic spread, as shown in Table 2. Lymphatic invasion, in particular, is a major prognostic factor for lymph node involvement.[19] This has also been confirmed in our study: Invasion of lymphatics was associated with a higher mean number of lymphatics and positive lymph node status.
The role that the main lymphangiogenic cytokine VEGF-C plays in the lymphatic spread remains another matter of controversy. It is generally agreed that VEGF-C is associated with increased tumoral LVD and lymph node metastasis in experimental and tumor implantation models.[20,21] However, the association of VEGF-C-induced lymphangiogenesis and nodal metastasis remains disputed.[6] In our study, VEGF-C was overexpressed in LN + tumors and correlated with a higher mean LVD, a finding that implied an induction of new lymphatics in both the peri- and intratumoral compartments. It is also interesting that cases with lymphatic invasion showed higher VEGF-C expression, indicating that VEGF-C possibly facilitated the entry of tumor cells in lymphatics. This could be accomplished by an increase in vessel permeability and vascular leakage.[22] It was also proposed that tumor-expressed VEGF-C interacts with the lymphatic endothelium, leading to an increase in the size of the lymphatics, thus providing an opportunity for the tumor cells to enter the lymphatic system.[23] Moreover, measurements of the total lymph flow showed an increased flow rate modulated by VEGF-C.[23]
Altogether, these data suggest that VEGF-C does indeed induce the formation of new lymphatics, in both the peri- and intratumoral compartments. Although some of these lymphatics may be immature and dysfunctional, the dual role of VEGF-C in vessel enlargement and permeability possibly enhances lymphatic invasion and nodal metastasis.
The presence of a chronic inflammatory infiltrate has been a common finding in our series. Inflammation has long been related to tumor progression and metastasis, and tumor-infiltrating lymphocytes are considered a prognostic factor for PSA-free survival in patients treated with radical prostatectomy.[24,25] Although their precise role remains unclear, both macrophages and T lymphocytes may contribute to this procedure via secretion of pro-inflammatory cytokines, including members of the VEGF family. Although inflammation has been extensively linked to angiogenesis (mainly via VEGF-A) little is known on its effect on lymphangiogenesis. There is evidence, however, that inflammation may increase the expression of VEGF-C and VEGFR-3, possibly by secretion of proinflammatory cytokines.[26,27] In our study the degree of inflammation has been associated with higher VEGF-C expression as well as with a higher mean LVD. However, the positivity of lymph nodes has not been influenced by the degree of inflammation. Determination of the inflammatory cell subtypes [CD4(+), CD8(+)] may shed light on the possible effect of the inflammatory infiltrate in the tumor studied; however, this is beyond the scope of the present study. Nevertheless, our findings suggest that chronic inflammation may induce the formation of lymphatics, possibly via VEGF-C overexpression; however, its effect on lymph node metastasis remains unclear.
A possible limitation of this study may lie in the semiquantitative method used for evaluation of VEGF-C expression; however, we have defined our Histoscore in as much detail as possible and evaluations have been done independently by two investigators. Another limitation may lie in the difficulty to classify cases in groups according to the Gleason score. Conventional scoring does not take into account the presence of more than two patterns.[28] We have used three groups in order to distinguish between grade 7 (3 + 4 or 4 + 3), due to the different biological behavior of these three groups. Other investigators use two groups, for example, low-high or 6-7 and 8-10.[8,12] These differences may account for the discrepancies in correlations between the Gleason score and parameters such as LVD. In our series, we have failed to show a statistically significant correlation between the Gleason score and mean LVD, as reported by others.[8] However, even when we divided cases according to Gleason score <6, 7, and ≥8, no significant differences were present in our results (data not shown). On the other hand, our findings may have potential clinical value. The positive correlation between the higher peritumoral mean LVD/lymphatic invasion and lymph node metastasis may be evaluated as a tool for predicting possible lymph node involvement. Especially in the era of laparoscopic and robotic-assisted radical prostatectomy without nodal dissection, assessing the mean LVD/lymphatic invasion in the pathology specimen may possibly identify those patients who need to be followed more intensively or even offered adjuvant treatment. Of course, larger studies with multivariate analysis are needed before firm conclusions can be drawn. It would be also very interesting to further assess the predictive value of the above-mentioned lymphatic parameters in needle biopsy specimens.
In summary, our results indicate that increased peritumoral (but not intratumoral) LVD in the tumor specimen is associated with lymph node metastasis. The podoplanin/D2-40 antigen is preferable to LYVE-1, as the expression of the latter is limited. Increased expression of VEGF-C is associated with higher LVD (in both intratumoral and peritumoral compartments) and with positive lymph node status, indicating a possible dual role in both lymphangiogenesis and lymphatic vessel invasion.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Hoffman RM. Randomized trial results did not resolve controversies surrounding prostate cancer screening. Curr Opin Urol. 2010;20:189–93. doi: 10.1097/MOU.0b013e3283383b55. [DOI] [PubMed] [Google Scholar]
- 2.Flanigan RC, McKay TC, Olson M, Shankey TV, Pyle J, Waters WB. Limited efficacy of preoperative computed tomographic scanning for the evaluation of lymph node metastasis in patients before radical prostatectomy. Urology. 1996;48:428–32. doi: 10.1016/S0090-4295(96)00161-6. [DOI] [PubMed] [Google Scholar]
- 3.Burkhard FC, Studer UE. Regional lymph node staging in prostate cancer: Prognostic and therapeutic implications. Surg Oncol. 2009;18:213–8. doi: 10.1016/j.suronc.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Gou HF, Chen XC, Zhu J, Jiang M, Yang Y, Cao D, et al. Expressions of COX-2 and VEGF-C in gastric cancer: Correlations with lymphangiogenesis and prognostic implications. J Exp Clin Cancer Res. 2011;30:14. doi: 10.1186/1756-9966-30-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botting SK, Fouad H, Elwell K, Rampy BA, Salama SA, Freeman DH, et al. Prognostic significance of peritumoral lymphatic vessel density and vascular endothelial growth factor receptor 3 in invasive squamous cell cervical cancer. Transl Oncol. 2010;3:170–5. doi: 10.1593/tlo.09292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trojan L, Rensch F, Voss M, Grobholz R, Weiss C, Jackson DG, et al. The role of the lymphatic system and its specific growth factor, vascular endothelial growth factor C, for lymphogenic metastasis in prostate cancer. BJU Int. 2006;98:903–6. doi: 10.1111/j.1464-410X.2006.06403.x. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK, Padera TP. Prevention and treatment of lymphatic metastasis by antilymphangiogenic therapy. J Natl Cancer Inst. 2002;94:785–7. doi: 10.1093/jnci/94.11.785. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Y, Opeskin K, Horvath LG, Sutherland RL, Williams ED. Lymphatic vessel density and lymph node metastasis in prostate cancer. Prostate. 2005;65:222–30. doi: 10.1002/pros.20288. [DOI] [PubMed] [Google Scholar]
- 9.Sands ME, Pollack A, Zagars GK. Influence of radiotherapy on node-positive prostate cancer treated with androgen ablation. Int J Radiat Oncol Biol Phys. 1995;31:13–9. doi: 10.1016/0360-3016(94)00324-E. [DOI] [PubMed] [Google Scholar]
- 10.Montironi R, Mazzucchelli R, Scarpelli M, Lopez-Beltran A, Mikuz G. Prostate carcinoma I: Prognostic factors in radical prostatectomy specimens and pelvic lymph nodes. BJU Int. 2006;97:485–91. doi: 10.1111/j.1464-410X.2005.05972.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee SK, Cho EY, Kim WW, Kim SH, Hur SM, Kim S, et al. The prediction of lymph node metastasis in ductal carcinoma in situ with microinvasion by assessing lymphangiogenesis. J Surg Oncol. 2010;102:225–9. doi: 10.1002/jso.21607. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda K, Horiguchi A, Asano T, Asano T, Hayakawa M. Prediction of lymphatic invasion by peritumoral lymphatic vessel density in prostate biopsy cores. Prostate. 2008;68:1057–63. doi: 10.1002/pros.20768. [DOI] [PubMed] [Google Scholar]
- 13.Roma AA, Magi-Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol. 2006;19:392–8. doi: 10.1038/modpathol.3800546. [DOI] [PubMed] [Google Scholar]
- 14.Soh S, Ishii T, Sato E, Akishima Y, Ito K, Baba S. Topographic distribution of lymphatic vessels in the normal human prostate. Prostate. 2005;63:330–5. doi: 10.1002/pros.20199. [DOI] [PubMed] [Google Scholar]
- 15.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–77. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson LA, Prevo R, Clasper S, Jackson DG. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J Biol Chem. 2007;282:33671–80. doi: 10.1074/jbc.M702889200. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Dumont DJ. Molecular mechanisms in lymphangiogenesis: Model systems and implications in human disease. Clin Genet. 2003;64:282–92. doi: 10.1034/j.1399-0004.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 18.Aishima S, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol. 2008;21:256–64. doi: 10.1038/modpathol.3800985. [DOI] [PubMed] [Google Scholar]
- 19.Baydar DE, Baseskioglu B, Ozen H, Geyik PO. Prognostic significance of lymphovascular invasion in clinically localized prostate cancer after radical prostatectomy. Sci World J. 2008;8:303–12. doi: 10.1100/tsw.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–98. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 21.Isaka N, Padera TP, Hagendoorn J. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 2004;64:4400–4. doi: 10.1158/0008-5472.CAN-04-0752. [DOI] [PubMed] [Google Scholar]
- 22.Lohela M, Saaristo A, Veikkola T. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost. 2003;90:167–84. doi: 10.1160/TH03-04-0200. [DOI] [PubMed] [Google Scholar]
- 23.Hoshida T, Isaka N, Hagendoorn J, DI tomaso e, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: Therapeutic implications. Cancer Res. 2006;66:8065–75. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 24.Lucia MS, Torkko KC. Inflammation as a target for prostate cancer chemoprevention: Pathological and laboratory rationale. J Urol. 2004;171:S30–4. doi: 10.1097/01.ju.0000108142.53241.47. [DOI] [PubMed] [Google Scholar]
- 25.Kärjä V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25:4435–8. [PubMed] [Google Scholar]
- 26.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: Development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–95. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Hamrah P, Chen L, Zhang Q, Dana MR. Novel expression of vascular endothelial growth factor receptor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am J Pathol. 2003;163:57–68. doi: 10.1016/S0002-9440(10)63630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. Update on the Gleason grading system for prostate cancer: Results of an international consensus conference of urologic pathologists. Adv Anat Pathol. 2006;13:57–9. doi: 10.1097/01.pap.0000202017.78917.18. [DOI] [PubMed] [Google Scholar]


