Abstract
Purpose
The aim of this study was to evaluate long-term outcome of the intraoperative and perioperative albendazole (ALB) treatment on the recurrence and/or secondary hydatidosis.
Methods
One hundred and one patients with hepatic hydatidosis were treated intraoperatively and perioperatively with ALB, in addition to surgery. Perioperative ALB treatment was given in a dose of 12-15 mg/kg/day. The ALB treatment was started 13.27 ± 14.34 days before the surgery, and it was continued for 4.39 ± 3.11 months postoperatively. A total of 1.7 µg/mL of ALB solution was used as a protoscolidal agent. The follow-up period was 134.55 ± 51.56 months.
Results
Four patients died, with only one death was secondary to hydatid disease (cerebral eccinococcus). There was only one recurrence (1%) of hepatic hydatidosis. Early and late morbidity rates were 8.91% and 7.92%, respectively.
Conclusion
Our results suggest that intraoperative and perioperative ALB is effective for the prevention of hepatic hydatidosis recurrence and/or secondary hydatidosis.
Keywords: Albendazole, Hepatic echinococcosis, Recurrence
INTRODUCTION
Benzoimidazole derivatives have been the drugs of choice for the medical treatment of echinococcosis since Bekhti's first report in 1977 [1]. Albendazole (ALB) is the most commonly used drug for the treatment of echinococcosis [2,3,4,5]. Medical treatment is effective in the prevention of secondary hydatidosis and/or hepatic hydatidosis recurrence.
Since 1995, we have treated hepatic hydatidosis by dual ALB treatment. Dual ALB treatment includes pre- and postoperative oral ALB therapy and intraoperative irrigation of the cystic cavity with ALB solution. The first 52 cases enrolled in this study have been previously reported elsewhere [6].
METHODS
In this retrospective study, data on 101 patients with hepatic hydatid disease treated by surgery and dual ALB treatment between December 1995 and June 2008 were reviewed.
Patients
Among the patients, two were admitted with recurrent hepatic hydatid disease. Both had previously undergone surgery for a hepatic hydatid cyst, one three years ago and one 30 years ago. All the patients were evaluated by a serology test and either an ultrasonography (US) or CT scan pre- and postoperatively. The cysts were classified according to Gharbi's classification [7]. Intraoperative US has been performed in all cases since 1997 [8].
ALB treatment protocol
A total of 1.7 µg /mL of ALB solution was used as a protoscolidal agent in the cystic cavity as previously reported [9,10]. Perioperative ALB therapy was performed in all cases. A dosage of 12-15 mg/kg/day of ALB was started preoperatively when the diagnosis of hepatic hydatid disease was established and continued postoperatively. During the ALB therapy, hematological and biochemical analyses were performed for all the patients. The treatment was discontinued in patients with elevated hepatic enzymes.
Postoperative follow-up
The patients were evaluated by serological tests and either a CT scan or USG on the fifth to seventh day and at the first, sixth, and twelfth months in the postoperative period. Patients were evaluated on an annual basis by US after the first year of follow-up.
All surviving patients were reached by phone for this study to eliminate bias. Biochemical and USG evaluations were done to update their current health status.
RESULTS
Sixty-two of the patients (61.38%) were female and 39 (38.61%) were male; with a mean age of 47.26 ± 15.78 years (range, 19-77 years). The follow-up period was 61-210 months (mean ± standard deviation, 134.55 ± 51.56 months).
The majority of the patients had solitary cyst (n = 70, 69.30%). Seventy-nine patients (78.21%) had cysts in the right lobe and 17 (16.83%) in the left lobe. Five patients (4.95%) had cysts in both lobes. One of the patients with bilateral hepatic hydatid disease had pelvic, peritoneal, and retroperitoneal cysts secondary to cyst rupture due to blunt abdominal trauma. Most of the cysts were grade II or III (n = 68, 67.32%) (Table 1).
Table 1.
Gradings and numbers of the cysts
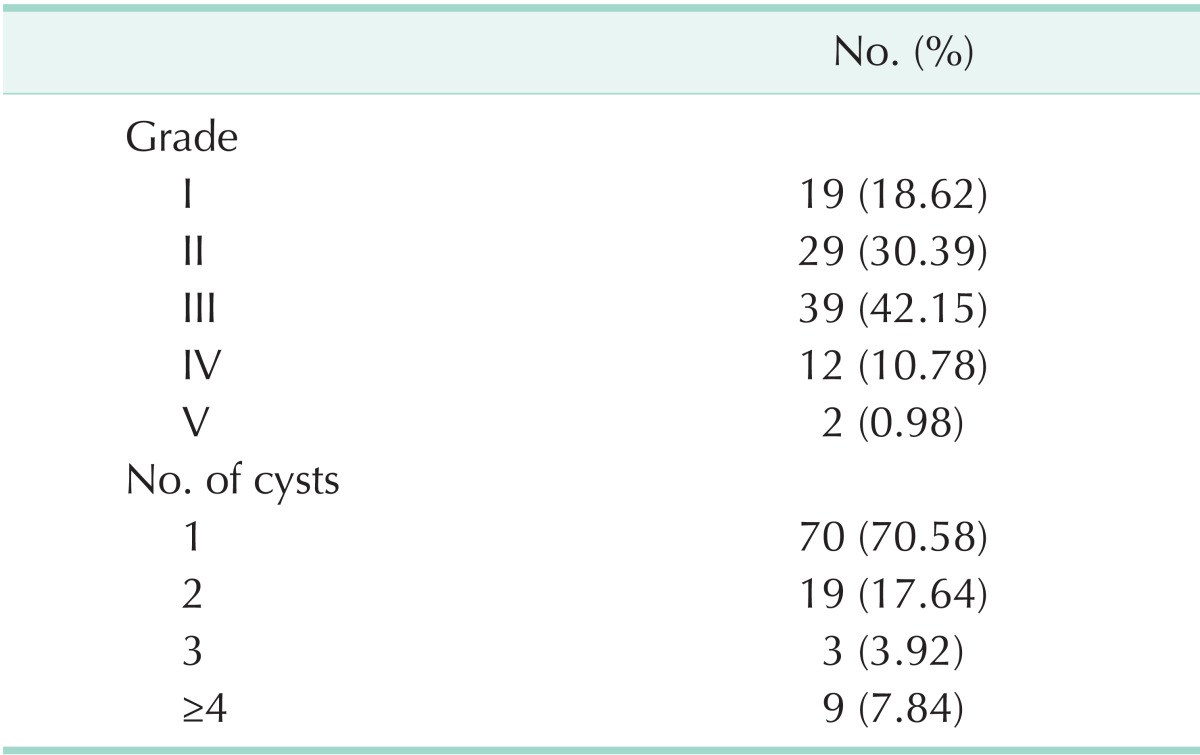
A latex agglutination test was done in 60 patients, and 38 (63.33%) were positive. An indirect hemaglutination test was done in 23 patients, with 11 (47.82%) positive results. Recently, IgG was studied in eight patients and found to be positive in three patients (37.5%). Eighty-seven patients (79.20%) were treated by drainage and omentoplasty with or without partial cystectomy. Eleven (10.89%) were operated video-laparoscopically. In four patients, surgical procedures began laparoscopically but were completed conventionally due to technical difficulties and posteriorly localization of the cysts.
Cysto-biliary communication was diagnosed in seven patients. One case was simple communication, and it was treated by intracystic suturing of the communication. The others were frank communications. Three had a large calibred choledochus and were treated by choledechoduodenostomy. Cysto-biliary communication was seen and sutured in one of the three patients. The remaining three patients had a normal calibred choledochus and were treated by T-tube drainage. Capittonage was applied in three cases, tube drainage in ten cases, and pericystectomy in one case (Table 2).
Table 2.
Surgical methods for the treatment of the cysts
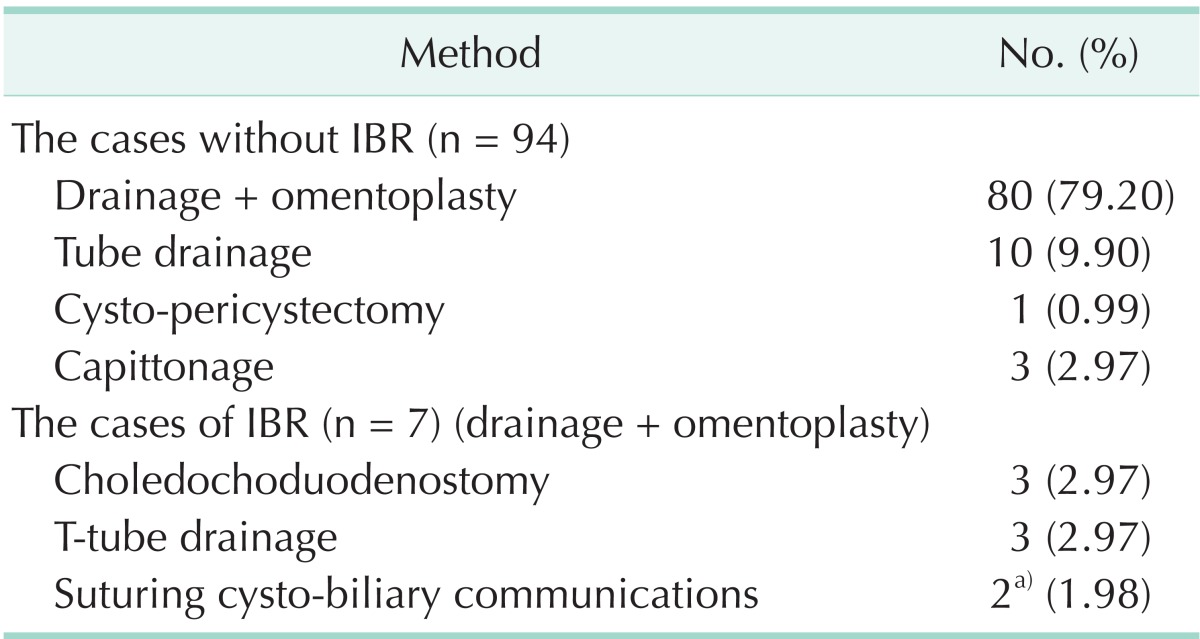
IBR, intrabiliary rupture.
a)In one case both choledochodudenostomy and suturing were applied.
The mean preoperative ALB therapy duration was 13.27 ± 14.34 days (range, 1-120 days). Most of the patients received ALB therapy for less than one month. One patient was admitted to the Department of General Surgery, Ondokuz Mayis University Medical Faculty on the 120th day of preoperative ALB treatment, which was started in another center.
The patients received ALB therapy for 1-24 months (mean ± standard deviation, 4.39 ± 3.11 months) postoperatively. Most of the patients received treatment for one to eight months. Two patients received postoperative ALB therapy for 12 and 24 months, respectively, because they had not observed our follow-up protocol until they were readmitted to our clinic 12 and 24 months later, respectively.
A total of 17 complications were encountered in 16 patients. The morbidity rate was 16.83%, most of which (n = 11, 10.89%) were due to cystic cavity infections or collections. All the patients with infectious complications received antibiotherapy. Six patients with a cystic cavity infection or biliary collection were treated by percutaneous drainage. Two infectious complications were misdiagnosed as recurrence, one at postoperative 18 months and one at 22 months. Celiotomy was performed in these cases. They were found to have an abscess of the cyst cavity, and drainage was performed. All the complications are shown in Table 3. The mean hospital stay was 12.8 ± 5.52 days (range, 5-45 days).
Table 3.
Complications and morbidity rates
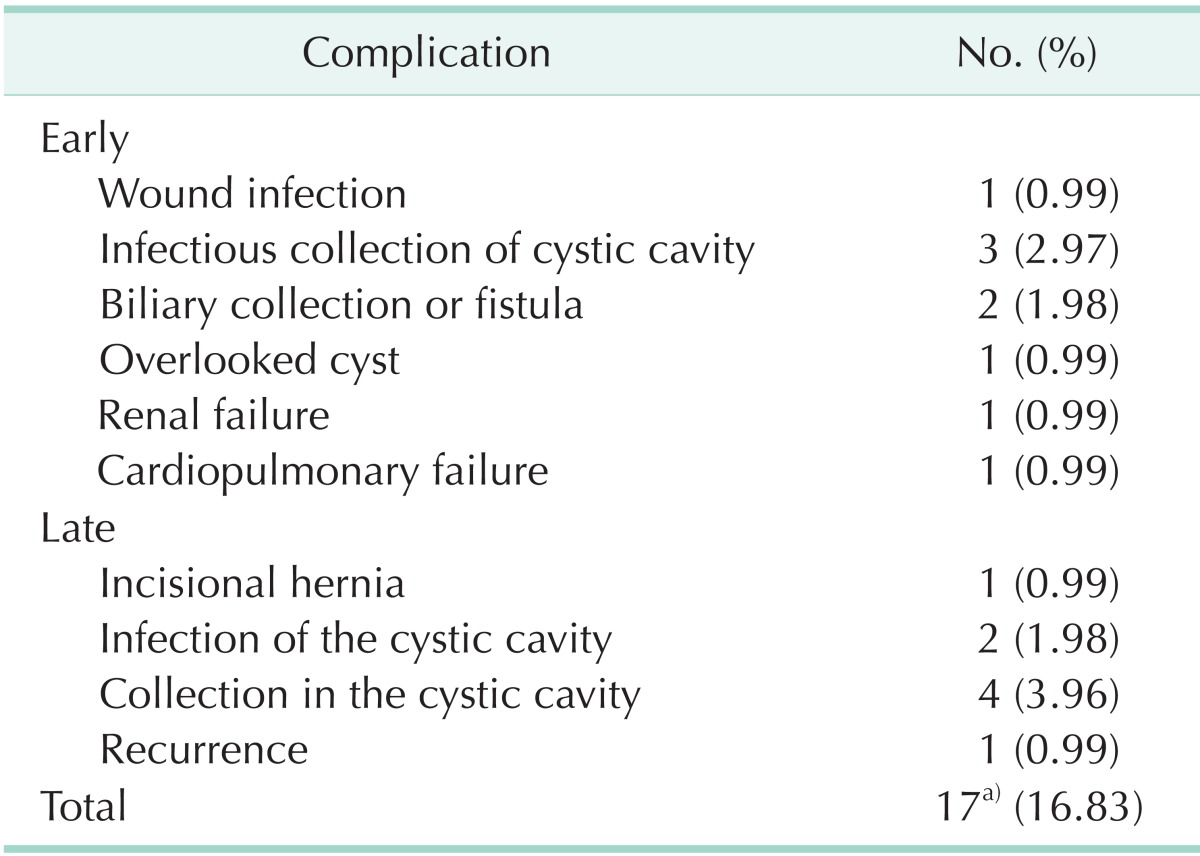
a)In a case, incisional hernia and cystic cavity infection coexisted.
Recurrence occurred only in one patient at postoperative month 78. This patient had a grade I cyst (15 cm in diameter) and was treated laparoscopically and received ALB for seven days preoperatively and three months postoperatively. The recurrent cyst was treated by drainage and omentoplasty.
Four patients (3.96%) died. One patient died in the early postoperative period due to cardio-pulmonary insufficiency. A 74-year-old male patient died due to a cerebral hydatid cyst and multiple organ failure in the postoperative second month. The third patient's death in the postoperative fifth month was secondary to renal insufficiency, which was not related to echinococcosis. The last patient died due to unknown causes in the postoperative third year (Table 4).
Table 4.
Objectives of the patients who died

ALT, AST, and ALP were mildly elevated in three patients. These enzymes values returned to normal levels after transient discontinuation of the ALB treatment. Two patients who received ALB for 12 and 24 months did not develop hepatotoxicity.
DISCUSSION
The recurrence of hepatic hydatidosis and/or secondary hydatidosis is a common problem after hydatid surgery. Recent studies have attributed differences in the rates of recurrence (0%-24%) to the surgical methods and/or medical treatment used [11,12,13,14,15,16]. In 1991, we reported a recurrence rate of 14.3% in 21 cases treated by tube drainage with no medical treatment [17]. In the present study, the recurrence rate decreased to 1% by dual treatment.
ALB inhibits ATP, pyruvate kinase, phosphoenolpyruvate-kinase, acid phosphatase, and alanine transferase and leads to a decrease in the glycogen content of the cyst wall. ALB causes cellular autolysis and degeneration in microthrics and microtubules, resulting in the death of scolices [18].
The pre-, post-, or perioperative use of ALB for the treatment of hydatid cysts has been studied, and an ALB dosage of 10-15 mg/kg/day was found to be effective in many of these studies [19,20,21]. The effectiveness of ALB was reported as 71.5%, 88.7%, and 97% by Horton [22], Liu et al. [23], and Chai et al. [24], respectively. The duration of ALB treatment for cyst sterilization remains controversial. Morris et al. [25] reported that preoperative ALB treatment for one month or a longer period sterilized 93.75% of cysts in an experimental model. The same study reported that 10 mg/kg/day of ALB for one week after inoculation reduced the formation of peritoneal cysts. Other studies reported successful outcomes after preoperative short-term (three to seven days) [7,24,25] or long-term (three weeks to one month) ALB treatment [21,25]. ALB usage was recommended for two or more months after surgical treatment or percutaneous drainage [4,5,6,20].
There are two biological metabolites of ALB in human organisms: albendazole sulfone and albendazole sulfoxide (ALSF). ALSF is the main metabolite, with high concentrations in cystic fluid and cyst wall, and it has more protoscolicidal effects. Plasma ALSF levels have been shown to range between 30 and 3,200 ng/mL after administration of 10 mg/kg of daily ALB [2,3,26,27]. Saimot et al. [2,5] reported 0.91 µg/mL and 0.8 µg/mL of ALSF in the cystic fluid and the cyst wall, respectively. The same study found that plasma concentrations over 900 ng/mL had a protoscolicidal effect. We previously reported that an ALSF solution in a concentration of 100 µg/mL exerted a protoscolicidal effect in 15 minutes [28].
The most common side effects of ALB are neutropenia due to bone marrow inhibition and elevation of liver enzymes [29]. Morris et al. [3] reported liver function abnormalities, including cholestatic icterus, in 18% of cases after ALB therapy for one month. Only one ALB-related death has been reported [30]. In this study, neither neutropenia nor any other hematological abnormality was detected in our patients. In three patients, ALT, AST, and ALP were mildly elevated but returned to normal levels following transient discontinuation of the ALB treatment.
The limitation of this study is its retrospective, nonrandomized design. Its strengths are its large patient population and long-term follow up by the same surgeon.
In conclusion, our results suggest that dual ALB treatment is safe and effective in the prevention of secondary hydatidosis and/or hydatid disease recurrence. Surgery must be combined with perioperative ALB medication for the effective treatment of hydatid disease.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bekhti A, Schaaps JP, Capron M, Dessaint JP, Santoro F, Capron A. Treatment of hepatic hydatid disease with mebedazole: preliminary results in four cases. Br Med J. 1977;2:1047–1051. doi: 10.1136/bmj.2.6094.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saimot AG, Meulemans A, Cremieux AC, Giovanangeli MD, Hay JM, Delaitre B, et al. Albendazole as a potential treatment for human hydatidosis. Lancet. 1983;2:652–656. doi: 10.1016/s0140-6736(83)92533-3. [DOI] [PubMed] [Google Scholar]
- 3.Morris DL, Dykes PW, Marriner S, Bogan J, Burrows F, Skeene-Smith H, et al. Albendazole--objective evidence of response in human hydatid disease. JAMA. 1985;253:2053–2057. doi: 10.1001/jama.253.14.2053. [DOI] [PubMed] [Google Scholar]
- 4.Khuroo MS, Dar MY, Yattoo GN, Zargar SA, Javaid G, Khan BA, et al. Percutaneous drainage versus albendazole therapy in hepatic hydatidosis: a prospective, randomized study. Gastroenterology. 1993;104:1452–1459. doi: 10.1016/0016-5085(93)90355-g. [DOI] [PubMed] [Google Scholar]
- 5.Saimot AG. Medical treatment of liver hydatidosis. World J Surg. 2001;25:15–20. doi: 10.1007/s002680020003. [DOI] [PubMed] [Google Scholar]
- 6.Polat C, Dervisoglu A, Hokelek M, Yetim I, Buyukkarabacak Y, Ozkutuk Y, et al. Dual treatment of albendazole in hepatic hydatidosis: new therapeutic modality in 52 cases. J Gastroenterol Hepatol. 2005;20:421–425. doi: 10.1111/j.1440-1746.2004.03535.x. [DOI] [PubMed] [Google Scholar]
- 7.Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatic liver. Radiology. 1981;139:459–463. doi: 10.1148/radiology.139.2.7220891. [DOI] [PubMed] [Google Scholar]
- 8.Dervisoglu A, Erzurumlu K, Tac K, Arslan A, Gursel M, Hokelek M. Should intraoperative ultrasonography be used routinely in hepatic hydatidosis? Hepatogastroenterology. 2002;49:1326–1328. [PubMed] [Google Scholar]
- 9.Erzurumlu K, Ozdemir M, Mihmanli M, Cevikbas U. The effect of intraoperative mebendazole-albendazole applications on the hepatobiliary system. Eur Surg Res. 1995;27:340–345. doi: 10.1159/000129418. [DOI] [PubMed] [Google Scholar]
- 10.Erzurumlu K, Sahin M, Selcuk MB, Yildiz C, Kesim M. Intracystic application of mebendazole solution in the treatment of liver hydatid disease. Preliminary report of two cases. Eur Surg Res. 1996;28:466–470. doi: 10.1159/000129492. [DOI] [PubMed] [Google Scholar]
- 11.Prousalidis J, Kosmidis CH, Fahantidis E, Harlaftis N, Aletras O. Surgical treatment of multiple cystic echinococcosis. HPB (Oxford) 2004;6:110–114. doi: 10.1080/16515320410026068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirelis CG, Bekiaridou KA, Bougioukas IG, Xanthoulis AI, Tsalkidou EG, Nannou G, et al. Long-term results of surgical treatment of hydatid disease. Acta Chir Belg. 2006;106:684–687. doi: 10.1080/00015458.2006.11679981. [DOI] [PubMed] [Google Scholar]
- 13.Aydin U, Yazici P, Onen Z, Ozsoy M, Zeytunlu M, Kilic M, et al. The optimal treatment of hydatid cyst of the liver: radical surgery with a significant reduced risk of recurrence. Turk J Gastroenterol. 2008;19:33–39. [PubMed] [Google Scholar]
- 14.Atmatzidis K, Koutelidakis I, Papaziogas B, Alexandrakis A, Chatzimavroudis G, Grigoriou M, et al. Primary hydatid cyst of the thigh. Chirurgia (Bucur) 2006;101:419–421. [PubMed] [Google Scholar]
- 15.Yagci G, Ustunsoz B, Kaymakcioglu N, Bozlar U, Gorgulu S, Simsek A, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg. 2005;29:1670–1679. doi: 10.1007/s00268-005-0058-1. [DOI] [PubMed] [Google Scholar]
- 16.Secchi MA, Pettinari R, Mercapide C, Bracco R, Castilla C, Cassone E, et al. Surgical management of liver hydatidosis: a multicentre series of 1412 patients. Liver Int. 2010;30:85–93. doi: 10.1111/j.1478-3231.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 17.Erzurumlu K, Yucel Y, Tezelman S. The effectiveness and late results of tube drainage in hepatic hydatidosis (an analysis of 21 cases) Med Bulletin of Ist Med Fac. 1991;54:283–290. [Google Scholar]
- 18.Xiao SH, Feng JJ, Guo HF, Jiao PY, Yao MY, Jiao W. Effects of mebendazole, albendazole, and praziquantel on fumarate hydratase, pyruvate kinase, and phosphoenolpyruvate carboxykinase of Echinococcus granulosus cyst wall harbored in mice. Zhongguo Yao Li Xue Bao. 1994;15:69–72. [PubMed] [Google Scholar]
- 19.Cakmakçi M, Sayek I. Prophylactic effect of albendazole in experimental peritoneal hydatidosis. Hepatogastroenterology. 1992;39:424–426. [PubMed] [Google Scholar]
- 20.Tsimoyiannis EC, Siakas P, Moutesidou KJ, Karayianni M, Kontoyiannis DS, Gossios KJ. Perioperative benzimidazole therapy in human hydatid liver disease. Int Surg. 1995;80:131–133. [PubMed] [Google Scholar]
- 21.Aktan AO, Yalin R. Preoperative albendazole treatment for liver hydatid disease decreases the viability of the cyst. Eur J Gastroenterol Hepatol. 1996;8:877–879. [PubMed] [Google Scholar]
- 22.Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79–93. doi: 10.1016/s0001-706x(96)00640-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Wang X, Wu J. Continuous long-term albendazole therapy in intraabdominal cystic echinococcosis. Chin Med J (Engl) 2000;113:827–832. [PubMed] [Google Scholar]
- 24.Chai J, Menghebat, Wei J, Deyu S, Bin L, Jincao S, et al. Observations on clinical efficacy of albendazole emulsion in 264 cases of hepatic cystic echinococcosis. Parasitol Int. 2004;53:3–10. doi: 10.1016/j.parint.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Morris DL, Chinnery JB, Hardcastle JD. Can albendazole reduce the risk of implantation of spilled protoscoleces? An animal study. Trans R Soc Trop Med Hyg. 1986;80:481–484. doi: 10.1016/0035-9203(86)90352-4. [DOI] [PubMed] [Google Scholar]
- 26.Penicaut B, Maugein P, Maisonneuve H, Rossignol JF. Pharmacokinetics and urinary metabolism of albendazole in man. Bull Soc Pathol Exot Filiales. 1983;76:698–708. [PubMed] [Google Scholar]
- 27.Marriner SE, Bogan JA. Pharmacokinetics of albendazole in sheep. Am J Vet Res. 1980;41:1126–1129. [PubMed] [Google Scholar]
- 28.Erzurumlu K, Hokelek M, Baris S, Sahin M, Birinci A, Amanvermez R, et al. Effect of albendazole sulfoxide solution on the scolices and the hepatobiliary system. Eur Surg Res. 1998;30:433–438. doi: 10.1159/000008610. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JF, Rausch RL. Mebendazole and alveolar hydatid disease. Ann Trop Med Parasitol. 1982;76:165–173. doi: 10.1080/00034983.1982.11687523. [DOI] [PubMed] [Google Scholar]
- 30.Avgerinos ED, Pavlakis E, Stathoulopoulos A, Manoukas E, Skarpas G, Tsatsoulis P. Clinical presentations and surgical management of liver hydatidosis: our 20 year experience. HPB (Oxford) 2006;8:189–193. doi: 10.1080/13651820500539495. [DOI] [PMC free article] [PubMed] [Google Scholar]


