Abstract
Background
Outcomes for patients in the second-line setting of advanced urothelial carcinoma (UC) are dismal. The recognized prognostic factors in this context are Eastern Cooperative Oncology Group (ECOG) performance status (PS) >0, hemoglobin level (Hb) <10 g/dl, and liver metastasis (LM).
Objectives
The purpose of this retrospective study of prospective trials was to investigate the prognostic value of time from prior chemotherapy (TFPC) independent of known prognostic factors. Design, setting, and participants: Data from patients from seven prospective trials with available baseline TFPC, Hb, PS, and LM values were used for retrospective analysis (n = 570). External validation was conducted in a second-line phase 3 trial comparing best supportive care (BSC) versus vinflunine plus BSC (n = 352).
Outcome measurements and statistical analysis
Cox proportional hazards regression was used to evaluate the association of factors, with overall survival (OS) and progression-free survival (PFS) being the respective primary and secondary outcome measures.
Results and limitations
ECOG-PS >0, LM, Hb <10 g/dl, and shorter TFPC were significant prognostic factors for OS and PFS on multivariable analysis. Patients with zero, one, two, and three to four factors demonstrated median OS of 12.2, 6.7, 5.1, and 3.0 mo, respectively (concordance statistic = 0.638). Setting of prior chemotherapy (metastatic disease vs perioperative) and prior platinum agent (cisplatin or carboplatin) were not prognostic factors. External validation demonstrated a significant association of TFPC with PFS on univariable and most multivariable analyses, and with OS on univariable analyses. Limitations of retrospective analyses are applicable.
Conclusions
Shorter TFPC enhances prognostic classification independent of ECOG-PS>0, Hb<10 g/ dl, and LM in the setting of second-line therapy for advanced UC. These data may facilitate drug development and interpretation of trials.
Keywords: Urothelial carcinoma, Second line, Prognosis, Time from prior chemotherapy, Hemoglobin, Liver metastasis, Performance status
1. Introduction
Second-line therapy for advanced urothelial carcinoma (UC) constitutes a substantial unmet need, with several agents demonstratingmarginal activity [1–13]. Vinflunine is approved in Europe based on a phase 3 trial that demonstrated statistically superior, limited activity for vinflunine plus best supportive care (BSC) compared to BSC alone in the eligible population (n = 357), with a median overall survival (OS) of 6.9 versus 4.3 mo (p = 0.040) [10].
Prognostic factors may confound the interpretation of phase 2 trials used to screen new agents. Three prognostic factors have been identified in the postplatinum second-line setting: Eastern Cooperative Oncology Group (ECOG) performance status (PS) >0, hemoglobin level (Hb) <10 g/dl, and presence of liver metastasis (LM) [14]. Four risk groups based on the presence of zero, one, two, or three prognostic factors demonstrated a median OS of 14.2, 7.3, 3.8, and 1.7 mo, respectively.
We hypothesized that time from prior chemotherapy (TFPC), a pragmatic measure of pace of disease, would provide significant prognostic information in advanced UC receiving second-line therapy. We pooled second-line, phase 2 clinical trials to study whether TFPC imparts a prognostic impact independent of ECOG-PS >0, Hb <10 g/dl, and LM. We also aimed to externally validate the findings in a phase 3 trial.
2. Patients and methods
2.1. Patient population
Individual patient data were obtained from 748 patients enrolled in 12 phase 2 trials (nine nonrandomized, three randomized) of second-line therapy for progressive, advanced UC, which were either published or presented at major conferences (Table 1) [3–9,12,15,16]. Prior chemotherapy was administered in the perioperative and/or metastatic disease settings. Trials or patients with missing ECOG-PS, Hb, LM, or TFPC data were ineligible. For external validation, the eligible population (n = 357) from the phase 3 trial comparing BSC with vinflunine plus BSC was used [10].
Table 1.
Eligibility criteria and evaluable patients in included trials of second-line therapy for progressive advanced urothelial cancer
| First author | Regimen | Patients, no. |
Evaluable for analysis, no. |
Allowed prior perioperative chemotherapy as only prior therapy |
Period allowed between prior perioperative chemotherapy and second-line therapy |
Required only one prior regimen in any setting |
Required prior therapy for metastatic disease |
Required prior platinum-based therapy |
|---|---|---|---|---|---|---|---|---|
| Albers [2] | Paclitaxel–gemcitabine† | 98 | 83* | Yes | Unlimited | Yes | No | Yes |
| Pili [11] | Pazopanib | 23 | 0* | Yes | Unlimited | No | No | Yes |
| Sternberg [8] | Paclitaxel–Gemcitabine | 41 | 0* | Yes | Unlimited | Yes | No | Yes |
| Sridhar [6] | Nab–paclitaxel | 48 | 48 | Yes | 1 yr | No | No | Yes |
| Galsky [15] | Pemetrexed | 13 | 0* | Yes | Unlimited | Yes | No | Yes |
| Choueiri [4] | Docetaxel plus placebo/vandetanib† | 152 | 147* | Yes | 2 yr | No | No | Yes |
| Wong [16] | Cetuximab with or without paclitaxel** | 39 | 39* | Yes | Unlimited | Yes | No | No |
| Petrylak [7] | Gefitinib | 31 | 0* | Yes | Unlimited | Yes | No | Yes |
| Beer [3] | Irinotecan | 45 | 0* | No | Not applicable | Yes | Yes | Yes |
| Vaughn [9] | Vinflunine | 151 | 151 | Yes | 1 yr | Yes | No | Yes |
| Culine [5] | Vinflunine | 57 | 56* | Yes | Unlimited | No | No | Yes |
| Stadler [12] | Volasertib | 50 | 46* | Yes | 2 yr | Yes | No | No†† |
| Total | Multiple | 748 | 570 | Variable | Variable | Variable | Variable | Variable |
Nab-paclitaxel = nanoparticle albumin-bound paclitaxel.
Randomized phase 2 trials with no significant differences in outcomes between arms.
Either some or all patients were not evaluable from these trials due to lack of baseline hemoglobin and/or liver metastasis status.
Trial had two noncomparative, randomized arms: cetuximab alone or cetuximab plus paclitaxel.
Although this trial did not explicitly require prior platinum therapy, all patients had received prior platinum therapy (one prior systemic cytotoxic therapy excluding targeted, biologic, or experimental agents was required).
2.2. Statistical methods
Using Fisher exact tests, Wilcoxon rank-sum tests, log-rank tests, or the Cochran-Armitage test for trend, characteristics of patients from phase 2 trials included in this analysis were compared with those excluded. TFPC was calculated from the last date the patient received prior chemotherapy to the date they were registered in the second-line trial, and defined using a priori selected cut-off points of 3-, 6-, 9-, and 12-mo, and as a continuous outcome with a logarithmic transformation. OS and PFS, the primary and secondary outcome measures respectively, were estimated using the Kaplan-Meier method. The association of TFPC with OS and PFS was evaluated on univariable analysis and on multivariable analysis after adjusting for ECOG-PS, Hb level, and LM, using Cox proportional hazards regression.
In trials using Karnofsky performance status (KPS), the values were converted to ECOG-PS by convention: KPS 100 was equivalent to ECOG 0, and KPS <100 equivalent to ECOG ≥1. Trial was included as a stratification factor throughout. The likelihood ratio χ2 statistic was calculated based on TFPC as a dichotomous factor with cut-off points from 1 to 15 mo. The cut-off point with the maximum χ2 statistic was deemed to have the optimal discrimination ability and was used for defining risk score. TFPC as a continuous variable was examined in multivariable models as supportive evidence of the biologic importance of TFPC. The number of poor prognostic factors was counted for each patient and the discriminatory ability for OS was evaluated using the concordance (c) statistic. To show improvement in prognostic accuracy of the new four-factor model compared with the old three-factor model, the guidelines described by Kattan and Nguyen were followed [17]. Internal validation was performed using 10 000 bootstrap samples (R software; R Project for Statistical Computing) and estimation of 95% bias-corrected and accelerated (BCa) confidence intervals (CI) were constructed to evaluate the estimated improvement in the c statistic when using the new risk model. We planned to externally validate the prognostic importance of TFPC on OS and PFS in the aforementioned phase 3 trial [10]. The primary analysis included both arms of the trial and the Cox models were stratified by treatment group. Supportive analyses were performed within each treatment group. All p values are two-sided.
3. Results
3.1. Patient characteristics
All data from five phase 2 trials were excluded due to missing PS, Hb, LM or TFPC data (Table 2). Of the remaining seven phase 2 trials, four were nonrandomized, one was randomized but noncomparative [16], and two were randomized [2,4], but no significant differences among the arms of these trials were reported. A total of 595 patients were enrolled in these seven trials, 25 of whom were excluded due to missing data. This resulted in 570 patients available for analysis, 443 (77.7%) of whom had date of death available; all included trials used Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 to define progression (Table 2). The reasons for patient exclusion were lack of TFPC (n = 88; 49%) or baseline Hb level (n = 139; 78%). ECOG-PS >0 (p < 0.001), Hb <10 g/dl (p < 0.001), and prior chemotherapy for metastatic disease (p = 0.006) were more common in excluded patients, and prior carboplatin therapy was more common in included patients (p = 0.041). There were no significant differences in TFPC (p = 0.17) or OS (p = 0.48) between included and excluded patients.
Table 2.
Overall characteristics of included and excluded patients for analysis
| Included patients | Excluded patients | p value | |
|---|---|---|---|
| Patients, No. | 570 | 178 | |
| Male patients, n/N (%) | 438/570 (76.8) | 131/178 (73.6) | 0.37 |
| Age, yr, mean (SD) | 65.1 (9.2) | 64.2 (10.6) | 0.32 |
| ECOG-PS ≥1, n/N (%) | 258/570 (45.3) | 103/155 (66.5) | <0.001 |
| Liver metastases, n/N (%) | 172/570 (30.2) | 48/172 (27.9) | 0.63 |
| Visceral metastases, n/N (%) | 333/570 (58.4) | 102/173 (59.0) | 0.93 |
| Anemia, n/N (%) | 83/570 (14.6) | 17/39 (43.6) | <0.001 |
| Prior cisplatin therapy, n/N (%) | 404/521 (77.5) | 60/75 (80.0) | 0.77 |
| Prior carboplatin therapy, n/N (%) | 159/521 (30.5) | 14/75 (18.7) | 0.041 |
| Prior chemotherapy for metastatic disease, n/N (%) | 312/522 (59.8) | 109/151 (72.2) | 0.006 |
| Time from last chemotherapy, mo, median (range) | 4.5 (0–103.4) | 4.3 (0.8–40.2) | 0.17 |
| Time from last chemotherapy, no. (%) | 0.30* | ||
| <3 mo | 195 (34.2) | 38 (42.2) | |
| 3 to <6 mo | 148 (26.0) | 21 (23.3) | |
| 6 to <12 mo | 137 (24.0) | 18 (20.0) | |
| ≥ 12 mo | 90 (15.8) | 13 (14.4) | |
| Censored for OS, n/N (%) | 127/570 (22.3) | 38/178 (21.4) | – |
| OS, median (95% CI) | 6.8 (6.2–7.6) | 7.1 (6.5–8.8) | 0.48** |
| 3 mo | 79.9 (76.3–83.0) | 72.6 (65.3–78.6) | |
| 6 mo | 54.8 (50.5–58.9) | 59.5 (51.8–66.4) | |
| 12 mo | 29.0 (25.0–33.1) | 30.7 (23.5–38.1) |
SD = standard deviation; ECOG-PS = Eastern Cooperative Oncology Group performance status; OS = overall survival; CI = confidence interval.
Cochran-Armitage test for trend.
Stratified log-rank test.
Baseline characteristics varied among different trials included for analysis: ECOG-PS >0 ranged from 31.8% to 67.9% and LM incidence ranged from 10.7% to 33.9%. Across all eligible trials, the median OS (95% CI) was 6.8 mo (6.2–7.6) and the range was 5.3 mo to 10.8 mo. While 127 (22.7%) patients were censored at the time of data extraction, 65 of these patients were from a single trial by Vaughn et al [9]. Patient characteristics of the phase 3 trial used for external validation have been published [10]. Of the 357 eligible patients, data for TFPC were missing for 5, rendering 352 patients evaluable for our analysis.
3.2. Prognostic impact of time from prior chemotherapy
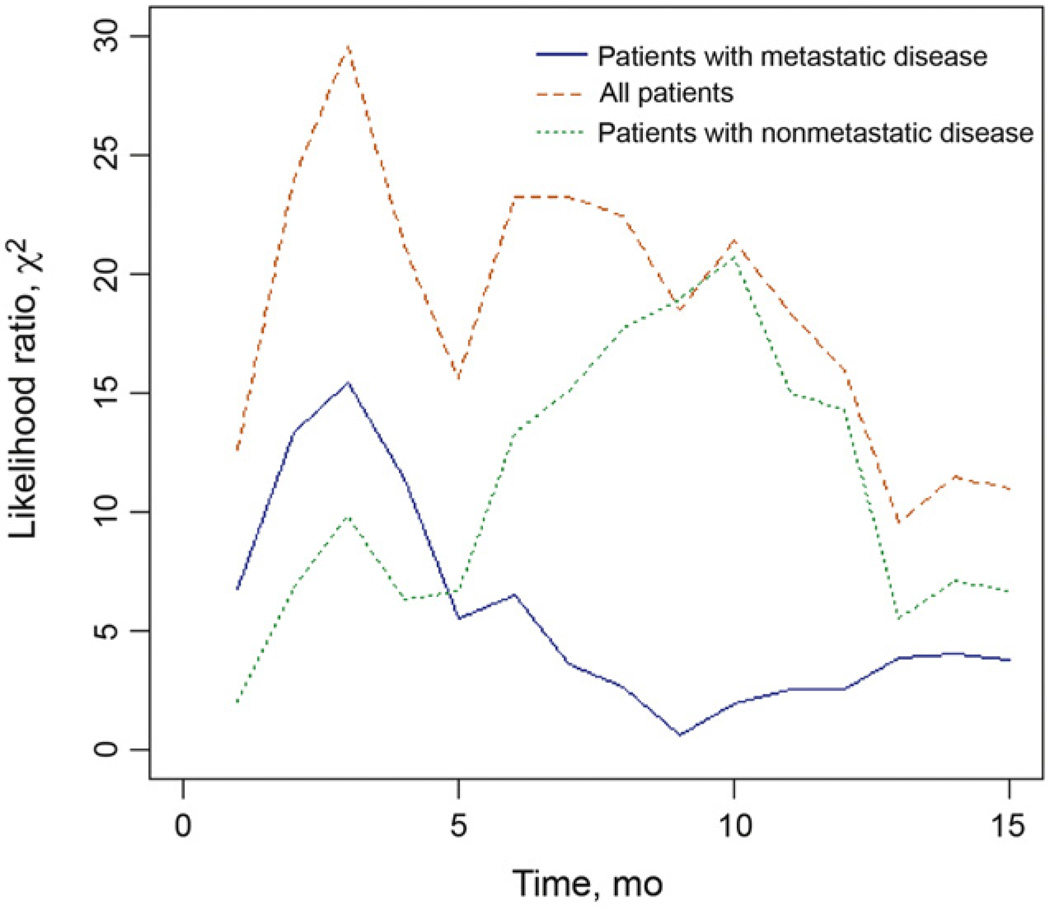
Shorter TFPC (regardless of definition), higher baseline ECOG-PS (>0), presence of liver or visceral metastases, and baseline Hb <10 g/dl were prognostic for decreased survival by univariable analysis (p < 0.001 for all) (Table 3). The optimal cut-off point in terms of discriminatory ability, as measured by the likelihood ratio χ2 statistic, was 3mo for all patients and those who received prior chemotherapy for metastatic disease (Fig. 1). For patients with nonmetastatic disease, the optimal cut-off point was 10 mo; however, χ2 statistics were similar between 6 and 12 mo.
Table 3.
Results from Cox proportional hazards regression analyses
| Factor | Factor type | Hazard ratio (95% CI) | p value |
|---|---|---|---|
| UNIVARIABLE | |||
| Time since last chemotherapy | <3movs ≥3 mo | 0.56 (0.46–0.69) | <0.001 |
| <6movs ≥6 mo | 0.61 (0.50–0.75) | <0.001 | |
| <9movs ≥9 mo | 0.61 (0.48–0.77) | <0.001 | |
| <12 mo vs ≥12 mo | 0.59 (0.44–0.77) | <0.001 | |
| Continuous (per mo) | 0.97 (0.96–0.99) | <0.001 | |
| Continuous (log scale) | 0.73 (0.66–0.80) | <0.001 | |
| Sex | Male vs female | 1.06 (0.85–1.32) | 0.63 |
| Age | Continuous (per yr) | 1.01 (1.00–1.02) | 0.29 |
| Performance status | ECOG ≥1 vs ECOG 0 | 2.03 (1.66–2.47) | <0.001 |
| Liver metastases | Yes vs no | 1.68 (1.36–2.07) | <0.001 |
| Visceral metastases | Yes vs no | 1.57 (1.28–1.93) | <0.001 |
| Anemia | <10 vs ≥10 g/dl | 2.25 (1.73–2.92) | <0.001 |
| Prior cisplatin therapy (n = 521) | Cisplatin vs no cisplatin | 0.90 (0.70–1.15) | 0.40 |
| Prior carboplatin therapy (n = 521) | Carboplatin vs no carboplatin | 1.22 (0.96–1.54) | 0.10 |
| Chemotherapy type (n = 522) | Metastatic vs neoadjuvant | 1.06 (0.86–1.31) | 0.57 |
| MULTIVARIABLE | |||
| Performance status | ECOG ≥1 vs ECOG 0 | 1.75 (1.42–2.16) | <0.001 |
| Liver metastases | Yes vs no | 1.54 (1.25–1.90) | <0.001 |
| Anemia | <10 vs ≥10 g/dl | 1.59 (1.21–2.09) | <0.001 |
| Time since last chemotherapy | Continuous (log scale) | 0.77 (0.70–0.86) | <0.001 |
| MULTIVARIABLE MODEL 2 | |||
| Performance status | ECOG ≥1 vs ECOG 0 | 1.79 (1.45–2.20) | <0.001 |
| Liver metastases | Yes vs no | 1.54 (1.25–1.90) | <0.001 |
| Anemia | <10 vs ≥10 g/dl | 1.60 (1.21–2.10) | <0.001 |
| Time since last chemotherapy | <3movs ≥3 mo | 0.63 (0.51–0.78) | <0.001 |
CI = confidence interval; ECOG = Eastern Cooperative Oncology Group.
Fig. 1.
Likelihood ratio χ2 statistics. The optimal discriminatory ability, measured by the likelihood ratio χ2-statistic, occurred when the cut-off point was 3 mo for all patients and those who received prior chemotherapy for metastatic disease. For nonmetastatic disease, the optimal cut-off point was 10 mo.
After adjusting for ECOG-PS >0 (hazard ratio [HR]: 1.75; 95% CI, 1.42–2.16), presence of LM (HR: 1.54; 95% CI, 1.25–1.90), and baseline Hb <10 g/dl (HR: 1.59; 95% CI, 1.21–2.09), TFPC as a continuous variable (log scale) remained a statistically significant factor (HR: 0.77, 95% CI, 0.70–0.86). Similar results were observed if TFPC was defined as a dichotomous factor based on a 3-mo cut-off point. The addition of TFPC increased the c statistic from 0.615 for a model with ECOG-PS, LM, and Hb <10 g/dl, to0.631 and 0.638 with TFPC as a dichotomous (<3 mo vs ≥3 mo) or continuous (log transformed) variable. No other characteristic was significant on multivariable analysis.
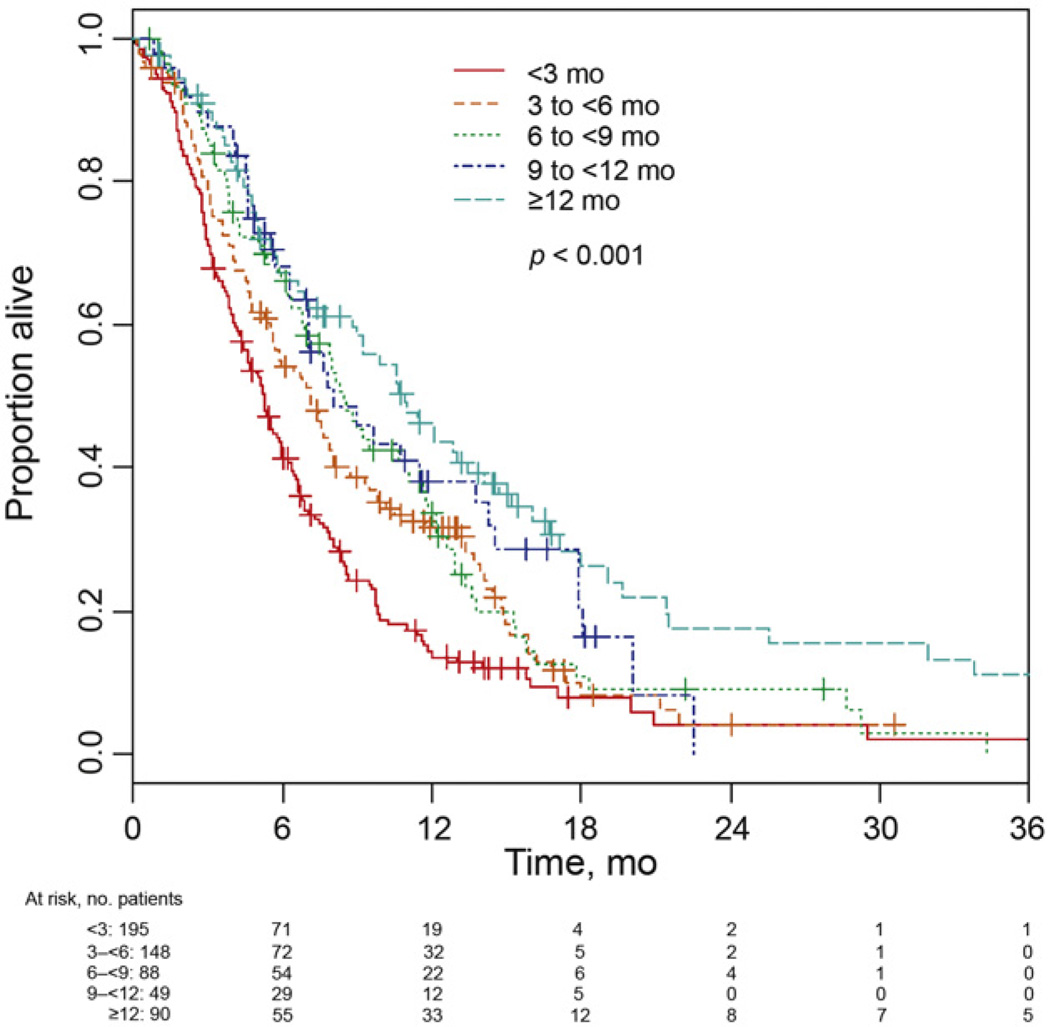
Median OS (95% CI) was 5.3 (4.4–5.9), 7.1 (5.6–8.0), 8.5 (6.8–11.1), 8.0 (6.3–13.8), and 10.9 (7.6–13.5) mo, respectively, for patients having TFPC <3mo (n = 195), 3 to <6mo (n = 148), 6 to <9 mo (n = 88), 9 to <12 mo (n = 49), and ≥ 12 mo (n = 90) (Fig. 2). The analysis correlating PFS (based on the individual trial definition of progression) with TFPC demonstrated similar results (data not shown).
Fig. 2.
Survival based on time from prior chemotherapy.
3.3. Prognostic risk model
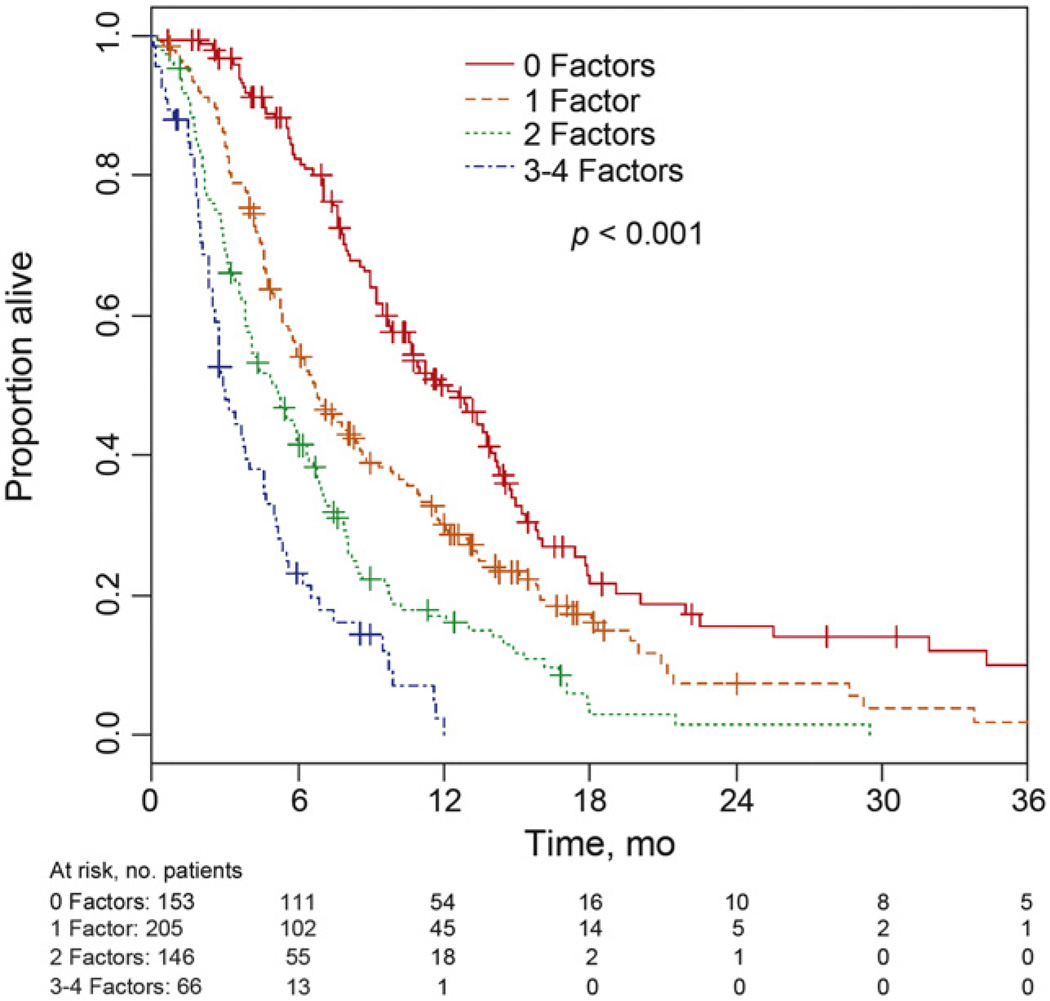
A total of 153, 205, 146, and 66 patients had, respectively, zero, one, two, and three to four poor prognostic factors (ie, PS>0, Hb<10 g/dl, LM, TFPC<3mo). Patients with three and four risk factors were combined into a single group because of the small number of patients (n = 53 and n = 13, respectively) in each group, and the similarity of their survival. The 6-mo (95% CI) OS was 82.4% (75.0–87.7), 54.2% (47.0–60.9), 41.4% (33.1–49.4), and 23.1% (13.5–34.2), respectively, for patients with zero, one, two, or three to four prognostic factors (Fig. 3). The corresponding median (95% CI) OS times were 12.2 (9.8– 13.8), 6.7 (5.7–8.0), 5.1 (3.9–6.0), and 3.0 (2.5–4.0) mo, respectively. The number of risk factors a patient had was significantly prognostic (p < 0.001).
Fig. 3.
Survival based on number of risk factors.
3.4. Improvement in prognostic accuracy and internal validation of the new prognostic model
As there are only four possible risk groups, it was difficult to assess calibration and clinical utility of the new model. Therefore, to compare the new, four-factor model with the previous three-factor model, we examined OS among those patients who had a change in risk classification. With the addition of TFPC, patients could only change risk groups by moving into a higher-risk group. Thus, 52 patients went from risk group 0 to risk group 1 (6-mo [95% CI] OS: 54.4% [39.7–67.0%]), 86 patients went from risk group 1 to risk group 2 (6-mo [95% CI] OS: 44.5% [33.4–55.1%]), and 44 patients went from risk group 2 to risk group 3 (6-mo [95% CI] OS: 23.7% [12.3–37.1%]). These values are similar to the estimated 6-mo OS of those patients in the higher-risk group who were not discordant, that is, the 6-mo (95% CI) OS for the 153, 60, and 22 patients with risk scores of 1, 2, and 3 in both models was 54.1% (45.7–61.8), 36.7% (24.7–48.7), and 21.6% (6.8–41.8), respectively. Thus, patients who were discrepant had OS more similar to the risk groups assessed by the new risk score. The mean (95% BCa CI) for the improvement in the c statistic using the four-factor versus three-factor model was 0.0159 (0.0057–0.0319).
3.5. External validation of prognostic impact of time from prior chemotherapy
Analysis of the phase 3, external validation dataset (n = 352) stratified by arm showed that TFPC, either continuous (with log transformation, p = 0.044) or dichotomized with cut-off points of 3 mo (p = 0.066), 6 mo (p = 0.018), and 9 mo (p = 0.026), was significantly associated with OS in univariable analyses. In multivariable analyses, TFPC (either continuous [HR: 0.93; 95% CI, 0.82–1.06; p = 0.29] or dichotomized at 3 mo [HR: 0.97; 95% CI, 0.78–1.21; p = 0.77]) was not independently significant. Additionally, the results of the analysis in the vinflunine plus BSC group were similar.
TFPC was significantly associated with PFS in univariable analyses as a continuous (log transformed, p = 0.009) or dichotomized variable with cut-off points of 3mo (p = 0.024), 6 mo (p = 0.002), and 9 mo (p = 0.001). Multivariable analyses showed a significant association of LM, ECOGPS> 0,Hb<10 g/dl, and TFPC with the cut-off points at TFPC of 6 mo (p = 0.020) and 9 mo (p = 0.014) and with the continuous log-transformed variable (p = 0.040), but not with a cut-off point of 3mo(p = 0.17).Median(95%CI) PFS for 62, 104, 123, and 63 patients in both treatment arms with zero, one, two, and three to four risk factors was 4.1 (2.8–5.4), 3.8 (2.9–4.6), 1.6 (1.4–2.1), and 1.4 (1.3–1.6)mo, respectively (p < 0.001). The analysis showed similar differentials in PFS when examining the vinflunine plus BSC group alone.
4. Discussion
A significant decrease in OS for patients with a shorter TFPC was observed independent of ECOG-PS>0, Hb<10 g/dl, and presence of LM in the largest, individual patient-level dataset of second-line therapy assembled to our knowledge (n = 570). A prognostic model was constructed with a c statistic of 0.638 with TFPC as a continuous variable and 0.631 with TFPC as < or ≥3 mo based on the presence of zero, one, two, or three to four factors. The median OS of these four groups (including TFPC cutoff of 3 mo) demonstrated significant divergence: 12.2, 6.7, 5.1, and 3.0 mo, respectively. This model further enhances the older three factor model, which had a c statistic of 0.615 in this dataset [14]. The mean (95% BCa CI) improvement in the c statistic of 0.0159 (0.0057–0.0319) is sufficient to pronounce this model useful for further evaluation, per Nguyen and Kattan [17]. Notably, treatment-free intervals are commonly used to stratify patients in other solid tumors. Moreover, shorter time from radical cystectomy to disease recurrence appears to be an independent prognostic factor [18,19].
Although the maximum dichotomy of median OS was observed by using a TFPC cut-off point of 3 mo, discrimination was observed across a range of cut-off points, and a continuous outcome obtained the greatest discrimination. Although a statistically significant improvement in the c statistic was observed with the new four-factor model compared with the previous three-factor model, the caveat is that it is unclear whether this level of an improvement is clinically relevant, as calibration and clinical utility of the new model were not assessable. However, for planning of future trials, stratifying patients by TFPC ≥3 or <3 mo appears reasonable. The convention is to initiate second-line therapy at the time of progression, and deferring chemotherapy >3 mo is not suggested by our data. It is noteworthy that neither prior platinum agent (cisplatin or carboplatin) nor chemotherapy setting (ie, for metastatic disease or in the perioperative/nonmetastatic setting) was prognostic. However, given the smaller numbers of patients that had received prior carboplatin or prior chemotherapy in the nonmetastatic setting, further work is needed to address both questions.
External validation analyses demonstrated a significant association of TFPC with both PFS and OS on univariable analyses in the vinflunine phase 3 trial (n = 352). However, multivariable analyses demonstrated numerical trends that were not statistically significant for the independent impact of TFPC on OS. In contrast, multivariable analyses for most of the definitions of TFPC did demonstrate statistically significant and independent associations with PFS, in addition to the associations for ECOG-PS >0, Hb <10 g/dl, and LM. A number of factors may have prevented the detection of an association of TFPC with OS in the phase 3 dataset. This trial consisted of a population that had all received prior chemotherapy for metastatic disease, with a median OS of 6.9 and 4.3moin the vinflunine plus BSC and BSC alone arms, respectively, compared to 6.8 mo among eligible phase 2 patients. Hence, these patients probably had more aggressive disease, which might have hampered the identification of TFPC as an independent prognostic factor for OS. In contrast, the pooled phase 2 dataset used to discover the impact of TFPC included 60% of patients who had received prior chemotherapy for metastatic disease. Intriguingly, more excluded patients from the combined discovery–phase 2 trial dataset had worse PS, anemia, and prior chemotherapy for metastatic disease compared to included patients. Therefore, it is possible that the prognostic model including TFPC applies better to patients with biologically more favorable disease. Moreover, 34%and29%of the patients in the BSC arm and vinflunine plus BSC arm in the phase 3 trial received subsequent chemotherapy, which may have confounded the detection of an association of TFPC with OS. The median follow-up time for the phase 3 trial was somewhat longer than may be expected in phase 2 trials (about 21–22 mo), resulting in more complete survival information (94% in the vinflunine trial compared with 78% among eligible phase 2 patients), which may be anticipated to capture relatively more non–cancer-relatedmortality. In aggregate, despite the independent impact on PFS but not OS in the external validation dataset, we believe our data suggest the incremental value of TFPC.
The pooled discovery–phase 2 dataset is limited by the heterogeneity of eligibility criteria, treatments, and outcomes (Table 1). These phase 2 trials generally allowed prior chemotherapy in a nonmetastatic setting (except one trial by Beer et al. [3]) and recurrence in this context was required to occur within varying periods (range: <1 yr to indefinite). Despite heterogeneity of therapy, there was limited evidence of activity. Variables of unclear importance (ie, prior cystectomy status), the reason for removal from first-line therapy (ie, for toxicity vs therapy completion) and response to first-line therapy were unavailable, although a longer TFPC may be considered to reflect a better quality of prior response. In addition, the frequency of imaging before starting second-line therapy may have varied but was unavailable. However, our study investigated the role of TFPC and not prior PFS as a prognostic factor and used OS (not PFS) as the primary clinical end point. Nevertheless, across a broad population with varying pretrial frequencies of imaging, TFPC may be more objective and may adequately capture pace of disease, number of cycles, and reason for stopping first-line therapy (eg, completion, toxicity, progression).
Our study also cannot be used to determine whether those with a longer TFPC may benefit from repeating platinum-based therapy. Nevertheless, we acquired individual patient-level data and data from well-conducted prospective trials regardless of therapy and definition of second-line therapy, which may enable these data to be broadly applicable.
5. Conclusions
TFPC conferred a significant impact on OS and PFS independent of ECOG-PS, Hb level, and presence of LM in patients receiving second-line therapy for advanced UC. This new four-factor model, based on the number of risk factors (zero, one, two, or three to four) may enhance the conduct and interpretation of clinical trials [20].
Acknowledgments
Financial disclosures: Joaquim Bellmunt certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: G. Sonpavde, N.J. Vogelzang, W.M. Stadler, P.H. O’Donnell, and D.J. Vaughn have received research support from Boehringer- Ingelheim. R Fougeray is an employee of Pierre-Fabre. T.K. Choueiri, A.Q. Qu, J.E. Rosenberg, and G. Sonpavde have received research support from AstraZeneca. N.D. James, D.J. Vaughn, and J.M. Bellmunt have received research support from Pierre-Fabre. N.D. James also has received speaker fees from Pierre-Fabre. Y.N. Wong has received research support from BMS. Y. Ko, S. Sridhar, and G. Sonpavde have received research support from Celgene. U.N. Vaishampayan and W.M. Stadler have received research support from GSK, and G. Sonpavde has received speaker fees from GSK. M.D. Galsky has received research support from Eli Lilly. G. Niegisch and P. Albers have received research support from Eli Lilly and BMS.
Funding/Support and role of the sponsor: None.
Footnotes
This research was presented in part at the poster discussion session of the American Society of Clinical Oncology annual conference held in June 2012 in Chicago, IL, USA, and was presented as a poster at the European Society for Medical Oncology Congress in September 2012 in Vienna, Austria.
Author contributions: Joaquim Bellmunt had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sonpavde, Pond, Bellmunt.
Acquisition of data: Pond, Fougeray, Sonpavde, Bellmunt.
Analysis and interpretation of data: Sonpavde, Fougeray, Choueiri, Qu, Vaughn, Niegisch, Albers, James, Wong, Ko, Sridhar, Galsky, Petrylak, Vaishampayan, Khan, Vogelzang, Beer, Stadler, O’Donnell, Sternberg, Rosenberg, Bellmunt.
Drafting of the manuscript: Sonpavde, Fougeray, Choueiri, Qu, Vaughn, Niegisch, Albers, James, Wong, Ko, Sridhar, Galsky, Petrylak, Vaishampayan, Khan, Vogelzang, Beer, Stadler, O’Donnell, Sternberg, Rosenberg, Bellmunt.
Critical revision of the manuscript for important intellectual content: Sonpavde, Fougeray, Choueiri, Qu, Vaughn, Niegisch, Albers, James, Wong, Ko, Sridhar, Galsky, Petrylak, Vaishampayan, Khan, Vogelzang, Beer, Stadler, O’Donnell, Sternberg, Rosenberg, Bellmunt.
Statistical analysis: Pond, Fougeray.
Obtaining funding: None.
Administrative, technical, or material support: Sonpavde, Pond, Bellmunt.
Supervision: Sonpavde, Pond, Bellmunt.
Other (specify): None.
References
- 1.Sonpavde G, Sternberg CN, Rosenberg JE, Hahn NM, Galsky MD, Vogelzang NJ. Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium. Lancet Oncol. 2010;11:861–870. doi: 10.1016/S1470-2045(10)70086-3. [DOI] [PubMed] [Google Scholar]
- 2.Albers P, Park SI, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99] Ann Oncol. 2011;22:288–294. doi: 10.1093/annonc/mdq398. [DOI] [PubMed] [Google Scholar]
- 3.Beer TM, Goldman B, Nichols CR, et al. Southwest Oncology Group phase II study of irinotecan in patients with advanced transitional cell carcinoma of the urothelium that progressed after platinumbased chemotherapy. Clin Genitourin Cancer. 2008;6:36–39. doi: 10.3816/cgc.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30:507–512. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culine S, Theodore C, De Santis M, et al. A phase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimen. Br J Cancer. 2006;94:1395–1401. doi: 10.1038/sj.bjc.6603118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sridhar SS, Canil C, Mukherjee SD, et al. A phase II study of single-agent nab-paclitaxel as second-line therapy in patients with metastatic urothelial carcinoma [abstract 279]. Poster presented at: American Society of Clinical Oncology Genitourinary Cancer Symposium; March 5–7, 2010; San Francisco, CA, USA. [Google Scholar]
- 7.Petrylak DP, Tangen CM, Van Veldhuizen PJ, Jr, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int. 2010;105:317–321. doi: 10.1111/j.1464-410X.2009.08799.x. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg CN, Calabro F, Pizzocaro G, Marini L, Schnetzer S, Sella A. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer. 2001;92:2993–2998. doi: 10.1002/1097-0142(20011215)92:12<2993::aid-cncr10108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase2 study. Cancer. 2009;115:4110–4117. doi: 10.1002/cncr.24460. [DOI] [PubMed] [Google Scholar]
- 10.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 11.Pili R, Qin R, Flynn PJ, et al. MC0553: a phase II safety and efficacy study with the VEGF receptor tyrosine kinase inhibitor pazopanib in patients with metastatic urothelial cancer [abstract 259] J Clin Oncol. 2011;29(Suppl 7) doi: 10.1016/j.clgc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadler WM, Vaughn D, Sonpavde G, et al. Clinical outcome of single agent volasertib (BI 6727) as second-line treatment of patients (pts) with advanced or metastatic urothelial cancer [abstract 4567] J Clin Oncol. 2011;29(Suppl 7) [Google Scholar]
- 13.Necchi A, Mariani L, Zaffaroni N, et al. Pazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trial. Lancet Oncol. 2012;13:810–816. doi: 10.1016/S1470-2045(12)70294-2. [DOI] [PubMed] [Google Scholar]
- 14.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 15.Galsky MD, Mironov S, Iasonos A, Scattergood J, Boyle MG, Bajorin DF. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs. 2007;25:265–270. doi: 10.1007/s10637-006-9020-9. [DOI] [PubMed] [Google Scholar]
- 16.Wong YN, Litwin S, Vaughn D, et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol. 2012;30:3545–3551. doi: 10.1200/JCO.2012.41.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen CT, Kattan MW. How to tell if a new marker improves prediction. Eur Urol. 2011;60:226–228. doi: 10.1016/j.eururo.2011.04.029. discussion 228–30. [DOI] [PubMed] [Google Scholar]
- 18.Rink M, Lee DJ, Kent M, et al. Predictors of cancer-specific mortality after disease recurrence following radical cystectomy. BJU Int. doi: 10.1111/j.1464-410X.2012.11433.x. In press. http://dx.doi.org/10.1111/j.1464-410X.2012.11433.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitra AP, Quinn DI, Dorff TB, et al. Factors influencing post-recurrence survival in bladder cancer following radical cystectomy. BJU Int. 2012;109:846–854. doi: 10.1111/j.1464-410X.2011.10455.x. [DOI] [PubMed] [Google Scholar]
- 20.Sonpavde G, Rosenberg JE, Hahn NM, et al. Suggestions for regulatory agency approval of second-line systemic therapy for metastatic transitional cell carcinoma. J Clin Oncol. 2010;28:e205–e207. doi: 10.1200/JCO.2009.27.1114. author reply e208. [DOI] [PubMed] [Google Scholar]