Abstract
Purpose
We aim to develop a protein microarray platform capable of presenting both natural and denatured forms of proteins for antibody biomarker discovery. We will further optimize plasma screening protocols to improve detection.
Experimental design
We developed a new covalent capture protein microarray chemistry using HaloTag fusion proteins and ligand. To enhance protein yield, we used HeLa cell lysate as an in vitro transcription translation system (IVTT). E. coli lysates were added to the plasma blocking buffer to reduce non-specific background. These protein microarrays were probed with plasma samples and autoantibody responses were quantified and compared with or without denaturing buffer treatment.
Results
We demonstrated that protein microarrays using the covalent attachment chemistry endured denaturing conditions. Blocking with E. coli lysates greatly reduced the background signals and expression with IVTT based on HeLa cell lysates significantly improved the antibody signals on protein microarrays probed with plasma samples. Plasma samples probed on denatured protein arrays produced autoantibody profiles distinct from those probed on natively displayed proteins.
Conclusions and clinical relevance
This versatile protein microarray platform allows the display of both natural and denatured proteins, offers a new dimension to search for disease-specific antibodies, broadens the repertoire of potential biomarkers, and will potentially yield clinical diagnostics with greater performance.
Keywords: Antibody, Autoantibody, Biomarker, Protein microarray, Denaturation
Antibodies are the products of the human adaptive immune response, which usually targets antigens from foreign pathogens in order to neutralize or destroy them. Disease-specific antibodies have great clinical utilities. The diagnosis and clinical evaluation of numerous infectious and autoimmune diseases rely on profiling serum antibodies against specific antigens. One special class of disease-specific antibodies is called autoantibody (AAb) because it targets self-proteins. In autoimmune diseases, AAbs play critical roles during disease pathogenesis. The discovery of AAbs in cancers against tumor associated antigens (TAAs) has generated great interest because of their potential use as early detection biomarkers[1]. The detailed mechanisms of how certain proteins become TAAs and trigger the immune response are still unclear, but the development of AAbs may relate to tumor antigen overexpression, mutation, or altered post-translational modification, etc. As AAbs are the responses of our body to the aberrant nature of cancer, they may serve as indicators of tumor initiation, treatment response, or disease prognosis[1]. Circulating AAbs are easy to access, very stable, and highly specific, making them particularly useful as potential serum cancer biomarkers.
The human immune system generates antibodies against both conformational and linear epitopes. Conformational epitopes are recognized by antibodies when the epitope domain is properly folded and may include amino acids from distant parts of the linear polypeptide brought together by secondary and tertiary folding. In contrast, linear epitopes comprise a single linear peptide, usually 7 amino acids long[2]. Linear epitopes may sometimes be buried inside a folded protein, preventing them from detection by antibodies. AAbs have been linked to both conformational and linear epitopes[3][4].
Protein microarrays displaying full-length human proteins enable screening humoral immune responses against thousands of antigens in parallel to search for disease-specific AAb biomarkers[5, 6], including cancer[7–9] and autoimmune diseases[10]. Conventional protein microarrays present proteins produced in and purified from bacteria, insect cells or yeast, which are then displayed on glass slides through various attachment chemistries including: amine reactive chemistry, anti-tag antibodies; and hydrophobic interactions (nitrocellulose coated slides) [11–15]. Low signals and high backgrounds have plagued detection sensitivity and specificity on protein microarrays probed with serum. This is particularly true in the case of AAbs where the responses to self-proteins are often weaker than those to foreign invaders. The backgrounds may be associated with the methods for protein production and/or the printing conditions.
Signals of AAb responses are usually related to the amount of immobilized proteins and the mechanism of epitope presentation. In this study, we aim to improve the detection of AAbs on microarrays by reducing background and improving specific signals. Protein microarrays generally display folded or semi-folded proteins, which could obscure some linear epitopes buried within the proteins. These protein microarrays may fail to identify AAbs that recognize such epitopes. There is a need to develop a platform that displays antigens in both native and denatured states.
To this end, we developed a versatile protein microarray platform capable of presenting proteins for antibody and AAb screening in both native and denatured states, as needed, that is based on our Nucleic Acid Programmable Protein Array (NAPPA) platform. NAPPA in its standard format expresses epitope-tagged proteins just-in-time from printed cDNA and captures them to the surface with an anti-tag antibody. Proteins on NAPPA are expected to be properly folded by virtue of the chaperone proteins used during expression and have been demonstrated to display appropriate protein-protein interactions and enzymatic activities [13, 14]. Thus standard NAPPA is a useful platform for displaying conformational epitopes; however, it was not clear if it could withstand denaturation. Indeed, after treatment of a standard NAPPA array with 125 mM Tris-Cl, 2% SDS, 100mM β-mercaptoethanol at 37°C for 30 min with mild agitation, we were unable to detect the protein using a protein specific antibody that recognizes a linear epitope, presumably because the protein was released from the slide surface (Figure 1B).
Figure 1.
HaloTag protein arrays can withstand harsh denaturing conditions. A. Picogreen staining of printed DNA (Left) and anti-HaloTag antibody detection of HaloTagged protein display (Right). The color scale for all protein array images is shown on right. B. Detection of p53 using an anti-p53 antibody on protein arrays where proteins were immobilized by GST/ anti-GST or HaloTag / HaloTag ligand with or without denaturation.
To solve this problem, we switched the immobilization chemistry from affinity capture to the covalent linkage between HaloTag and its ligand. HaloTag is a modified haloalkane dehalogenase developed to covalently bind to halogenated alkanes[15]. We hypothesized that protein microarrays with covalent immobilization chemistry would withstand harsh denaturation treatment without losing proteins from the slide surface.
To test this hypothesis, we designed and constructed pJFT7-nHalo vector that bears a Gateway™ death cassette next to an N-terminal fusion HaloTag protein (Supplementary Figure S1). This vector has a T7 promoter and an encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) sequence upstream of the gene of interest. We then transferred 69 genes from our DNASU repository (supporting information Table S1) from donor vector (pDONR221) to pJFT7-nHalo through Gateway™ LR reactions. DNA was miniprepped using a customized protocol as described[14].
To manufacture protein microarrays, for each gene, the printing mix of BSA, HaloTag ligand, BS3 and DNA plasmid was prepared at concentrations of 3.7 mg/ml, 0.5 mM, 5 mM and ~1000 ng/µl, respectively, at a final volume of 30 µl. DNA samples were incubated overnight at 4°C before arrayed onto aminosilane coated slides by Genetix QArray2 with 350 µm solid pins. Each protein was fused to the n-terminal HaloTag. Just-in-time, in vitro expressed proteins were immobilized in situ to co-spotted HaloTag ligand that was linked to the slide surface using the amine chemistry. Picogreen staining of printed DNA and anti-HaloTag antibody detection of expressed proteins were carried out subsequently for quality control (Figure 1A).
In order to determine whether the protein microarrays displaying protein captured via covalent linkage would survive harsh washing conditions, we treated the slides with denaturing conditions and detected p53 using DO-1 monoclonal anti-p53 antibody, which recognizes a normally exposed linear epitope QETFSDLWKL on p53[16]. As a control, we did the exact same treatment on standard NAPPA which uses anti-GST antibody as the capture reagent. Similar p53 levels were detected in both denatured and non-denatured HaloTag-based protein microarrays, whereas p53 signal disappeared after denaturation on GST-tag NAPPA (Figure 1B).
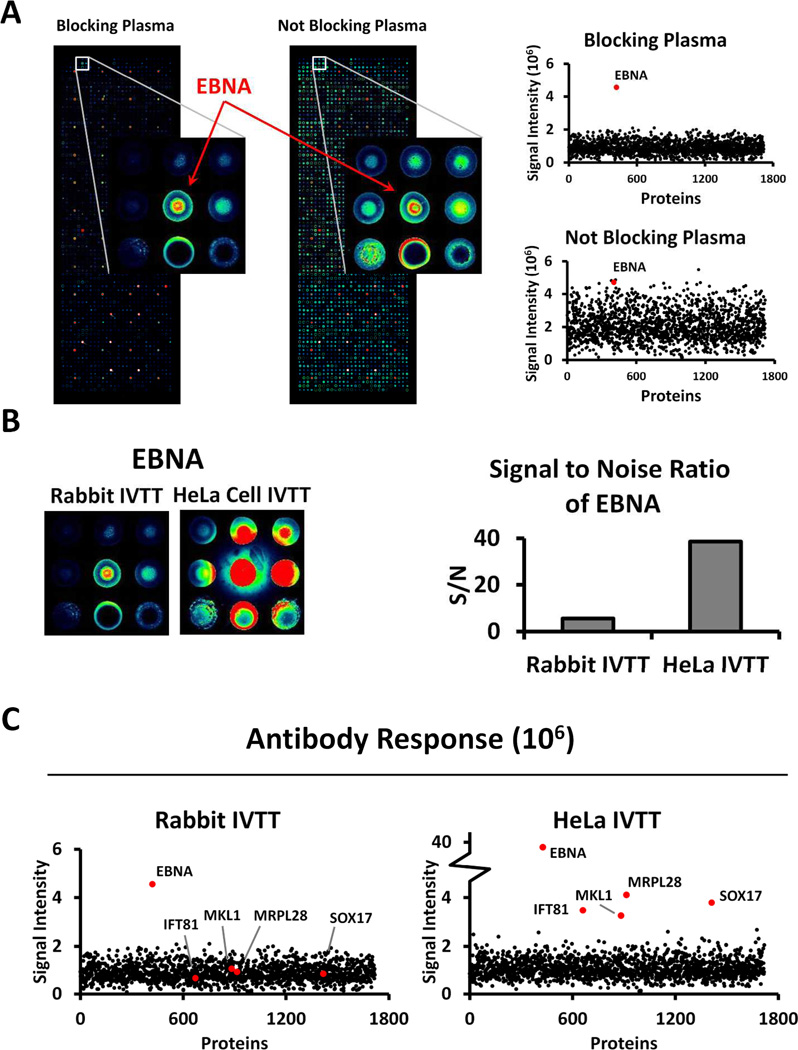
During serum screening experiments, approximately 10% of samples show generalized background signals on all features with intensities that correlate with the serum sample (data not shown). Such background signals are particularly evident on features with DNA, and less on features with printing buffer only, and they do not correlate with the amount of displayed protein. To address this issue, we tested the possibility of using E. coli lysates as a blocking reagent for plasma, because it has been reported to reduce background signal[17] (Figure 2A). We selected a plasma sample known to have high background and probed two identical NAPPA slides displaying around 2000 full-length human proteins as well as a domain of the Epstein-Barr virus nuclear antigen (EBNA) as a positive control. (In our experience, about 90% of individuals have sero-reactivity to EBNA[18].) The proteins on the slides were produced by in vitro transcription and translation (IVTT) using rabbit reticulocyte lysate as a source of ribosomes. One of them was probed with plasma pre-blocked with E. coli lysate, the other one without blocking served as control. In the right panel of Figure 2A, the EBNA signal is shown in red. Although the “true signal” represented by the EBNA response was unchanged, the background signal on the other proteins was significantly reduced when blocked with E. coli lysate. We speculated that trace amounts of bacterial proteins carried over through the DNA miniprep might contribute to this background in individuals who had immune responses to these E. coli proteins.
Figure 2.
Optimization of the plasma screening protocol by blocking plasma samples with E.coli lysate and using HeLa IVTT expression system. A. Blocking plasma with E.coli lysate before probing onto arrays could reduce background signals. Two identical arrays were probed with the same plasma sample. Left, plasma pre-blocked by E.coli lysate; right, no pre-blocking. Plots of antibody response signal intensity of all protein spots are shown on the right: top, plasma pre-blocked by E.coli lysates; bottom, no pre-blocking. B. HeLa IVTT improved the detection of antibody response. Zoomed-in images of antibody response to EBNA on NAPPA arrays expressed by either Rabbit IVTT or HeLa IVTT are shown on the left. Experiment condition was the same as that in Figure 2A, with plasma pre-blocked by E.coli lysate. Signal to noise ratio plot of EBNA antibody responses is shown on the right. C. Plots of antibody response signal intensity of all protein spots. Increased autoantibody responses against several human proteins when using HeLa IVTT expressed NAPPA arrays were detected (shown in red dots).
A common system used for the coupled transcription and translation of proteins has been rabbit reticulocyte-based IVTT mix (Promega), which has the advantage of using mammalian ribosomes and chaperones for protein production. This system is highly efficient, and has succeeded at producing thousands of different proteins, leading to the identification of disease-specific antibodies in cancers and infectious disease[8, 19, 20]. A new mammalian cell free expression system derived from cultured human HeLa cells (HeLa IVTT; Thermo Scientific) was made available recently. We found that E. coli lysate blocking also worked with HeLa cell lysates (Supplementary Figure S2). We also compared its performance with rabbit IVTT for plasma autoantibody detection after blocking plasma with E. coli lysate. We probed the same plasma sample onto two identical protein microarrays expressed with either rabbit IVTT or HeLa IVTT. Arrays expressed with HeLa IVTT gave much higher EBNA response (Figure 2B). The signal to noise ratio (EBNA signal intensity : median signal intensity of all spots on one slide) was calculated for each system and compared. As indicated in Figure 2B, we achieved a 7 fold increase of signal to noise ratio in the HeLa IVTT. Furthermore, some AAbs showed reactivity only on the HeLa IVTT expressed slide. Signal intensities for proteins IFT81, MKL1, MRPL28, SOX17 (red dots) were greater than the background in the HeLa IVTT expressed slide, but they were hidden in the background on the rabbit IVTT expressed slide (Figure 2C).
We then applied the optimized AAb screening protocol to evaluate the effect of denaturation on AAb detection. AAb profiles for the same plasma sample on denatured and non-denatured HaloTag NAPPA arrays were compared. For each condition, an additional “mock-expressed” slide, which used HeLa IVTT lacking T7 polymerase (thus incapable of expressing proteins), was used as a negative control to accurately measure background signals unrelated to protein expression. AAb responses were calculated by subtracting spot intensities on slides without protein expression from those on slides with expression. Spots with calculated signals less than 0 were set to 0. We detected AAb response changes against several proteins when comparing denatured and non-denatured slides. The AAb response against TPD52 (red dots) was lower in the denatured slide (Figure 3A; suggesting a conformational epitope), whereas AAb responses against MYC and CCNA1 (green dots) were higher (suggesting linear epitopes). Images of antibody responses to CCNA1 on both denatured and non-denatured HaloTag protein microarrays are shown in Figure 3B as an example (AAb response to TPD52 and MYC are shown in the Supplementary Figure S3). It is obvious that AAb responses to CCNA1 were much stronger when the protein was denatured. Denaturation of proteins may expose hidden linear epitopes as well as unfold conformational epitopes that only exist in the non-denatured form. TPD52 may lose its epitope after denaturation, whereas MYC and CCNA1 may gain their epitopes through denaturation. The adaptation of HaloTag to protein microarrays not only maintains its utility for studying of proteins where conformational presentation is needed, but also, more importantly, enables applications where denatured proteins are preferred. Combining the information from both natural and denatured protein microarrays may increase both the diagnostic sensitivity and the number of potential biomarkers, as more responses can be discovered.
Figure 3.
NAPPA using covalent capture chemistry enables the detection of AAb against denatured proteins. A. AAb response profile against proteins on denatured NAPPA was different from that on non-denatured NAPPA. Red squares, AAb response decreased after denaturation. Green dots, AAb response increased after denaturation. B. An example of different AAb response between the native protein array and the denatured protein array. NAPPA requires transcription of cDNA by T7 polymerase into mRNA before translation. T7 polymerase was not added to arrays labeled with “not expressed” to assess non-specific background signals.
In conclusion, we have developed a protein microarray platform where proteins are covalently linked to the matrix. We demonstrated that protein microarrays using the HaloTag and its ligand immobilization chemistry could survive denaturing buffer condition and produce distinct AAb response profiles from the non-denatured protein microarrays when probed with plasma samples. We recognize that more work needs to be done to analyze the differences of AAb profiles systematically between denatured and native protein microarrays using a larger sample size and determine its utility in identifying AAb biomarkers with better clinical performance. We believe that this method can easily extend beyond AAbs detection in cancers to automimmune and infectious diseases. Moreover, covalent attachment of proteins on the matrix for denaturation can also be adapted to other types of protein microarrays.
Supplementary Material
Statement of clinical relevance.
In addition to their protective roles, circulating antibodies serve as diagnostic and prognostic markers for many diseases. One class, autoantibodies (AAb), shows particular promise for the detection of cancer. Antibodies target both conformational and linear epitopes, the latter sometimes requiring denaturation for detection. Protein microarrays provide a high-throughput multiplexed platform to characterize antibody responses in diseases. However, conventional protein microarrays are designed to display folded proteins and cannot withstand denaturing treatments. To this end, we developed a protein microarray platform that is capable of displaying proteins in both native and denatured states depending on defined experimental protocols. This allows the discovery of antibody biomarkers with potentially greater clinical performance that recognize different epitopes.
Acknowledgments
We would like to thank Carlos Morales Betanzos for advice on preparing the figures. We would also like to thank Eliseo Mendoza Garcia for helping with manufacturing NAPPA. The research relevant to this paper was supported by a grant from the Early Detection Research Network (5U01CA117374-02) (J.L. and J.Q.).
Samples from the Polish breast cancer study (PBCS) were collected under IRB protocol# OH99CN040, and supported by Intramural Research Funds of the National Cancer Institute, Department of Health and Human Services, USA. Written consent was obtained from all human subjects under institutional review board approval.
The PBCS would like to thank Montserrat Garcia-Closas, Louise Brinton, Mark Sherman, Jolanta Lissowska, Neonila Szeszenia-Dabrowska, Beata Peplonska, Witold Zatonski for their efforts in launching and conduct of the study.
Abbreviations
- NAPPA
Nucleic Acid Programmable Protein Array
- AAb
Autoantibody
- TAA
Tumor Associated Antigen
- IVTT
in vitro transcription translation system
- EBNA
Epstein-Barr virus nuclear antigen
Footnotes
The authors have declared no conflict of interest.
References
- 1.Piura E, Piura B. Autoantibodies to tailor-made panels of tumor-associated antigens in breast carcinoma. J Oncol. 2011;2011:982425. doi: 10.1155/2011/982425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larman HB, Zhao Z, Laserson U, Li MZ, et al. Autoantigen discovery with a synthetic human peptidome. Nat Biotechnol. 2011;29:535–541. doi: 10.1038/nbt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BJ, Namchuk M, Bugawan T, Fu Q, et al. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD(65), distinguish stiff-man syndrome from insulin-dependent diabetes-mellitus. J Exp Med. 1994;180:595–606. doi: 10.1084/jem.180.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz HL, Chandonia JM, Kash SF, Kanaani J, et al. High-resolution autoreactive epitope mapping and structural modeling of the 65 kDa form of human glutamic acid decarboxylase. J Mol Biol. 1999;287:983–999. doi: 10.1006/jmbi.1999.2655. [DOI] [PubMed] [Google Scholar]
- 5.Kijanka G, Murphy D. Protein arrays as tools for serum autoantibody marker discovery in cancer. J Proteomics. 2009;72:936–944. doi: 10.1016/j.jprot.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran N, Srivastava S, Labaer J. Applications of protein microarrays for biomarker discovery. Proteomics Clin Appl. 2008;2:1444–1459. doi: 10.1002/prca.200800032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu J, Madoz-Gurpide J, Misek DE, Kuick R, et al. Development of natural protein microarrays for diagnosing cancer based on an antibody response to tumor antigens. J Proteome Res. 2004;3:261–267. doi: 10.1021/pr049971u. [DOI] [PubMed] [Google Scholar]
- 8.Anderson KS, Sibani S, Wallstrom G, Qiu J, et al. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J Proteome Res. 2011;10:85–96. doi: 10.1021/pr100686b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam MJ, Madoz-Gurpide J, Wang H, Lescure P, et al. Molecular profiling of the immune response in colon cancer using protein microarrays: occurrence of autoantibodies to ubiquitin C-terminal hydrolase L3. Proteomics. 2003;3:2108–2115. doi: 10.1002/pmic.200300594. [DOI] [PubMed] [Google Scholar]
- 10.Hueber W, Utz PJ, Steinman L, Robinson WH. Review Autoantibody profiling for the study and treatment of autoimmune disease. Arthritis Res. 2002;4:290–295. doi: 10.1186/ar426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacBeath G, Schreiber SL. Printing proteins as microarrays for high-throughput function determination. Science. 2000;289:1760–1763. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Bilgin M, Bangham R, Hall D, et al. Global analysis of protein activities using proteome chips. Science. 2001;293:2101–2105. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran N, Hainsworth E, Bhullar B, Eisenstein S, et al. Self-assembling protein microarrays. Science. 2004;305:86–90. doi: 10.1126/science.1097639. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran N, Raphael JV, Demirkan G, Fuentes MG, et al. High density self-assembling functional protein arrays. Nat Methods. 2008;5:535–538. doi: 10.1038/nmeth.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurst R, Hook B, Slater MR, Hartnett J, et al. Protein-protein interaction studies on protein arrays: effect of detection strategies on signal-to-background ratios. Anal Biochem. 2009;392:45–53. doi: 10.1016/j.ab.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Stephen CW, Helminen P, Lane DP. Characterisation of epitopes on human p53 using phage-displayed peptide libraries: insights into antibody-peptide interactions. J Mol Biol. 1995;248:58–78. doi: 10.1006/jmbi.1995.0202. [DOI] [PubMed] [Google Scholar]
- 17.Vigil A, Chen C, Jain A, Nakajima-Sasaki R, et al. Profiling the humoral immune response of acute and chronic Q fever by protein microarray. Mol Cell Proteomics? 2011;10:M110.006304. doi: 10.1074/mcp.M110.006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran N, Anderson KS, Raphael JV, Hainsworth E, et al. Tracking humoral responses using self assembling protein microarrays. Proteomics Clin Appl. 2008;2:1518–1527. doi: 10.1002/prca.200800034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montor WR, Huang J, Hu Y, Hainsworth E, et al. Genome-wide study of Pseudomonas aeruginosa outer membrane protein immunogenicity using self-assembling protein microarrays. Infect Immun. 2009;77:4877–4886. doi: 10.1128/IAI.00698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright C, Sibani S, Trudgian D, Fischer R, et al. Detection of Multiple Autoantibodies in Patients with Ankylosing Spondylitis Using Nucleic Acid Programmable Protein Arrays. Mol Cell Proteomics. 2012;11:M9.00384. doi: 10.1074/mcp.M9.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.