Abstract
Dexamethasone is frequently administered to the developing fetus to accelerate pulmonary development. The purpose of the present study was to determine if prenatal dexamethasone programmed a progressive increase in blood pressure and renal injury in rats. Pregnant rats were given either vehicle or 2 daily intraperitoneal injections of dexamethasone (0.2 mg/kg body weight) on gestational days 11 and 12, 13 and 14, 15 and 16, 17 and 18, or 19 and 20. Offspring of rats administered dexamethasone on days 15 and 16 gestation had a 20% reduction in glomerular number compared with control at 6 to 9 months of age (22 527±509 versus 28 050±561, P<0.05), which was comparable to the percent reduction in glomeruli measured at 3 weeks of age. Six- to 9-month old rats receiving prenatal dexamethasone on days 17 and 18 of gestation had a 17% reduction in glomeruli (23 380±587) compared with control rats (P<0.05). Male rats that received prenatal dexamethasone on days 15 and 16, 17 and 18, and 13 and 14 of gestation had elevated blood pressures at 6 months of age; the latter group did not have a reduction in glomerular number. Adult rats given dexamethasone on days 15 and 16 of gestation had more glomeruli with glomerulosclerosis than control rats. This study shows that prenatal dexamethasone in rats results in a reduction in glomerular number, glomerulosclerosis, and hypertension when administered at specific points during gestation. Hypertension was observed in animals that had a reduction in glomeruli as well as in a group that did not have a reduction in glomerular number, suggesting that a reduction in glomerular number is not the sole cause for the development of hypertension.
Keywords: glucocorticoids, hypertension, gestational, glomerular filtration rate, kidney
Glucocorticoids are often administered to pregnant women to accelerate fetal pulmonary maturation and prevent respiratory distress syndrome.1–4 However, there is evidence that prenatal administration of steroids may have adverse effects on the developing fetus with consequences in later life.5–9 Daily administration of prednisone, used as a treatment for infertility in humans, resulted in infants that are small for gestational age.5 Similar findings have been demonstrated in rodents.5–7
Administration of dexamethasone to pregnant rats produces adverse effects on the developing kidney.6,7,9 Rats born to pregnant animals that received daily dexamethasone throughout gestation had a 50% reduction in the number of nephrons and a 30% reduction in glomerular filtration rate when they were studied at 60 days of age.7 Rats exposed to daily steroids during development also had significant hypertension at 2 months of age.6,7 Similarly, offspring of ewes given prenatal glucocorticoids have hypertension as adults.10–14 However, rats that were the product of mothers receiving daily steroids had severe intrauterine growth retardation, which is also a predisposing factor for the development of hypertension and renal disease in later life.15–18
We have recently examined the effect of 2 daily injections of prenatal dexamethasone (0.2 mg/kg per day) in rats.9 We chose this dose to be comparable to that administered to humans to accelerate pulmonary maturation. Two daily doses of dexamethasone did not produce intrauterine growth retardation, unlike the above studies, in which more doses of dexamethasone were administered.6,7 In addition, we found no effect on the numbers of rats in the litter or on the length of gestation with prenatal dexamethasone.9 Administration of dexamethasone on either days 15 and 16 or 17 and 18 resulted in a significant reduction in nephrons when the rats were arbitrarily studied at 2 months of age. Both the male and female rats that received prenatal dexamethasone on days 15 and 16 of gestation had hypertension, whereas only the male rats that received dexamethasone on days 17 and 18 of gestation had hypertension. Rats that received dexamethasone on days 11 to 12, 13 to 14, 19 to 20, and 20 to 21 were unaffected. Thus, there appears to be a critical time of gestation that prenatal dexamethasone has its deleterious effects that cause hypertension and a reduction in nephron number.
A major shortcoming of our previous study was the fact that rats that received prenatal dexamethasone were studied at only one time point, which left several unanswered questions. It was unclear if prenatal dexamethasone affected renal development or caused a reduction in nephrons by programming the developing animal to have accelerated and progressive glomerular senescence. It was unclear whether the hypertension was manifest in neonates and whether the severity of the hypertension increased as the animal aged. It was also unclear if the reduction in nephron number was the sole cause for the hypertension. The present study examined rats as neonates and at 6 to 9 months of age to address some of these questions. We found that there is a reduction in nephron number in neonatal rats but that only female rats have an elevated blood pressure. In 6- to 9-month-old rats we found that male rats that received dexamethasone on days 13 and 14, 15 and 16, and 17 and 18 of gestation have hypertension but that a reduction in nephron number is not the sole cause for the hypertension. Finally, we show that prenatal dexamethasone can lead to glomerulosclerosis.
Methods
Animals
The pregnant rats arrived at our institution at least 2 days before initiation of the study. Pregnant rats received either intraperitoneal vehicle or dexamethasone (0.2 mg/kg body wt) daily on gestational days 11 and 12, 13 and 14, 15 and 16, 17 and 18, or 19 and 20. We have previously shown that these dexamethasone protocols do not affect litter size and do not cause intrauterine growth retardation. Both male and female rats were studied. Prenatal dexamethasone resulted in comparable differences from control rats in both male and female rats in each of the protocols studied, and the results were therefore combined except for blood pressure and renal histology.
Measurement of Glomerular Filtration Rate
Inulin clearance was performed in a fashion similar to that previously described.9,19
Measurement of Blood Pressure
Blood pressure was measured with an IITC Model 179 Blood Pressure Analyzer. Six-month-old animals were placed in a Lucite tube, and a tail-cuff blood pressure cuff was inflated several times daily on the 4 days before the actual measurement of blood pressure. Three-week-old animals were placed in a Styrofoam tube and trained as above and then returned to their mothers. On the day of the actual blood pressure measurement, there were at least 4 determinations, and the mean of these values was used as the blood pressure for that rat.
Glomerular Number
Glomerular number was determined by using a variation of the technique of Damadian et al, with significant modifications.9,20–22 Alcian blue is superior to india ink to stain glomeruli.20,22 Six- to 9-month-old rats received an intra-arterial infusion of 0.2 mL per 100 g body weight of 5% alcian blue (Aldrich Chemical Company) in normal saline over a period of 1 minute, using a femoral arterial catheter placed above the renal arteries after measurement of glomerular filtration rate. A second 0.2 mL per 100 g body weight bolus of alcian blue was then administered 5 to 7 minutes after the first infusion. For the 3-week-old rats, an intra-aortic infusion of 2.5% alcian blue was administered. The kidneys were then removed 5 minutes after the second infusion and processed as recently described.9
Histology and Glomerular Size
Kidneys from 8-month-old control rats and rats that received prenatal dexamethasone on 15 and 16 and 17 and 18 days of gestation or vehicle were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin and with periodic acid–Schiff stain. All of the glomeruli in a cross section of kidney (100 to 200 glomeruli) from each animal were examined for evidence of glomerulosclerosis. Glomerulosclerosis was defined as segmental or global collapse of the peripheral capillary loops, often associated with capsular adhesions. There were at least 3 kidneys from different animals examined in each group. The kidneys were examined without operator knowledge of whether they were from control or dexamethasone-treated rats.
Statistical Analysis
Values are expressed as mean±SEM. ANOVA with post hoc Student-Newman-Keuls test, unless otherwise noted, or unpaired Student t test for studies examining 2 groups, was used to determine statistical significance except for studies examining number of sclerotic glomeruli, in which χ2 analysis was used.
An expanded Methods section can be found in an online supplement available at http://www.hypertensionaha.org.
Results
Effect of Prenatal Dexamethasone on Glomerular Number at 3 Weeks of Age
To determine if prenatal dexamethasone caused a reduction in glomerular number in infant rats, we counted the number of glomeruli in rats at 3 weeks of age. We examined the effect of dexamethasone administered at days 15 and 16 of gestation, since this was previously determined to cause the greatest reduction in glomerular number when the rats were studied at 2 months of age.9 As shown in Figure 1, there was a 14% reduction in glomerular number at 3 weeks of age (P<0.001). Thus, 3-week-old rats exposed to prenatal dexamethasone have a reduction in nephron number consistent with a reduction in nephron number in the perinatal period.
Figure 1.
Effect of prenatal dexamethasone on number of glomeruli in 3-week-old rats. Pregnant rats were administered dexamethasone (0.2 mg/kg body wt) or vehicle on gestational days 15 and 16. At 3 weeks of age, the number of glomeruli were counted by using alcian blue to stain the glomeruli followed by acid digestion. Number of glomeruli were reduced in animals that received dexamethasone at 15 and 16 compared with control. There were 8 rats in each group. Prenatal dexamethasone caused a significant reduction in the number of glomeruli at 3 weeks of age (P<0.01).
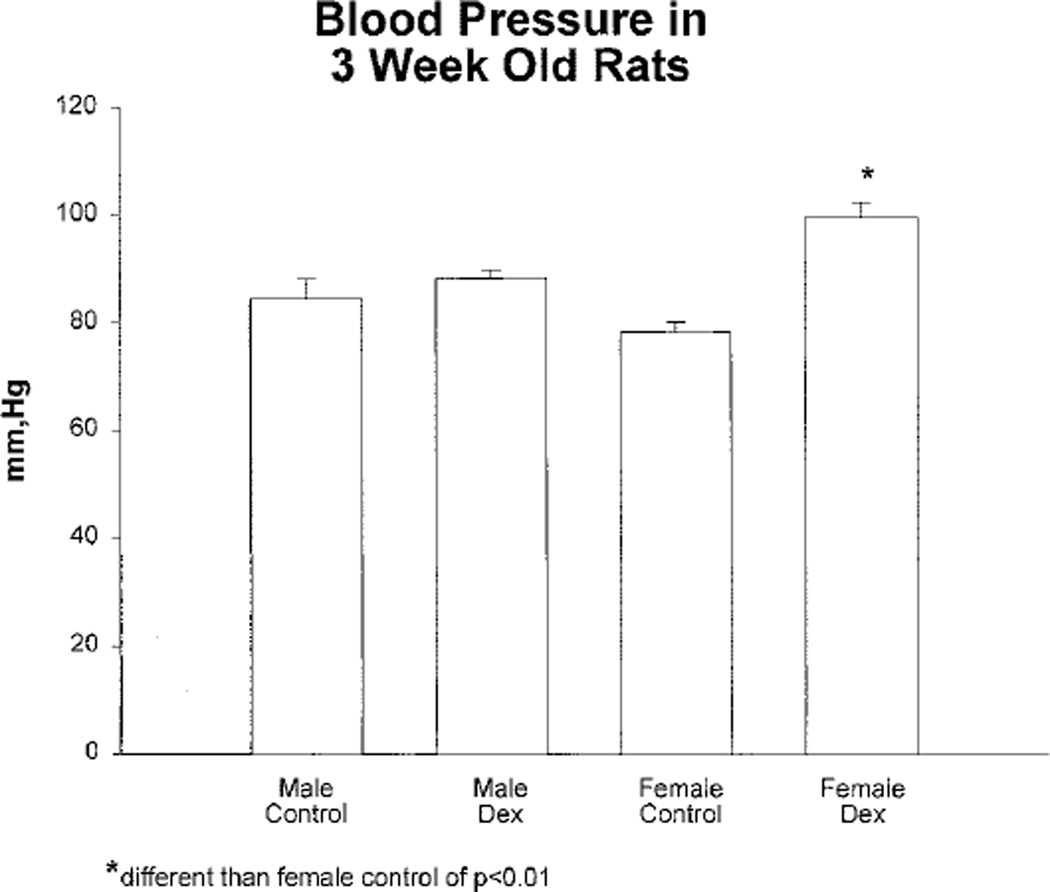
Effect of Prenatal Glucocorticoids on Blood Pressure at 3 Weeks of Age
The effect of prenatal dexamethasone, administered at days 15 and 16 of gestation, on systolic blood pressure is shown on Figure 2. We studied rats that were given dexamethasone on days 15 and 16 of gestation because this was previously shown to cause the greatest rise in blood pressure in rats studied at 2 months of age. There was a small increase in blood pressure in male rats that was not statistically significant. Female rats that received prenatal dexamethasone had a higher blood pressure than female control rats (P<0.01).
Figure 2.
Effect of prenatal dexamethasone on systolic blood pressure in male and female rats measured at 3 weeks of age. There was no significant difference in blood pressure in control male rats and male rats that received prenatal dexamethasone on days 15 and 16 of gestation. However, the difference in female rats was significant (P<0.001). There were at least 9 rats in each group.
Effect of Prenatal Glucocorticoids on Number of Glomeruli and Glomerular Filtration Rate at 6 to 9 Months of Age
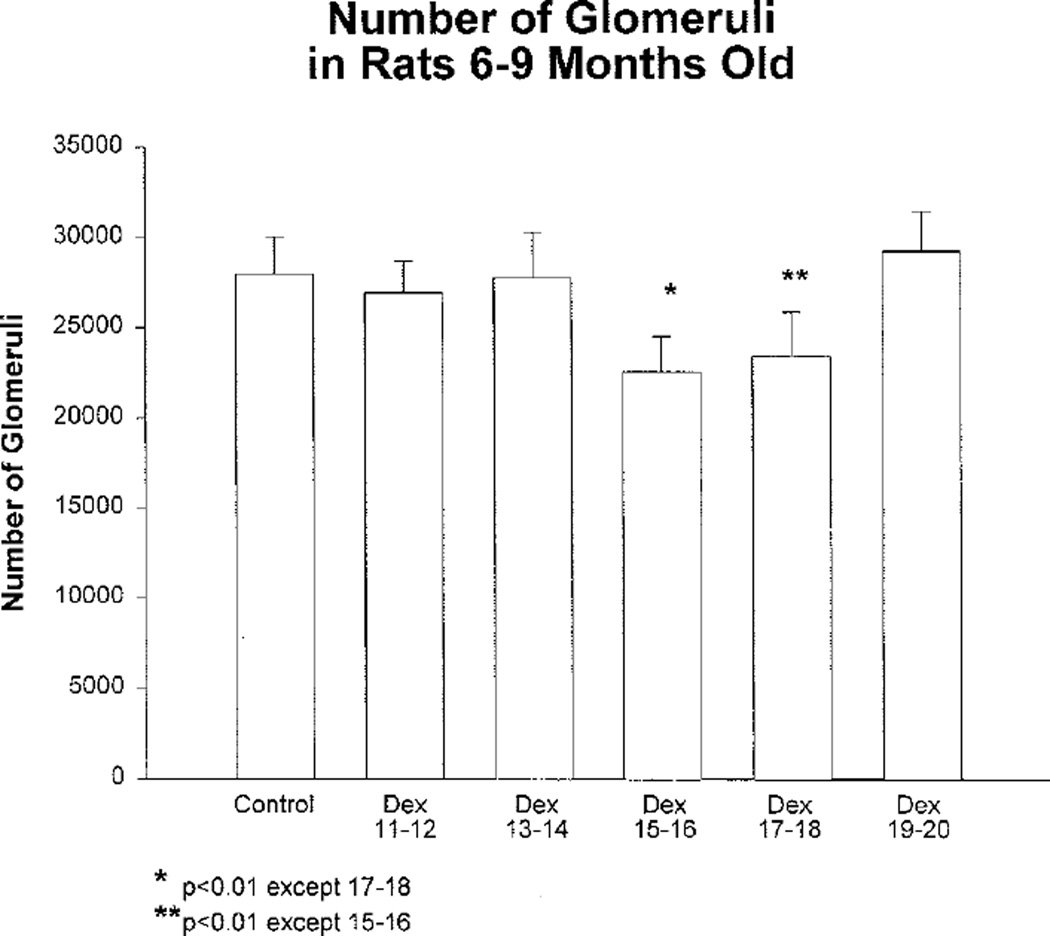
The effect of prenatal dexamethasone on glomerular number was determined in rats at 6 to 9 months of age that were administered dexamethasone on days 11 and 12, 13 and 14, 15 and 16, 17 and 18, and 19 and 20. Rats were studied at 7.6±0.2, 7.6±0.3, 8.3±0.2, 8.6±0.1, and 7.6±0.3 months, respectively. Control rats were studied at 8.4±0.2 months. The 11 and 12 dexamethasone rats were slightly younger (7.6±0.2 months) compared with control rats (8.4±0.2 months, P<0.05). There was no difference in age between the control rats and the other groups. As shown in Figure 3, the results demonstrate that there was a 20% and 17% reduction in the number of glomeruli when dexamethasone was administered on days 15 and 16 and days 17 and 18 of gestation, respectively, compared with control rats (P<0.05). These results were virtually identical to those found when animals were studied at 2 months of age.9
Figure 3.
Effect of prenatal dexamethasone on number of glomeruli in 6- to 9-month-old rats. Pregnant rats were administered dexamethasone (0.2 mg/kg body wt) on gestational days 11 and 12, 13 and 14, 15 and 16, 17 and 18, 19 and 20, 20 and 21, or vehicle. Number of glomeruli was reduced in animals that received dexamethasone either at 15 and 16 or 17 and 18 days of gestation. There were at least 12 rats in each group.
As is shown in Figure 4A, there was no reduction in glomerular filtration rate when the rats were analyzed at 6 to 9 months of age. There was also no difference in glomerular filtration rate when factored per body weight as shown in Figure 4B.
Figure 4.
A, Effect of prenatal dexamethasone on glomerular filtration rate (GFR). Glomerular filtration rate was measured in anesthetized 6- to 9-month-old rats that received either prenatal dexamethasone or vehicle. GFR was measured with [methoxy-3H] inulin. There were at least 11 rats in each group. B, Effect of prenatal dexamethasone on GFR factored per 100 g body weight.
Effect of Prenatal Glucocorticoids on Blood Pressure at 6 Months of Age
The effect of prenatal dexamethasone on systolic blood pressure was determined in rats at 6 months of age that were administered dexamethasone on days 11 and 12, 13 and 14, 15 and 16, 17 and 18, and 19 and 20 of gestation. There was a difference in male and female rats at this age, as we found in our previous study so male and female rats were analyzed independently. The results are shown in Figure 5. Male rats had an increase in blood pressure if administered dexamethasone on days 13 and 14, 15 and 16, and 17 and 18 days of gestation. It should be noted that the 13 and 14 dexamethasone male group had an elevated blood pressure, whereas there was no difference in the number of glomeruli 27 568±387 in controls versus 28 545±972 in the 13 and 14 male dexamethasone group. Thus prenatal dexamethasone can increase blood pressure independent of a reduction in glomerular number. There was no effect of prenatal dexamethasone on blood pressure in female rats.
Figure 5.
Systolic blood pressures in 6-month-old rats that received prenatal dexamethasone or vehicle. Blood pressure was taken in trained rats using a tail cuff. Male rats (A) that received prenatal dexamethasone on days 13 and 14, 15 and 16, and 17 and 18 had elevated blood pressure compared with control rats. Female rats (B) were not hypertensive. There are at least 10 male rats in each group. There were 8 female rats in each group except days 11 and 12 and days 19 and 20, in which there were 5 rats.
Renal Histology
Histology of kidneys from an 8-month-old control male rat and a male rat that received prenatal dexamethasone on days 15 and 16 of gestation are shown in Figure 6. Male rats that had received prenatal dexamethasone on days 15 and 16 of gestation had an increase in interstitial fibrosis and glomerulosclerosis. The number of glomeruli with evidence of glomerulosclerosis is shown in the Table. As can be seen, male rats in all groups had more sclerotic glomeruli than female rats. The numbers of sclerotic glomeruli in both male and female rats that received dexamethasone on days 15 and 16 of gestation were greater than in control rats (P<0.001).
Figure 6.
A, Female control kidney at 8 months of age showing segmental sclerosis in 1 of 4 glomeruli. Note capillary collapse and adhesion to capsule. The 3 other glomeruli show no significant abnormalities (periodic acid Schiff, ×100). B, Male whose mother received dexamethasone on days 15 and 16 of gestation. Segmental sclerosis is present in 3 glomeruli. There is interstitial fibrosis and tubular atrophy and a mild lymphocytic infiltrate (periodic acid Schiff, ×100).
Number of Sclerotic Glomeruli Divided by the Total Number Counted
| Gender | Control | Prenatal Dex 15 and 16 |
Prenatal Dex 17 and 18 |
|---|---|---|---|
| Male, n (%) | 88/556 (15.8) | 182/457 (39.8)* | 122/650 (18.8)† |
| Female, n (%) | 22/612 (3.6) | 46/342 (13.5)* | 31/615(5.0) |
Dex indicates dexamethasone.
P<0.001,
P=0.09 vs control.
Discussion
The present study examined the effect of prenatal dexamethasone on glomerular number in neonatal and adult rats. Although we had previously found that prenatal dexamethasone resulted in hypertension and a reduction in nephron number at 2 months of age, this study extends the previous observations significantly and provides clues to how prenatal dexamethasone reduces nephron number and produces an elevated blood pressure in adult rats.
The fetus is normally protected from maternal steroids by placental 11β-hydroxysteroid dehydrogenase.23 Pregnant rats exposed to carbenoxalone, an inhibitor of placental 11β-hydroxysteroid dehydrogenase, have offspring in which hypertension later develops.24,25 However, pregnant rats given carbenoxolone during pregnancy but adrenalectomized to protect the fetuses from maternal glucocorticoids were normotensive as adults.24 Pregnant rats that ingest a low protein diet have low levels of 11β-hydroxysteroid dehydrogenase and have offspring that develop hypertension as adults.26,27 Pregnant rats that ingest a low protein diet but also receive metyrapone, an inhibitor of glucocorticoid synthesis, for the first 14 days of gestation have offspring with normal blood pressure.27 Dexamethasone is a poor substrate for 11β-hydroxysteroid dehydrogenase, which allows fetal exposure to the administered glucocorticoid.
The mechanism for the hypertension resulting from limited exposure to dexamethasone has yet to be elucidated. The endowment of nephrons at birth has been proposed to be a factor affecting blood pressure in adults.15,16,28 Human neonates with intrauterine growth retardation have a reduced number of nephrons28 and are predisposed to developing hypertension as adults.15–18 The mechanism mediating the hypertension associated with congenital oligonephropathy is controversial. A reduction in nephron number could impair renal sodium excretion resulting in elevated blood pressure.15,16,28 However, it should be noted that in the present study, there was no effect of prenatal dexamethasone on glomerular filtration rate or when the glomerular filtration was factored for body weight.
There is increasing evidence that the hypertension seen in rat neonates born to mothers who ingested a low protein diet during pregnancy is mediated by a dysregulation of the renin-angiotensin system.29–31 Rats born to mothers that ingested a low protein diet have an elevated plasma ACE activity,29,30 and their blood pressure is normalized when treated with an ACE inhibitor.29 Recent studies by Woods et al31 demonstrated that renin mRNA and intrarenal angiotensin II levels were lower in rats that were the offspring of mothers that ingested a low protein diet than control rats. This group hypothesizes that reduced fetal and neonatal intrarenal angiotensin II results in impaired renal development and the reduced nephron number leads to hypertension in adult.31 There is also evidence that maternal dietary protein deprivation results offspring with upregulation of the bumetanide-sensitive cotransporter and the thiazide-sensitive cotransporter, 2 transporters important in renal sodium absorption.32
Our data suggest that a reduction in nephron number is not the sole factor causing the hypertension in rats exposed to prenatal dexamethasone. Although there is by and large a concordance with a reduction in nephron number and the development of hypertension in rats exposed to prenatal dexamethasone, there is only a ≈20% reduction in nephron number in the 15 and 16 and 17 and 18 dexamethasone group. It is hard to imagine that this small reduction in nephron number is responsible for the hypertension. Furthermore, unlike our previous study examining rats at 2 to 3 months of age, we find that at 6 to 9 months, the 13 and 14 male dexamethasone group had hypertension despite the fact that there was no reduction in glomerular number in this group. Thus this study demonstrates that there has to be other factors besides a reduction in nephron number that causes the hypertension with prenatal dexamethasone.
The other interesting factor relating to the hypertension noted in this and our previous study was that there was a difference in hypertension between male and female rats exposed to prenatal dexamethasone. At 2 to 3 months of age, only the female 15 and 16 prenatal dexamethasone group had hypertension compared with 15 and 16 and 17 and 18 in the male rats. In this study, at 6 months of age, the male rats that received prenatal dexamethasone on days 13 and 14 also were hypertensive. The female rats that received prenatal dexamethasone were not hypertensive at 6 months of age yet had hypertension at 2 months. The reason for the amelioration in hypertension is unknown. It is also of interest that only the female rats at 3 weeks of age that received prenatal dexamethasone on days 15 and 16 of life had elevated blood pressures, whereas the male rats did not. The reason for this disparity is unclear at present but underlies the importance of studying both genders.
In our previous and current study, we found that prenatal dexamethasone administered on days 15 and 16 of gestation resulted in a reduction in glomerular number compared with control.9 There were several possible causes for the reduction in glomerular number. The reduction in glomerular number may result from intrauterine injury resulting in a paucity in glomerular number from the time of birth or a progressive reduction in glomerular number either caused by hypertension or programmed accelerated glomerular senescence. We found a 14% reduction in glomerular number in 3-week-old animals treated with dexamethasone at days 15 and 16 of gestation and a 20% reduction in animals studied at 6 to 9 months of age. The fact that we were able to measure a reduction in glomerular number in infant rats suggests that the damage probably was due to an intrauterine insult. It should be mentioned that the method used to measure glomerular number in this study has not been validated in young rats. The total number glomeruli in control 3-week-old rats were less than that in adult control rats, even though nephrogenesis is complete. Thus, it is likely that not all the glomeruli in the 3-week-old rats were counted. Nonetheless, we were able to demonstrate that there is a significant reduction in glomeruli at this age.
There was also a difference in glomerulosclerosis between our previous study and the current one. Focal segmental glomerulosclerosis was rare at 2 months of age, and there was no difference in control and dexamethasone-treated rats. However, one of the most important findings in the current study there was a statistically significant increase in glomerulosclerosis in both male and female rats who were exposed to prenatal dexamethasone on days 15 and 16 of gestation. This demonstrates that prenatal dexamethasone can cause glomerular injury in rats.
Perspectives
This study demonstrates that prenatal dexamethasone administered at specific times of gestation results in a reduction in glomerular number in infant as well as adult rats. The hypertension was manifested at 3 weeks of age in female rats but not male rats. In older rats, male rats that received prenatal dexamethasone at specific times during gestation had more severe hypertension than female rats. This study extends our previous findings to show that intrauterine exposure to dexamethasone can program the rat to develop glomeru-losclerosis. Finally, the hypertension was not solely due to the reduction in glomerular number since the reduction was modest and there were hypertensive males in the 13 and 14 dexamethasone group that did not have a reduction in glomerular number. The relevance of this study to humans is unclear at present. Although the 2 daily doses of dexamethasone were administered using a comparable dose per kg body weight as used in humans to accelerate pulmonary maturation, the development of the rat kidney is significantly different than the human kidney. Nephrogenesis ends by about 34 weeks of gestation in the human, but rats continue to form new nephrons until approximately 1 week after birth. Thus the time during gestation and when glucocorticoids affect rat renal development may be quite different in the human. Whether prenatal dexamethasone in humans increases the likelihood of developing hypertension and renal disease is unknown.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease grant DK-41612 (M.B.).
References
- 1.Ballard P, Granberg LP, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest. 1975;56:1548–1554. doi: 10.1172/JCI108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley P. Promoting pulmonary maturity. In: Chalmers IM, Keirse KJNC, editors. Effective Care in Pregnancy. Oxford, UK: Oxford University Press; 1989. pp. 746–764. [Google Scholar]
- 3.NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 4.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature rats. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 5.Reinisch JM, Simon NG, Karow WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 6.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CRW. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 7.Celci G, Kistner A, Aizman R, Eklof A, Ceccatelli S, De Santiago A, Jacobson SK. Prenatal dexamethasone causes oligonephronia, sodium retention and a higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Epstein MF, Farrell PM, Sparks JW, Pepe G, Driscoll SG, Chez RA. Maternal betamethasone and fetal growth and development in the monkey. Am J Obstet Gynecol. 1977;127:261–263. doi: 10.1016/0002-9378(77)90465-3. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci. 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- 11.Dodic M, Peers A, Moritz K, Hantzis V, Wintour EM. No evidence for HPA reset in adult sheep with high blood pressure due to short prenatal exposure to dexamethasone. Am J Physiol Regul Comp Physiol. 2002;282:R343–R350. doi: 10.1152/ajpregu.00222.2001. [DOI] [PubMed] [Google Scholar]
- 12.Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002;16:1017–1026. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- 13.Dodic M, Baird R, Hantzis V, Koukoulas I, Moritz K, Peers A, Wintour EM. Organs/systems potentially involved in one model of programmed hypertension in sheep. Clin Exp Pharmacol Physiol. 2001;28:952–956. doi: 10.1046/j.1440-1681.2001.03556.x. [DOI] [PubMed] [Google Scholar]
- 14.Edwards LJ, Coulter CL, Symonds ME, McMillen IC. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clin Exp Pharmacol Physiol. 2001;28:938–941. doi: 10.1046/j.1440-1681.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 15.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23:171–175. [PubMed] [Google Scholar]
- 16.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure: less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 17.Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 19.Cogan MG, Liu F-Y. Metabolic alkalosis in the rat: evidence that reduced glomerular filtration rather than enhanced tubular bicarbonate reabsorption is responsible for maintaining the alkalotic state. J Clin Invest. 1983;71:1141–1160. doi: 10.1172/JCI110864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankir L, Hollenberg NK. In vivo staining of the kidney with Alcian blue: an adjunct to morphological and physiological studies. Renal Physiol. 1983;6:151–155. doi: 10.1159/000172894. [DOI] [PubMed] [Google Scholar]
- 21.Damadian RV, Shwayri E, Bricker NS. On the existence of non-urine forming nephrons in the diseased kidney of the dog. J Lab Clin Med. 1965;65:26–39. [PubMed] [Google Scholar]
- 22.Gilbert T, Lelievre-Pegorier M, Malienou R, Meulemans A, Merlet-Benichou C. Effects of prenatal and postnatal exposure to gentamicin on renal differentiation in the rat. Toxicology. 1987;43:301–313. doi: 10.1016/0300-483x(87)90089-8. [DOI] [PubMed] [Google Scholar]
- 23.Edwards CRW, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 24.Langley-Evans SC. Maternal carbenoxolone treatment lowers birth-weight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci. 1997;93:423–429. doi: 10.1042/cs0930423. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay RS, Lindsay RM, Edwards CRW, Seckl JR. Inhibition of 11β-Hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 26.Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CRW, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 27.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: the fetal flaw in essential hypertension? Kidney Int Suppl. 1996;55:S30–S34. [PubMed] [Google Scholar]
- 29.Langley-Evans SC, Jackson AA. Captopril normalizes systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol. 1995;110A:223–338. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 30.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci. 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- 31.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Manning M, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol, Renal Physiol. 2002;283:F202–F206. doi: 10.1152/ajprenal.00358.2001. [DOI] [PubMed] [Google Scholar]