Abstract
Purpose
Extended liver resection may provide long-term survival in selected patients with Bismuth type IV hilar cholangiocarcinoma (HCCA). The purpose of this study was to identify anatomical factors that predict curative-intended resection.
Methods
Thirty-three of 159 patients with Bismuth type IV HCCA underwent major hepato-biliary resection with curative intent (CIR) between 2000 and 2010. Disease extent and anatomical variations were analyzed as factors enabling CIR.
Results
CIR ratio with hilar trifurcation bile duct variation (13/16) was significantly higher than that with other bile duct variation types (18/25). Hilum to left second bile duct confluence and tumor infiltration over left second bile duct confluence lengths in right-sided CIR were significantly shorter than those lengths in left-sided CIR (10.8 ± 4.9 and 2.7 ± 0.8 mm vs. 16.5 ± 8.4 and 7.0 ± 5.3 mm, respectively). Left-sided CIR patients had a marginally higher proportion of tumors invading ≤5 mm over the right second confluence than that in right-sided CIR patients (13/17 vs. 6/16; P = 0.061). The 3-year survival rate after CIR (28%) was significantly higher than after non-CIR (6.1%).
Conclusion
We recommend the criteria of CIR as bile duct variation type, length of hilum to contralateral second bile duct confluence, and extent of tumor infiltration over the second confluence for Bismuth type IV HCCA.
Keywords: Klatskin's tumor, Bismuth type IV, Surgery, Anatomy
INTRODUCTION
Hilar cholangiocarcinoma (HCCA), also known as Klatskin's tumor, is a relatively rare tumor typically affecting the hepatic duct confluence. Complete surgical resection is the only therapeutic strategy offering the chance of a cure to patients with HCCA [1,2,3,4]. Recently, the use of aggressive surgical management, including major hepatectomy, has increased tumor resectability and has improved long-term results for patients [1,4].
HCCA is classified on the basis of the involvement of the hepatic confluence into four types according to Bismuth's scheme [5]. A type IV lesion is a combination of Bismuth type IIIa and IIIb lesions, in which the tumor invades the second-order bile duct tributaries of both hepatic ducts [6]. In the past, type IV lesions had been considered unresectable [7,8], with that opinion being included in the American Joint Committee on Cancer (AJCC) cancer staging manual (7th edition) [9].
Although extended liver resection may provide long-term survival in patients with HCCA, there are few reports on factors influencing resectability of Bismuth type IV HCCA. The aim of this study was to identify anatomical factors that predict curative-intended resection in patients with Bismuth type IV HCCA.
METHODS
Patient selection
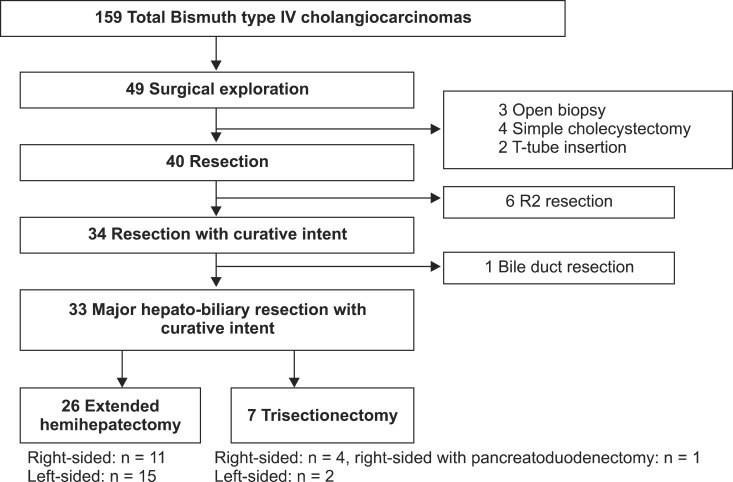
With approval from Institutional Review Board of Seoul National University Hospital (IRB No. H-1112-025-388), data for 159 patients with Bismuth type IV HCCA who were evaluated at our institute between 2000 and 2010 were entered into a prospectively maintained database. Of the patients, 119 had unresectable disease including distant metastasis, extensive lymph node metastasis, or involvement of major vessels except ipsilateral involvement of hepatic artery or portal vein branch to the resected hepatectomy side. Of the remaining 40 patients, data for those patients who underwent the current standard treatment of HCCA (major hepatectomy plus extrahepatic bile duct resection and regional lymphadenectomy) were examined. Thirty-three patients who underwent major hepato-biliary resection with curative intent (CIR) were enrolled (Fig. 1). All of those patients were confirmed Bismuth type IV HCCA after pathologic examination.
Fig. 1.
Patients with Bismuth type IV hilar cholangiocarcinoma selected for curative-intended resection.
Imaging analysis
Imaging was performed before any intervention on the bile duct, with the exception of patients in whom biliary drains had been placed before referral (18/159, 11.3%). In all patients evaluated for inclusion, CT examinations had been performed on multidetector CT (MDCT) scanners [10,11]. MRI or MR cholangiopancreatography imaging was performed for 92 patients (57.9%) on a 1.5-T superconducting system [12]. Direct cholangiograms were obtained for 142 patients (89.3%) using endoscopic retrograde cholangiopancreatography or percutaneous transhepatic cholangiography [12]. We estimated the length of longitudinal tumor extent along the biliary tree by evaluating the extent of the irregular stricture on cholangiograms or by evaluating the extent of the bile duct wall thickening on MDCT or MRI images. Measurements of longitudinal tumor extent and anatomical variation are shown in Fig. 2. Measurements of longitudinal tumor extent and anatomical variation were reviewed by an experienced radiologist.
Fig. 2.
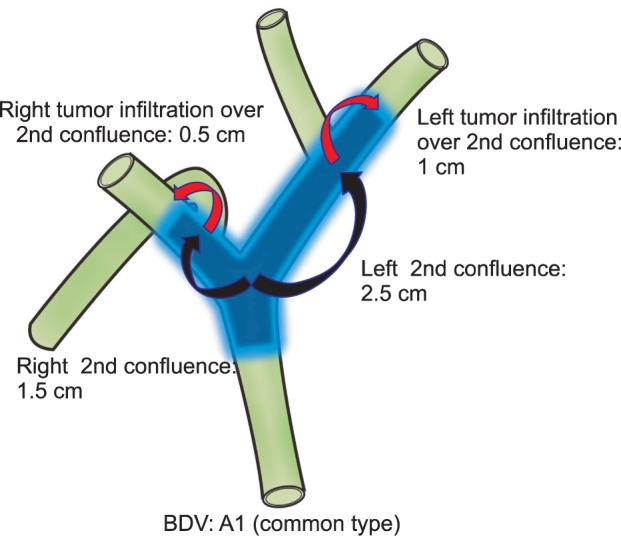
Longitudinal measure of tumor extent in bile duct variation type A1. BDV, bile duct variation.
Definition of anatomical variation
Huang et al. [13] proposed a system for bile duct variations (BDVs). Type A1 was defined as common (right and left hepatic duct junction), type A2 (hilar trifurcation type) as a common junction of right anterior hepatic duct, right posterior hepatic duct (RPHD), and left hepatic duct (LHD), type A3 as an aberrant drainage of RPHD to the left main duct, type A4 as an aberrant drainage of RPHD to the main hepatic duct, and type A5 as aberrant drainage of RPHD to the cystic duct [13]. In the present study, BDVs were grouped according to this classification. Also, we used the hepatic artery variation classification system proposed by Hiatt et al. [14]. In this classification scheme, type 1 was defined as common, type 2 as having a replaced or accessory left hepatic artery arising from the left gastric artery, type 3 as having a replaced or accessory right hepatic artery originating from the superior mesenteric artery, type 4 as having a double-replaced pattern, and type 5 as having the entire common hepatic artery originating as a branch of the superior mesenteric artery. Finally, our classification of portal venous variation was based on the Cheng classification [15], in which type I was defined as having a normal portal bifurcation, type II as having a portal trifurcation, type III as having the left portal vein originating from the right anterior portal branch, and type IV as having the right anterior portal branch originating from the left portal vein.
Surgical procedures
After laparotomy and the exclusion of distant metastasis, all of the following surgical procedures included a regional lymphadenectomy at the right side of the celiac artery, and removal of all tissues in the hepatoduodenal ligament except the portal vein and the hepatic artery. The type of resection was determined by the relative location and extent of the tumor. When the tumor was predominantly located in the right- or left-perihilar bile ducts, or tumor involvement in the liver parenchyma, unilateral hepatic artery, or when portal vein was observed on the preoperative images, an extended right- or left-hemihepatectomy, or trisectionectomy including caudate lobectomy combined with bile duct resection was performed (Fig. 1). As commonly used, we defined R0 resection as having complete tumor removal, R1 resection as having microscopic residual ductal disease, and R2 resection as having macroscopic remnant ductal carcinoma. Intraoperative evaluation of the proximal (hepatic)-side and/or distal (duodenal)-side ductal margins was performed by using frozen sections in all patients. Operative specimens were submitted for permanent histopathology. When the distal-side ductal margin was positive, additional resection of the intrapancreatic bile duct or pancreatoduodenectomy was performed, as far as was possible in principle. When the proximal-side ductal margin was positive, additional resection of the hepatic duct was performed, if possible.
Adjuvant treatment
Except in patients with poor performance status or those who refused chemoradiotherapy (CRT), we performed adjuvant CRT with 5-fluorouracil (5-FU) radiosensitizer after patients were informed of the prognosis and of the effects of each treatment modality. None of the patients received chemotherapy alone as adjuvant treatment. Treatment consisted of a 40-Gy dose to the tumor given in 10 daily fractions (as intravenous bolus of 5-FU) over a 2-week period. In some cases, patients received 5-FU monthly over a period of 1 year after CRT [16].
Statistical analyses
The data was analyzed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). Continuous and normally distributed variables are presented as the medians and range. Continuous parameters in each group were compared using the independent t test or the Mann-Whitney U test, and categorical parameters using the chi-square test or Fisher exact test. Survival curves were constructed using the Kaplan-Meier method and differences in survival were evaluated using the log-rank test. P-values of 0.05 or less were considered statistically significant.
RESULTS
Clinicopathological analysis of CIR of Bismuth type IV HCCA
Median cohort age was 60 years (range, 24-79 years) and the male to female ratio was 1.8:1. Twenty-one of 33 patients underwent R0 resection. Preoperative PVE was performed in 10 patients. The surgical procedures included right-sided resection (16/33) and left-sided resection (17/33). Median follow-up duration was 14.9 months (range, 0-51.0 months).
Anatomical variation in patients with Bismuth type IV HCCAs
The percentage of BDV in patients with CIR was significantly higher than in non-CIR patients. Among the variant BDV types, the rate of type A2 BDV in patients with CIR was higher than that in non-CIR patients. However, no significant differences were found between CIR and non-CIR patients in terms of hepatic arterial variations, or portal venous variations (Table 1).
Table 1.
Comparison of anatomical variations between CIR and non-CIR patient
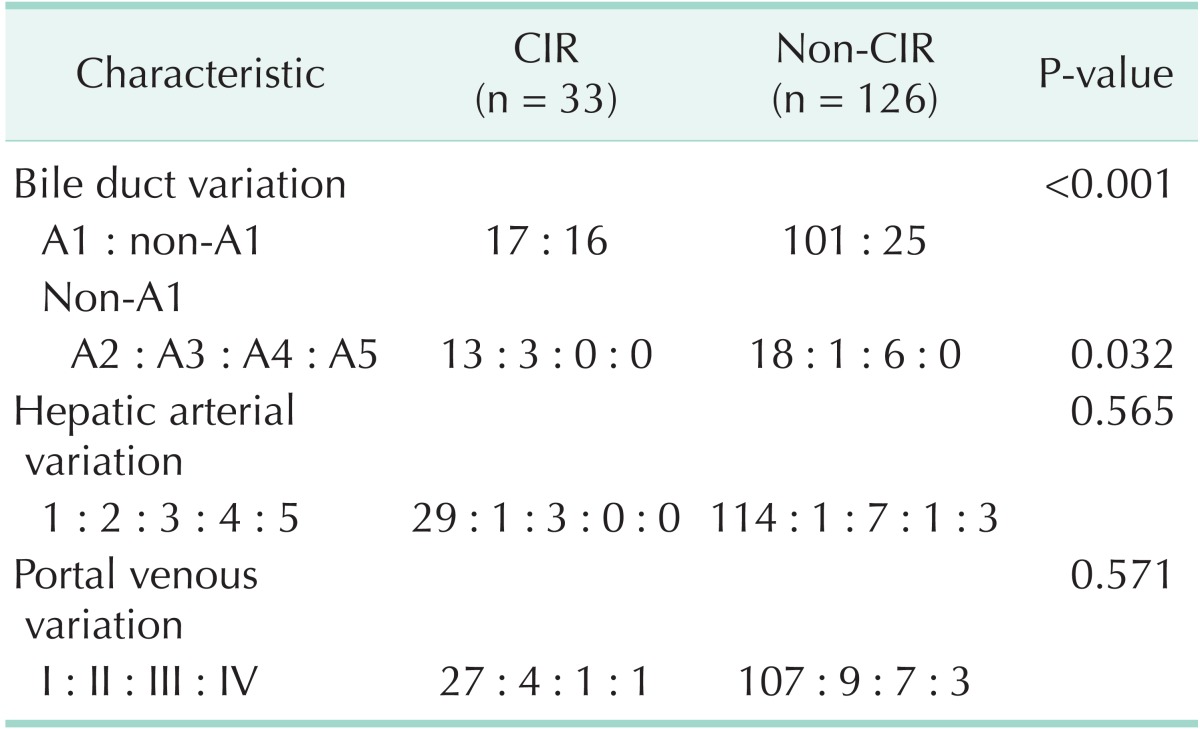
CIR, major hepato-biliary resection with curative intent.
Analysis of length of hilum to second bile duct confluence and length of tumor infiltration
The mean length from the hilum to the left second bile duct confluence and the length of tumor infiltration over the ipsilateral second bile duct confluence in right-sided CIR patients were significantly shorter than those in left-sided CIR patients (Table 2). Similarly, the proportion of left-sided CIR patients with tumor infiltration of <5 mm over the right second confluence was greater than that in right-sided CIR patients, but the difference was only of marginal significance (P = 0.061) (Table 3).
Table 2.
Length of hilum to 2nd bile duct confluence and extent of tumor infiltration in CIR patients
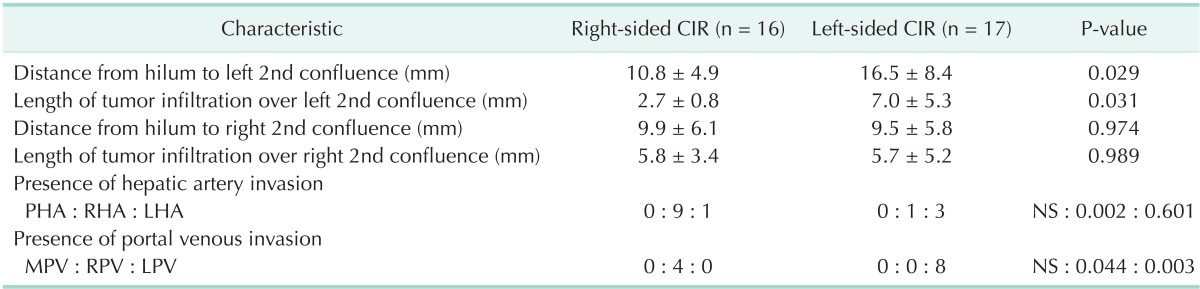
CIR, major hepato-biliary resection with curative intent; PHA, proper hepatic artery; RHA, right hepatic artery; LHA, left hepatic artery; MPV, main portal vein; RPV, right portal vein; LPV, left portal vein; NS, not significant.
Table 3.
Length of tumor infiltration over 2nd bile duct confluence in CIR patients
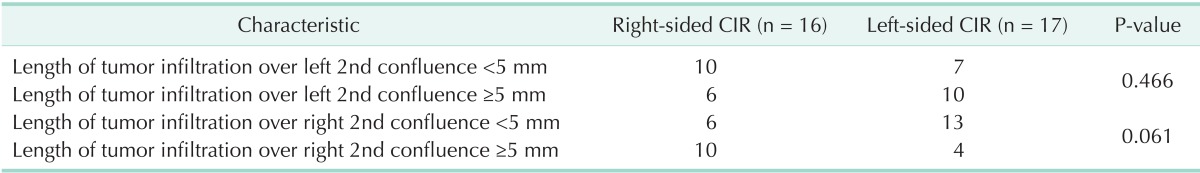
CIR, major hepato-biliary resection with curative intent.
Survival analysis of Bismuth type IV HCCAs
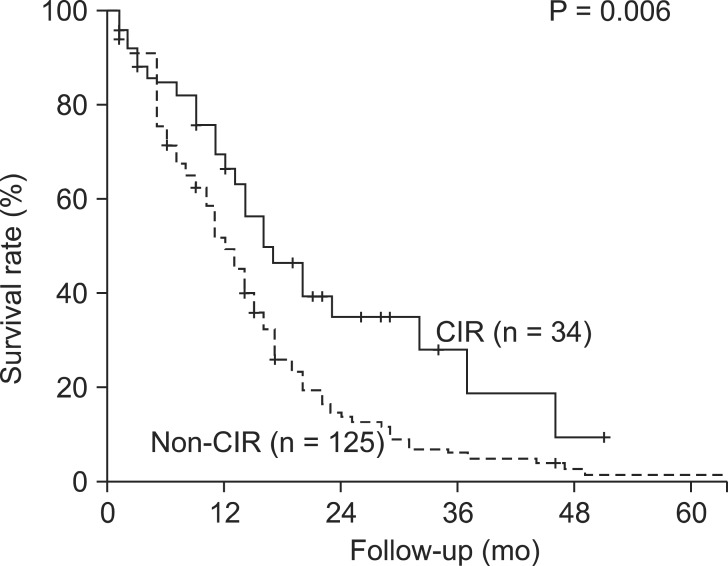
Overall survival of CIR and non-CIR patients with Bismuth type IV HCCA was determined. Median survival duration and 3-year survival rate of CIR and non-CIR patients were 16 months and 28.0% vs. 12 months and 6.1%, respectively (P = 0.006) (Fig. 3). Survival after surgical resection was significantly better in CIR patients with adjuvant CRT than in those without CRT (Table 4). There were no significant differences in survival on the basis of tumor invasion (AJCC T category), lymph node metastasis, histologic differentiation, presence of perineural invasion, combined portal vein resection, preoperative portal vein embolization (PVE), type of operation, or resection status (Table 4).
Fig. 3.
Survival rate at follow-up in patients with Bismuth type IV hilar cholangiocarcinomas. CIR, major hepato-biliary resection with curative intent.
Table 4.
Survival analysis in patients with Bismuth type IV hilar cholangiocarcinomas who underwent curative-intended resection
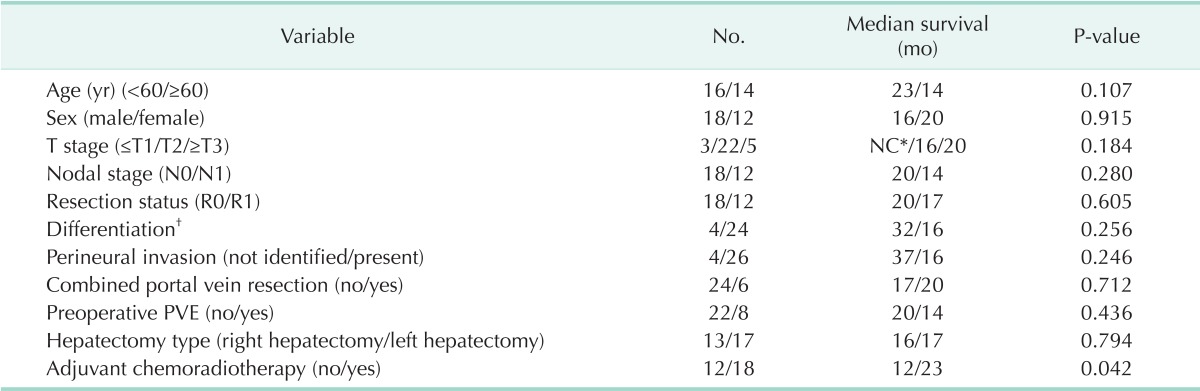
PVE, portal vein embolization; *NC, not calculated (Three in-hospital mortality [two hepatic failure and one pulmonary embolism]) were excluded; †papillary or well-differentiated/moderate- or poorly differentiated.
DISCUSSION
Even though diagnosis of Bithmuth type IV HCCA has markedly improved with advanced imaging modalities, patients with Bithmuth type IV HCCA have not been considered as candidates for curative resection [7,17,18]. However, recent reports have suggested that certain patients with Bismuth type IV HCCA are able to undergo curative resection [8,17,18]. In the present study, 33 of 159 patients (20.8%) underwent CIR. Among those, most patients (21/33) had negative surgical margins (R0 resection).
The proportion of CIR patients with BDV types A2 to A4 (especially, A2 hilar trifurcation BDV) was markedly higher than that in patients with common bile ducts (type A1) (Table 1). A2 BDV has typically short lengths of first-order bile duct compared to type A1 BDV. As a result, Bismuth type IV HCCA with A2 type BDV is actually similar to Bismuth types IIIa or IIIb, right- or left- sided hepatic resection as CIR could be possible.
In the present study, the distance from the hilum to the left second bile duct confluence and the extent of tumor infiltration over the left second bile duct confluence in right-sided CIR patients were significantly shorter than those lengths in left-sided CIR patients (Table 2). Also, the proportion of left-sided CIR patients with tumor infiltration of less than 5 mm over the right second bile duct confluence was marginally larger than that in right-sided CIR (Table 3). This result may be because the relatively short length of the first-order bile duct makes the anatomical characteristics of Bismuth type IV similar to those of Bismuth types IIIa or IIIb. Thus, we suggest that basing the selection of the operative procedure on of the length of the first-order bile duct and on the tumor extension over the second confluence along the bile duct is crucial for patients with Bismuth type IV HCCA.
With regard to vascular variation, we assumed that not only some type of hepatic artery variation, such as types 2, 3, or 4, but also some type of portal venous variation, such as type III or IV, had potential benefit for performing CIR due to the relatively close anatomical proximity between tumor and vasculatures. However, presently there were no significant differences in CIR rate among vascular variations (Table 1). This might be due to our relatively small study cohort or to there being no actual contribution of those variables to the resectability of Bismuth type IV HCCA. As a result, we suggest that further study is needed to determine the diagnostic usefulness of vascular variation.
Several reports including our previous report indicate that adjuvant CRT might be useful in patients with HCCA [19,20,21,22]. In the present study, there was a survival gain in patients who received adjuvant CRT compared to non-CRT patients (Table 4). Thus, we believe that adjuvant CRT after surgical resection can be beneficial in Bismuth type IV HCCA patients.
Recent studies have established that the important prognostic factors for long-term survival in HCCA patients are negative surgical margins, lymph node status, and tumor differentiation grade [3,20,21,23,24]. Among these, the most consistently reported independent determinant for long-term survival after HCCA resection is the surgical margin status of the resected bile duct [3,20,21,23,24]. In the present study, there was no significant difference in survival related to resection status (Table 4). However, patients who underwent CIR showed more prolonged survival than that in non-CIR patients (Fig. 3). This result can be explained because surgical resection with a positive margin has a better prognosis if adjuvant treatment, such as CRT, is combined with surgical resection [19,21,22].
To obtain a negative resection margin, a more extensive hepatic resection is essential in HCCA. Some authors suggest that an extensive hepatic resection, such as performing a right or left trisectionectomy with caudate lobectomy, may be an effective surgical procedure that can achieve a low mortality rate and high pathological curability for HCCA [25,26]. However, in-hospital deaths from postoperative hepatic failure have been the greatest contributor to poor survival rates [2,25]. As a partial solution to this poor survival rate, preoperative PVE is widely used to minimize the risk of liver failure when extensive liver resection is anticipated [4,7]. Here, however, there was no indication of a survival gain with implementation of preoperative PVE (Table 4). As a result, we suggest that preoperative PVE should be selectively used, perhaps in patients with an expected fingerling future liver remnant.
This study has several limitations. The first is the existence of inaccuracies in the performance of imaging analyses. Imaging analysis was performed in 18 patients after placing a drainage catheter in the bile duct. The presence of such a catheter may have limited the accurate evaluation of the enhancing or intraluminal lesion secondary to luminal collapse or there may be some interfering effect related to tube's presence. Second, although modern imaging techniques provide sufficient details to identify clearly the longitudinal and radial extents of the tumor [27,28], a subset of patients had unexpectedly extensive tumor infiltration along the biliary tree. This may be related to a submucosal extension along the bile duct, as is often observed in the periductal-infiltrating and nodular-infiltrating types of HCCA [10]. In contrast, there was overestimation of tumor extent from preoperative imaging modalities in some patients. Periductal fibroplastic and inflammatory reactions may cause such overestimation, particularly if adjacent to a major vascular structure, as the cicatrizing effect may lead to strictures without adventitial invasion [29,30]. For further evaluation of this potential source of limitation, we will analyze an ongoing prospective study of longitudinal tumor extent when it is assessed by preoperative imaging and pathologic examination.
With careful selection, patients with Bismuth type IV HCCA can be considered as candidates for CIR with an expectation of prolonged survival. Presence of type A2 BDV, the length of the hilum to the contralateral second bile duct confluence, and the extent of tumor infiltration over the second confluence of right- or left-dominant Bismuth type IV HCCA were the important factors for selection of a CIR procedure.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (No. 1120310).
The authors thank Dr. Kyung-Sik Ahn, Department of Radiology, Korea University College of Medicine, Seoul, Korea, for imaging analysis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250:210–218. doi: 10.1097/SLA.0b013e3181afe0ab. [DOI] [PubMed] [Google Scholar]
- 2.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratti F, Cipriani F, Ferla F, Catena M, Paganelli M, Aldrighetti LA. Hilar cholangiocarcinoma: preoperative liver optimization with multidisciplinary approach. Toward a better outcome. World J Surg. 2013;37:1388–1396. doi: 10.1007/s00268-013-1980-2. [DOI] [PubMed] [Google Scholar]
- 5.Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39–47. doi: 10.1007/BF01658484. [DOI] [PubMed] [Google Scholar]
- 6.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- 7.Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208:134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki R, Kondo T, Oda T, Murata S, Wakabayashi G, Ohkohchi N. Impact of three-dimensional analysis of multidetector row computed tomography cholangioportography in operative planning for hilar cholangiocarcinoma. Am J Surg. 2011;202:441–448. doi: 10.1016/j.amjsurg.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 10.Seo H, Lee JM, Kim IH, Han JK, Kim SH, Jang JY, et al. Evaluation of the gross type and longitudinal extent of extrahepatic cholangiocarcinomas on contrast-enhanced multidetector row computed tomography. J Comput Assist Tomogr. 2009;33:376–382. doi: 10.1097/RCT.0b013e318184f3f7. [DOI] [PubMed] [Google Scholar]
- 11.Ryoo I, Lee JM, Park HS, Han JK, Choi BI. Preoperative assessment of longitudinal extent of bile duct cancers using MDCT with multiplanar reconstruction and minimum intensity projections: comparison with MR cholangiography. Eur J Radiol. 2012;81:2020–2026. doi: 10.1016/j.ejrad.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Choi JY, Lee JM, Lee JY, Kim SH, Lee MW, Han JK, et al. Navigator-triggered isotropic three-dimensional magnetic resonance cholangiopancreatography in the diagnosis of malignant biliary obstructions: comparison with direct cholangiography. J Magn Reson Imaging. 2008;27:94–101. doi: 10.1002/jmri.21038. [DOI] [PubMed] [Google Scholar]
- 13.Huang TL, Cheng YF, Chen CL, Chen TY, Lee TY. Variants of the bile ducts: clinical application in the potential donor of living-related hepatic transplantation. Transplant Proc. 1996;28:1669–1670. [PubMed] [Google Scholar]
- 14.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50–52. doi: 10.1097/00000658-199407000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng YF, Huang TL, Lee TY, Chen TY, Chen CL. Variation of the intrahepatic portal vein; angiographic demonstration and application in living-related hepatic transplantation. Transplant Proc. 1996;28:1667–1668. [PubMed] [Google Scholar]
- 16.Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim TY, et al. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer. 2008;8:374. doi: 10.1186/1471-2407-8-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Chen Y, Chen H. Application of portal parenchyma-enterostomy after high hilar resection for Bismuth type IV hilar cholangiocarcinoma. Am Surg. 2010;76:182–187. [PubMed] [Google Scholar]
- 18.Endo I, Shimada H, Sugita M, Fujii Y, Morioka D, Takeda K, et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery. 2007;142:666–675. doi: 10.1016/j.surg.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Gwak HK, Kim WC, Kim HJ, Park JH. Extrahepatic bile duct cancers: surgery alone versus surgery plus postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2010;78:194–198. doi: 10.1016/j.ijrobp.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Q, Luo X, Zhang B, Jiang X, Yi B, Wu M. Predictive factors for prognosis of hilar cholangiocarcinoma: postresection radiotherapy improves survival. Eur J Surg Oncol. 2007;33:202–207. doi: 10.1016/j.ejso.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, et al. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 2005;241:77–84. doi: 10.1097/01.sla.0000150166.94732.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Kim SW, Bang YJ, Heo DS, Ha SW. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2002;54:414–419. doi: 10.1016/s0360-3016(02)02952-8. [DOI] [PubMed] [Google Scholar]
- 23.Liu CL, Fan ST, Lo CM, Tso WK, Lam CM, Wong J. Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 2006;93:1488–1494. doi: 10.1002/bjs.5482. [DOI] [PubMed] [Google Scholar]
- 24.Wakai T, Shirai Y, Moroda T, Yokoyama N, Hatakeyama K. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005;103:1210–1216. doi: 10.1002/cncr.20906. [DOI] [PubMed] [Google Scholar]
- 25.Paik KY, Choi DW, Chung JC, Kang KT, Kim SB. Improved survival following right trisectionectomy with caudate lobectomy without operative mortality: surgical treatment for hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1268–1274. doi: 10.1007/s11605-008-0503-1. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu H, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251:281–286. doi: 10.1097/SLA.0b013e3181be0085. [DOI] [PubMed] [Google Scholar]
- 27.Aloia TA, Charnsangavej C, Faria S, Ribero D, Abdalla EK, Vauthey JN, et al. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg. 2007;193:702–706. doi: 10.1016/j.amjsurg.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155–164. doi: 10.1055/s-2004-828892. [DOI] [PubMed] [Google Scholar]
- 29.Chryssou E, Guthrie JA, Ward J, Robinson PJ. Hilar cholangiocarcinoma: MR correlation with surgical and histological findings. Clin Radiol. 2010;65:781–788. doi: 10.1016/j.crad.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Kim BS, Joo SH, Kim GY, Joo KR. Aggressive hilar inflammatory myofibroblastic tumor with hilar bile duct carcinoma in situ. J Korean Surg Soc. 2011;81(Suppl 1):S59–S63. doi: 10.4174/jkss.2011.81.Suppl1.S59. [DOI] [PMC free article] [PubMed] [Google Scholar]