Abstract
Background
Public awareness campaigns could address risk factors for melanoma to reinforce their sun protection message. The objective of this study is to prioritise risk factors associated with malignant melanoma (MM) to improve public awareness.
Method
Design: A cross-sectional study with retrospective data analysis from 2004 to 2010.
Setting: Western Australian Melanoma Advisory Service (WAMAS), a tertiary referral multidisciplinary organisation providing MM management advice. WAMAS data files were analysed with histologically confirmed cutaneous MM. Forty- seven patients had two or more melanomas, but the patient file was counted only once. Six MM data files with missing or incomplete information were excluded.
Main outcome measures: The number of naevi, blood relatives with MM, and previous sunburns were the primary variables collected.
Results
The results showed that 70.9 per cent (268/378) had previous sunburn; 40.2 per cent (152/378) had multiple naevi; and 22.5 per cent (85/378) had a positive family history. In the 110 MM data files not associated with sunburn, multiple naevi and a positive family history represented 34.5 per cent (38/110) and 20.0 per cent (22/110), respectively.
Conclusion
The results confirm the findings of previous studies that multiple naevi and a positive family history are important risk factors associated with MM. We suggest that MM can be detected earlier and its mortality decreased by focusing on these high-risk groups who are not targeted by current public awareness campaigns.
Keywords: Risk factors, melanoma, prevention
What this study adds:
-
What is known about this subject?
Increased naevus numbers and family history of melanoma are known risk factors for MM. It is not known if public awareness campaigns highlighting these facts or targeted skin screening of these groups could result in earlier detection of melanoma and therefore lower the incidence of metastatic disease.
-
What new information is offered in this study?
Risk factors for the development of MM are well known, but not communicated to the general public. The public is told to avoid excessive sun exposure as the predominant preventative measure. This study examines the medical records of patients with melanoma referred to the Western Australian Melanoma Advisory Service with reference to naevus numbers and family history of melanoma. Many patients with these risk factors would have had their melanomas detected earlier had they known their risk was higher than that of the general population.
-
What are the implications for research, policy, or practice?
It is hoped that better public awareness campaigns may lead to earlier detection of melanoma with an attendant better prognosis.
Background
Malignant melanoma (MM) is considered to be one of the most preventable forms of cancer and is associated with multiple risk factors. The literature identifies sunburn, multiple naevi, and a positive family history to be the most significant of these;1–3 however, their impact as predictors of MM development is poorly understood.
In Western Australia, MM is a major public health problem. The incidence of MM has continued to rise over the last two decades making it the most rapidly increasing cancer in the Caucasian population.4
In 2011, in Western Australia MM was the third most common cancer overall; the most common cancer in men aged 15–39 years and second only to breast cancer in women of this age group. In this state melanoma was the fourth leading cancer causing death in men overall. Between 2000–2011 in Western Australia, the average Breslow thickness of melanoma increased from 0.6mm to 0.65mm, and the percentage of thicker melanoma (levels III, IV, and V) increased in both men and women. From 2002–2011, there has been a modest decrease in the incidence of melanoma.5
Current public awareness campaigns directed at the whole population (primary prevention) focus only on sun exposure and the prevention of sunburn. They do not address these other established risk factors that have been well known among medical professionals for the past 30 years,6-8 and the link with multiple naevi was first documented in 1857.9
The increase in average Breslow thickness of melanoma in Western Australia in this period suggests later presentation of a patient with a melanoma and may indicate these public awareness campaigns could be improved.
The National Health and Medical Research Council does not recommend community screening for melanoma.10 The Melanoma Network, the Cancer Council Australia, the Royal Australian College of General Practitioners, and others endorse this stance. Public awareness campaigns as well as advertising from chains of commercial skin cancer and mole clinics warn against the danger of melanoma and implicitly suggest that screening of any individual is a worthwhile initiative. In Western Australia many employers, including local government authorities, employ mobile skin cancer screening organisations staffed by general practitioners or nurses to screen employees at workplaces.11
Targeted screening—that is, examining individuals with multiple naevi and a family history of melanoma—has been conducted previously with encouraging results,12 yet universal screening seems to be widely conducted despite expert advice to the contrary. If expert groups have concluded that universal screening is unnecessary, then it follows that the cost involved constitutes a waste of health resources.
The landmark paper in 1988 by English and Armstrong13 showed that 54 per cent of melanoma cases arose in a subgroup of just 16 per cent of the population. This group comprised those with increased naevus numbers, time spent outdoors age 10–24, history of non-melanoma skin cancer, family history of melanoma, and arrival in Australia younger than 10 years.
In the intervening 25 years since this paper was published little has happened to improve education of the public about melanoma.
Numerous studies have assessed the relative risk of developing melanoma with absolute or localised numbers of naevi. A 1984 study by Holman et al. showed that more than 10 naevi on the arms was linked with an 11.3 relative risk (RR) for melanoma.14 An Italian study in 1995 demonstrated a RR of 2.6 for 10–30 total body count of naevi,15 and a study by Holly et al. in 19876 demonstrated that 4.4 was the relative risk for total naevus count of 26– 50.
Aims
The objective of this study is to prioritise melanoma risk factors: sunburn, multiple naevi, and a positive family history. It is hypothesised that additional public health campaigns targeting high-risk people (secondary prevention) with multiple naevi and a positive family history of MM would improve public awareness, increase the early detection of thinner MM lesions, and thereby minimise MM-associated morbidity and mortality.
Sun exposure and sunburn—widely accepted as the primary cause of MM10 even though the exact mechanism remains unclear11,12 and essentially the only message promoted in awareness campaigns—may not be as strong a predictor of MM development as previously thought. It appears that in certain high-risk people without a history of sunburn, MM development is likely, and early detection (rather than prevention) must be a focus of campaigns.
Methods
This cross-sectional study allowed the retrospective collection and analysis of all data files referred to the Western Australia Melanoma Advisory Service (WAMAS) with histologically confirmed cutaneous MM of any thickness diagnosed between 2004 and 2010. WAMAS, a multidisciplinary unit established by the Western Australian Health Department and St John of God Hospital, Subiaco, provides comprehensive advice regarding the management of MM. Their data files represent approximately 10 per cent of the state’s total melanomas.
Sunburn, multiple naevi, and a positive family history were the main variables collected. Multiple melanomas were each recorded as separate data files and included in the data collection. MM data files with missing or incomplete information were excluded from the study. A control group was not included as it was outside the scope of the study.
Data were obtained from forms completed by patients and dermatologists at WAMAS. All data was stored electronically on the WAMAS database and extracted as complete files. Statistical analysis was performed using Pivot tables in Excel spreadsheets to determine the proportions of each risk factor in the sample population. The results were summarised and presented as text and figures.
This low-risk design meant that there was no contact with any participants in the study. All participants had given consent for their information to be used for future research. Approval was obtained from the Human and Research Ethics Committees (HREC) of St John of God Health Care (SJGHC), Subiaco (Ref: 413) and The University of Notre Dame (UNDA), Fremantle. Privacy and confidentiality were maintained at all times in accordance with standard research protocols and section 95A of the Privacy Act 1988 (Cth).
Multiple naevi
Multiple naevi refer to more than 20 melanocytic naevi. In this study, melanocytic naevi are defined as “light to dark brown or black pigmented macules or papules, 2mm or greater in diameter, reasonably well defined, darker in colour than surrounding skin and not having features of freckles, solar lentigines, seborrheic keratoses, cafe-au-lait spots, or non- melanocytic lesions”.13 A dermatologist examined each patient and total naevi were counted and categorised into groups (<20, 20–50, 50–100, 100–200, 200–500, >500). Acquired and congenital melanocytic naevi were both included in the counts. The number of naevi is believed to parallel an increasing risk of melanoma.6
A positive family history refers to participants that have any blood relative (first-degree or second-degree) with past or present cutaneous MM. Participants were categorised according to their knowledge of family history (Yes, No, Unsure). If present, it was further categorised according to the number of blood relatives with melanoma (1, 2, 3, 4, 5, >5). Data were also collected on hair colour (brown, blonde, red) and eye colour (blue, hazel, brown).
Sunburn
Sunburn refers to a previous sun exposure sufficient to produce sunburn with or without blistering of the skin. Participants were categorised into groups (Yes, No). If present, it was further categorised according to the number of occasions this occurred (0, 1–5, 5–10, 10–20, >20). Data was also collected on skin type (I–III are pale with a tendency to easily burn and poor ability to tan; IV– VI are dark with tendency to easily tan and poor ability to burn).3
Results
Three hundred and seventy eight MM data files were analysed from a total of 431 obtained from WAMAS between 2004 and 2010. Of these, 57.1 per cent (216/378) were males and the average age was 56.8 years (range 17–87 years).
Sunburn 70.9 per cent (268/378), multiple naevi 40.2 per cent (152/378), and a positive family history 22.5 per cent (85/378) are highly prevalent risk factors associated with MM. Figure 1 demonstrates this in a simple bar graph while Figure 2 demonstrates this in a Venn diagram conveying the subsequent overlap of each risk factor which may co-exist in the same data file.
Figure 1. Bar graph of risk factors.

Figure 2. Venn diagram of risk factors.

Positive family history
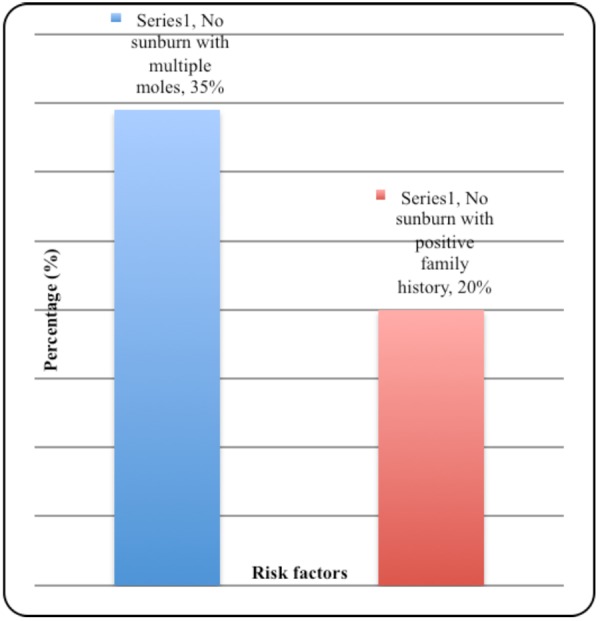
In the 85 MM data files with a positive family history, 50.6 per cent (43/85) had blue eyes, 32.9 per cent (28/85) had red or blonde hair, and 88.2 per cent (75/85) had skin type I–III. In the 110 MM data files not associated with sunburn, 34.5 per cent (38/110) had multiple naevi, and 20.0 per cent (22/110) had a positive family history. Figure 3 demonstrates this in a simple bar graph.
Figure 3. Bar graph of positive family history.
Discussion
This study shows that in addition to sunburn, having multiple naevi and a positive family history are important risk factors associated with MM. It suggests that MM development may be predicted in specific populations according to these risk factors.
We acknowledge that sunburn is a significant risk factor associated with MM. This is highlighted by the 70.1 per cent (268/378) prevalence in this study and supports the role of primary prevention strategies to limit sun exposure. However, the retrospective assessment of sun exposure and potential for recall bias, coupled with the difficulty in differentiating sunburn history, sun exposure habits, ability to tan, and other phenotypic factors, suggest this could be an overestimation.16,17
Furthermore, approximately 20 per cent of the MM globally occurs in non-Caucasians that have skin types III– VI with a resistance to sunburn20 or develop at sites rarely exposed to sun.21 This suggests that sunburn is not an essential component of MM development and our study supports this with sunburn being absent in 29.1 per cent (110/378) of the MM data files.
In view of the increasing incidence of MM4 and the “Slip, slop, slap” campaigns that may inadvertently suggest there is a “safe” sun tan,22 we believe that sun exposure should not be the sole focus of public awareness, and it appears previous literature reviews concur.23,24
We suggest that new multi-strategic campaigns targeting people based on the risk factors identified in this study, need to be introduced. We postulate that this would optimise public awareness of MM and lead to early detection,19 especially in high-risk groups with multiple naevi and/or a positive family history who are ignorant of the fact that they have a heightened risk of developing melanoma.
Evidence to support this new approach to public awareness is well documented in previous literature: the total number of naevi is the most important independent risk factor for MM development;16 the relative risk of MM increases linearly with rising numbers of naevi corresponding to a 4.4–10.7 times higher risk in individuals with more than 25 naevi compared to individuals without multiple naevi;6 there is a potential for genetic predisposition to MM development;25 sunburn is highly dependent on skin type, a trait which is largely inherited, usually occurring in people with blue eyes, blonde/red hair, and skin types I–III that are more likely to burn rather than tan.3,26
This implies that the 40.2 per cent (152/378) of MM patients in this study who had more than 20 naevi, and the 22.5 per cent (85/378) with a positive family history —50.6 per cent (43/85) with blue eyes, 32.9 per cent (28/85) with blonde or red hair, and 88.2 per cent (75/85) with skin types I–III—could have been identified as high-risk for MM development prior to their diagnosis.
WAMAS is often identified as dealing with complex and more advanced disease so that the cases included here may not be representative of MM in Western Australia generally as the majority of MM in this state are thin melanomas.
Conclusion
Although the risk factors for melanoma outlined here have been known to the medical profession for many years, the public has not been informed. Future public awareness campaigns which target patients with a family history of melanoma and patients with multiple naevi to reinforce the well worn “sun smart” message may help with earlier detection of melanoma and lessen morbidity and mortality of this disease.
ACKNOWLEDGEMENTS
We thank Julie Teraci, nurse co-coordinator at Western Australian Melanoma Advisory Service (WAMAS), for entering patient and doctor information into the database from 2004- 2010. We also thank Prof. Kathryn Hird and A/Prof. Ilse O’Farrell, research co-coordinators at the University of Notre Dame Australia (UNDA), for overseeing the conduct of the research project and Professor Dallas English, University of Melbourne for his input.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
ETHICS COMMITTEE APPROVAL
Human and Research Ethics Committees (HREC) of St John of God Health Care (SJGHC), Subiaco (Ref: 413)
Please cite this paper as: Williams C, Quirk C, Quick A. Melanoma: A new strategy to reduce morbidity and mortality. AMJ 2014, 7, 7, 266–271.http//dx.doi.org/10.4066/AMJ.2014.1949
References
- 1.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: Common and atypical naevi. Eur J Cancer. 2005 Jan;41(1):28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005 Jan;41(1):45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic change and phenotypic factors. Eur J Cancer. 2005 Sep;41(14):2040–59. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Mar V, Wolfe R, Kelly J. Predicting melanoma risk for the Australian population. Australas J Dermatol. 2011;52:109–16. doi: 10.1111/j.1440-0960.2010.00727.x. [DOI] [PubMed] [Google Scholar]
- 5.Threlfall TJ, Thompson JR. Cancer incidence and mortality in Western Australia, 2011. Department of Health, Western Australia, Perth; 2013. Statistical Series Number 97. [Google Scholar]
- 6.Holly EA, Kelly JW, Shpall S, Chiu SH. Number of melanocytic naevi as a major risk factor for malignant melanoma. J Am Acad Dermatol. 1987;17(3):459–68. doi: 10.1016/s0190-9622(87)70230-8. [DOI] [PubMed] [Google Scholar]
- 7.English DR, Armstrong BK. Identifying people at high risk of cutaneous malignant melanoma: Results from a case- control study in Western Australia. BMJ. 1988;296:1285–88. doi: 10.1136/bmj.296.6632.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes AR, Weinstock MA, Fitzpatrick TB, Mihm MC Jr., Sober AJ. Risk factors for cutaneous melanoma: A practical method of recognizing predisposed individuals. JAMA. 1987;258:3146–54. [PubMed] [Google Scholar]
- 9.Bennett JP, Hall P. Moles and melanoma: A history. Ann R Coll Surg. Engl. 1994;76(6):373–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. Cancer Council Australia and Australian Cancer Network. Sydney and New Zealand Guidelines Group, Wellington. 2008 [Google Scholar]
- 11.Health on the Move (AU). Transportable skin cancer screen (Internet) 2013. [cited 2013 Nov 10]. Available from: Available from: http://www.healthmove.com.au. [Google Scholar]
- 12.Williams HA, Fritschi L, Reid A, Beauchamp C, Katris P. Who attends skin cancer screening in Western Australia? Results from the Lions Cancer Institute Skin Cancer Screening Program. Aust NZ J Public Health. 2006;30(1):75–80. doi: 10.1111/j.1467-842x.2006.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer. A review of human carcinogens. Part D Radiation IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, France; 1992 [Google Scholar]
- 14.Holman C, Armstrong BK. Pigmentary Traits ethnic origin, benign naevi, and family history for Cutaneous Malignant Melanoma J. Natl Cancer Inst. 1984;72(2):257–66. [PubMed] [Google Scholar]
- 15.Carli P, Biggeri A, Gianotti B. Malignant Melanoma in Italy: risks associated with common and clinically atypical naevi. J Am Acad Dermatol. 1995;32:734–39. doi: 10.1016/0190-9622(95)91451-x. [DOI] [PubMed] [Google Scholar]
- 16.Bauer J, Garbe C. Acquired Melanocytic Naevi as Risk factor for Melanoma Development. A Comprehensive Review of Epidemiological Data. Pigment Cell Melanoma Res. 2003;16:297–306. doi: 10.1034/j.1600-0749.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman D, Green A. Melanoma and Sunburn. Cancer Causes Control. 1994;5:564–72. doi: 10.1007/BF01831385. [DOI] [PubMed] [Google Scholar]
- 18.English DR, McLennan R, Rivers JK, Harrison SA, Lewis AE, Tate BJ. Epidemiological Studies of Melanocytic Naevi: Protocol for Identifying and Recording Naevi Int. Agency Res Cancer, Lyon, France, IARC Internal Rep. 90(002) [Google Scholar]
- 19.Giles G, Armstrong B, Burton R, Staples M. Thursfield. Has Mortality from Melanoma Stopped Rising in Australia? Analysis of trends between 1931 and 1994. BMJ. 1996;312:1121–25. doi: 10.1136/bmj.312.7039.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong BK, Kricker A. How Much Melanoma is Caused by Sun Exposure? Proceedings of the IARC Monographs of the Evaluation of Carcinogenic Risks to Humans. 1993;13(6):395–401. [Google Scholar]
- 21.Rivers JK. Melanoma. Lancet. 1996;347:803–07. doi: 10.1016/s0140-6736(96)90873-9. [DOI] [PubMed] [Google Scholar]
- 22.Dutch Cancer Society. Outdoors and Indoors: sun wisely. Report on the ‘Sensible Sunbathing’ consensus meeting held under the auspices of the Dutch Cancer Society. Utrecht, 1995 Oct 6. Holland: Dutch Cancer Society, 1995. [Google Scholar]
- 23.Slevin T, Clarkson J, English D. Skin Cancer Control in Western Australia: Is It Working and What Have We Learned? Radiation Protection Dosimetry. 2000;91(1-3):303–06. [Google Scholar]
- 24.Stratigos A, Katsambas AD. The Value of Screening in Melanoma. J Clin Dermatol. 2009;27:10–25. doi: 10.1016/j.clindermatol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 25.McMeniman E, De’Ambrosis K, De’Ambrosis B. Risk Factors in a Cohort of Patients With Multiple Primary Melanomas. Australas J of Dermatol. 2010;51:254–57. doi: 10.1111/j.1440-0960.2010.00674.x. [DOI] [PubMed] [Google Scholar]
- 26.Rampen FH. Risk Factors in Melanoma. Lancet. 1995;345(8946):397–98. doi: 10.1016/s0140-6736(95)90390-9. [DOI] [PubMed] [Google Scholar]


