Abstract
Rationale
Initial lab studies suggest that adolescent drinkers crave alcohol when presented with alcohol cues. Whether this effect generalizes to the natural environment, however, remains unknown, and studies have not examined whether craving predicts drinking among youths.
Objectives
This study builds on existing research by pairing controlled lab-based cue reactivity assessments with data collected in the natural environment using ecological momentary assessment (EMA) methods. We examined whether alcohol cues evoke craving among adolescent drinkers in the lab and natural environment, and tested the clinical relevance of craving during adolescence by examining the prospective association between craving and alcohol use.
Methods
Non-treatment-seeking adolescent drinkers (N = 42; ages 15 to 20 years) completed a lab-based cue reactivity assessment followed by a 1-week EMA monitoring period. During the EMA period, youth were prompted randomly throughout the day to record momentary data on craving and contextual factors (e.g., alcohol cues, peers present).
Results
Alcohol cues elicited craving in the lab, and this effect generalized to the natural environment, especially among adolescents with more alcohol problems. In addition, craving predicted subsequent drinking levels in the natural environment.
Conclusions
This study demonstrates the utility of pairing lab paradigms with EMA methods to better characterize adolescents’ reactivity to alcohol cues. Results implicate craving as a clinically meaningful motivator for drinking among adolescents and highlight a potentially important target of pharmacological or behavioral intervention.
Keywords: Adolescents, Alcohol, Craving, Cue Reactivity, Ecological Momentary Assessment, Alcohol Problems
Craving is a chief motivational determinant of drug use in most contemporary theoretical models of addiction, including alcoholism (Drummond 2001). Indeed, reviews of the empirical literature consistently conclude that craving holds clinical importance for understanding and treating alcohol addiction (Tiffany & Wray 2012). Clinical trials generally show a positive association between craving and risk for relapse or subsequent drinking levels (Bottlender & Soyka 2004; Flannery et al. 2001; 2003; Rohsenow et al. 1994). Lab studies consistently show that alcohol cues evoke craving and physiological reactivity among adults under controlled conditions, with greater responsiveness among individuals with alcohol dependence (Carter & Tiffany 1999; Monti et al. 1987) and heavier drinkers (Ihssen et al. 2011). Consequently, craving is often a focal point of treatment (O’Brien 2005) and the most recent revision of the Diagnostic and Statistical Manual of Mental Disorders introduced craving as a new criterion to advance clinical detection of pathological drinking along an alcohol use disorder (AUD) continuum (American Psychiatric Association 2013).
Despite considerable research with adults, our knowledge of craving during adolescence is based on only a handful of studies. This dearth of empirical data among youths is notable given that adolescence is a key period in the onset of alcohol use and in the development of pathological drinking (Merikangas & McClair 2012; Swendsen et al. 2012). Initial survey data from community-based youths suggest that craving is common in this age group (Deas et al. 2001; 2005; Martin et al. 1995). These early findings were supported by several lab studies of alcohol cue reactivity in adolescent drinkers, with results showing stronger effects among youths with more alcohol-related problems. For example, Tapert and colleagues (2003) found that adolescents with AUDs had greater craving in response to alcohol pictures than non-AUD controls during a functional magnetic resonance imaging protocol. Others studied reactivity to in vivo alcohol cues and found adolescents with alcohol dependence had greater cue-elicited craving compared to drinkers without an AUD (Thomas et al. 2005). A similar study found photographs of alcohol elicited craving among community-based adolescent drinkers, with stronger reactions associated with heavier drinking histories (Curtin et al. 2005). On the whole, research suggests that adolescents crave alcohol when faced with drinking cues, and this effect appears more pronounced among youths with greater alcohol problems. It remains unknown, however, whether lab findings generalize to the natural environment and whether craving is clinically relevant in this age group such that it prospectively predicts drinking behavior.
In this study we paired lab and ecological momentary assessment (EMA) methods to characterize the nature and function of alcohol craving in adolescent drinkers. Specifically, we tested whether alcohol cues elicit craving responses in adolescent drinkers under experimentally controlled conditions and, if so, whether this effect generalizes to the natural environment and prospectively predicts how much alcohol youths consume. We hypothesized that adolescent drinkers would show increases in craving and physiological arousal when exposed to in vivo alcohol cues compared to water cues in the lab, and that this effect would be more pronounced among youths with more severe alcohol-related problems. We expected this effect to generalize to the natural environment, such that adolescents would experience a greater likelihood and severity of craving when in the presence of alcohol cues in their daily lives compared to situations where alcohol cues are not present. We examined both the likelihood and severity of craving in the field because we anticipated infrequent reports of craving based on previous studies utilizing EMA methods to assess craving for alcohol among adults (Litt et al. 2000; Lukasiewicz et al. 2005). Similar to the lab portion of the study, we expected this effect would be more pronounced among adolescents with more several alcohol-related problems. We also tested the hypothesis that higher daily average craving recorded in the natural environment would be associated with higher volumes of alcohol consumption on a given day. Finally, we examined the ecological validity of lab-based cue reactivity by testing the associations between craving in the lab and craving and drinking recording via EMA methods in the field. These associations have not been studied in adolescents and, to our knowledge, only one study examined these effects in adults and found modest correlations between craving recorded in the lab and craving in the field among adults in treatment for alcohol dependence (Litt et al. 2000).
Methods
Participant Selection
Participants were 42 adolescent drinkers (ages 15–20 years) recruited from the community for a pharmacotherapy study on drinking and reactions to alcohol. Eligible youths consumed alcohol ≥ 2 times per week in the past 30 days and were able to read simple English for EMA purposes. Youths were excluded for the following reasons: prepubescent, history of alcohol treatment or treatment-seeking; opiate use in the past 30 days or opiate use disorder; positive toxicology screen for narcotics, amphetamines, sedative hypnotics, or opiates; clinically significant alcohol withdrawal; actively suicidal or psychotic; and medical conditions or medications that contraindicated taking the medication studied in the larger trial. Females were ineligible if they were pregnant, nursing, or unwilling to use birth control.
Procedures
The study was fully described to adolescents and, if younger than 18 years, to their parents. Consent was obtained from 18–20 year olds and from the parents of minors; assent was obtained from minors. Following screening, eligible participants completed a lab-based cue reactivity assessment (CRA) followed by a premedication EMA period of approximately one week (M = 6.2 days; SD = 1.5), which constitutes the assessment period for this study. No instructions were given to reduce or otherwise alter drinking habits. The Brown University institutional review board approved this study.
Lab procedures
The CRA followed standard procedures (Miranda et al. 2008), which began with a 3-min acclimation period during which participants sat quietly. Acclimation was followed by a 3-min water cue trial, wherein participants were exposed to a glass of water accompanied by its commercially labeled bottle to control for exposure to a potable liquid. Participants then underwent a 3-min relaxation period followed by two consecutive 3-min alcohol cue trials. Two alcohol trials were administered to ensure a stable assessment of alcohol cue reactivity and were averaged together for analyses (Rohsenow et al. 2000). During alcohol cue exposure, participants were presented with a glass of their most commonly consumed alcoholic beverage accompanied by its commercially labeled bottle. The order of trials was not counterbalanced due to known carryover effects (Monti et al. 1987). During each trial, participants were instructed to hold and sniff the beverage for 5 seconds when auditory tones were presented. Thirteen tones were presented with variable intervals during each 3-min block to ensure all participants received equivalent olfactory exposure. Audio-recorded instructions afforded standardization across participants and observation through a one-way mirror ensured compliance. At the end of each 3-min period, participants rated their urge to drink on an 11-point Likert Scale. Physiological measures, which included mean arterial pressure (MAP) and heart rate measured in beats per minute (BPM), were continuously assessed during each 3-min period using a Criticare© Scholar II 507EP blood pressure monitor. All sessions took place in the afternoon or early evening on the participant’s typical drinking day.
EMA procedures
Participants were taught to use our EMA program, which was implemented on handheld wireless devices (Omnia; Samsung Electronics, Ridgefield Park, NJ). Response options included visual analog bars (converted to discrete point scales), multiple checkboxes when more than one option was appropriate (e.g., activities), and categorical checkboxes when only one response was warranted (e.g., location). Other features enhanced the usability of the program, such as an alarm-clock feature, set by youth, to avoid assessments while sleeping. Data were transmitted wirelessly to our lab daily.
Participants initiated momentary assessments upon waking (morning reports) and before and after consuming each alcoholic drink (drink reports). Participants also completed assessments in response to audible prompts (random assessments) delivered at randomly selected times once within each 3-hour block (e.g., 3 p.m. to 6 p.m.) throughout the day except when participants were sleeping or otherwise unable to respond to the device (e.g., driving). This study focuses primarily on data collected during random assessments, with daily drinking levels culled from morning reports. Data from drink reports were excluded from the present study to avoid the confounding effects of alcohol intoxication and to best compare lab and field data.
Participants identified the presence of alcohol at random assessments by selecting one of three response options (not visible, visible directly [bottle, glass, etc.], or visible indirectly [television, advertisement, etc.]). We dichotomized alcohol cues as not present versus directly or indirectly present. Contextual variables were also recorded to include as time-varying covariates in analyses. Our EMA software date and time stamped each entry. We dichotomized each entry according to whether they were made on a weekend (6 p.m. on Friday through 6 p.m. on Sunday) to examine the influence of weekends on our dependent measures. Time of day was represented by four categories, with 6 p.m. to midnight serving as the reference category and the remaining categories representing exclusive 6-hr blocks. To control for whether entries were recorded on drinking days versus nondrinking days, each day was retrospectively dichotomized as a drinking (1) or nondrinking (0) day. Participants also indicated their location by selecting one of eight response options (home, friend’s house, others’ house, school, work, public place, vehicle, other location). Each option was coded as a binary variable indicating whether it was selected; home served as the reference category. Due to infrequent recordings in some locations, school and work were merged into one category and “others’ house” was merged with the “other location” category. Participants also indicated who accompanied them at the time of each entry by selecting all applicable options from a menu (mother, father, brother, sister, child, other relative, boy/girlfriend, friend, teacher, other, no one). We coded whether participants were with peers (boy/girlfriend or friend), non-peers, or alone; alone served as the reference category.
Measures
Alcohol craving
Craving in the lab and field was assessed with the same single-item measure to allow for the most direct comparison of craving across settings. Participants rated their urge to drink on an 11-point visual analog scale from no urge (0) to strongest ever (10). This measure is widely used in lab and EMA research (Miranda et al. 2008; Ray et al. 2010).
Alcohol use
Baseline drinking was assessed using the 90-day timeline follow-back interview (TLFB; Sobell & Sobell 1992). Alcohol use during the trial was assessed by EMA and the TLFB. EMA data obtained from the morning reports were our primary measure of drinking, with missing data culled from the TLFB. Morning reports included both the type of alcohol consumed and the volume reported in standard drinks, which served as our units for analyses.
Person-level variables
Demographic and clinical information was collected at baseline. For descriptive purposes, AUD diagnoses were derived using the Kiddie Schedule for Affective Disorders for School-Age Children (Kaufman et al. 1997). Interviewers received systematic training and achieved a high level of inter-rater reliability (kappa > 0.90) prior to conducting interviews independently. Diagnostic decisions were based on adolescents’ reports and made by case consensus. Participants also completed the 23-item Rutgers Alcohol Problem Index (RAPI; White & Labouvie 1989) to provide a continuous measure of alcohol problems used in analyses.
Data Analytic Strategy
Analyses were performed using the SPSS statistical package, version 20.0 (IBM, Armonk, NY). Our hypotheses regarding cue reactivity in the lab were tested using generalized estimating equation (GEE) models to examine the main and interactive effects of cue type (water versus alcohol) and severity of alcohol-related problems (i.e., RAPI scores) on craving and physiological reactivity (BPM, MAP) while controlling for age, sex, and baseline drinking levels. GEE models are essentially regression equations that account for the nesting of multiple observations within participants while controlling for autocorrelation (Zeger et al. 1988). We compared several covariance structures using the quasi-likelihood under the independence model criterion (Pan 2001) to select the optimum working correlation matrix. Models for lab data used an autoregressive AR1 structure, assumed a normal link function, and coded cue type with an orthogonal contrast (− 0.5 for water cues versus 0.5 for alcohol cues). Continuous variables were standardized to ease interpretation of results; the model coefficients represent differences in standard deviation units associated with the predictors (effect size d).
Our next set of analyses tested our hypothesis that alcohol cue exposure predicts craving among adolescents in the natural environment, and that this effect is more pronounced among youths with more severe alcohol-related problems. Analyses were restricted to random assessments recorded prior to drinking each day to eliminate the confounding effects of intoxication. We first tested whether alcohol cue exposure predicted the likelihood that adolescents experience craving. In this analysis, craving was categorized as a binary dependent variable (no craving = 0, any craving = 1). We then conducted a separate model to predict craving intensity. The dependent variable was the continuous measure of craving recorded during random assessments, which was transformed (logarithmically) due to positive skewness. In both models, an autoregressive AR1 structure best fit the data. We used a logit link function for binary outcome data and a normal link function when the dependent measure was continuous. All models examined the main and interactive effects of alcohol cue exposure (present vs. not present) and severity of alcohol-related problems (RAPI scores) on craving while controlling for person- (i.e., age, sex, baseline drinking levels) and occasion-level covariates (i.e., time of day, drinking day, weekend, social context, location). Cue type was coded with an orthogonal contrast (− 0.5 for water cues versus 0.5 for alcohol cues) and continuous variables were standardized. We included the likelihood and intensity of craving in response to alcohol cues in the lab in the respective models to examine whether lab-based cue reactivity predicted cue reactivity in the natural environment. The Bonferroni method controlled for inflation of Type I error by adjusting the threshold of significance (α = 0.05) for each hypothesized effect on the likelihood and severity of craving (Dar et al. 1994), yielding a modified threshold of significance (α = 0.025).
Our final set of analyses tested whether craving prospectively predicts alcohol use. First, we examined whether craving recorded in the lab predicts the subsequent frequency and quantity of alcohol use in the field during the monitoring period. Specifically, we tested the main and interactive effects of craving recording following alcohol-cue exposure in the lab and severity of alcohol-related problems on percent drinking days (frequency) on the average number of standard drinks consumed each drinking day (quantity) in the natural environment while controlling for person-level covariates (i.e., craving recorded in response to the water-cue trial, age, sex, and baseline drinking levels). Models used an autoregressive AR1 structure and the Bonferroni method controlled for inflation of Type I error by adjusting the threshold of significance (α = 0.05) for each hypothesized effect (modified α = 0.025).
We then tested whether daily average craving levels in the field prospectively predict the volume of alcohol use each day. Days were sorted according to adolescents’ individual social schedules (e.g., 8am to 3am) rather than calendar days. We examined the main and interactive effects of craving and severity of alcohol-related problems on drinking while controlling for person- (i.e., age, sex, baseline drinking levels) and occasion-level (weekend status) covariates. Other occasion-level covariates (i.e., time of day, social context, location) were not included due to an inability to compute meaningful daily summary scores given considerable within-day changes in these variables. Given our interest in person-level effects (i.e., how much an individual’s drinking levels change in accordance with his or her craving level), we disentangled within-person day-to-day variation in craving and drinking from the effects of between-person variability in typical craving levels and drinking (Palta 2003). Specifically, we entered daily averages of craving recorded at random assessments (within-person effect) and each participant’s average craving across the entire period (between-person effect) into the model. An unstructured covariance structure best fit the data and the model used a normal link function.
Results
Descriptive Analyses
Table 1 presents characteristics of the sample. All participants completed the lab CRA and contributed EMA data to the analyses. Physiological data were missing from one participant. Participants completed 85.6% of the random assessments (N = 954 reports), with an average of 22.7 (SD = 6.4) completed by each participant. Of these reports, 71 (8.2%) were recorded after drinking and thus excluded from analyses. An additional 18 (1.9%) were removed due to technological errors, leaving a total of 865 random assessments for analyses. Adolescents were more likely to report that alcohol was visible while at home, on weekends, in the evening (6pm – 5:59am), and while with peers (see Table 2). Alcohol was less likely visible in public locations, in the morning (6am – 11:59am), and while with others who were not peers. In terms of drinking, participants consumed alcohol on 34.4% of study days, with an average of 4.88 (SD = 5.88) standard drinks per drinking day.
Table 1.
Baseline Participant Characteristics by Sex: Percentage or Mean (With Standard Deviation in Parentheses)
| Variable | Males (n = 20) |
Females (n = 22) |
Overall (N = 42) |
|---|---|---|---|
| Age | 18.4 (1.4) | 18.6 (0.9) | 18.5 (1.2) |
| Race | |||
| Caucasian | 75.0 | 72.7 | 73.8 |
| African-American | 5.0 | 13.6 | 9.5 |
| American Indian | 5.0 | 0.0 | 2.4 |
| Asian/Pacific Islander | 10.0 | 13.6 | 11.9 |
| Hispanica | 25.0 | 9.1 | 16.7 |
| Alcohol abuse | 25.0 | 18.2 | 21.4 |
| Alcohol dependent | 50.0 | 54.5 | 52.4 |
| RAPI | 6.9 (6.3) | 9.2 (8.7) | 8.1 (7.6) |
| Cigarette Smoker | 35.0 | 31.8 | 33.3 |
| Drinking daysb | 27.2 (13.4) | 28.5 (18.0) | 27.9 (15.8) |
| Drinks per drinking dayb | 5.6 (3.6) | 4.7 (3.8) | 5.1 (3.7) |
| Heavy drinking daysb | 11.8 (9.1) | 16.4 (20.4) | 14.2 (16.0) |
Note.
Ethnicity and race were not mutually exclusive;
Derived from the 90-day Timeline Follow-Back interview conducted at baseline; RAPI = Rutgers Alcohol Problem Index (higher scores indicate greater severity of alcohol-related problems)
Table 2.
Comparisons of Contextual Variables by Alcohol Cue Exposure in the Natural Environment
| Alcohol Cues | ||||||
|---|---|---|---|---|---|---|
| Not Visible (n = 677) |
Visible (n = 188) |
|||||
| Contextual Variable | n | % | n | % | χ2 | p |
| Time of day | ||||||
| 12 a.m. – 5:59 a.m. | 35 | 5.2 | 18 | 9.6 | 4.96 | .026 |
| 6 a.m. – 11:59 a.m. | 146 | 21.6 | 24 | 12.8 | 7.22 | .007 |
| 12 p.m. – 5:59 p.m. | 262 | 38.7 | 65 | 34.6 | 1.07 | .302 |
| 6 p.m. – 11:59 p.m. | 234 | 34.6 | 81 | 43.1 | 4.61 | .032 |
| Drinking day | 181 | 26.7 | 53 | 28.2 | 0.16 | .691 |
| Weekend | 179 | 26.4 | 65 | 34.6 | 4.81 | .028 |
| Social context | ||||||
| Peers present | 273 | 40.3 | 93 | 49.5 | 5.04 | .025 |
| Nonpeers only | 141 | 20.8 | 22 | 11.7 | 8.01 | .005 |
| Alone | 263 | 38.8 | 73 | 38.8 | 0.00 | .996 |
| Location | ||||||
| Friend’s house | 61 | 9.0 | 23 | 12.2 | 1.74 | .187 |
| School or work | 93 | 13.7 | 21 | 11.2 | 0.85 | .357 |
| Public location | 123 | 18.2 | 12 | 6.4 | 15.52 | < .001 |
| Vehicle | 61 | 9.0 | 14 | 7.4 | 0.45 | .500 |
| Other location | 55 | 8.1 | 20 | 10.6 | 1.18 | .278 |
| Home | 284 | 41.9 | 98 | 52.1 | 6.18 | .013 |
Note. Percentages are calculated within visibility category.
Effects of Alcohol Cues on Craving in the Laboratory
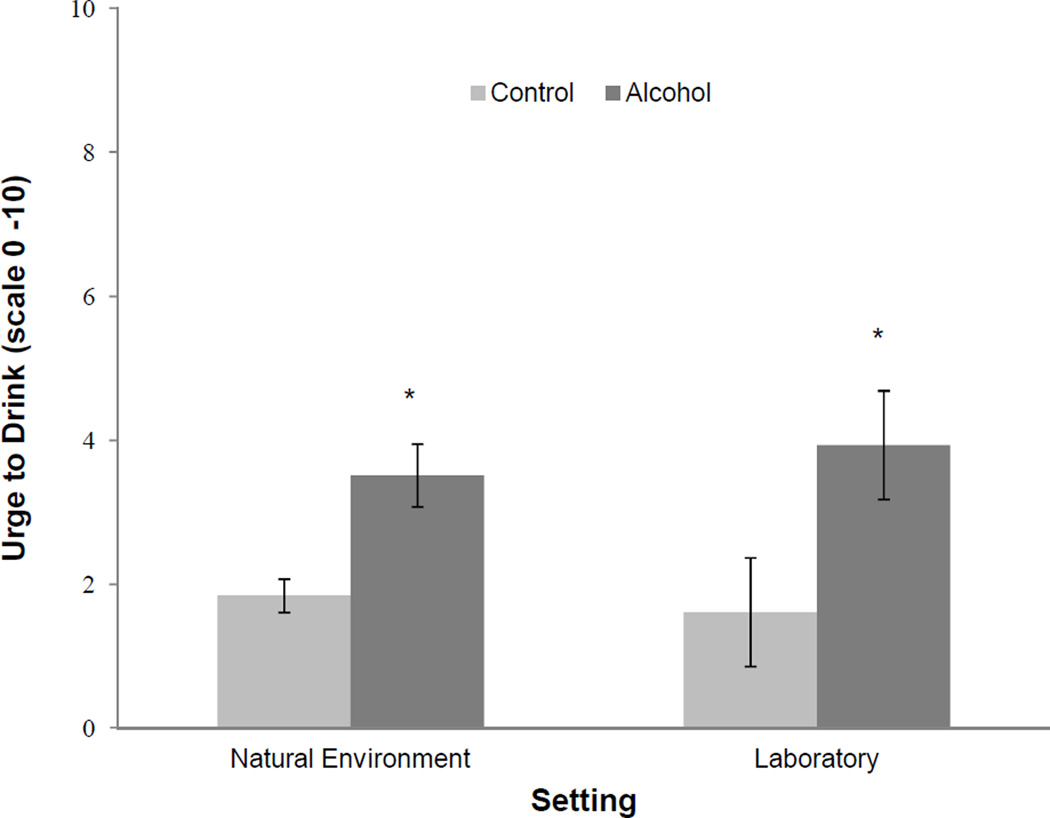
As shown in Table 3 and Figure 1, we found a main effect of cue type on craving, such that alcohol cues increased craving relative to water cues, with a large magnitude effect size (d = 0.86). Contrary to hypotheses, alcohol cue exposure did not affect BPM or MAP, and neither the main nor interactive effects of alcohol-problem severity predicted craving or physiological reactivity. Baseline drinking rates and age predicted craving such that participants with a higher drinking levels and younger participants experienced greater craving.
Table 3.
Summary of GEE Models Predicting Reactivity to Alcohol Cues in the Lab
| Dependent Measures Recorded in the Lab | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Craving | Heart Rate (beats per minute) | Mean Arterial Pressure | |||||||
| Predictor | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| Cue type | 0.86 | [0.65, 1.07] | < .001 | − 0.04 | [− 0.18, 0.10] | .581 | 0.18 | [− 0.01, 0.36] | .066 |
| RAPI | 0.05 | [− 0.13, 0.23] | .581 | − 0.12 | [− 0.48, 0.24] | .519 | 0.03 | [− 0.25, 0.30] | .856 |
| Cue Type × RAPI | 0.16 | [− 0.09, 0.41] | .204 | 0.08 | [− 0.06, 0.22] | .252 | 0.05 | [− 0.12, 0.21] | .583 |
| Age | − 0.19 | [− 0.35, − 0.03] | .017 | 0.17 | [− 0.02, 0.35] | .073 | 0.26 | [0.05, 0.47] | .017 |
| Sex (female) | − 0.20 | [− 0.59, 0.20] | .325 | − 0.42 | [− 1.02, 0.18] | .173 | − 0.95 | [− 1.49,− 0.41] | .001 |
| Drinking days (%)a | 0.47 | [0.32, 0.61] | < .001 | 0.02 | [− 0.26, 0.29] | .916 | − 0.11 | [− 0.44, 0.22] | .515 |
Note. Dependent measures are continuous and standardized. In all models, cue type was coded with an orthogonal contrast (− 0.5 for water cues versus 0.5 for alcohol cues). Continuous predictors were standardized prior to entry in all models; the reported coefficients represent standardized effects (effect size d). GEE = generalized estimating equation; RAPI = Rutgers Alcohol Problem Index (higher scores indicate greater severity of alcohol-related problems); CI = confidence interval;
derived from the 90-day Timeline Follow-Back interview conducted at baseline.
Fig. 1.
Comparison of cue-elicited craving reported in the natural environment and in the lab. ‘Alcohol’ bars refer to cases in which alcohol was reported as visible in the natural environment and trials involving in vivo alcohol cue exposure in the lab. ‘Control’ bars refer to cases in which alcohol was not visible in the natural environment and trials involving water cue exposure in the lab.
Effects of Alcohol Cues on Craving in the Natural Environment
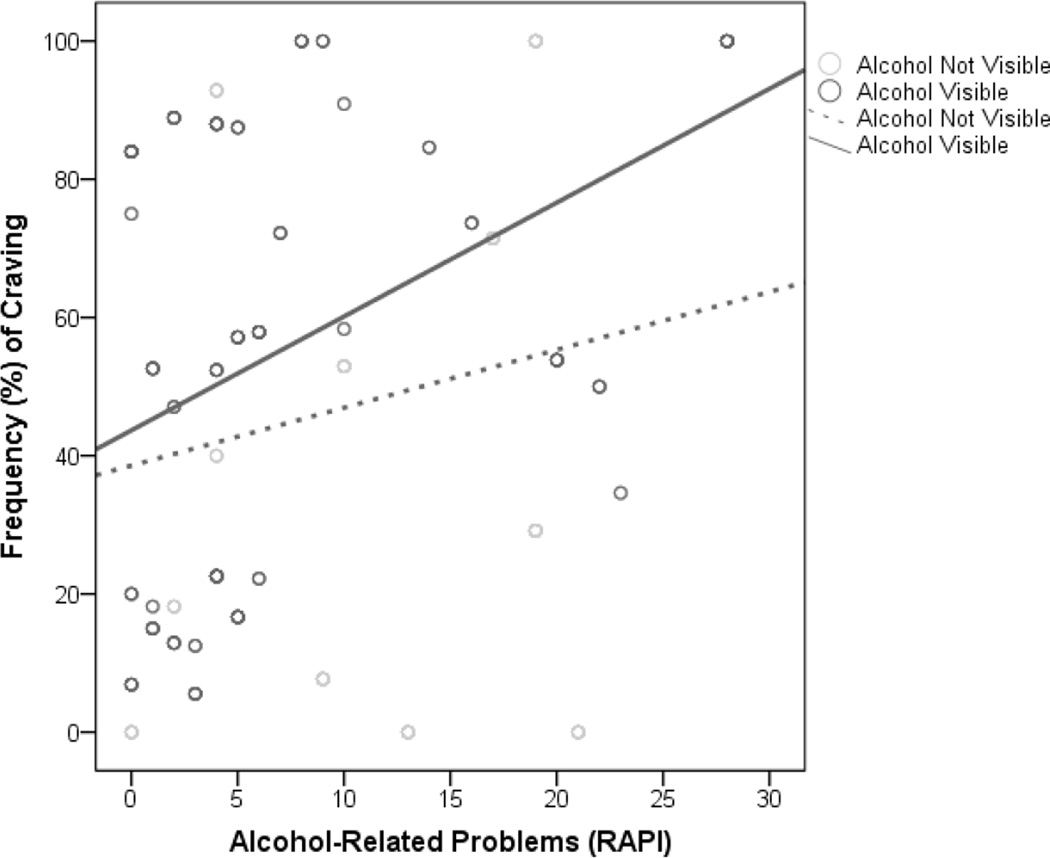
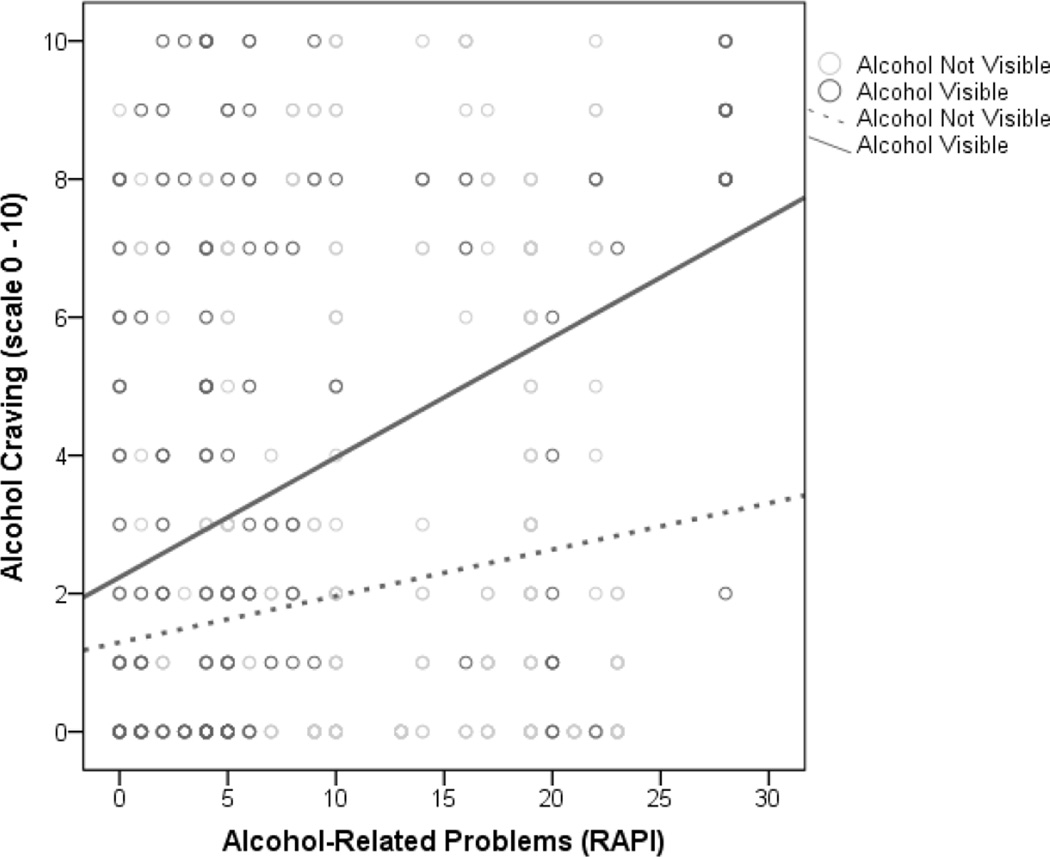
As hypothesized, there was a main effect of alcohol cue visibility on the likelihood of craving in the field, such that the presence of alcohol cues predicted a greater likelihood of craving (see Table 4). There was a main effect of alcohol problem severity such that greater RAPI scores predicted higher likelihoods of craving. As expected, these main effects were subsumed under a significant Cue Type × RAPI interaction such that adolescents with greater alcohol problems were more likely to experience craving in the presence of alcohol cues (see Table 4 and Figure 2). Results also revealed a main effect of alcohol cue visibility on the intensity of craving in the field, such that participants reported higher levels of craving when in the presence of alcohol cues, with a medium magnitude effect size (d = 0.42; see Table 4). There was a main effect of alcohol problem severity on craving intensity, with those reporting more problems experiencing more craving. As hypothesized, the Cue Type × RAPI interaction was also significant, such that adolescents with more alcohol-related problems had higher levels of craving in the presence of alcohol cues (see Table 4 and Figure 3). Finally, both the likelihood and intensity of craving experienced in the lab predicted the likelihood and intensity of craving in the natural environment, respectively (see Table 4).
Table 4.
Summary of GEE Models Predicting Momentary Craving From Alcohol Cues and Occasion- and Person-Level Covariates
| Dependent Measures | ||||||
|---|---|---|---|---|---|---|
| Likelihood of Cravinga | Craving Intensityb | |||||
| Predictor | Exp(B) | 95% CI | p | β | 95% CI | p |
| Cue type | 3.01 | [1.80, 5.03] | <.001 | 0.42 | [0.20, 0.64] | <.001 |
| RAPI | 2.09 | [1.24, 3.51] | .005 | 0.26 | [0.10, 0.42] | .002 |
| Cue type× RAPI | 2.11 | [1.23, 3.62] | .007 | 0.25 | [0.05, 0.46] | .015 |
| Occasion-level covariates | ||||||
| Time of day | ||||||
| 12 a.m. – 5:59 a.m. | 0.81 | [0.39, 1.72] | .589 | − 0.14 | [− 0.44, 0.16] | .361 |
| 6 a.m. – 11:59 a.m. | 0.42 | [0.24, 0.73] | .002 | − 0.49 | [− 0.67, − 0.30] | <.001 |
| 12 p.m. – 5:59 p.m. | 0.75 | [0.53, 1.06] | .101 | − 0.22 | [− 0.33, − 0.10] | <.001 |
| 6 p.m. – 11:59 p.m. (reference) | 1.00 | [—,—] | — | 0.00 | [—,—] | — |
| Drinking day | 1.98 | [1.23, 3.07] | .003 | 0.21 | [0.04, 0.38] | .014 |
| Weekend | 0.90 | [0.64, 1.28] | .567 | − 0.09 | [− 0.23, 0.05] | .222 |
| Social context | ||||||
| Peers present | 1.31 | [0.87, 1.97] | .197 | 0.20 | [0.05, 0.34] | .009 |
| Nonpeers only | 1.06 | [0.61, 1.84] | .829 | − 0.08 | [− 0.24, 0.09] | .363 |
| Alone (reference) | 1.00 | [—,—] | — | 0.00 | [—,—] | — |
| Location | ||||||
| Friend’s house | 1.05 | [0.46, 2.38] | .909 | 0.14 | [− 0.23, 0.51] | .462 |
| School or Work | 0.50 | [0.32, 0.78] | .003 | 0.02 | [− 0.21, 0.25] | .857 |
| Public location | 0.84 | [0.57, 1.24] | .388 | 0.15 | [0.01, 0.28] | .029 |
| Vehicle | 0.94 | [0.59, 1.52] | .812 | 0.38 | [0.13, 0.64] | .003 |
| Other location | 0.87 | [0.52, 1.46] | .602 | − 0.01 | [− 0.18, 0.15] | .871 |
| Home (reference) | 1.00 | [—,—] | — | 0.00 | [—,—] | — |
| Person-level covariates | ||||||
| Age | 0.70 | [0.42, 1.16] | .164 | − 0.02 | [− 0.20, 0.15] | .805 |
| Sex (female) | 0.87 | [0.37, 2.03] | .742 | − 0.12 | [− 0.45, 0.22] | .486 |
| Drinking days (%)c | 0.66 | [0.40, 1.08] | .097 | − 0.15 | [− 0.36, 0.06] | .152 |
| Lab craving (alcohol cues) | 2.93 | [1.37, 6.23] | .005 | 0.31 | [0.14, 0.48] | <.001 |
Note. Continuous predictors were standardized prior to entry in both models. In both models, cue type was coded with an orthogonal contrast (− 0.5 for assessments recorded when alcohol was not visible versus 0.5 for assessment recorded when alcohol was visible). GEE = generalized estimating equation; RAPI = Rutgers Alcohol Problem Index (higher scores indicate greater severity of alcohol-related problems); CI = confidence interval;
Binary outcome (0 when participants reported no urge to drink versus 1 when participants reported any urge to drink).
Continuous and standardized outcome;
Derived from the 90-day Timeline Follow-Back interview conducted at baseline; coefficients for craving intensity represent the standardized difference between situations when alcohol cue were visible versus not visible (effect size d).
Fig. 2.
Observed frequencies of experiencing craving from individual measures of alcohol-related problems (RAPI) as a function of alcohol visibility in the natural environment. Best fitting lines for cases in which alcohol was visible or not visible are illustrated.
Fig. 3.
Observed intensities of craving (0–10 scale) from individual measures of alcohol-related problems (RAPI) as a function of alcohol visibility in the natural environment. Best fitting lines for cases in which alcohol was visible or not visible are illustrated.
Several occasion-level variables also predicted craving levels. Drinking days predicted greater likelihood and intensity of craving. Time of day also influenced craving, such that participants were less likely to experience craving during the morning (i.e., 6 a.m. to 11:59 a.m.), and when they did, it was less intense. A similar negative association between time of day and craving intensity was found for the hours between 12 p.m. and 5:59 p.m. In terms of location, participants were less likely to experience craving while at school or work relative to home. Location influenced craving intensity as youths reported higher levels of craving while in a vehicle and in public locations. Although the presence of peers did not influence the likelihood that participants experienced craving, it did predict more intense levels of craving.
Effects of Craving on Drinking in the Natural Environment
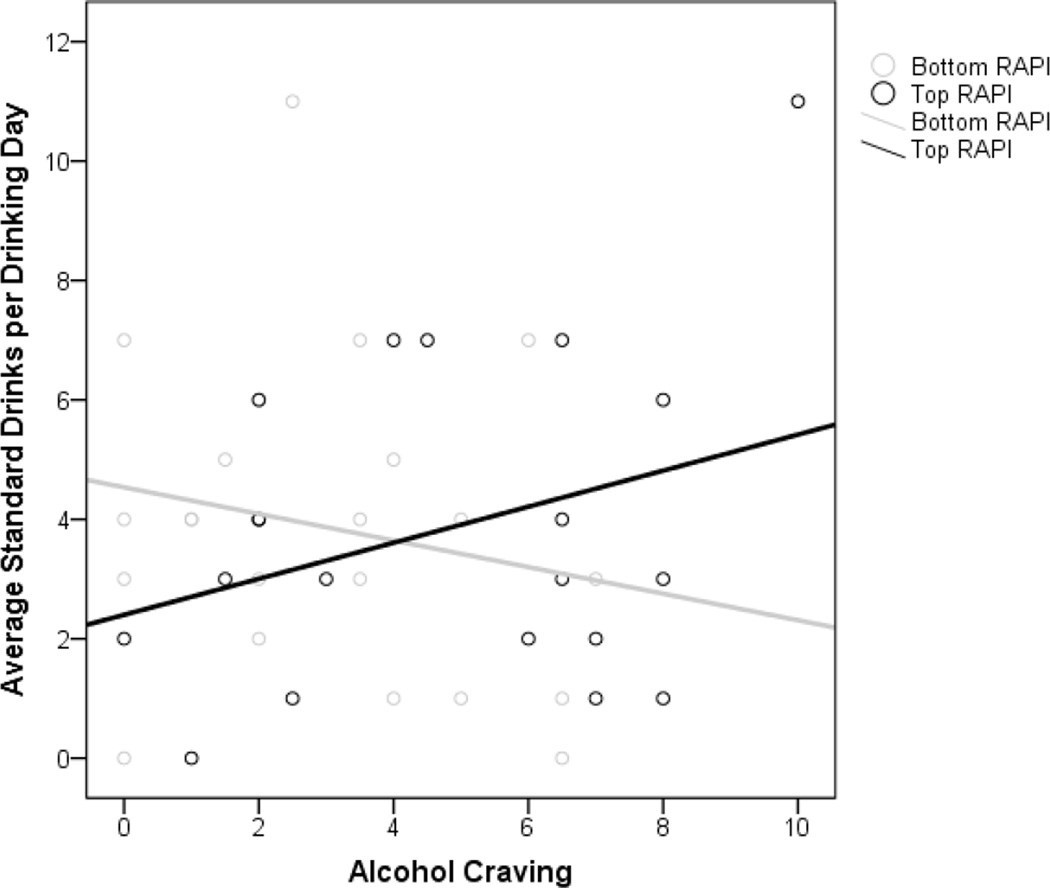
As shown in Table 5, the main effects of craving in the lab on the frequency or quantity of drinking in the natural environment were not significant. The Craving × RAPI interaction was significant, however, such that greater craving during alcohol cue reactivity in the lab predicted higher average volumes of alcohol consumption in the natural environment among adolescents with more alcohol-related problems (see Figure 4). The Craving × RAPI interactive effect predicting the frequency of drinking (i.e. percent drinking days) was not significant.
Table 5.
Summary of GEE Model Predicting Alcohol Consumption in the Natural Environment From Craving in the Lab
| Dependent Measures | ||||||
|---|---|---|---|---|---|---|
| Percent Drinking Daysa | Average Drinks per Drinking Daya | |||||
| Model and Predictor | β | 95% CI | p | β | 95% CI | p |
| Craving (alcohol trials) | 0.40 | [− 0.05, 0.86] | .079 | − 0.15 | [− 0.52, 0.21] | .405 |
| RAPI | 0.21 | [− 0.02, 0.44] | .073 | 0.19 | [− 0.06, 0.44] | .146 |
| Craving (alcohol trials) × RAPI | 0.07 | [− 0.14, 0.29] | .503 | 0.29 | [0.04, 0.54] | .022 |
| Person-level covariates | ||||||
| Craving (water trial) | − 0.14 | [− 0.57, 0.30] | .538 | − 0.01 | [− 0.39, 0.37] | .971 |
| Age | 0.20 | [− 0.09, 0.49] | .181 | − 0.27 | [− 0.51, − 0.03] | .025 |
| Sex (female) | − 0.08 | [− 0.68, 0.52] | .795 | − 0.25 | [− 0.78, 0.28] | .363 |
| Baseline percent drinking daysb | − 0.08 | [− 0.59, 0.44] | .766 | — | [—,—] | — |
| Baseline average drinks per dayb | — | [—,—] | — | 0.12 | [− 0.33, 0.57] | .600 |
Note. Continuous predictors were standardized prior to entry in both models. GEE = generalized estimating equation; RAPI = Rutgers Alcohol Problem Index (higher scores indicate greater severity of alcohol-related problems); CI = confidence interval;
Continuous and standardized outcome based on drinking behavior during the overall monitoring period;
Derived from the 90-day Timeline Follow-Back interview conducted at baseline; coefficients represent the standardized effects of predictors on drinking outcomes (effect size d).
Fig. 4.
Observed intensities of craving (0–10 scale) from individual measures of alcohol-related problems (RAPI) as a function of craving following alcohol cue exposure in the lab. Best fitting lines for the ‘Bottom’ and ‘Top’ RAPI groups (median split used to distinguish groups) are illustrated.
As hypothesized, higher daily average craving levels predicted greater volumes of alcohol consumption (see Table 6). This finding reflects a within-subjects effect across the monitoring period and indicates that when adolescents experienced higher average levels of craving throughout the day they drink greater quantities of alcohol subsequently that day. Results also revealed a negative association between craving levels averaged across the monitoring period (i.e., between-subject effect) and the volume of alcohol consumption on a given day, however this relationship was only negative when daily average levels of craving were included in the model as the two craving variables were highly correlated resulting in a reversal paradox (Tu et al. 2008). As expected, severity of alcohol problems was positively predictive of alcohol consumption.
Table 6.
Summary of GEE Model Predicting Daily Volume of Alcohol Consumption From Craving in the Natural Environment and Occasion- and Person-Level Covariates
| Predictor | β | 95% CI | p |
|---|---|---|---|
| Daily average craving | 0.34 | [0.12, 0.55] | .002 |
| Overall average craving | − 0.31 | [− 0.51, − 0.11] | .002 |
| RAPI | 0.16 | [0.03, 0.29] | .020 |
| RAPI × Overall average craving | 0.07 | [− 0.12, 0.26] | .467 |
| RAPI × Daily average craving | 0.03 | [− 0.22, 0.27] | .835 |
| Occasion-level covariate | |||
| Weekend | 0.09 | [− 0.15, 0.33] | .448 |
| Person-level covariates | |||
| Age | − 0.03 | [− 0.09, 0.04] | .430 |
| Sex (female) | − 0.16 | [− 0.36, 0.04] | .119 |
| Drinks per drinking day (Mean)a | 0.08 | [− 0.01, 0.18] | .075 |
Note. Continuous predictors were standardized prior to entry in both models. GEE = generalized estimating equation; RAPI = Rutgers Alcohol Problem Index (higher scores indicate greater severity of alcohol-related problems); CI = confidence interval;
Derived from the 90-day Timeline Follow-Back interview conducted at baseline; coefficients represent the standardized effects of predictors on drinking outcomes (effect size d).
Discussion
This study examined whether alcohol cues evoke craving among adolescent drinkers in the lab and in the natural environment, and tested the clinical relevance of craving during this developmental period by examining the prospective association between craving and alcohol use. Our lab results replicated previous findings that adolescent drinkers experience increases in craving when exposed to alcohol cues (Curtin et al. 2005; Thomas et al. 2005), with a large magnitude effect size (d = 0.86). In contrast, we did not find an effect of alcohol cue exposure on physiological reactivity among adolescents. Although there are modest effects of cue reactivity on physiological measures among adults (Carter & Tiffany 1999), our findings are consistent with previous work that found no effect of alcohol cue exposure on heart rate among adolescent alcoholics (Thomas et al. 2005). Of particular importance, our findings extend lab-based research by demonstrating alcohol cues also predict craving among adolescents in their natural environment. Notably, this effect was robust (d = 0.42) even when controlling for occasion-level covariates (i.e., peer presence, weekend, etc.). These results provide support for the hypothesis that alcohol cues elicit craving among adolescent drinkers and indicate that the magnitude of this effect is comparable to those observed in adults (d = 0.53, Carter & Tiffany 1999). The inclusion of lab and field data also allowed us to directly compare craving across settings. The ecological validity of lab-based cue reactivity was supported by the finding that the likelihood and intensity of craving experienced in the lab predicted the likelihood and intensity of craving experienced in the natural environment.
A central finding of this study was that severity of alcohol-related problems moderated the effects of alcohol cues on craving in the natural environment. Specifically, alcohol cues were robust predictors of craving among adolescents with more severe pathological drinking habits but had negligible effects on craving among youths with few alcohol problems. Despite a similar pattern of results in the lab, the interaction between cue type and alcohol problem severity was not significant in the lab. It seems likely that the large number of repeated observations collected in the field afforded greater power to detect this moderating effect. Future lab research with larger samples is needed to clarify this possibility. Nonetheless, our observation that cue-induced craving in the natural environment is especially salient for youths further along the pathological drinking continuum supports the validity of craving as a clinically significant indicator of pathological drinking among adolescents.
Our findings also showed that craving is clinically meaningful among adolescents by demonstrating that higher average daily craving levels were associated with higher volumes of subsequent alcohol use. This effect was upheld while controlling for between-participant differences in average craving across the monitoring period to ensure that a positive association between craving and drinking was not the product of heavier drinking participants who crave more in general. This finding is consistent with other demonstrations of positive associations between craving and drinking outcomes when measured in real time or on a daily basis (Leeman et al. 2009; Litt et al. 2000; Oslin et al. 2009). These findings build on existing research that typically tests associations between craving assessed at a single time point and drinking outcomes over extended monitoring periods (Bottlender & Soyka 2004; Flannery et al. 2001; 2003) by using real-time assessments to examine prospective associations between daily variations in craving and drinking behavior. Notably, craving recorded in the lab predicted how much alcohol youths consumed when they drank in the natural environment during the EMA monitoring period. This effect was only observed, however, among youths with greater alcohol-related problems. On the whole, these findings support the inclusion of craving as a clinically significant diagnostic indicator of alcohol addiction among adolescents. Moreover, these findings suggest that adolescents with alcohol problems may benefit from pharmacological and psychosocial treatments that target craving.
This study also showed that occasion-level variables other than alcohol cues predicted craving in the field. Namely, the presence of peers was associated with greater levels of craving intensity. Among nicotine craving studies, peers are conceptualized as distal cues that elicit craving similar to proximal cues among smokers (Conklin et al. 2013). In terms of alcohol, however, few studies have examined the potential of peers to influence craving. Peer selection and peer influences are generally explored more directly as determinants of drinking outcomes in adolescents (Ali & Dwyer 2010; Mercken et al. 2012), with adolescents often imitating the drinking behavior of peers (Larsen et al. 2010). This study demonstrates the potential for peers to not only influence drinking directly, but also to influence craving. The likelihood and intensity of craving were also both greater on drinking days and in the evening (6pm to 11:59pm).
Several considerations should be noted regarding our findings. Although field data afforded considerable statistical power to test within-subjects effects, our modest sample size limited our ability to explore between-subjects comparisons on potentially relevant variables, such as alcohol dependence. Replication in a larger sample with greater attention to individual difference characteristics is important. Similarly, our modest sample size may have hindered our ability to detect cues effects on cardiovascular measures, as adult studies show such effects are small compared to subjective craving (Carter & Tiffany 1999). Interestingly, effect sizes for physiological measures in this study were similarly small. In addition, our sample consisted of mostly heavy drinkers and we excluded treatment seekers. It remains unclear whether our findings extend to adolescents seeking treatment or social drinkers. Additional research is needed to clarify the impact of abstinence on craving and to assess whether the current findings are directly relevant to treatment providers. Finally, the study of craving in the field may have presented a self-selection problem in that, unlike cue exposure in the lab, the amount of alcohol cue exposure varied across participants. It is noteworthy, however, that cue effects in the field were robust even when controlling for person-level covariates.
On balance, this is the first study to test the effects of alcohol cues on craving among adolescents in the natural environment, as well as the first to prospectively examine the association between craving and future drinking in this age group. Our findings implicate craving as an important clinical characteristic among adolescents and demonstrate the utility of pairing lab paradigms with EMA methods to better characterize reactivity to alcohol cues.
Acknowledgments
The National Institute of Alcohol Abuse and Alcoholism at the National Institutes of Health supported this work (AA017273, AA07850). We thank Alexander Blanchard, Jason Frezza, Jacqueline Lee, Bethany Rallis, and Justin Souliere for their assistance with this project.
Footnotes
The authors have no conflicts of interest to disclose.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed., Text Revision. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Ali MM, Dwyer DS. Social network effects in alcohol consumption among adolescents. Addict Behav. 2010;35:337–342. doi: 10.1016/j.addbeh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol Alcohol. 2004;39:357–361. doi: 10.1093/alcalc/agh073. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Conklin CA, Salkeld RP, Perkins KA, Robin N. Do people serve as cues to smoke? Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, Barnett NP, Colby SM, Rohsenow DJ, Monti PM. Cue reactivity in adolescents: Measurement of separate approach and avoidance reactions. J Stud Alcohol. 2005;66:332–343. doi: 10.15288/jsa.2005.66.332. [DOI] [PubMed] [Google Scholar]
- Dar R, Serlin RC, Omer H. Misuse of statistical test in three decades of psychotherapy research. J Consult Clin Psychol. 1994;62:75–82. doi: 10.1037//0022-006x.62.1.75. [DOI] [PubMed] [Google Scholar]
- Deas D, Roberts J, Grindlinger D. The utility of DSM-IV criteria in diagnosing substance abuse/dependence in adolescents. J Subst Abuse. 2005;10:10–21. [Google Scholar]
- Deas D, Roberts J, Randall CL, Anton RF. Adolescent obsessive-compulsive drinking scale: An assessment tool for problem drinking. J Natl Med Assoc. 2001;93:92–103. [PMC free article] [PubMed] [Google Scholar]
- Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, Volpicelli JR. Alcohol craving predicts drinking during treatment: An analysis of three assessment instruments. J Stud Alcohol. 2003;64:120–126. doi: 10.15288/jsa.2003.64.120. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Roberts AJ, Cooney N, Swift RM, Anton RF, Rohsenow DJ. The role of craving in alcohol use, dependence and treatment. Alcohol Clin Exp. 2001;25:299–308. [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex. 2011;21:1408–1415. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present version and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Larsen H, Engels RC, Souren PM, Granic I, Overbeek G. Peer influence in micro-perspective: imitation of alcoholic and non-alcoholic beverages. Addict Behav. 2010;35:49–52. doi: 10.1016/j.addbeh.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Corbin WR, Fromme K. Craving predicts within session drinking behavior following placebo. Pers Individ Dif. 2009;46:693–698. doi: 10.1016/j.paid.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Cooney ND, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz M, Benyamina A, Reynaud M, Falissard B. An in vivo study of the relationship between craving and reaction time during alcohol detoxification using the ecological momentary assessment. Alcohol Clin Exp. 2005;29:2135–2143. doi: 10.1097/01.alc.0000191760.42980.50. [DOI] [PubMed] [Google Scholar]
- Martin C, Kaczynski N, Maisto S, Bukstein O, Moss H. Patterns of DSM-IV alcohol abuse and dependence symptoms in adolescent drinkers. J Stud Alcohol. 1995;56:672–680. doi: 10.15288/jsa.1995.56.672. [DOI] [PubMed] [Google Scholar]
- Mercken L, Steglich C, Knibbe R, Vries H. Dynamics of friendship networks and alcohol use in early and mid-adolescence. J Stud Alcohol Drugs. 2012;73:99–110. doi: 10.15288/jsad.2012.73.99. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, McClair VL. Epidemiology of substance use disorders. Hum Genet. 2012;131:779–789. doi: 10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary lab study. Alcohol Clin Exp. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: A possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Cary M, Slaymaker V, Colleran C, Blow FC. Daily ratings measures of alcohol craving during an inpatient stay define subtypes of alcohol addiction that predict subsequent risk for resumption of drinking. Drug Alcohol Depend. 2009;103:131–136. doi: 10.1016/j.drugalcdep.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta M. Quantitative Methods in Population Health: Extensions of Ordinary Regression. Hoboken, NJ: John Wiley & Sons, Inc.; 2003. [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the mu-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010;119:115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone’s effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol. 2000;109:738–742. [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Wunschel SM, Abrams DB. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychological and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J, Merikangas KR. Use and abuse of alcohol and illicit drugs in US adolescents: results of the National Comorbidity Survey-Adolescent Supplement. Arch Gen Psychiatry. 2012;69:390–398. doi: 10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg B, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Deas D. Alcohol cue reactivity in alcohol dependent adolescents. J Stud Alcohol. 2005;66:354–360. doi: 10.15288/jsa.2005.66.354. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YK, Gunnell D, Gilthorpe MS. Simpson’s paradox, Lord’s paradox, and suppression effects are the same phenomenon – the reversal paradox. Emerg Themes Epidemiol. 2008;5 doi: 10.1186/1742-7622-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie ER. Towards the assessment of adolescent problem drinking in adolescence. J Stud Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models of longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]