Abstract
Background
Patients with inflammatory bowel disease (IBD) are at increase risk for bone loss and fractures. Therefore, in the present study, we examined the effect of experimental IBD on bone health.
Methods
We used a murine model of colitis, H. hepaticus-infected IL-10 deficient animals. Molecular and histological properties of bone and intestine were examined to identify the immunopathological consequences of colitis in male and female mice.
Results
At 6 weeks post-infection we observed significant trabecular bone loss in male but surprisingly not in female mice. This was true for both distal femur and vertebral locations. In addition, H. hepaticus infection suppressed osteoblast markers only in males. Consistent with effects on bone health, male mice with H. hepaticus infection had more severe colitis as determined by histology and elevated levels of inflammatory cytokines in the colon. While H. hepaticus levels in the stool appeared similar in male and female mice 1-week after infection, by 6-weeks H. hepaticus levels were greater in male mice, indicating that H. hepaticus survival and virulence within the GI tract could be gender-dependent.
Conclusion
In summary, H. hepaticus induced colitis severity and associated bone loss is gender regulated, possibly as a result of gender-specific effects on H. hepaticus colonization in the mouse GI tract and the consequent immunopathologic responses.
Keywords: bone, osteoblast, colitis, gender, intestine, inflammation
INTRODUCTION
The inflammatory bowel diseases (IBD) Crohn’s disease and ulcerative colitis are the most common chronic gastrointestinal illness in children and adolescence. Approximately 1.4 million Americans have IBD, and more than 30,000 new cases are diagnosed in the United States annually. IBD is a key risk factor for bone loss and fractures in children1–7 as well as adults8–13. Multiple factors are thought to contribute to IBD effects on bone including nutritional status3,14–19, reduced physical activity/muscle1,20, glucocorticoid use1 and inflammatory cytokines21,22.
The skeleton is a highly dynamic tissue that is regulated by local, systemic, and environmental cues that modify osteoblast (bone formation) and/or osteoclast (bone resorption) activities and IBD impacts bone regulation at all levels: environmentally through intestinal barrier breaks and/or altered gut microbial composition, systemically through circulation of gut immune cells and cytokines throughout the body, and locally by causing inflammation of extra-intestinal organs (such as the bone marrow). Chronic inflammatory diseases are in general associated with increased risk for osteoporosis23. Treatment of these diseases with steroids can further contribute to bone loss and fracture risk in IBD8,24,25, however low bone density is evident in males recently diagnosed with IBD prior to steroid use26 and young adults with IBD exhibit little or no correlation between steroid use and bone density status. Many studies demonstrate that IBD is associated with increased production of pro-inflammatory factors such as TNFα, IL-1β, IL-17 and IL-6. Treatment of colitis patients with infliximab, a TNFα inactivating antibody, can be successful in treating colitis and improves BMD (spine and femur) in Crohn’s patients 21,27–29. Most studies do not address changes in bone architecture, lineage selection, gene expression or local cytokine expression.
Multiple mouse strains with targeted immune alterations such IL-2−/−30 and IL-10−/−31 develop colitis and thus are used as models of IBD. Intestinal luminal microbes are needed for the development of colitis in these genetic models30,32,33. Enterohepatic Helicobacter species (EHS) exist in the intestinal tract and livers of humans, other mammals and birds 34–36. It was subsequently found that H. hepaticus infection of mice with altered immune function could trigger inflammatory bowel disease. Re-derivation of these strains to Helicobacter-free status will reduced or eliminate the development of colitis while challenge with H. hepaticus trigger the development of colitis37–40. Two days after infection H. hepaticus is a minor component of the microbiota but after 14 days it becomes the dominant member of the microbial community41. H. hepaticus infection induces Th1 and Th17 immune responses marked by elevated interferon gamma (IFNγ) and IL-17 levels, respectively42.
IBD affects both males and females1 although the magnitude of bone loss is suggested to differ by gender43. Specifically, a cross sectional study of 51 patients with IBD found that gender (being male) was negatively associated with spine and femur T-scores in ulcerative colitis patients43, suggesting that the extent of disease-induced bone loss was related to gender. In the present study, we examined the effect of H. hepaticus induced colitis in IL-10−/− mice on gastrointestinal and bone health to determine if there is a gender difference in bone loss and disease severity. Our findings indicate that male mice display greater bone loss and disease severity compared to females infected with H. hepaticus and suggest different pathologic and inflammatory responses to colitis in this model.
MATERIALS AND METHODS
Mice
To examine the effect of experimental IBD on bone health, 14-week-old IL-10−/− male and female littermate mice were infected with H. hepaticus and examined 6 weeks later. Age-matched non-infected littermates (controls) were sham-infected with sterile tryptic soy broth. To examine the influence of estrogen deficiency, IL-10−/− mice were ovariectomizd at 12 weeks of age and gavaged with Helicobacter hepaticus at 14 weeks and examined 6 weeks later. Adult mice, at the plateau of their growth rate, were used to reduce the contribution of confounding effects on bone growth; also, at this age bone length has stabilized allowing comparative analyses among animals. Breeding pairs of Helicobacter-free C57BL/6 IL-10−/− were housed in a specific pathogen free environment and given autoclaved food, bedding, and water. Cage changes were performed in a laminar flow hood. Experimental mice were transferred to the University Research Containment Facility at Michigan State University at 12 weeks and housed under the same conditions as the breeding pairs. Animals were housed up to 4 animals per cage in SPF conditions and were negative (by PCR analysis) for Helicobacter prior to the experiment (data not shown). Mice were maintained on a 12:12-h light-dark cycle at 23°C and had food and water ad libitum. The Michigan State University Institutional Animal Care and Use Committee approved all animal procedures.
Bacteria
Helicobacter hepaticus was obtained from American Type Culture Collection (ATCC 51449; Manassas, VA). H. hepaticus was maintained on tryptic soy agar (TSA) supplemented with 5% sheep blood (HemoStat Laboratory, Dixon, CA). H. hepaticus cultures were maintained at 37C in a microaerobic environment generated in vented GasPak jars without catalyst that were evacuated to −20mmHg and equilibrated with a gas mixture consisting of 80% N2, 10% CO2, and 10% H2.
Murine infection with H. hepaticus
Cultured H. hepaticus was harvested from agar plates and resuspended in a small volume of sterile tryptic soy broth. The inoculum optical density (OD, at 600 nm wave length) was measured and diluted with sterile tryptic soy broth to an OD of 1.0–2.0. Male and female littermate mice at 14 weeks of age mice were inoculated with a single dose of bacterial suspension in a volume of 0.3 ml. Bacteria were introduced directly to the stomach with a 24-guage ball-tipped gavage needle. Control mice were inoculated with sterile tryptic soy broth.
Detection of H. hepaticus in mouse feces
Colonization was confirmed 1 week after infection. DNA was isolated from fecal pellets using DNeasy Kit (Qiagen, Valencia, CA). Single-stage PCR amplification was performed with primers (B38) 5’ GCA TTT GAA ACT GTT ACT CTG 3’, and (B39) 5’ CTG TTT TCA AGC TCC CC 3’, which produce a 417 bp amplicon used to detect H. hepaticus44. PCR analysis was performed using 5 µl DNA, 20 pmol of each primer and iQ SYBR Green Supermix (BioRad, Hercules, CA). Cycling condition were 30 cycles of 30 seconds at 95°C, 45 seconds at 54°C, and 45 seconds at 72°C. PCR products were visualized by agarose gel electrophoresis.
Cecum histology and scoring
To monitor and confirm colitis induction, a scoring system was developed based on previous reports 45–47 utilizing live mouse measures and cecum histology. Disease status in live mice evaluated fecal pellet consistency (0–10, ranging from normal to loose to bloody diarrhea), appearance of rectal bleeding, and weight loss. For histomorphometric evaluation, paraffin embedded cecum sections were stained with hematoxylin-eosin and imaged at 4X, 10X and 25X magnification. At high magnification, three areas were imaged, coded and analyzed/scored blinded to the condition and gender of the mouse. Active disease was evaluated by measures of crypt distortion, numbers of lymphoid aggregates, numbers of lymphoid cells and neutrophils, and mucosal inflammation/ulceration severity.
Crypt distortion measurements were obtained at the ileocecocolic junction and were scored as follows: 0, straight crypts; 2, crypts with slight distortion; 4, distorted crypts with mild hyperchromasia with fewer goblet cells; 6, larger areas of pronounced crypt distortion, more pronounced hyperchromasia with very few goblet cells; 8, crypt drop-out, marked hyperchromasia and no goblet cells. In each section the number of lymphoid aggregates were counted and the abundance of lymphoid cells was scored as follows: 0, 0–10 lymphoid cells counted; 2, 11–30 cells; 4, 31–100; and 6, more than 100 lymphoid cells, respectively and counted within the submucosal layer of the cecum. Neutrophils were scored by 0, no neutrophils present; 5, few neutrophils; and 10, numerous neutrophils in section. Disease severity was examined using the entire cecum region and was scored as follows: 0, normal; 2, mild, small focal areas (1–5 crypts) of disease/inflammation limited to the lamina propria; 4, moderate, multifocal with areas of disease/inflammation extended into the submucosa; 6, severe with ulcers covering large areas of mucosa less than 20 crypts wide; 8, very severe with ulcers covering large areas of mucosa more than 20 crypts; and 10, extremely severe with ulcers covering large areas of mucosa more than 20 crypts and signs of bacteria or fungal invasion observed. Total overall disease score was obtained by adding the live mouse scores with the values obtained from the histological analyses. To further confirm colitis, RNA was extracted from the proximal colon to assess cytokine expression levels as previously described48 and noted below. Similarly, tissue was processed for cytokine protein levels.
Helicobacter-infection was detected histologically on silver-stained sections stained by the Steiner method. Sections were scored as number of positive crypts per visual field (>100 crypts counted per mouse cecum sample), 4 photographs per cecal section. The percentage of infected crypts was a scoring value. The number of visualized Helicobacter organisms per crypt were also counted and used as a score and this ranged from 1 to 5. In the cases of heavily infected male cecum crypts, the score of 10 was given to each crypt that had too numerous bacteria to count. The total Steiner stain score was the percentage of infected crypts added to the score of Helicobacter organisms.
Elisa assay
To determine the tissue cytokine levels colons were snap-frozen in liquid nitrogen and homogenized in PBS. TNF-α, IFN-γ, and IL-17A levels in homogenates were analyzed using ELISA kits from eBioscience, Inc. Cytokine levels were normalized to the total protein and expressed as pg/mg of total protein as described before49.
RNA analysis
Immediately following euthanasia tibias were cleaned of muscle and connective tissue, snap frozen in liquid nitrogen and stored at −80°C. Frozen tibias were crushed under liquid nitrogen conditions with a Bessman Tissue Pulverizer (Spectrum Laboratories, Rancho Dominguez, CA). RNA was isolated using TriReagent (Molecular Research Center, Cincinnati, OH), and integrity assessed by formaldehyde-agarose gel electrophoresis. cDNA was synthesized by reverse transcription using Superscript II Reverse Transcriptase Kit and oligo dT(12–18) primers (Invitrogen, Carlsbad, CA) and amplified by real-time PCR with iQ SYBR Green Supermix (BioRad, Hercules, CA), and gene specific primers were synthesized by Integrated DNA Technologies (Coralville, IA). Hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA levels do not fluctuate with disease were used as an internal control. Amplicon specificity was confirmed by melting curve, size, and sequence analysis. Primers for real-time PCR were designed or previously described: Runx2, OC, AP, Trap5b, HPRT48. ColI (5’-AGAAAGGATCTCCTGGTGCTGAT-3’) and (5’-AAGCCTCTTTCTCCTCTCTGACC-3’) and OPG (5’GAAGAAGATCATCCAAGACATTGAC-3’) and (5’-TCCATAAACTGAGTAGCTTCAGGAG-3’). RankL (5’-TTTGCAGGACTCGACTCTGGAG-3’) and (5’-TCCCTCCTTTCATCAGGTTATGAG-3).
µCT bone imaging
Fixed femurs were scanned using a GE Explore Locus microcomputed tomography (µCT) system at a voxel resolution of 20 µm obtained from 720 views. Beam angle of increment was 0.5, and beam strength was set at 80 peak kV and 450 µA. Each run consisted of control (broth treated) and H. hepaticus treated bones, and a calibration phantom to standardize grayscale values and maintain consistency. On the basis of autothreshold and isosurface analyses of multiple bone samples, a fixed threshold (800) was used to separate bone from bone marrow. Trabecular bone analyses were performed in a region of trabecular bone defined at 0.17 mm (~ 1% of the total length) distal to the growth plate of the proximal femur extending 2 mm toward the diaphysis excluding the outer cortical bone. Trabecular bone mineral content, bone volume fraction, thickness, spacing, and number values were computed by a GE Healthcare MicroView software application for visualization and analysis of volumetric image data. Cortical measurements were performed in a 2 X 2 X 2 mm cube centered midway down the length of the bone using a threshold of 1000 to separate bone from marrow.
Femur histomorphometry and dynamic measures
Femurs were fixed in 10% formalin and transferred to 70% ethanol after 24 h. Fixed samples were processed on an automated Thermo Electron Excesior tissue processor for dehydration, clearing, and infiltration using a routine overnight processing schedule. Samples were then embedded in Surgipath-embedding paraffin on a Sakura Tissue Tek II-embedding center. Paraffin bocks were sectioned at 5 µm on a Reichert Jung 2030 rotary microtome. Slides were stained for TRAP activity and counterstained with hematoxylin according to manufacturer protocol (387A-1KT, Sigma, St. Louis, MO). Osteoblast and osteoclast surface area was measured and expressed as a percentage of total bone surface in the femur trabecular region ranging from the growth plate to 2 mm distal.
For dynamic histomorphometric measures of bone formation, mice were injected intraperitoneally with 200 µl of 10 mg/ml calcein (Sigma, St. Louis, MO, USA) dissolved in sterile saline at 7 and 2 days prior to harvest. L3-L4 vertebrae were fixed in formalin at time of harvest then transferred to 70% ethanol 48 hours later. Vertebrae were then embedded, sectioned and examined under UV light. Five images were taken and the distance between the calcein lines (bone formation rate, BFR) and their length along the bone surface was measured and used to calculate mineral apposition rate (MAR).
Statistical analysis
All measurements are presented as the mean ± SE. Statistically significant (α = 0.05) effects of H. hepaticus or gender or H. hepaticus X gender (which would indicate gender altering H. hepaticus effects or vice versa) were determined using factorial (two-way) analysis of variance (ANOVA) with SPSS statistical software (Chicago, IL). Student’s t-test (assuming equal variance) using Microsoft Excel (Microsoft, Redmond, WA) was also used to determine significance where noted. For categorical, ordinal data comparisons (such as disease severity, crypt distortion or Steiner staining scores), two-tailed Mann-Whitney U-tests were used (http://vassarstats.net/index.html) to determine statistical significance at p < 0.05.
RESULTS
Male and female littermate IL-10−/− mice were infected with H. hepaticus at 14-weeks of age and harvested at 20 weeks of age to assess the impact of this model on bone density and architecture at an age where bone remodeling predominates over growth (REF?). Both femur and vertebrae were examined since these sites can differ in responses to treatments/disease. As expected, femurs from males had more trabecular bone volume fraction and mineral content and density, trabecular number, trabecular thickness and cortical bone area than female femurs (Figure 1; Table 1). Similar to other models of colitis48, H. hepaticus infection resulted in a significant decrease in male trabecular bone parameters such as bone volume fraction (BV/TV −37%), but only slightly decreased most cortical parameters (not reaching statistical significance). The outer cortical perimeter, however, did decrease significantly after infection. Surprisingly, compared to the males, none of the female bone parameters (trabecular or cortical) were affected by H. hepaticus infection (Figure 1; Table 1). Normalization of femur bone volume fraction values by individual mouse weights, to correct for differences in body weight, still showed similar findings (Figure 1). Two-way ANOVA analysis identified gender and H. hepaticus as having a significant (p<0.001) effect on femur bone volume fraction and identified a significant effect caused the interaction of gender X H. hepaticus (p<0.001).
Figure 1. Helicobacter hepaticus infection causes loss of distal femur trabecular bone volume.
A. Representative microcomputed tomography isosurface images of distal femur trabecular bone isolated from control and treated mice. B. Femur trabecular bone volume fraction (corrected for weight loss). n≥16. Data values are averages ± SE. **p<0.001, ***p<0.0001.
Table 1. Femoral uCT parameters.
Analysis of femoral trabecular and cortical bone parameters by microcomputed tomography.
| Female | Male | ||||
|---|---|---|---|---|---|
| Control | H. hep | Control | H. hep | % Diff | |
|
Trabecular |
|||||
| BVF% | 10.8 ± 0.1 | 10.8 ± 0.1 | 34.2 ± 2.2 * | 21.6 ± 1.5 *# | 37↓ |
| BMC (mg) | 0.36 ± 0.01 | 0.36 ± 0.02 | 0.66 ± 0.03 * | 0.49 ± 0.02 *# | 26↓ |
| BMD (mg/cc) | 119 ± 5 | 120 ± 5 | 217 ± 9 * | 160 ± 7 *# | 26↓ |
| Tb.N (1/mm) | 2.64 ± 0.16 | 2.66 ± 0.14 | 6.48 ± 0.17 * | 4.91 ± 0.25 *# | 24↓ |
| Tb.Th (µm) | 4.11 ± 0.14 | 4.00 ± 0.13 | 5.22 ± 0.25 * | 4.34 ± 0.15 *# | 17↓ |
| Tb.Sp (µm) | 35.4 ± 2.1 | 35.5 ± 2.1 | 10.4 ± 0.6 * | 17.2 ± 1.3 *# | 65↑ |
|
Cortical |
|||||
| Tt.Ar (mm2) | 1.97 ± 0.02 | 1.96 ± 0.03 | 2.46 ± 0.04 * | 2.33 ± 0.04 * | |
| Ct.Ar (mm2) | 1.12 ± 0.02 | 1.15 ± 0.03 | 1.26 ± 0.04 * | 1.23 ± 0.03 * | |
| Ma.Ar (mm2) | 0.86 ± 0.02 | 0.81 ± 0.02 | 1.20 ± 0.06 * | 1.10 ± 0.03 * | |
| Ct.Ar/Tt.Ar | 56.6± 0.7 | 59.0 ± 1.0 | 52.0 ± 2.0 * | 52.7 ± 0.7 * | |
| Ct.Th (mm) | 27.2 ± 0.6 | 28.4 ± 0.8 | 27.2 ± 0.8 | 27.2 ± 0.5 | |
| Inner P (mm) | 3.48 ± 0.04 | 3.38 ± 0.04 | 4.20 ± 0.10 * | 4.01 ± 0.05 * | |
| Outer P (mm) | 5.15 ± 0.02 | 5.13 ± 0.04 | 5.90 ± 0.06 * | 5.67 ± 0.01 *# | 4↓ |
The distal femoral trabecular bone and diaphysis were examined. BV/TV, bone volume fraction; BMC, bone mineral content; BMD, bone mineral density; Tb.N., trabecular number; Tb. Th., trabecular thickness; Tb. Sp., trabecular spacing; Tt. Ar., total area; Ct. Ar., cortical area; Ma. Ar., marrow area; Ct. Ar./Tt. Ar., cortical area fraction; Ct. Th., cortical thickness; Inner P, inner perimeter; Outer P, outer perimeter. Values are averages SE;
p<0.05 compared to female controls;
p<0.05 compared to corresponding gender control.
Examination of vertebrae trabecular structure did not identify a difference between control males and control females (Figure 2; Table 2). While vertebrae can display unique responses to treatments/disease, H. hepaticus infection caused somewhat similar changes/trends in vertebrae to that seen in femurs. Specifically, H. hepaticus significantly reduced vertebral trabecular bone volume only in male mice (-46%) but not in female mice (Figure 2). Two-way ANOVA analysis identified H. hepaticus as having a major effect on vertebral bone volume (p<0.05) and that the interaction of H. hepaticus and gender displayed a trend toward affecting vertebral bone volume (p=0.06). Other vertebral trabecular parameters were also altered in male but not female mice (Table 2).
Figure 2. Helicobacter hepaticus infection causes vertebral trabecular loss in male, but not female mice.
A. Representative microcomputed tomography isosurface images of the L3 vertebrae isolated from control and treated mice. B. Bone volume fraction (corrected for weight loss). n=5. Data values are averages ± SE. ^p<0.05, *p<0.01.
Table 2. Vertebral uCT and histomorphometry measures.
Analysis of vertebral trabecular bone histomorphometry and structural parameters.
| Female | Male | ||||
|---|---|---|---|---|---|
| Control | H. hep | Control | H. hep | % Diff | |
|
Trabecular |
|||||
| BVF % | 28.2 ± 2.5 | 25.6 ± 2.7 | 31.8 ± 3.9 | 17.0 ± 3.0 *# | 46 ↓ |
| BMC (mg) | 0.52 ± 0.02 | 0.52 ± 0.04 | 0.53 ± 0.02 | 0.44 ± 0.02# | 17 ↓ |
| BMD (mg/cc) | 260 ± 16 | 251 ± 14 | 227 ± 10 | 197 ± 10 * | |
| Tb.N (1/mm) | 6.04 ± 0.30 | 5.65 ± 0.37 | 7.16 ± 0.42 | 5.18 ± 052# | 28 ↓ |
| Tb.Th (µm) | 46.3 ± 2.2 | 45.0 ± 2.4 | 43.9 ± 3.1 | 32.0 ± 2.5 *# | 27 ↓ |
| Tb.Sp (µm) | 121 ± 12 | 135 ± 14 | 97 ± 11 | 166 ± 20# | 71 ↑ |
|
Histomorphometry |
|||||
| Osteoblast % | 12.2 ± 0.6 | 11.2 ± 0.8 | 8.7 ± 0.8 * | 7.0 ± 0.9 * | |
| Osteoclast % | 5.6 ± 1.0 | 8.7 ± 1.0 | 3.4 ± 0.4 | 5.2 ± 1.2 | |
| MAR (µm/day) | 1.41 ± 0.12 | 1.13 ± 0.07 | 1.19 ± 0.06 | 1.34 ± 16 | |
| BFR (µm3/µm2/day) | 0.19 ± 0.05 | 0.20 ± 0.04 | 0.14 ± 0.01 | 0.15 ± 0.04 | |
The L3 vertebrae were examined. BV/TV, bone volume fraction; BMC, bone mineral content; BMD, bone mineral density; Tb.N., trabecular number; Tb. Th., trabecular thickness; Tb. Sp., trabecular spacing; Tt. Ar., total area; Ct. Ar., cortical area; Ma. Ar., marrow area; Ct. Ar./Tt. Ar., cortical area fraction; Ct. Th., cortical thickness; Inner P, inner perimeter; Outer P, outer perimeter. Values are averages SE;
p<0.05 compared to female controls;
p<0.05 compared to corresponding gender control.
Tibial RNA was used to determine if markers of osteoblast (bone anabolic cells) or osteoclast (bone catabolic cells) differentiation were affected by H. hepaticus. HPRT, which is not modulated by colitis or inflammation, was used as a housekeeping gene. Osteoblast maturation is characterized by their ability to secrete type 1 collagen-rich extracellular matrix during early stages of maturation and by markers of osteoblast lineage/differentiation such as expression of alkaline phosphatase and osterix. All three of these genes were reduced in the bones of male mice treated with H. hepaticus (Figure 3), but not in the bones of female mice treated with H. hepaticus, consistent with the gender-dependent bone loss after infection. Osteoclast activity markers, TRAP5b, and osteoclast regulators RANKL (activator) and OPG (inhibitor) were also examined. TRAP5b levels did not differ among conditions (Figure 3), consistent with TRAP5b serum levels (not shown). RANKL levels displayed a trend toward decreasing in male treated mice (p=0.08) and OPG levels were significantly decreased in male treated mice. To further assess the impact of H. hepaticus on osteoblasts and osteoclasts, cell surface area in trabecular bone regions were measured by histomorphometry. H. hepaticus infection did not cause any significant changes in osteoblast or osteoclast surface area or mineral apposition rate. We did notice that H. hepaticus treated female mice demonstrated a trend toward increased osteoclast number and decreased MAR (Table 2); the consequence of this is not clear as the female mice did not lose bone.
Figure 3. Osteoblast and osteoclast markers are decreased in male treated mice.
RNA was extracted from frozen tibia and made into cDNA by reverse transcription. Primers specific to ColI (collagen I), AP (alkaline phosphatase), Osx (osterix), Trap5b (tartrate resistant alkaline phosphatase), RANKL, and OPG (osteoprotegrin) were used. Values are expressed relative to HPRT, which is a housekeeping control gene. Data values are averages ± SE. n≥16. * p≤0.05 determined by Student’s t-test.
With the supposition that colitis severity could differ between genders and in this way contribute to gender differences in bone pathology, general body and intestinal parameters were examined. As expected, H. hepaticus infected male mice lost a significant amount of body weight (-5%; Figure 4). In contrast, infected female mice did not lose weight. Correspondingly, infected male mice lost tibialis muscle mass compared to control males (-8%; Figure 4) while female mice did not lose muscle mass. Two-way ANOVA analysis indicated that gender and H. hepaticus infection had a significant effect on body and muscle mass and that a gender X H. hepaticus effect was significant for muscle mass and nearly significant for body mass. Subcutaneous fat pad weight did not significantly change in either male or female mice (not shown). As expected, muscle and body mass was strongly impacted (p<0.001) by gender, with control male mice having more compared to control female mice.
Figure 4. H. hepaticus infection causes significant body weight and muscle mass loss in male but not female mice.
Body weight, and tibialis muscle weight taken at 20 weeks of age. Mice treated with H. hepaticus for 6 weeks or control broth. n≥16 per group. Data values are averages ± SE. *p<0.01, **p<0.001, ***p<0.0001.
Next we examined colitis severity at 6 weeks using cecum histology and scoring (Figure 5) where the total disease score was based on the additive total of crypt/mucosal structure, lymphoid aggregate number, lymphoid cell number, neutrophil presence, ulcer severity scores and assessment of live animals (see Methods for scoring specifics). While control male and female mice had similar scores (7.0 ± 2 versus 7.2 ± 2, respectively), H. hepaticus treated mice had elevated disease severity scores (Figure 5). Most interestingly, the total disease score for H. hepaticus treated male mice was nearly two fold greater than treated female mice (38.9 ± 6.00 versus 21.6 ± 4.49, respectively). Two-way ANOVA indicated a strong gender, H. hepaticus, and gender X H. hepaticus effect on the level of disease severity (all displayed a p < 0.05). Increased disease severity in male versus female mice was evident for all individual parameters including mucosal structure (6.0 ± 0.6 versus 3.6 ± 0.3, respectively) and lymphoid aggregates (4.5 ± 0.9 versus 2.4 ± 0.6, respectively).
Figure 5. Histological examination of H. hepaticus treated mice.
The ileocecocolic junction of control and H. hepaticus treated mice at 20 weeks were examined by histology and scored according to protocol (see methods). A. Representative photographs of histological images of the ileocecocolic junction of control and Helicobacter hepaticus treated mice. B. Total disease score. Data values are averages ± SE. n=10 mice per group. *p<0.01, and **p<0.001 by 2-tailed Mann-Whitney U-test.
To further assess and quantitate disease severity colon cytokine levels were measured. Consistent with the cecum disease score, the colon of male mice treated with H. hepaticus displayed a significant increased cytokine production whereas the infected female mice did not show elevated cytokine production (Figure 6). Specifically levels of TNFα, produced by several immune and non-immune cell types, were increased in male but not female mouse colon (152 ± 51 versus 40.0 ± 10, respectively) and were affected by disease and displayed a trend to be influenced by gender and gender X H hepaticus (p<0.06). Based on previous report that H. hepaticus infection induces a Th1 and Th17 immune response, we also measured interferon gamma (IFNγ) and IL-17 levels, respectively42. Consistent with a Th1 and Th17 response, IFNγ and IL-17A levels were elevated in H. hepaticus treated male but not female mouse colon. Two-way ANOVA determined that IFNγ and IL-17 was regulated by gender, H. hepaticus infection and the combination of both parameters (gender X H. hepaticus).
Figure 6. Male H. hepaticus treated mice have increased colon cytokine protein levels compared to female mice.
Protein levels of TNFa, IL-17a, and IFNg, expressed as pg/mg of tissue, were determined in isolated colon tissue samples. n=5 per group. Data values are average ± SE. ^ p<0.05.
In our study, male and female mice were infected with the same quantity of H. hepaticus, however variations in the amount of H. hepaticus retained and thriving within the GI tract could impact disease severity (higher levels being associated with greater disease severity). Examination of H. hepaticus levels in mouse stool one-week post infection indicated similar levels of the bacteria were present in both male and female mice (Figure 7). However, 6 weeks after infection, H. hepaticus DNA levels were greater in the colons of male compared to female mice, suggesting that H. hepaticus did not thrive over time in the female mice (Figure 7). This was supported by greater Steiner staining of helicobacter bacteria in male versus female colon histology sections. Thus, greater levels of H. hepaticus and disease severity are associated with increase bone loss. We further carried out regression analyses looking at male and female relationships to disease score; results could not be combined/pooled due to large gender differences- male bone and body parameters are significantly greater than female mice. Regression analyses and pearson’s correlation coefficients indicated a significant relationship between disease severity and bone loss (femur BV/TV) (Figure 8) as well as weight, fat pad and muscle loss in male mice but not female mice.
Figure 7. Confirmation of H. hepaticus infection in mice.
A. PCR analysis was used to confirm bacterial infection in mice 1 week after gavage with Helicobacter hepaticus. At 6 weeks post-infection, the presence of H. hepaticus was determined in isolated colon DNA. B. Steiner staining indicates the presence of Helicobacter hepaticus in cecal crypts. *p<0.01by 2-tailed Mann-Whitney U-test. Photographs are representative of cecums isolated from female and male mice infected with Helicobacter hepaticus.
Figure 8. Cecum inflammation of male but not female mice correlates significantly with decreased body weight, tibialis muscle mass decrease, and decreased femur bone volume fraction.
Regression analyses of selected general and bone parameters relative to disease score. Each dot represents an individual mouse. Note that male and female axes differ since general body parameters as well as the extent of inflammation display significant gender differences. Pearson’s correlation coefficient and significance are noted for individual graphs. p<0.05 is considered significant.
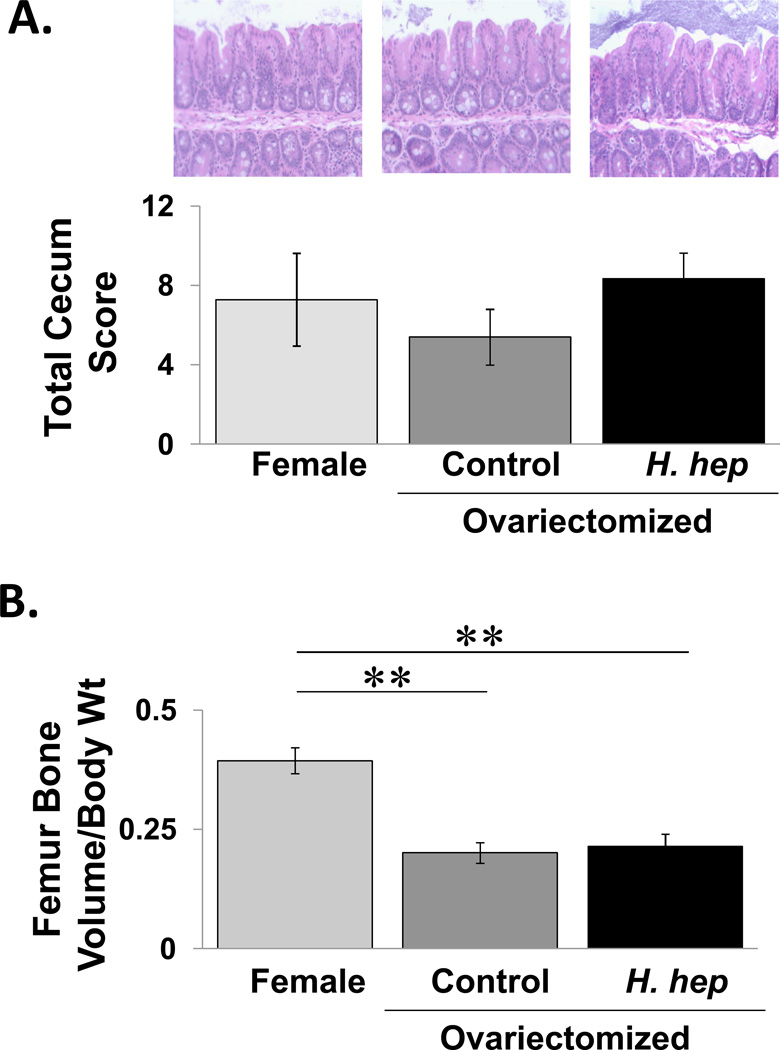
To determine if loss of sex steroids could make female mice more vulnerable to H. hepaticus infection and bone loss, littermate female mice were ovariectomized and given H. hepaticus. Ovariectomy did not cause cecal inflammation in the IL-10 deficient female mice. Interestingly, infection of ovariectomized mice with H. hepaticus did not increase cecum inflammation. Consistent with the literature, analysis of femoral trabecular bone volume (Figure 9) and other parameters (not shown) indicate that ovariectomy induces significant trabecular bone loss, by more than 50%, while infection with H. hepaticus did not cause further bone loss.
Figure 9. Effect of H. hepaticus infection on cecal inflammation and bone volume in ovariectomized mice.
Mice were ovariectomizd at 12 weeks of age and gavaged with Helicobacter hepaticus at 14 weeks. At 20 weeks the mice were examined. Conditions are non-ovariectomized female (light gray bar, n=4), ovariectomized mice (control, medium gray bar, n=5) and H. hepaticus infected ovariectomized mice (dark gray bar, n=9). A) The ileocecocolic junction region was assessed histologically (representative images shown) and scored (blinded protocol) (see methods). Data values are averages ± SE. No statistical significance found using 2-tailed Mann-Whitney U-test. B) Microcomputed tomographical analysis of the distal femur trabecular bone volume fraction. Data values are averages ± SE. **p<0.001, ***p<0.0001 by 2-tailed Student’s t-test.
DISCUSSION
Our findings demonstrate that H. hepaticus infected male mice display greater bone loss and cecal inflammation (disease severity) compared to female mice. In clinical studies, IBD is clearly a risk factor for bone loss and fractures in children1–7 and adults8–13, but the risk factors for this outcome are not completely clear. Our finding that there is a gender component to bone loss is somewhat surprising, since there are few clinical reports describing the contribution of gender to the extent of bone loss in IBD but the findings are variable. One study indicates that for ulcerative colitis patients, but not Crohn’s patients, male gender is negatively correlated with bone density and that male patients have significantly lower lumbar spine and distal femur T-scores than female patients43. However, another study found no significant differences in gender or age between fracture and non-fracture IBD patients, but older age, female sex and osteoporosis are associated with hospitalization for fractures in IBD patients50. The variations in findings suggest a complexity in the relationship between gender and IBD induced bone loss.
Analyses of bone markers (histomorphometry and RNA analyses) in the male mice support a reduction in osteoblast activity. Our findings of suppressed osteoblast activity are supported by studies in our laboratory and others, which indicate a reduction in bone formation in other mouse models of IBD48,51. Suppressed osteoblast activity was also observed in a study conducted in young (8 and 12 week) male IL-10 deficient compared to wild type mice52; the same study found that some of the mice, which were housed under a standard (not pathogen free) environment, developed spontaneous colitis and that this was associated with greater bone loss, consistent with our findings in male IL-10 deficient mice infected with H. hepaticus. Regarding osteoclast activity, we did not observe suppression in osteoclast markers, which would have suggested an overall reduction in bone remodeling as has been reported clinically53. Other animal studies show variable results with regard to osteoclast activity51,52. In addition to differences in mouse age among the studies, we found that osteoclast regulators, both activators and inhibitors, were suppressed in the male mice and may possibly negate any directional effect in our study.
Our studies demonstrate in the H hepaticus infected-IL-10 deficient mouse colitis model that disease severity is greater in adult male compared to female mice, which correlates with the extent of bone loss. Clinically, there is a relationship between IBD severity and/or duration and bone loss and fractures13,43,54, which is not surprising given that IBD can cause weight loss, reduced nutrient intake as well as systemic inflammation. A few clinical studies have directly examined the contribution of gender to colitis severity. Some studies do not demonstrate a relationship between gender and disease severity, but note that age is reciprocally related to disease severity55,56. Others have identified female IBD patients as having a lower remission rate and less immunosuppressive medications than males57. Thus, the contribution of gender to IBD severity is not completely clear and difficult to assess at the mechanistic level clinically, since variations in factors such as diet, genetics, gut microbiota and sex hormone levels can confound interpretations.
Mouse models of colitis allow identification of potential mechanisms regulating disease severity and complications such as bone loss. H. hepaticus induced colitis is one model where factors contributing to disease severity are being identified including signaling factors such as SMAD358, gut permeability and Th1 inflammatory activity59. Our studies utilize H. hepaticus infected IL-10−/− mice the C57BL/6 background. Interestingly, IL-10 deficient mice in this background are less prone to develop H. hepaticus-induced colitis compared to IL-10 deficient Balbc or 129 mice, which develop moderate and severe colitis, respectively42. The mechanisms accounting for the different strain responses to H. hepaticus have yet to be identified but likely involve variations in immune responses. Studies have also shown that certain host genetic loci, such as Cdcs160 and Nod259, can affect H. hepaticus virulence/severity.
Consistent with a Th1 and Th17 immune response H. hepaticus infection male mice displayed increases in gut interferon gamma (IFNγ) and IL-17 levels, respectively42. This was not seen in female mice. Studies indicate that elevated cytokines are associated with disease severity42, thus the lack of cytokine induction in female H. hepaticus infected mice is consistent with the reduced disease severity. Thus male gender increases the severity of H. hepaticus induced colitis in C57BL/6 mice. Livingston et al. also examined the role of gender in H. hepaticus induced colitis in A/JCr mice, but in contrast to our studies, female A/JCr mice displayed greater severity than males.61 Differences in mouse strain (A/JCr versus C57BL/6), mouse age of infection (3 weeks of age versus 14 weeks of age in our study), and length of inoculation (3 months versus 1.5 months) likely contribute to the different results seen in the two studies. This reinforces the role of age and mouse strain on responses to H. hepaticus infection and colitis severity.
How could gender influence disease severity? Our findings indicate that H. hepaticus survival and virulence within the GI tract is gender dependent. Differences in the intestinal environment could contribute to this and include differences in gut immune-surveillance, gut acidity, and even bile acid composition. The latter can affect the growth and proteome of H. hepaticus in a way that could alter virulence, inflammation, host adaptation and colonization location62. Regression analysis indicates that the disease score, an indicator of intestinal inflammation, is negatively correlated with bone volume in male mice. This was not observed in female mice. One possible cause of this gender difference is that a high/threshold disease score may be necessary to initiate bone loss since the majority of infected male mice displayed a disease score well above female mice (an average of 39 for males versus 21 for females).
The lack of visible bone loss in H. hepaticus infected ovariectomized mice suggests that estrogen may not be not required for the protective effects at this age and time point. However, this interpretation should be taken with caution for two main reasons. First, it is possible that the significant bone loss in the ovariectomized mice prevents observation of additional reductions (from an already low bone volume). Second, estrogenic effects (immune, neurological, bile) can be permanent or take time to deplete in adult mice, therefore the ovariectomized mice could still be benefiting from previous estrogen effects. The lack of bone loss in H. hepaticus infected ovariectomized mice correlates with the lack of cecal inflammation, consistent with pro-inflammatory cytokines being required to induce IBD in this model63. Our studies suggest that female mice are protected from gut inflammation or alternatively male mice, with testosterone, are more susceptible to infection. Understanding the role of sex steroids in regulating bone loss caused by H. hepaticus is difficult because interpretations have to made in light of bone loss that already occurs in the absence of estrogen or testosterone. Taken together, the data indicate that long-term infection by H. hepaticus in the GI tract is reduced in female compared to male mice and could contribute to differences in disease severity and ultimately bone loss. The role of sex steroids in this process is complex and a focus of ongoing studies.
ACKNOWLEDGEMENT
The authors thank Jing Zhang for her critical review of the manuscript, and the Investigative Histology Laboratory in the Department of Physiology, Division of Human Pathology at Michigan State University for their assistance with histological analyses. These studies were supported by funding from the Crohn’s and Colitis Foundation of America.
Footnotes
Authors have nothing to disclose.
REFERENCES
- 1.Burnham JM, Shults J, Semeao E, Foster B, Zemel BS, Stallings VA, Leonard MB. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19(12):1961–1968. doi: 10.1359/JBMR.040908. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester FA, Davis PM, Wyzga N, Hyams JS, Lerer T. Are activated T cells regulators of bone metabolism in children with Crohn disease? J Pediatr. 2006;148(4):461–466. doi: 10.1016/j.jpeds.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Semeao EJ, Jawad AF, Zemel BS, Neiswender KM, Piccoli DA, Stallings VA. Bone mineral density in children and young adults with Crohn's disease. Inflamm Bowel Dis. 1999;5(3):161–166. doi: 10.1097/00054725-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Gokhale R, Favus MJ, Karrison T, Sutton MM, Rich B, Kirschner BS. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology. 1998;114(5):902–911. doi: 10.1016/s0016-5085(98)70309-9. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester FA, Wyzga N, Hyams JS, Davis PM, Lerer T, Vance K, Hawker G, Griffiths AM. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(1):42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester FA. IBD and skeletal health: children are not small adults! Inflamm Bowel Dis. 2005;11(11):1020–1023. doi: 10.1097/01.mib.0000188341.96726.15. [DOI] [PubMed] [Google Scholar]
- 7.Semeao EJ, Stallings VA, Peck SN, Piccoli DA. Vertebral compression fractures in pediatric patients with Crohn's disease. Gastroenterology. 1997;112(5):1710–1713. doi: 10.1016/s0016-5085(97)70055-6. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 9.Bartram SA, Peaston RT, Rawlings DJ, Walshaw D, Francis RM, Thompson NP. Mutifactorial analysis of risk factors for reduced bone mineral density in patients with Crohn's disease. World J Gastroenterol. 2006;12(35):5680–5686. doi: 10.3748/wjg.v12.i35.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjarnason I, Macpherson A, Mackintosh C, Buxton-Thomas M, Forgacs I, Moniz C. Reduced bone density in patients with inflammatory bowel disease. Gut. 1997;40(2):228–233. doi: 10.1136/gut.40.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollak RD, Karmeli F, Eliakim R, Ackerman Z, Tabb K, Rachmilewitz D. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol. 1998;93(9):1483–1490. doi: 10.1111/j.1572-0241.1998.468_q.x. [DOI] [PubMed] [Google Scholar]
- 12.van Hogezand RA, Hamdy NA. Skeletal morbidity in inflammatory bowel disease. Scand J Gastroenterol Suppl. 2006;(243):59–64. doi: 10.1080/00365520600664276. [DOI] [PubMed] [Google Scholar]
- 13.van Staa TP, Cooper C, Brusse LS, Leufkens H, Javaid MK, Arden NK. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003;125(6):1591–1597. doi: 10.1053/j.gastro.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Siffledeen JS, Siminoski K, Steinhart H, Greenberg G, Fedorak RN. The frequency of vitamin D deficiency in adults with Crohn's disease. Can J Gastroenterol. 2003;17(8):473–478. doi: 10.1155/2003/391308. [DOI] [PubMed] [Google Scholar]
- 15.Siffledeen JS, Fedorak RN, Siminoski K, Jen H, Vaudan E, Abraham N, Steinhart H, Greenberg G. Randomized trial of etidronate plus calcium and vitamin D for treatment of low bone mineral density in Crohn's disease. Clin Gastroenterol Hepatol. 2005;3(2):122–132. doi: 10.1016/s1542-3565(04)00663-9. [DOI] [PubMed] [Google Scholar]
- 16.Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr. 2002;76(5):1077–1081. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 17.Vaisman N, Dotan I, Halack A, Niv E. Malabsorption is a major contributor to underweight in Crohn's disease patients in remission. Nutrition. 2006;22(9):855–859. doi: 10.1016/j.nut.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118(5):1950–1961. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duggan P, O'Brien M, Kiely M, McCarthy J, Shanahan F, Cashman KD. Vitamin K status in patients with Crohn's disease and relationship to bone turnover. Am J Gastroenterol. 2004;99(11):2178–2185. doi: 10.1111/j.1572-0241.2004.40071.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee N, Radford-Smith G, Taaffe DR. Bone loss in Crohn's disease: exercise as a potential countermeasure. Inflamm Bowel Dis. 2005;11(12):1108–1118. doi: 10.1097/01.mib.0000192325.28168.08. [DOI] [PubMed] [Google Scholar]
- 21.Paganelli M, Albanese C, Borrelli O, Civitelli F, Canitano N, Viola F, Passariello R, Cucchiara S. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006 doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- 22.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armour KE, Van THRJ, Grabowski PS, Reid DM, Ralston SH. Evidence for a pathogenic role of nitric oxide in inflammation-induced osteoporosis. J Bone Miner Res. 1999;14(12):2137–2142. doi: 10.1359/jbmr.1999.14.12.2137. [DOI] [PubMed] [Google Scholar]
- 24.Vestergaard P, Krogh K, Rejnmark L, Laurberg S, Mosekilde L. Fracture risk is increased in Crohn's disease, but not in ulcerative colitis. Gut. 2000;46(2):176–181. doi: 10.1136/gut.46.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compston J. Management of glucocorticoid-induced osteoporosis. Nature reviews. Rheumatology. 2010;6(2):82–88. doi: 10.1038/nrrheum.2009.259. [DOI] [PubMed] [Google Scholar]
- 26.Sakellariou GT, Moschos J, Berberidis C, Mpoumponaris A, Kadis S, Molyvas E, Kouklakis G. Bone density in young males with recently diagnosed inflammatory bowel disease. Joint Bone Spine. 2006;73(6):725–728. doi: 10.1016/j.jbspin.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein M, Irwin S, Greenberg GR. Maintenance infliximab treatment is associated with improved bone mineral density in Crohn's disease. Am J Gastroenterol. 2005;100(9):2031–2035. doi: 10.1111/j.1572-0241.2005.50219.x. [DOI] [PubMed] [Google Scholar]
- 28.Miheller P, Muzes G, Zagoni T, Toth M, Racz K, Tulassay Z. Infliximab therapy improves the bone metabolism in fistulizing Crohn's disease. Dig Dis. 2006;24(1–2):201–206. doi: 10.1159/000091299. [DOI] [PubMed] [Google Scholar]
- 29.Pazianas M, Rhim AD, Weinberg AM, Su C, Lichtenstein GR. The effect of anti-TNF-alpha therapy on spinal bone mineral density in patients with Crohn's disease. Ann N Y Acad Sci. 2006;1068:543–556. doi: 10.1196/annals.1346.055. [DOI] [PubMed] [Google Scholar]
- 30.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 32.Morrissey PJ, Charrier K. Induction of wasting disease in SCID mice by the transfer of normal CD4+/CD45RBhi T cells and the regulation of this autoreactivity by CD4+/CD45RBlo T cells. Res Immunol. 1994;145(5):357–362. doi: 10.1016/s0923-2494(94)80200-9. [DOI] [PubMed] [Google Scholar]
- 33.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66(11):5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solnick JV, Schauer DB. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev. 2001;14(1):59–97. doi: 10.1128/CMR.14.1.59-97.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.On SL, Hynes S, Wadstrom T. Extragastric Helicobacter species. Helicobacter. 2002;7(Suppl 1):63–67. doi: 10.1046/j.1523-5378.7.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 36.Fox JG. The non-H pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002;50:273–283. doi: 10.1136/gut.50.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chin E, Dangler CA, Fox JG, Schauer DB. Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor alpha/beta mutant mice. Comp Med. 2000;50:586–594. [PubMed] [Google Scholar]
- 38.Erdman S, Fox JG, Dangler CA, Feldman D, Horwitz BH. Typhlocolitis in NF-kappa B-deficient mice. J Immunol. 2001;166(3):1443–1447. doi: 10.4049/jimmunol.166.3.1443. [DOI] [PubMed] [Google Scholar]
- 39.Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65(8):3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, Viney JL, Maggio-Price L. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G764–G778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- 41.Kuehl CJ, Wood HD, Marsh TL, Schmidt TM, Young VB. Colonization of the cecal mucosa by Helicobacter hepaticus impacts the diversity of the indigenous microbiota. Infection and immunity. 2005;73(10):6952–6961. doi: 10.1128/IAI.73.10.6852-6961.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal immunology. 2011;4(1):22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ardizzone S, Bollani S, Bettica P, Bevilacqua M, Molteni P, Bianchi Porro G. Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn's disease and ulcerative colitis. J Intern Med. 2000;247(1):63–70. doi: 10.1046/j.1365-2796.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 44.Young VB, Knox KA, Pratt JS, Cortez JS, Mansfield LS, Rogers AB, Fox JG, Schauer DB. In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infect Immun. 2004;72(5):2521–2527. doi: 10.1128/IAI.72.5.2521-2527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bleich A, Mahler M, Most C, Leiter EH, Liebler-Tenorio E, Elson CO, Hedrich HJ, Schlegelberger B, Sundberg JP. Refined histopathologic scoring system improves power to detect colitis QTL in mice. Mamm Genome. 2004;15(11):865–871. doi: 10.1007/s00335-004-2392-2. [DOI] [PubMed] [Google Scholar]
- 46.Pratt JS, Sachen KL, Wood HD, Eaton KA, Young VB. Modulation of host immune responses by the cytolethal distending toxin of Helicobacter hepaticus. Infect Immun. 2006;74(8):4496–4504. doi: 10.1128/IAI.00503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2(3):541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 48.Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1020–G1029. doi: 10.1152/ajpgi.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patial S, Shahi S, Saini Y, Lee T, Packiriswamy N, Appledorn DM, Lapres JJ, Amalfitano A, Parameswaran N. G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced NFkappaB activation in primary macrophages and modulates inflammation in vivo in mice. Journal of Cellular Physiology. 2011;226(5):1323–1333. doi: 10.1002/jcp.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. Fracture-associated hospitalizations in patients with inflammatory bowel disease. Digestive Diseases and Sciences. 2011;56(1):176–182. doi: 10.1007/s10620-010-1433-9. [DOI] [PubMed] [Google Scholar]
- 51.Hamdani G, Gabet Y, Rachmilewitz D, Karmeli F, Bab I, Dresner-Pollak R. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone. 2008;43(5):945–950. doi: 10.1016/j.bone.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Dresner-Pollak R, Gelb N, Rachmilewitz D, Karmeli F, Weinreb M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology. 2004;127(3):792–801. doi: 10.1053/j.gastro.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Sylvester FA, Wyzga N, Hyams JS, Davis PM, Lerer T, Vance K, Hawker G, Griffiths AM. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflammatory bowel diseases. 2007;13(1):42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 54.Reffitt DM, Meenan J, Sanderson JD, Jugdaohsingh R, Powell JJ, Thompson RP. Bone density improves with disease remission in patients with inflammatory bowel disease. European journal of gastroenterology & hepatology. 2003;15(12):1267–1273. doi: 10.1097/00042737-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Roth LS, Chande N, Ponich T, Roth ML, Gregor J. Predictors of disease severity in ulcerative colitis patients from Southwestern Ontario. World journal of gastroenterology : WJG. 2010;16(2):232–236. doi: 10.3748/wjg.v16.i2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee GJ, Kappelman MD, Boyle B, Colletti RB, King E, Pratt JM, Crandall WV. Role of Gender in The Treatment And Clinical Outcomes of Pediatric Patients With Inflammatory Bowel Disease. Journal of pediatric gastroenterology and nutrition. 2012 doi: 10.1097/MPG.0b013e318266241b. [DOI] [PubMed] [Google Scholar]
- 57.Blumenstein I, Herrmann E, Filmann N, Zosel C, Tacke W, Bock H, Dignass A, Hartmann F, Zeuzem S, Stein J, Schroder O. Female patients suffering from inflammatory bowel diseases are treated less frequently with immunosuppressive medication and have a higher disease activity: a subgroup analysis of a large multi-centre, prospective, internet-based study. Journal of Crohn's & colitis. 2011;5(3):203–210. doi: 10.1016/j.crohns.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 58.McCaskey SJ, Rondini EA, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI. Increased presence of effector lymphocytes during Helicobacter hepaticus-induced colitis. World journal of gastroenterology : WJG. 2012;18(13):1459–1469. doi: 10.3748/wjg.v18.i13.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas A, Liu YJ, Hao L, Mizoguchi A, Salzman NH, Bevins CL, Kobayashi KS. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14739–14744. doi: 10.1073/pnas.1003363107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchler G, Wos-Oxley ML, Smoczek A, Zschemisch NH, Neumann D, Pieper DH, Hedrich HJ, Bleich A. Strain-specific colitis susceptibility in IL10-deficient mice depends on complex gut microbiota-host interactions. Inflammatory bowel diseases. 2012;18(5):943–954. doi: 10.1002/ibd.21895. [DOI] [PubMed] [Google Scholar]
- 61.Livingston RS, Myles MH, Livingston BA, Criley JM, Franklin CL. Sex influence on chronic intestinal inflammation in Helicobacter hepaticus-infected A/JCr mice. Comparative medicine. 2004;54(3):301–308. [PubMed] [Google Scholar]
- 62.Okoli AS, Raftery MJ, Mendz GL. Effects of human and porcine bile on the proteome of Helicobacter hepaticus. Proteome science. 2012;10:27. doi: 10.1186/1477-5956-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, Cheever AW, Sher A. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infection and immunity. 2001;69(7):4232–4241. doi: 10.1128/IAI.69.7.4232-4241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]