Abstract
Purpose
To investigate the recently discovered association between gadolinium based MRI contrast agents and the development of nephrogenic systemic fibrosis (NSF) in patients with chronic kidney disease or acute kidney injury.
Materials and Methods
A systematic review of the PubMed database and publicly available patient databases was performed to characterize NSF and its possible association with exposure to gadolinium based MRI contrast agents.
Results
Data from case series reports, NSF patient databases, NSF case reporting to the Federal Drug Administration (FDA) after gadolinium contrast agent exposure, and retrospective case-control studies suggest a strong association between the use of gadolinium based MRI contrast agents and the subsequent development of NSF in patients with renal disease. These data also suggest that the risk of NSF depends on the degree of renal dysfunction, dose of contrast agent, gadolinium contrast agent stability, and severity of concomitant illness. Thus, the occurrence of NSF after gadolinium contrast agent exposure may vary from negligible up to 2–5% in select high-risk clinical situations.
Conclusions
MRI utilizing gadolinium based contrast agents must be undertaken judiciously in patients with renal dysfunction carefully weighing, on a case by case basis, the benefits of MRI and the risk of NSF as well as the disadvantages of undergoing alternative imaging studies or foregoing imaging studies.
Keywords: gadolinium, NSF, Nephrogenic Fibrosing Dermopathy, NFD
The prevalence of kidney disease is rising and selecting imaging studies in renal disease patients is challenging leading to a growing clinical problem. Historically, contrast-induced nephropathy has limited contrasted computed tomography (CT) studies, while magnetic resonance imaging (MRI) studies have been considered “safe” in patients with chronic kidney disease (CKD) or acute kidney injury (AKI). A recently discovered association between gadolinium based MRI contrast agents and the development of nephrogenic systemic fibrosis (NSF) in patients with severely reduced renal function has cast doubt on the dogma of MRI safety in these patients and has further complicated decision making regarding imaging in this population. Herein for the practicing urologist, we will review the history and clinical features of nephrogenic systemic fibrosis, its association with gadolinium based MRI contrast agents, and suggested approaches to imaging studiesin patients with renal dysfunction in light of these new data.
Nephrogenic Systemic Fibrosis: A New Devastating Disease
Nephrogenic systemic fibrosis is a new disease entity initially discovered in 1997 and documented in the landmark case series by Cowper and colleagues in 2000 as a scleromyxoedema-like cutaneous disease in dialysis patients.1 This case series reported 15 dialysis patients presenting with extensive thickening and hardening of the skin with brawny hyperpigmentation primarily affecting the extremities. Skin biopsy of affected areas revealed a unique pattern of dense, haphazardly arranged collagen bands and an increased number of fibroblast-like spindle cells in the dermis often extending deep into the subcutaneous tissue. The nascent disorder was coined nephrogenic fibrosing dermopathy (NFD) to succinctly describe its pathology and association with renal disease.2
NFD clearly carries significant morbidity and mortality. Most patients suffer from neuropathic pain in affected areas and develop flexion contractures from skin thickening and tightening, which limits mobility occasionally over the course of weeks. Patient deaths have been reported related to immobility and debilitation and the associated infectious complications such as sepsis from pressure ulcer infections or aspiration pneumonia.3–5 Furthermore, autopsies of NFD patients have suggested a systemic fibrotic process with skeletal muscle, pleural, pericardial, myocardial, and pulmonary fibrosis that may also contribute to morbidity and mortality.6 In light of these data, the disease was renamed nephrogenic systemic fibrosis (NSF) to emphasize its systemic manifestations.
From an epidemiologic perspective, the emergence of a clinically unique disease restricted to dialysis patients immediately raised the possibility of a causal relationship between the dialytic process and the development of NSF. However, subsequent case reports documented NSF in patients with severe CKD or AKI who did not receive dialytic therapy.7–9 Although about 90% of cases to date have been reported in dialysis patients, NSF associates with renal dysfunction and not dialysis per se.3, 10 No cases of NSF in patients with a glomerular filtration rate (GFR) > 30 mL/min have been described. One study suggested that NSF may occur in patients with an estimated GFR > 30 mL/min.11 Closer examination suggests that these patients exhibited AKI, where non-steady state serum creatinine levels invalidate approaches used to estimate GFR based on serum creatinine. Moreover, the vast majority of AKI results in a GFR < 30 mL/min irrespective of serum creatinine. Thus, NSF becomes evident when GFR falls below 30 mL/min in either AKI or CKD and its incidence correlates with the degree of renal dysfunction being most common in end-stage renal disease (ESRD) patients.
While NSF is rare, its exact prevalence is unclear. As of the end of 2006, the Yale NSF Registry, the largest patient database, consisted of slightly over 200 cases.10 The U.S. Food and Drug Administration (FDA) and the Pharmacovigilance Working Party in Europe have each collected about 90 NSF cases.12, 13 Thus, there are at most about 400–500 reported cases of NSF worldwide as of the end of 2006. Many NSF cases, particularly milder manifestations, may remain unreported.
Identifying Links Between Gadolinium Based MRI Contrast Agents and NSF
The emergence of NSF in a small percentage of severe CKD or AKI patients suggests an inciting agent or exposure may be responsible. In early 2006, Grobner reported a case series of 5 dialysis patients who developed NSF weeks after administration of a gadolinium based MRI contrast agent (GBMCA) suggesting an association between these agents and NSF.14 Grobner’s report then spawned a flurry of case reports throughout 2006 and 2007, initially in Europe and then in the U.S., connecting NSF with prior GBMCA exposure.11, 15–18 Retrospective analysis of the Yale NSF Registry revealed that GBMCA exposure predated NSF in over 95% of cases by weeks to months.10 These reports suggested that a very small minority of NSF cases occurred without preceding GBMCA exposure.10, 19
The proportion of patients developing NSF after GBMCA use in patients with renal dysfunction is around 2–5% in single center cohorts.11, 15, 18, 20 The number of MRI contrast studies in kidney disease or hemodialysis patients in large populations has not been directly reported. Based on market research analyses, it can be conservatively estimated that at least 50,000 MRI contrast studies have been conducted in hemodialysis patients in the U.S. during the last 10 years based on the total number of MRI contrast studies (at least 50 million) and the proportion of ESRD patients in the U.S. (about 0.1%).21 Given that there are at most 500 reported cases of NSF worldwide and fewer in the U.S. alone, the proportion of patients developing reported NSF after gadolinium based contrast agent exposure in hemodialysis patients is probably no more than 1%. While we acknowledge these estimates are crude, they suggest that the 2–5% estimates of NSF incidence after GBMCA exposure based on single center cohorts may be inflated. The reporting of previously unrecognized cases of NSF could raise all estimates.
The latency between GBMCA exposure and developing NSF is variable. In most patients, the lag period is typically weeks to months, but a small subset of patients have prolonged lag periods extending to 1–2 years.16, 20 Some argue that these cases reflect delayed recognition of NSF or slowly evolving biologic events after GMBCA exposure leading to clinically evident NSF. But equally possible is that cases with prolonged lag periods represent NSF occurring independently of unrelated, distant GBMCA exposure. Unfortunately, no current data can differentiate these interpretations, which could weaken the association between GBMCA exposure and NSF.
GBMCA dose correlates with the occurrence and/or severity of NSF. Most studies note that NSF develops more frequently in patients after MRI studies using high contrast dose.11, 15, 20, 22 Magnetic resonance angiography (MRA) studies typically utilize two to three times the contrast dose compared to conventional MRI studies particularly when utilized to image abdominal and peripheral vascular territories. 23 Despite widespread off-label use, the FDA has never approved a gadolinium based MRI contrast agent for MRA studies.24 In many cohorts, the majority of NSF cases have been noted after intra-abdominal and peripheral vascular MRA studies.11, 15, 17, 20 The discovery of NSF in 1997 and its increasing prevalence may temporally correlate with the increasing use of non-cerebral MRA studies starting in the mid to late 1990’s. NSF also occurs more commonly with high cumulative GBMCA dose exposure after multiple contrasted MRI studies within a short period of time.11, 20 Other reports have also found an association between GBMCA dose and the clinical severity of subsequent NSF.19, 22
The case control studies conducted thus far do not allow for precise quantification of absolute NSF risk after GBMCA exposure given their inherent shortcomings including small numbers, retrospective analysis, and well-described forms of statistical bias. The burden of proof qualitatively supports a strong association between GBMCA administration and developing NSF. Given the devastating nature of NSF, the qualitative data warrants a change in clinical practice towards avoiding contrasted MRI studies in patients with severely reduced renal function.
Pathophysiologic Basis for Gadolinium Induced NSF
Gadolinium based MRI contrast agents are chemical chelates with a gadolinium ion bound by a linear or cyclic molecule. Since free gadolinium ions are toxic and poorly excreted, GBMCA safety completely rests on the ability of the chelate molecule to prevent gadolinium release after intravenous administration. In animals and normal human subjects, gadolinium based MRI contrast agents release presumably safe, minute quantities of gadolinium ion with subsequent tissue deposition particularly into liver and bone.25 However, the kidneys solely excrete these agents and their half-life prolongs from 1–2 hours to over 30 hours with severe renal dysfunction.25–27 Renal failure establishes a situation where gadolinium based MRI contrast agents reside within patients for long periods of time potentially allowing for toxic levels of gadolinium ion release and tissue deposition.
Preliminary evidence suggests that NSF may represent a form of gadolinium ion toxicity. First, skin biopsies from NSF patients have demonstrated gadolinium ion deposits in areas of abnormal fibrosis.28, 29 Second, NSF risk highly correlates with the severity of renal failure and GBMCA dose, which determine the duration and intensity of GBMCA exposure and therefore the extent of gadolinium ion release. Finally, the development of NSF after GBMCA exposure may be proportional to the propensity of the chelate molecule to release gadolinium ion after administration.
Gadodiamide (Omniscan™; GE Healthcare, Princeton, NJ), gadoversetamide (OptiMARK®; Mallinckrodt, Hazelwood, MO), gadopentetate (Magnevist®; Bayer Healthcare Pharmaceuticals, Montville, NJ), gadoteridol (ProHance®; Bracco Diagnostics, Inc., Princeton, NJ), and gadobenate (MultiHance®; Bracco Diagnostics, Inc., Princeton, NJ) are the five approved MRI contrast agents in the United States. Linear chelates, in general, are less stable compared to cyclic chelates and the rank order for chelate instability and gadolinium ion release is Omniscan > Magnevist > MultiHance > ProHance. OptiMARK, the newest agent on the market, has been less studied but is biochemically similar to Omniscan.25, 27 Most case reports suggest that the vast majority of NSF cases are associated with Omniscan, the agent with the least chelate stability. The Yale NSF registry has reported that 90% of their cases are linked to Omniscan administration.10 Furthermore, the 2–5% incidence of NSF after GBMCA exposure in single center studies may be higher than the nationwide incidence, since most centers with NSF cohorts solely utilized Omniscan, while the U.S. as a whole uses a mixture of agents.
Alternatively, the association of NSF with Omniscan may be related to more common use of this agent. Overall market share data does not support this hypothesis as Magnevist holds about 50% of the U.S. and worldwide market share followed by Omniscan at 20–30% with the remaining market share almost equally split amongst ProHance, MultiHance, and OptiMARK.21 Based on its FDA approval for high dose central nervous system MRI, Omniscan may be preferentially utilized for high-risk, high contrast dose MRI applications, which would explain a disproportionate association with NSF.30 Definitive data to refute this possibility are currently unavailable.
The correlation between gadolinium chelate instability and NSF extends beyond Omniscan. Up to 10% of NSF cases in the Yale registry have been associated with the use of Magnevist and OptiMARK, while no cases have been associated with MultiHance or ProHance.10 While Magnevist associated NSF cases may correlate with its large market share, OptiMARK, an agent biochemically similar to Omniscan, has a market share less than or equal to MultiHance or ProHance and is not approved for high dose applications. Chelate instability would explain the increased incidence of OptiMARK related NSF when compared to MultiHance or ProHance. The recent report of a dialysis patient developing NSF after six ProHance contrasted MRI studies in one year suggests that no GBMCA is free from associated NSF risk.31 This risk most likely correlates with chelate instability and cumulative contrast dose.
GBMCA exposure is critical but not sufficient for the development of NSF. Intravenous iron and erythropoietin administration, secondary hyperparathyroidism, and pro-coagulant and “proinflammatory” states such as the peri-operative period have been weakly associated with NSF and may act as instigating factors in the presence of gadolinium based MRI contrast agents.3, 5, 8, 11, 22, 32 Through unclear mechanisms, GBMCA administration leads to significant iron mobilization raising serum iron levels and transferrin saturation.25, 33 Iron may “bump” gadolinium off the GBMCA chelate molecule as well as deposit alongside gadolinium within tissues to enhance toxicity. Skin biopsies from NSF patients have also demonstrated significant iron deposition.29 High doses of erythropoietin may promote iron mobilization or simply act as a marker for pro-inflammatory states. Hyperphosphatemia and significant secondary hyperparathyroidism may promote gadolinium tissue deposition since phosphate binds gadolinium and causes it to precipitate into tissues. Finally, pro-coagulant and pro-inflammatory states could generate a cytokine milieu that promotes tissue fibrosis in the presence of deposited gadolinium.
NSF Prevention
Given the strong association between GBMCA exposure and NSF, the most important measure to prevent NSF is to avoid contrasted MRI studies in patients with severely reduced renal function. If GBMCA administration is absolutely necessary, one may contemplate measures designed to minimize prolonged exposure to the agent. In ESRD patients, hemodialysis does effectively remove gadolinium based MRI contrast agents clearing about 75%, 95%, and 99% on the first, second, and third hemodialysis sessions respectively.34 Conversely, peritoneal dialysis does not effectively remove these contrast agents.26 Hemodialysis after contrasted MRI studies may reduce the incidence of NSF. However, NSF has been reported after contrasted MRI studies in patients undergoing daily hemodialysis.15 These patients underwent relatively low efficiency hemodialysis 9–21 hours after GBMCA administration.35 Presumably, high efficiency hemodialysis immediately after GBMCA administration is required to prevent NSF, since hemodialysis cannot efficiently remove gadolinium already deposited within tissues. Hemodialysis initiation in a patient with severe renal dysfunction (GFR < 30 mL/min) for the sole purpose of preventing NSF after GBMCA exposure comes with significant risk associated with vascular access placement and complications associated with hemodialysis initiation. GBMCA removal requires high efficiency hemodialysis, which may risk complications such as dialysis dysequilibrium in a pre-dialysis, severe chronic kidney disease patient. While hemodialysis seems promising as a preventive maneuver, it has several practical limitations and has not been definitively proven to prevent NSF after GBMCA exposure.
Utilizing high stability gadolinium based contrast agents may reduce the release of gadolinium and its subsequent tissue deposition. The American College of Radiology recommends against the use of Omniscan in patients with any degree of renal dysfunction.36 We would also avoid OptiMARK given its biochemical similarity to Omniscan. Minimizing contrast dose both during individual studies as well as cumulative dose over time represents an obvious way of reducing NSF risk. Unfortunately, a “safe” interval between contrasted MRI studies cannot be determined from the currently available data.
FDA Warnings and Recommendations
The FDA issued its first alert and began requesting adverse event reports in June, 2006 with the report of NSF cases after Omniscan administration in Europe. It has issued revised alerts in December, 2006 and May, 2007 reflecting the continuing flux of the field. The most recent May, 2007 alert warns against using any of the five approved gadolinium based MRI contrast agents in patients with acute or chronic renal failure with GFR < 30 mL/min “unless the information is essential and cannot be obtained” with other imaging techniques.24 If contrasted MRI is considered in an at-risk patient, the patient should be warned about the risks of NSF and its clinical features and contrast dose should be minimized. The FDA suggests conducting hemodialysis immediately after GBMCA administration and on the subsequent 1–2 days to hasten GBMCA clearance in chronic hemodialysis patients.24
Approach to Imaging Studies in Kidney Disease Patients
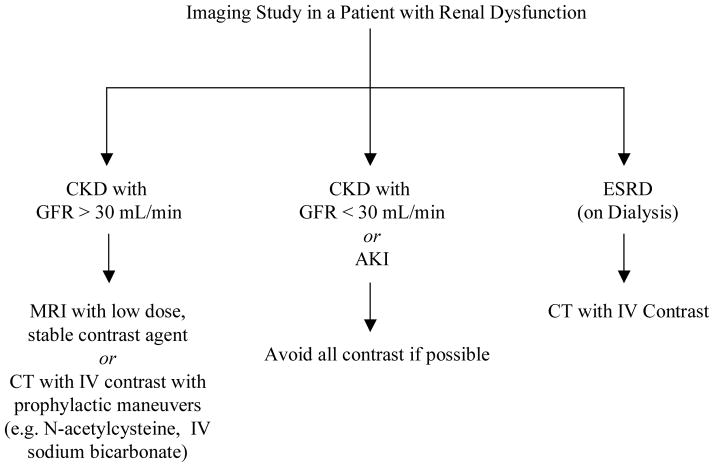
Our approach to imaging in kidney disease patients is to initially stratify patients into three basic risk categories: 1) Stable, mild to moderate CKD patients with an estimated GFR > 30 mL/min, 2) Dialysis patients, and 3) Patients with AKI or stable, severe CKD patients with an estimated GFR < 30 mL/min not on dialysis. Decisions regarding imaging modality and patient education are then considered within this stratification scheme (Figure 1).
Figure 1.
Decision-Making on Imaging Studies in Patients with Renal Dysfunction.
Stable patients with mild to moderate CKD (GFR > 30 mL/min) can probably undergo contrasted MRI studies using low dose, high stability contrast agents without significant contraindication. Caution should be exercised to ensure renal function stability. Patients even with early AKI may be at significant risk for NSF after GBMCA administration.
ESRD patients should not receive gadolinium based MRI contrast agents except in extraordinary cases as these patients represent the population at greatest risk for NSF. In most cases, contrasted CT imaging carries less risk as the nephrotoxic effects of conventional IV contrast are mitigated by the functionally anephric state of most ESRD patients. If contrasted MRI is contemplated, hemodialysis should be planned immediately after the study and on the subsequent 1–2 days to maximize GBMCA clearance. Informed consent of patients including the possible risk of NSF and the unproven benefit of preventive hemodialysis should be obtained. Peritoneal dialysis patients should not receive gadolinium based MRI contrast agents unless temporary hemodialysis is planned after imaging.
The most difficult decisions regarding imaging studies occur in patients with severe CKD (GFR < 30 mL/min) or AKI not on dialysis. These patients have an undefined but discrete risk of developing NSF after contrasted MRI studies and alternative imaging approaches may also incur significant risk. Contrasted CT studies risk worsening renal failure due to contrast-induced nephropathy, which may precipitate the need for hemodialysis. For instance, a validated clinical risk score for contrast-induced nephropathy after percutaneous coronary intervention suggests that a 75 year-old diabetic patient with stage IV CKD (GFR 15–30 mL/min) incurs a 25% risk for contrast-induced nephropathy with about 1% requiring hemodialysis.37 Measures to prevent contrast-induced nephropathy, such as oral N-acetylcysteine (Mucomyst®; Bristol-Myers Squibb, New York, NY) and IV sodium bicarbonate infusion, appear efficacious in study groups, but their ability to prevent significant renal failure and particularly hemodialysis in this tenuous patient population is not fully quantified.38, 39 More importantly, patients with contrast-induced nephropathy have poorer in-hospital and long-term outcomes particularly if they require chronic hemodialysis.40 Thus, we recommend that gadolinium based MRI contrast studies and probably all contrasted studies generally be avoided in these patients. In non-emergent situations, clinicians should collaborate extensively with radiologists to ensure non-contrasted imaging techniques have been completely exhausted. In many cases, radiologists may use alternative imaging techniques such as ultrasound or nuclear imaging or new modifications of non-contrasted MRI sequences, such as time of flight analysis for MRA, to provide adequate clinical information. If a contrasted MRI study is contemplated, patients should be counseled on the clinical features and risk of NSF, given the opportunity to choose an alternative imaging study or no study, and provided the option of post-MRI hemodialysis.
For the practicing urologist, renal mass imaging is the most commonly utilized MRI application. Although high resolution CT imaging with conventional IV contrast is equivalent to contrasted MRI for renal lesions, many clinicians preferentially use contrasted MRI in patients with moderate or severe renal dysfunction and/or large kidney resections to minimize the risk of contrast-induced nephropathy.41 In patients with a GFR < 30 mL/min at high NSF risk after GBMCA administration, this approach will need to be changed. Patients on dialysis can simply undergo contrasted CT imaging, while pre-dialysis patients will require strategies that avoid any form of IV contrast.
CT or MRI characterization of renal masses, particularly complex cystic lesions, as benign or possibly malignant rests primarily on contrast enhancement of the mass.41 Without contrasted imaging, several clinical strategies may be utilized. First, resection of equivocal masses can provide definitive diagnosis and treatment. Unfortunately, a tenuous pre-dialysis patient is exposed to surgical risk and possible precipitation of dialysis for minimal benefit with the resection of a benign mass. Second, percutaneous renal core needle biopsy may provide a diagnosis with less risk compared to surgery. Depending on institutional expertise, recent evidence suggests that renal mass biopsy may be safe and diagnostic in select clinical situations.42, 43 Third, clinicians may opt for serial surveillance using non-contrasted imaging with enlarging masses prompting surgical resection. Finally, if the risk of invasive procedures or delayed diagnosis with surveillance outweighs the risk of conventional contrast or GBMCA exposure, then contrasted CT or MRI may be undertaken with appropriate precautions and informed consent. Currently, no evidence exists to suggest one strategy is superior to another in the approach to renal masses where contrasted imaging is limited.
Conclusions
NSF is a rare, but devastating disease. Although associative from a rigorous perspective, the newly discovered link between NSF and gadolinium based MRI contrast agent exposure in patients with renal dysfunction raises the depressing prospect of morbidity and mortality related to well-intentioned radiologic studies, but also raises the hope of curtailing NSF in the future. A major barrier to this hope is physician and patient education. In this paper, we have provided the practicing urologist a framework to approach contrasted MRI studies in patients with renal dysfunction carefully weighing the risk of NSF against the benefit of the study or the disadvantages of alternative approaches.
References
- 1.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 2.Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383. doi: 10.1097/00000372-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 3.DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19:191. doi: 10.1111/j.1525-139X.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 4.Grobner T, Prischl FC. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72:260. doi: 10.1038/sj.ki.5002338. [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan S, Shah SV. New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol. 2007;18:2636. doi: 10.1681/ASN.2007060645. [DOI] [PubMed] [Google Scholar]
- 6.Ting WW, Stone MS, Madison KC, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903. doi: 10.1001/archderm.139.7.903. [DOI] [PubMed] [Google Scholar]
- 7.Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45:56. doi: 10.1111/j.1365-4632.2005.02701.x. [DOI] [PubMed] [Google Scholar]
- 8.Mackay-Wiggan JM, Cohen DJ, Hardy MA, Knobler EH, Grossman ME. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease) J Am Acad Dermatol. 2003;48:55. doi: 10.1067/mjd.2003.78. [DOI] [PubMed] [Google Scholar]
- 9.Swartz RD, Crofford LJ, Phan SH, Ike RW, Su LD. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563. doi: 10.1016/s0002-9343(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 10.Cowper SE. [Accessed October 24, 2007.];Nephrogenic Fibrosing Dermopathy [NFD/NSF Website] 2001–2007 Available at http://www.icnfdr.org.
- 11.Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. [Accessed October 24, 2007.];Information for Healthcare Professionals: Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging Scans. 2006 Available at http://www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200612HCP.htm.
- 13.Pharmacovigilance Working Party. [Accessed November 18, 2007.];Increased risk of nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis and gadolinium-containing MRI contrast agents. 2007 Available at http://www.ismrm.org/special/MHRA_Report.pdf.
- 14.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 15.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol. 2007;188:586. doi: 10.2214/ajr.06.1094. [DOI] [PubMed] [Google Scholar]
- 16.Cheng S, Abramova L, Saab G, Turabelidze G, Patel P, Arduino M, et al. Nephrogenic Fibrosing Dermopathy Associated with Exposure to Gadolinium-Containing Contrast Agents - St. Louis, Missouri, 2002 – 2006. Morbidity and Mortality Weekly Report. 2007;56:137. [PubMed] [Google Scholar]
- 17.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 18.Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2:264. doi: 10.2215/CJN.03921106. [DOI] [PubMed] [Google Scholar]
- 19.Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature. Am J Transplant. 2007;7:2425. doi: 10.1111/j.1600-6143.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- 20.Collidge TA, Thomson PC, Mark PB, Traynor JP, Jardine AG, Morris ST, et al. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology. 2007;245:168. doi: 10.1148/radiol.2451070353. [DOI] [PubMed] [Google Scholar]
- 21.Frost, Sullivan . In: North American MRI Contrast Media Market. Published June 25, 2007. Frost, Sullivan, editors. Vanderbilt University Walker Library; Nashville, TN: [Accessed November 2, 2007.]. http://www.frost.com. [Google Scholar]
- 22.Marckmann P, Skov L, Rossen K, Heaf JG, Thomsen HS. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm261. [DOI] [PubMed] [Google Scholar]
- 23.Goyen M, Ruehm SG, Debatin JF. MR-angiography: the role of contrast agents. Eur J Radiol. 2000;34:247. doi: 10.1016/s0720-048x(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration. [Accessed October 24, 2007.];Information for Healthcare Professionals: Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging. 2007 Available at http://www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm.
- 25.Idee JM, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol. 2006;20:563. doi: 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 26.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol. 1998;5:491. doi: 10.1016/s1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]
- 27.Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80:73. doi: 10.1259/bjr/17111243. [DOI] [PubMed] [Google Scholar]
- 28.Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56:27. doi: 10.1016/j.jaad.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 29.High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56:21. doi: 10.1016/j.jaad.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 30.Runge VM, Knopp MV. Off-label use and reimbursement of contrast media in MR. J Magn Reson Imaging. 1999;10:489. doi: 10.1002/(sici)1522-2586(199909)10:3<489::aid-jmri35>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Hayes E. Bracco reports case of NSF with gadolinium contrast agent ProHance. [Accessed November 18, 2007.];Diagnostic Imaging. 2007 Available at http://www.dimag.com/showNews.jhtml?articleID=201802091.
- 32.Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;57:725. doi: 10.1016/j.jaad.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Swaminathan S, Horn TD, Pellowski D, Abul-Ezz S, Bornhorst JA, Viswamitra S, et al. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med. 2007;357:720. doi: 10.1056/NEJMc070248. [DOI] [PubMed] [Google Scholar]
- 34.Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42:339. doi: 10.1080/028418501127346756. [DOI] [PubMed] [Google Scholar]
- 35.Broome DR, Cottrell AC, Kanal E. Response to “Will dialysis prevent the development of nephrogenic systemic fibrosis after gadolinium-based contrast administration? AJR Am J Roentgenol. 2007;189:W234. doi: 10.2214/AJR.07.2919. [DOI] [PubMed] [Google Scholar]
- 36.Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, Jr, Froelich JW, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 37.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 38.Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 39.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 40.Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant. 2006;21:i2. doi: 10.1093/ndt/gfl213. [DOI] [PubMed] [Google Scholar]
- 41.Israel GM, Bosniak MA. Renal imaging for diagnosis and staging of renal cell carcinoma. Urol Clin North Am. 2003;30:499. doi: 10.1016/s0094-0143(03)00019-3. [DOI] [PubMed] [Google Scholar]
- 42.Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology. 2005;236:441. doi: 10.1148/radiol.2362040218. [DOI] [PubMed] [Google Scholar]
- 43.Maturen KE, Nghiem HV, Caoili EM, Higgins EG, Wolf JS, Jr, Wood DP., Jr Renal mass core biopsy: accuracy and impact on clinical management. AJR Am J Roentgenol. 2007;188:563. doi: 10.2214/AJR.06.0220. [DOI] [PubMed] [Google Scholar]