Abstract
Purpose
The aim of this study was to compare the therapeutic effects of radiofrequency ablation (RFA) and hepatic resection (HR) with regards to procedural morbidity, mortality, overall survival (OS) and disease-free survival (DFS) rates in hepatocellular carcinoma (HCC) patients.
Methods
Retrospective studies were performed based on the medical records of 129 patients who underwent curative HR, and 57 who patients received RFA for HCC, between 2005 and 2009. The inclusion criteria of HCC were the presence of three or fewer nodules 3 cm or less in diameter or a single nodule of 5 cm or less.
Results
The 1-, 3- and 5-year OS rates in the HR group were 91.3%, 78.8%, and 64.9%, compared to 94.4%, 74.0%, and 74.0% in the RFA group, with no significant difference between the two groups (P = 0.725). The estimated 1- and 3-year DFS rates were 70.0% and 53.0% in the HR group and 65.2% and 24.7% in the RFA group, respectively. The DFS rates of HR group were significantly higher than RFA group (P = 0.015). Multivariate analysis identified that recurrence (P = 0.036) and portal hypertension (P = 0.036) were associated with OS and that portal hypertension (P = 0.048) and increased serum α-FP (P = 0.008) were the factors significantly associated with DFS.
Conclusion
HCC within Milan criteria should consider hepatectomy as the primary treatment if the patient's liver function and general conditions are good enough to undergo surgical operation. But in that RFA revealed similar overall survival to HR, RFA can be an alternative therapy for patients who are eligible for surgical resection.
Keywords: Hepatocellular carcinoma, Catheter ablation, Hepatectomy, Survival rate
INTRODUCTION
It has been reported that liver cancer is the fifth most common malignant tumor worldwide and the fourth most common malignant cancer nationwide [1], and according to domestic statistics of South Korea, five-year survival rates from hepatocellular carcinoma (HCC) was 15.3% [2], a relatively low survival rate compared to its high incidence. Liver cirrhosis is the factor with the most decisive influence on the incidence of HCC, and about 90% of patients with HCC have chronic infections of hepatitis B or C virus, known as the basal diseases of cirrhosis [3].
Hepatectomy, radiofrequency thermal ablation (RFA), transcatheter arterial chemoembolization, and liver transplantation were conducted for the treatment of HCC, and hepatic function reserve is an important consideration in selecting treatment methods for HCC. Of the current treatment methods of HCC, hepatectomy has been known as a radical treatment method [4,5,6]. RFA had been introduced as an alternative therapy for hepatectomy, but recently, it has also been used as the primary treatment method of small HCC according to the status of patients. Liver transplantation (LT) has been recognized as ideal therapy of primary HCC, because it can treat basal cirrhosis as well as HCC [7]. But hepatic resection (HR) revealed similar survival and recurrence to LT in the setting of well compensated cirrhosis and early HCC [8]. Also, thanks to a shortage of donors, high medical expenses, and restriction of criteria, LT is not generally undergone as the primary therapy of HCC.
In cases where the possibility of postoperative complication caused by hepatic failure induced from cirrhosis is high, where HR is impossible due to the development of intrahepatic multiple mass at the time of diagnosis of HCC, and where the cancer mass is located in an area where surgical approach or resection is impossible, an operation cannot be performed [9], and just 9%-27% of HCC patients can receive hepatectomy [10,11,12,13,14]. Thus, several nonsurgical therapies including transcatheter arterial chemoembolization, percutaneous ethanol injection and RFA have been used as an alternative to surgery. Of these therapies, percutaneous ethanol injection and RFA were regarded as optimal treatment modalities for small HCC [15]. Furthermore, RFA has been reported to achieve the optimal efficacy of treatment for small HCC [14,16,17]. Since its first introduction in 1996, RFA has been used mainly as an alternative therapy for the treatment of HCC and recently, it considered as first-line treatment of small HCC even when eligible for HR. According to recent studies, the pre-eminence of HR over RFA for small HCCs was suspected in that HR failed to demonstrate superiority in terms of overall survival (OS) and disease-free survival (DFS), also higher occurrence of major complications after surgery were reported [18,19]. However, as rising early detection of small HCC due to generalization of ultrasound screening for patients at risk of HCC and the preference for noninvasive therapy have been rising, RFA has widely been performed as first-line treatment modality of small HCC.
Therefore, this study examined the efficacies of HR and RFA for small HCC, conducted at a single hospital, for the same period through the comparison of complications, recurrences, OS rate, and DFS rate. In addition, it also investigated the factors affecting the prognosis of HCC.
METHODS
Patients
Patients who were diagnosed with HCC and underwent HR or RFA as their first treatment modalities at Chonnam National University Hospital from Jan. 1, 2005 to Dec. 31, 2009 were included in this study. Since the patients who were subject to the RFA were limited to the relatively small size of HCC, only the cases applicable to RFA were sampled from cases that underwent HR for the comparison of two the treatment methods. Namely, patients with three or fewer nodules 3 cm or less in diameter or a single nodule of 5 cm or less were selected. Patients who underwent HR and RFA simultaneously were excluded because the influence of the treatment methods on recurrences and period of survival could not be distinguished. Retrospective study was conducted on the basis of the medical records of 129 consecutive patients who underwent HR and 57 who underwent RFA for the treatment of HCC.
Written informed consent was obtained from all the patients before HR and RFA. This study was approved by the Institutional Review Board of the ethical committee of Chonnam National University Hospital and also granted waiver for individual patient consent in the view point of being evaluated retrospectively (2012-173).
The diagnosis of HCC
HCC was diagnosed using liver biopsy, imaging study, and serum α-FP. All 129 patients of HR group confirmed HCC histopathologically after HR and 5 cases (8.7%) of RFA group were diagnosed through ultrasound-guided fine needle aspiration cytology before procedure. Of the diagnoses in the RFA group through noninvasive approaches [20], (1) 4 cases (7.0%) were equivalent to α-FP of the patients with risk factors including cirrhosis, chronic hepatitis B, and chronic hepatitis C showed an increase of 200 ng/dL or more with typical HCC findings of early arterial enhancement and early wash-out at portal-delayed phase in dynamic CT or dynamic MRI, (2) 42 cases (73.9%) reported α-FP of 200 ng/dL or less but typical HCC findings both in dynamic CT and dynamic MRI, and (3) 11 cases (19.3%) indicated typical findings of HCC in dynamic CT or dynamic MRI of 2 cm or more in size with cirrhosis.
Clinical characteristics
Baseline evaluation included gender, age, presence or absence of cirrhosis, HBsAg, anti-HCV, albumin, AST, ALT, α-FP, total bilirubin, PT, platelet count, indocyanine green retention rate at 15 minutes (ICG R-15), Child-Pugh class, presence or absence of portal hypertension, size and number of HCC, length of hospital stay, complications, and aspects of recurrence of all patients. Diagnosis of cirrhosis in HR group was based on final histopathologic report. But determination of cirrhosis in RFA group was done by integrating radiologic findings suggestive of cirrhosis such as diffuse liver nodularity, change of echotexture, atrophy or hypertrophy of parenchyma and signs of portal hypertension. Of patients diagnosed with cirrhosis, portal hypertension was diagnosed when (1) gastric or esophageal varix was found by gastroduodenoscopy or conspicuous findings in abdominal imaging study or (2) a conspicuous splenomegaly was observed by abdominal imaging study with decreased the platelet count below 100,000/mm3 [21].
In order to apply the same criteria to both groups, the size of the HCC was measured with the maximum diameter of mass from the abdominal CT conducted before treatment procedure. Complications were confined to cases of surgical complication grade II or more, which require medication, surgical and radiologic intervention [22].
Recurrence
Local recurrence was defined as the cases in which recurrence was found around resection margin in HR group and recurrence was found at the periphery of the ablated zone in subsequent CT scans after complete ablation was confirmed in RFA group. Intrahepatic distant recurrence was defined as the cases in which recurrence was observed at a region not adjacent to the primary lesion within same segment or in different segments.
Survival
The concluding time of OS was based on the date of death in cases of death and on the date of the most recent follow-up visit in cases of survival. The concluding time of DFS was based on the date of the confirmation of the recurrence by imaging test in cases of recurrence and on the date of the last imaging test in cases of nonrecurrence. OS and DFS were computed monthly with the period from the date of surgical operation or RFA to the concluding date of OS and DFS, respectively.
Methods of hepatectomy
Of the patients who were diagnosed with clinical HCC and eligible to undergo HR considering the location and the distribution of tumor, the patients who were not limited in hepatic reserve to undergo HR at the preoperative test were selected for HR. The range of hepatectomy was determined by considering ICG R-15 results and position of tumor, and anatomical resection was performed if possible. Forty-four of 129 patients (34.1%) underwent wedge resection, 29 (22.5%) segmentectomy, 33 (25.6%) bisegmentectomy, and 23 (17.8%) major HR. Eight of 129 patients (6.2%) underwent laparoscopic hepatectomy, and 121 (93.8%) were treated with open hepatectomy. All patients were finally diagnosed with HCC by histopathological study. Three patients showed no findings of residual tumor infiltration at the resection margin grossly, but microscopic finding revealed the margin positive at the resection margin.
Methods of RFA
Patients with the deterioration of liver function over Child-Pugh class B, patients belonging to high-risk groups of HR due to poor general conditions and patients expressing their wishes to reject the surgical operation in spite of recommended hepatectomy were selected as the subjects of the study. Fifty of 59 patients (84.7%) who underwent RFA were treated with percutaneous approach, and 9 patients (15.3%) were treated with RFA after laparotomy under general anesthesia, which included 8 patients who gave up hepatectomy after diagnostic laparotomy and converted to RFA and 1 patient treated with RFA after laparotomy for the surgical operation of choledocholithiasis. Every procedure of RFA was performed by interventional radiologist regardless of place - whether operation room or ultrasonography suite. RFA instruments were used as: CC-I (Radionics, Burlington, MA, USA) with clustered cooled-tip needle (17 gauge, electrode length of 2-3 cm). For percutaneous intervention, 2 mL of Fentanyl (Fentanyl, Hana Pharmacy, Seoul, Korea) was administered for sedation and local anesthesia from skin to hepatic capsule was conducted along to expected electrode insertion route with 2% Lidocane HCl (Lidocaine, Gwangmyeong Pharmacy, Seoul, Korea) and radiofrequency electrode was inserted into the center of targeting HCC under real time ultrasound guidance. RFA was performed with consecutive activation mode and a 17-G dual clustered cooled-tip electrode was introduced to index tumor. RF current was conducted through from generator with settings to deliver maximal power in the automatic impedance control mode. RFA was applied for 8-12 minutes continuously. End point of RFA was determined mainly according to ultrasound imaging of fully covered of index tumor by hyperechoic ablated zone. Tumors larger than a diameter of 3.5 cm required multiple overlapping ablations [23]. There was no premature stop of procedure caused by pain discomfort. To prevent hemorrhage and seeding along the track, the electrode was drawn back with thermocoagulation of inserted track.
Dynamic CT was conducted on the day after intervention, and when low attenuation shown in the images of arterial and portal phases contained enough to cover the index HCC and there was no contrast enhancement near the region of thermal therapy, patient discharge was planned without additional sessions of RFA. But if tumoral enhancement was detected, additional sessions were performed for residual tumor. One of 57 patients (1.7%) showed incomplete necrosis, and one week later, RFA was performed again to achieve complete necrosis. The completeness of RFA was confirmed with dynamic CT performed 1 month after procedure.
Follow-up
After treatments HR and RFA, dynamic CT, serum α-FP and liver function test were regularly conducted every 2-6 months after discharge from hospital, and cases of suspected recurrence were confirmed by dynamic MRI. In case of recurrence, rehepatectomy, transcatheter arterial chemoembolization, or RFA was performed selectively.
Statistical analysis
Comparison of nominal variables was conducted with the chi-square test and the analysis of continuous variables used the independent t-test. The analysis of the survival employedthe Kaplan-Meier method and the significance of differences between groups was compared with the log-rank test. The Cox regression model was used for the univariate and the multivariate analysis of the factors affecting the prognosis of HCC. Multivariate analysis was conducted with all factors of P < 0.100 in the univariate. All statistical analyses were performed with the statistical program SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) and P < 0.05 was considered as a statistically significant value.
RESULTS
Patients' characteristics (Table 1)
Table 1.
Characteristics of patients with HR and RFA
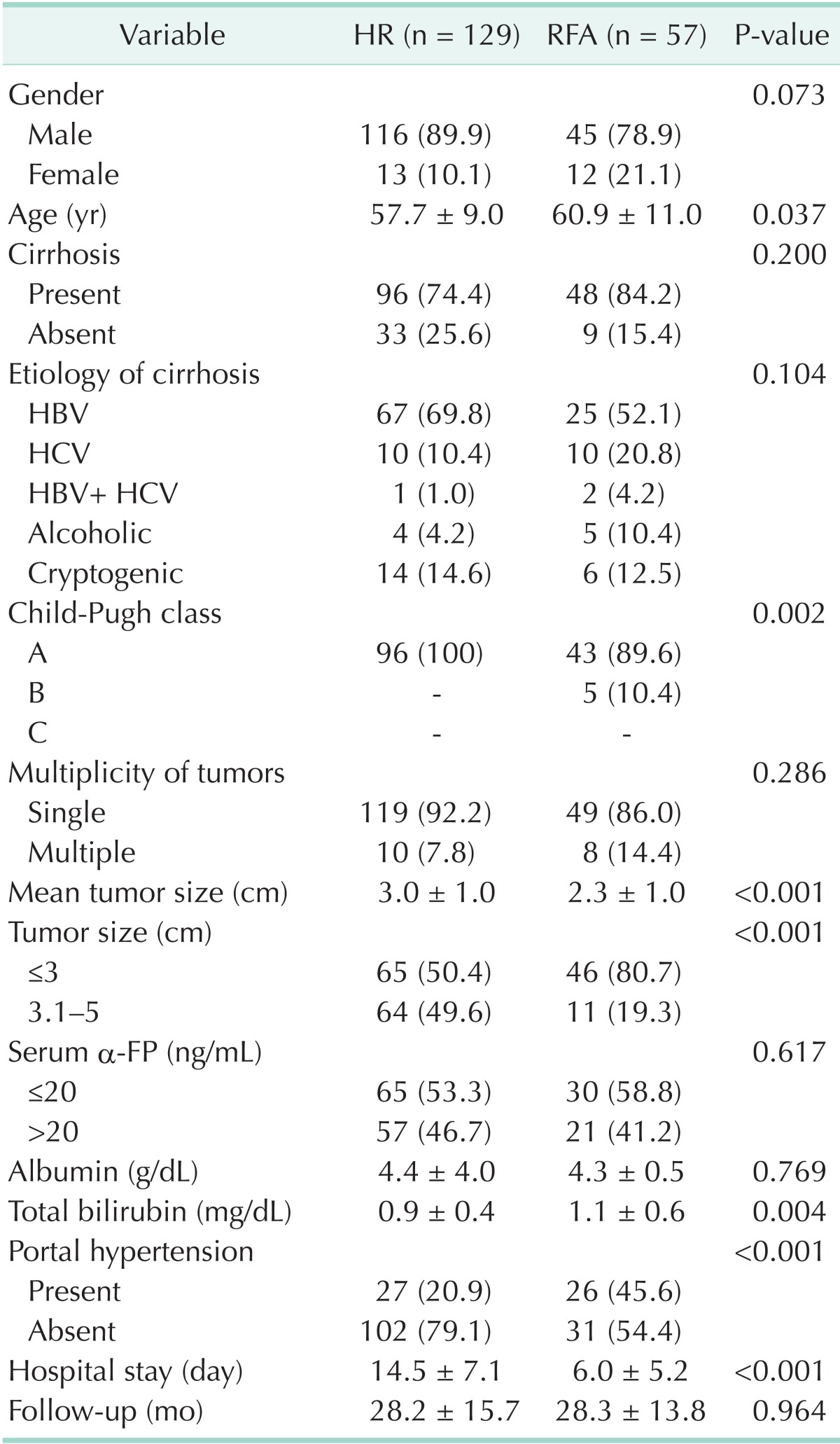
Values are presented as number (%) or mean ± standard deviation.
HR, hepatic resection; RFA, radiofrequency ablation.
The male to female ratio of total HCC cases was 6.4:1, with there being more male patients than female patients (P < 0.001), and there was no difference in the sex ratio between the two groups (P = 0.254). Cirrhosis was found in 96 patients of the HR group (74.4%) and 48 of the RFA group (84.2%). In the etiology of liver cirrhosis, hepatitis B infection was the most common infection in both groups at 69.8% and 52.1%, respectively, and there was no significant difference in the percentages of hepatitis B infection (P = 0.104). In Child-Pugh class, which can estimate the progress of cirrhosis, the entire HR group was diagnosed as class A, but 43 of the RFA group (89.6%) was diagnosed as class A and 5 (10.4%) as class B, and it was found that cirrhosis had progressed further in the RFA group (P = 0.002). The mean diameters of the HCC were 3.0 ± 1.0 cm and 2.3 ± 1.0 cm in the HR group and the RFA group, respectively, and the tumors of the RFA group were smaller than those of the HR group (P < 0.001). There was no difference in the serum α-FP distributions in the two groups after dividing them into over and below serum α-FP 20 ng/mL (P = 0.617) and total bilirubin was higher in the RFA group at 1.1±0.6 g/dL than in the HR group at 0.9 ± 0.4 g/dL (P = 0.004). There were more patients who had portal hypertension in the RFA group (P < 0.001). The mean follow-ups between the two groups were 28.2 and 28.3 months for the HR group and the RFA group, respectively, and there was no difference between the two groups (P = 0.964). The mean lengths of hospital-stay were 14.5 days in the HR group and 6.0 days in the RFA group, demonstrating longer stay in the HR group (P < 0.001).
Complications
The percentages of incidence of complications was 17.8% in the HR group and 10.5% in the RFA group, higher in the HR group, but there was no statistical difference (P = 0.206) (Table 2). Three patients (2.3%) died of hepatic failure and ensuing disseminated intravascular coagulation after surgical operation (1 right hepatectomy case, 1 segmentectomy case, and 1 wedge resection case).
Table 2.
Complications and mortality of HR and RFA
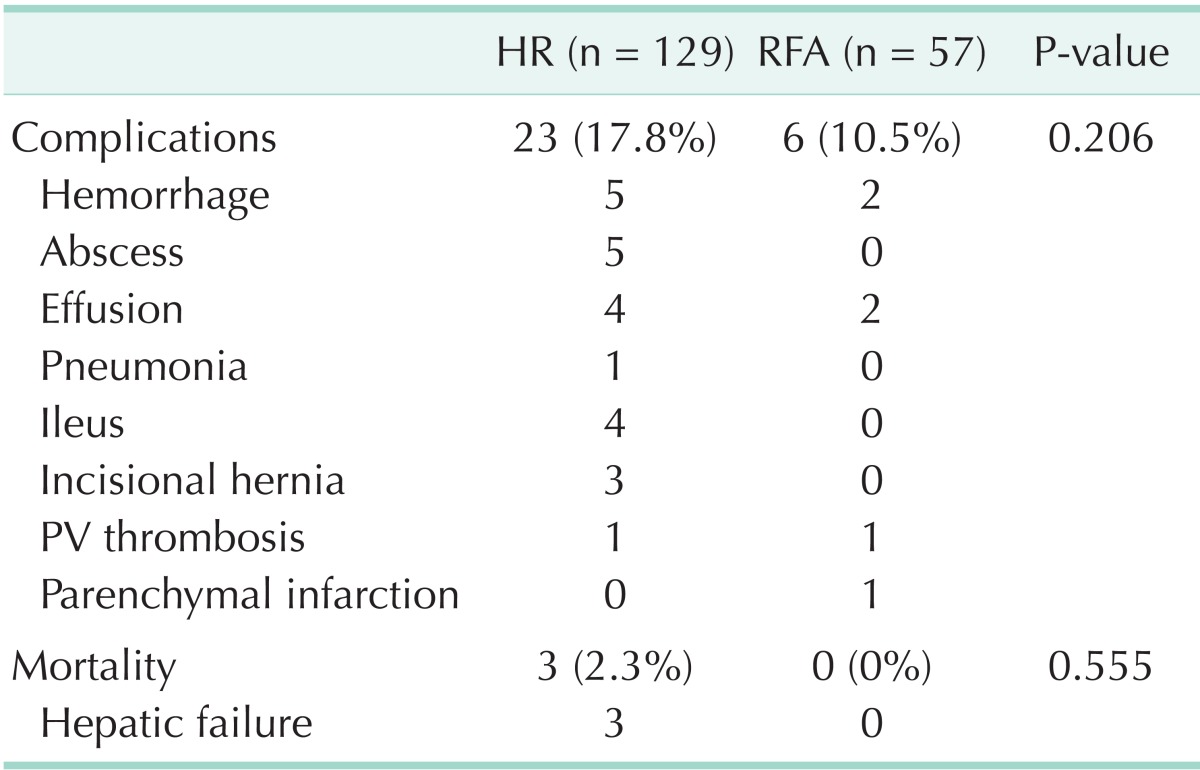
HR, hepatic resection; RFA, radiofrequency ablation; PV, portal vein.
Recurrence (Table 3)
Table 3.
Recurrence of HCC after HR and RFA
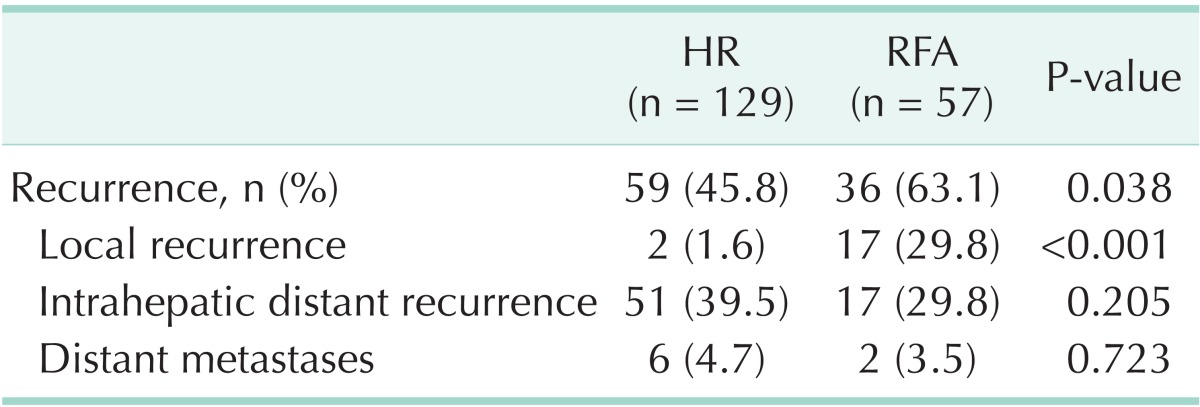
HCC, hepatocellular carcinoma; HR, hepatic resection; RFA, radiofrequency ablation.
Fifty-nine of 129 patients (45.8%) treated with HR reported recurrence, and remote recurrence was the most frequent type of recurrence at 11 cases (39.5%). Thiry-six of 57 patients (63.1%) treated with RFA reported recurrence and showed higher recurrence rates than the HR group (P = 0.038). Local recurrence was found in 17 patients of the RFA group (29.8%) and 2 patients of the HR group (1.6%), significantly higher in the RFA group (P < 0.001). Of the local recurrence in RFA group, 10 of 17 recurrence cases (58.8%) reported recurrence within 1 year and 7 (41.2%) within 2 years, and 2 local recurrence cases of the HR group were within 1 year.
OS rates and DFS rates (Table 4)
Table 4.
Overall and disease-free survival of HR and RFA
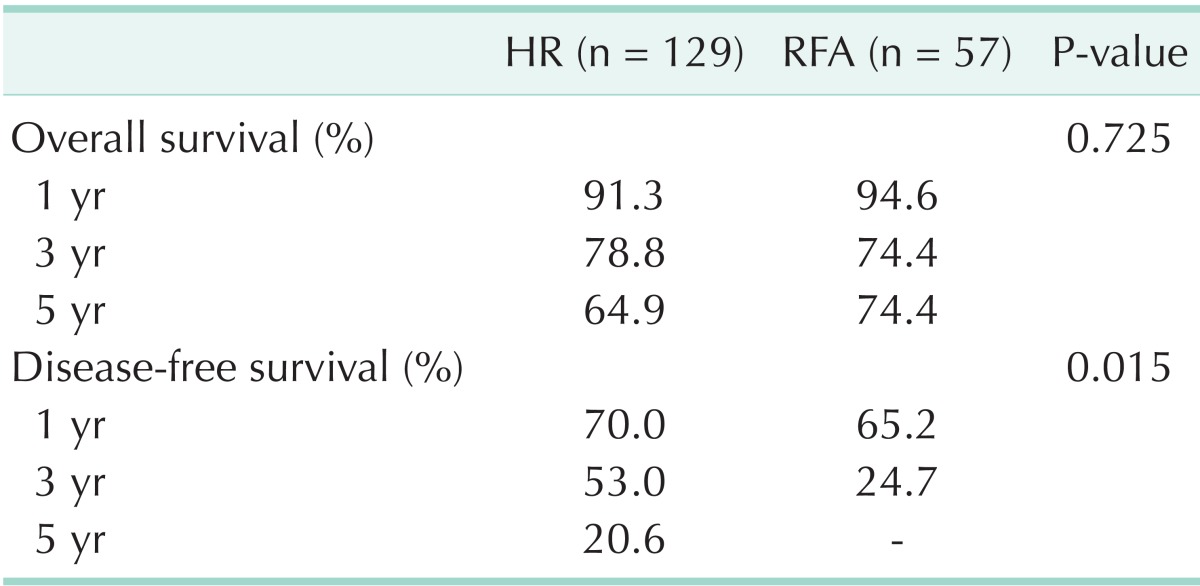
HR, hepatic resection; RFA, radiofrequency ablation.
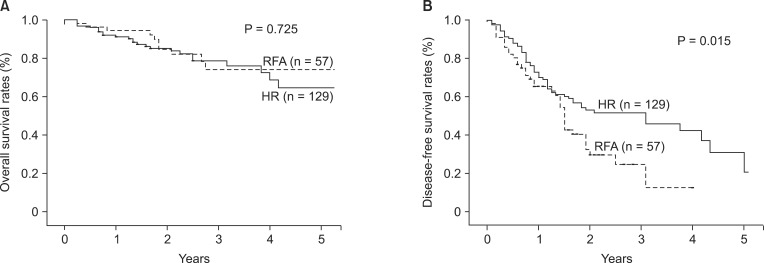
The 1-, 3- and 5-year OS rates in the HR group were 91.3%, 78.8%, and 64.9%, compared to 94.6%, 74.4%, and 74.4% in the RFA group, and the mean OS was 50.7 ± 2.1 and 53.2 ± 3.0 months, respectively, with no difference in the OS between the two groups (P = 0.725) (Fig. 1A).
Fig. 1.
Overall (A) and disease-free survival (B) of all patients in the two treatment groups. (A) The overall survival were not significantly different (P = 0.725), in the two treatment groups. (B) But disease-free survival was significantly higher in hepatic resection (HR) group (P = 0.015). RFA, radiofrequency ablation.
The 1- and 3-year DFS rates in the HR group were 70.0% and 53.0%, compared to 65.2% and 24.7% in the RFA group, respectively. The DFS rates of HR group was significantly higher than RFA group in this study (P = 0.015) (Fig. 1B).
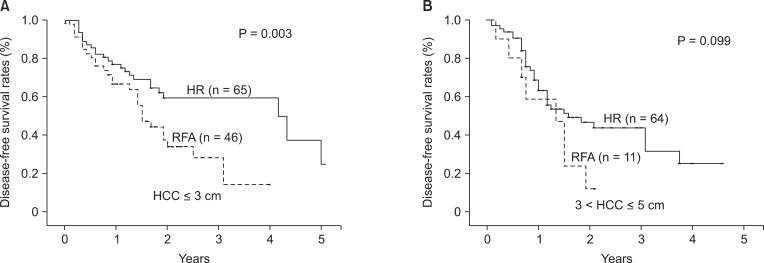
Additionally, DFS was estimated in dividing subgroups such as HCC of 3 or less centimeters (Fig. 2A) and ranging from 3 to 5 centimeters (Fig. 2B). Mean DFS was 38.5 ± 3.4 months for HR group of 3 or less centimeters of HCC and 22.0 ± 2.6 for the RFA group, with significantly longer DFS in the HR group (P = 0.003). DFS in the subgroup ranging from 3 to 5 centimeters showed higher tendency in the HR group over RFA group, but could not reach a significant difference (P = 0.099). OS in the subgroups below and over 3 cm of HCC showed no difference between the two groups (P = 0.838 and P = 0.682, respectively).
Fig. 2.
Disease-free survival of patients with hepatocellular carcinoma (HCC ≤ 3 cm (A) and 3 cm < HCC ≤ 5 cm (B) in the two treatment groups. (A) Disease-free survival was significantly higher in resection group (P = 0.003). (B) Disease-free survival was higher in hepatic resection (HR) group but the difference did not reach statistical significance (P = 0.099). RFA, radiofrequency ablation.
Prognostic factors of HCC
Factors affecting the OS were analyzed based on the entire 186 subjected patients (Table 5). Univariate analysis reported that recurrence (P = 0.021), total bilirubin (P = 0.013), and portal hypertension (P < 0.001) were analyzed as significant prognostic factors, but multivariate analysis showed that recurrence (P = 0.036) and portal hypertension (P = 0.036) were independent prognostic factors affecting the OS.
Table 5.
Analysis of prognostic factors associated with overall survival
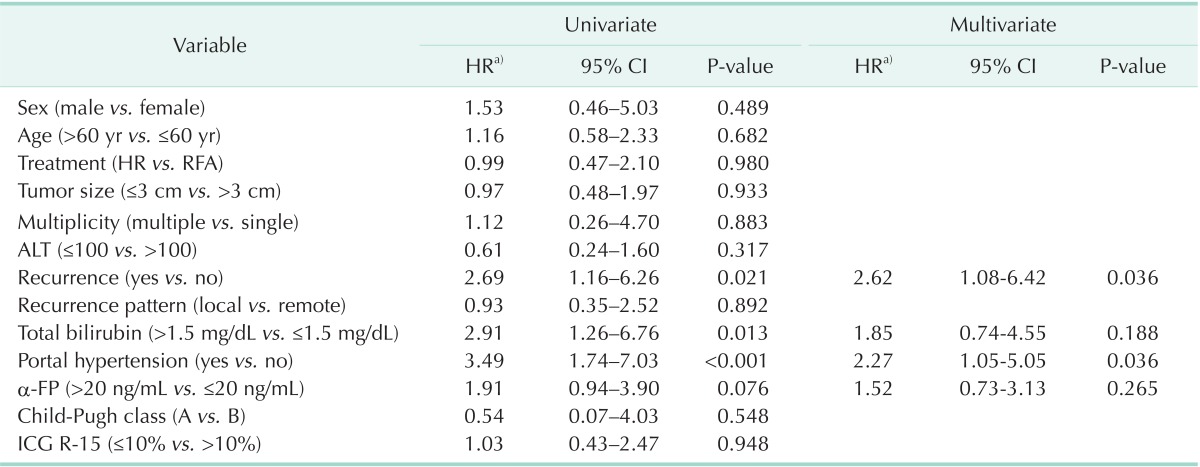
CI, confidence interval; HR, hepatic resection; RFA, radiofrequency ablation; ICG R-15, indocyanine green retention rate at 15 minutes.
a)Hazard ratio.
As a result of univariate analysis of the factors affecting DFS, treatment methods (P = 0.009), number of tumor (P = 0.006), total bilirubin (P = 0.046), portal hypertension (P < 0.001), and α-FP (P < 0.001) were analyzed as the significant prognostic factors, but multivariate analysis reported that portal hypertension (P = 0.048) and α-FP (P = 0.008) were the significant independent prognostic factors of DFS (Table 6).
Table 6.
Analysis of prognostic factors associated with disease-free survival
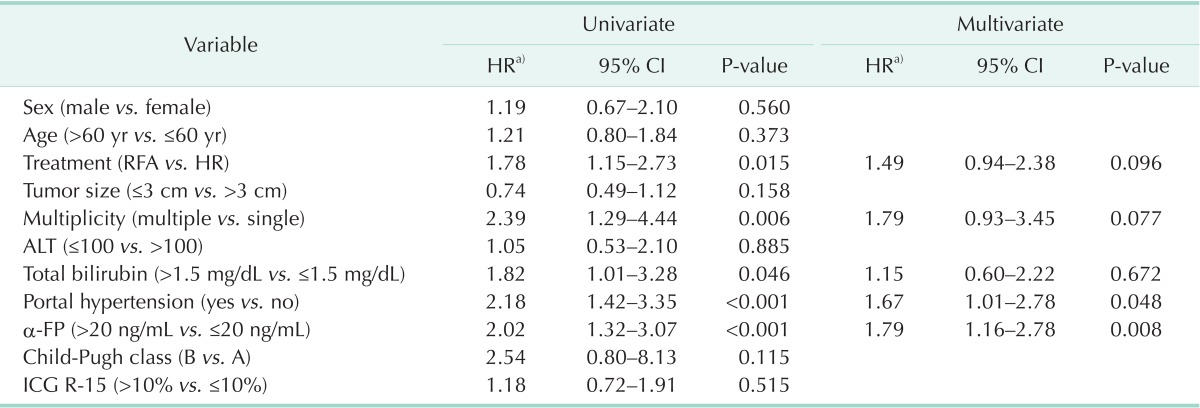
CI, confidence interval; HR, hepatic resection; RFA, radiofrequency ablation; ICG R-15, indocyanine green retention rate at 15 minutes.
a)Hazard ratio.
DISCUSSION
Staging of tumors in most solid cancers is deeply related to prognosis, but the prognosis of HCC is closely related to basal liver state [24,25], as well as HCC stage. In addition, a variety of complementary treatment modalities to HR have been used due to limitations in treatment methods depending on the existence of basal liver diseases including cirrhosis. RFA has been widely known as the most effective therapy for small HCC among current nonsurgical, locoregional treatment methods [14,15,16,17], and in particular, it has been reported that its therapeutic achievement for HCC of 2 or less centimeters in the size of tumor was similar to that of surgical HR [19]. But there are only a few studies that compared treatment effects between HR and RFA and, in particular, only one randomized controlled study was reported. Chen et al. [26] conducted a randomized controlled study with 180 patients diagnosed with HCC with a single nodule of 5 or less centimeters and reported that the treatment efficacies of RFA and HR for 4-year survival was similar in the two groups at 68% and 64%, respectively. But Ueno et al. [27] questioned the objectivity of the study by Chen et al. [26] in that its follow-up period was relatively short at 2 years and that the 4-year survival from RFA and HR was 70% or less.
Our study demonstrated that HR for HCC of 5 or less centimeters showed no significant increase OS compared to RFA, but an extension of DFS was expected (P = 0.015). We should estimate the reason of this discordance between OS and DFS that death from HCC was influenced not only by treatment modality or recurrence of malignancy itself but also by another factor represented as cirrhosis [28].
RFA has some advantages compared to HR such as being less invasive, having a relatively rapid recovery period, and short hospital stay. But it also has shortcomings such as more frequent local recurrence after treatment than HR [29]. This study confirmed three cases of mortality due to postoperative complications of hepatectomy although there was no statistical significance. Furthermore, HR group indicated higher incidences of complications compared to RFA. In addition, HR has weaknesses such as a longer hospital stay and a longer recovery period after operation.
The local recurrence rate of RFA group in this study was higher at 29.8% than HR group at 1.6% (P < 0.001). Mulier et al. [29] argued that the diameter of HCC was the greatest influential factor on local recurrence, and while HCC of 3 or less centimeters in the diameter showed a local recurrence rate of 3.6% in the first year after RFA, while HCC of 3-5 cm showed 21.7%.
Ng et al. [30] suggested in their analysis of correlation between recurrence type and survival that cases of local recurrence indicated lower OS than that of no recurrence but that it showed higher OS than cases of remote recurrence or metastasis. However, this study showed no difference in the OS between the local recurrence group and the intrahepatic distant recurrent or remote metastasis group (P = 0.892).
Ueno et al. [27] conducted a study with patients who met the Milan criteria to compare an RFA group that was divided into a percutaneous treatment group and a surgical approach group with an HR group and found that the surgically approached RFA group showed much higher DFS (P = 0.001) and OS (P = 0.010) than the percutaneously approached group. There was no statistical significance in comparison to the HR group, but the surgically approached RFA group indicated a higher OS. Our study conducted RFA with nine patients through surgical approaches, but since the sample size was small, statistical analysis was not conducted.
As a result of analyzing the factors affecting OS, recurrence and portal hypertension were confirmed as independent risk factors (Table 5), and portal hypertension and serum α-FP were independent risk factors related to DFS (Table 6). It suggests that the prognosis of HCC is involved with the conservation of liver function as well as the radical treatment itself.
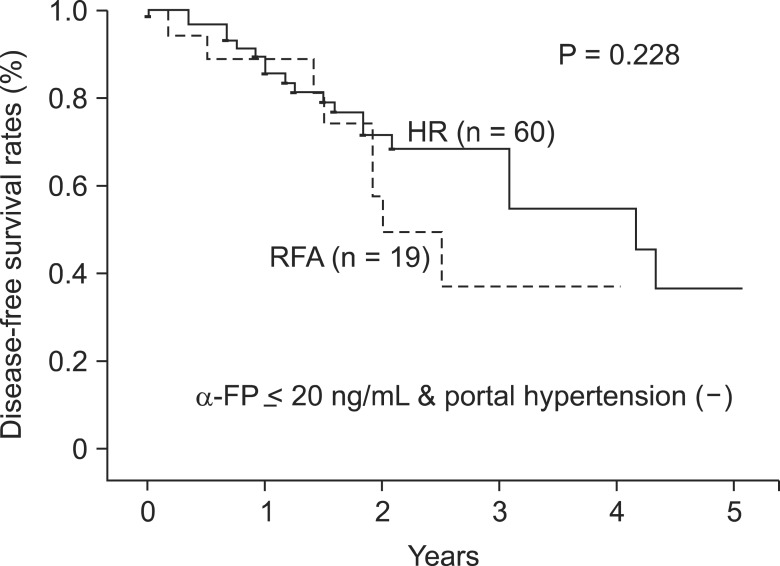
However, portal hypertension and α-FP, which were analyzed as independent prognostic factors affecting DFS in this study, were unfavorably distributed in the RFA group than in the HR group as shown in the Table 1 (P < 0.001 and P = 0.617, respectively). Accordingly, patients with α-FP of 20 ng/mL or less and no portal hypertension were reselected for subgroup analysis, and consequently, the authors could not find any statistical difference in DFS between HR and RFA subgroup (P = 0.228) (Fig. 3).
Fig. 3.
Disease-free survival of patients with α-FP below 20 ng/mL and absence of portal hypertension in the two treatment groups: Disease-free survival was higher in hepatic resection (HR) group but the difference did not reach statistical significance (P = 0.228). RFA, radiofrequency ablation.
Yet, this study has several limitations; First, this study was performed retrospectively and under a nonrandomized design. Second, treatment groups were not well matched in terms of well admitted prognostic factors of HCC such as α-FP level, Child-pugh class, bilirubin level and size of HCC, being biased unfavorably for RFA group with exception of size of tumor. Third, diagnosis of HCC and cirrhosis of patients in RFA group were not based on histopahologic evidence but on radiologic and clinical findings.
In conclusion, when hepatectomy was conducted to treat small HCC, a more significant extension of DFS could be expected than RFA. Thus, HCC with three or fewer nodules 3 cm or less in diameter or a single nodule of 5 cm or less should be performed hepatectomy as the primary treatment if the patient's liver function and general conditions are good enough to undergo surgical operation. Despite a higher recurrence rate, RFA was revealed to have similar OS as HR for the treatment of HCC within the Milan criteria. And in terms of less invasiveness, RFA has advantages over HR. So, RFA can be an alternative therapy for patients who are eligible for surgical resection.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health & Welfare. Annual report of cancer incidence (2005) and survival (1993-2005) in Korea. Seoul: Ministry of Health & Welfare; 2008. [Google Scholar]
- 3.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. European Association for the Study of the Liver. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 5.Livraghi T. Guidelines for treatment of liver cancer. Eur J Ultrasound. 2001;13:167–176. doi: 10.1016/s0929-8266(01)00129-x. [DOI] [PubMed] [Google Scholar]
- 6.Marin-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13–27. doi: 10.1016/s1040-8428(02)00213-5. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 8.Margarit C, Escartin A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242–1251. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 9.Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479–1486. doi: 10.1002/1097-0142(20010415)91:8<1479::aid-cncr1155>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 11.Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma: an audit of 343 patients. Ann Surg. 1995;221:291–298. doi: 10.1097/00000658-199503000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67–76. doi: 10.1007/s10534-002-0714-y. [DOI] [PubMed] [Google Scholar]
- 15.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda M, Okada S, Ueno H, Okusaka T, Kuriyama H. Radiofrequency ablation and percutaneous ethanol injection in patients with small hepatocellular carcinoma: a comparative study. Jpn J Clin Oncol. 2001;31:322–326. doi: 10.1093/jjco/hye063. [DOI] [PubMed] [Google Scholar]
- 17.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 18.Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247–252. doi: 10.1097/01.mcg.0000152746.72149.31. [DOI] [PubMed] [Google Scholar]
- 19.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 20.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients: mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260–271. doi: 10.1148/radiol.2321030821. [DOI] [PubMed] [Google Scholar]
- 24.Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 26.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, et al. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16:359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 28.Ueno S, Tanabe G, Nuruki K, Oketani M, Komorizono Y, Hokotate H, et al. Prognosis of hepatocellular carcinoma associated with Child class B and C cirrhosis in relation to treatment: a multivariate analysis of 411 patients at a single center. J Hepatobiliary Pancreat Surg. 2002;9:469–477. doi: 10.1007/s005340200058. [DOI] [PubMed] [Google Scholar]
- 29.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng KK, Poon RT, Lo CM, Yuen J, Tso WK, Fan ST. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008;12:183–191. doi: 10.1007/s11605-007-0276-y. [DOI] [PubMed] [Google Scholar]