Abstract
Objectives
This study aims to examine whether changes in short-term exposures to particulate matter are associated with changes in lung function, breath rate, and blood pressure among healthy adults and whether smoking status modifies the association.
Methods
We took advantage of the artificially controlled changes in air pollution levels that occurred during the 2008 Olympic Games in Beijing, China and conducted a panel study of 201 Beijing residents. Data were collected before, during, and after the Olympics, respectively. Linear mixed-effects models and generalized estimating equation models were used to compare measurements of peak expiratory flow, breath rate, blood pressure across the three time points.
Results
The mean values of peak expiratory flow were 346.0 L/min, 399.3 L/min, and 364.1 L/min over the three study periods. Peak expiratory flow levels increased in 78% of the participants when comparing the during- and pre- Olympics time points, while peak expiratory flow levels decreased in 80% of participants for the post- and during-Olympic periods comparison. In subgroup analyses comparing the during -Olympic to pre-Olympic time points, we found a larger percentage change in peak expiratory flow (+17%) among female, younger and non-smoking participants than among male, elderly and smoking participants (+12%). The percentage of participants with a fast breath rate (>20/min) changed from 9.7%, to 4.9%, to 30.1% among females, and from 7.9%, to 2.6%, to 27.3% among males over the three time points respectively. The changes on blood pressure over the three study periods were not very clear, although there is an increase in diastolic pressure and a decrease in pulse pressure among males during the games.
Conclusions
The results suggest that exposure to different air pollution levels has significant effects on respiratory function. Smoking, age and gender appear to modify participants’ biological response to changes in air quality.
Keywords: Air pollution, Peak expiratory flow, Breath rate, Blood pressure, Panel study
1. Introduction
Exposure to ambient air pollution has been linked to various health effects including impaired cardiopulmonary function, respiratory and cardiovascular diseases, cancers, and all-cause mortality (Boffetta 2006; Franchini and Mannucci 2007; Mannino and Buist 2007). Air-borne particulate matter (PM) is a complex mixture of solid and liquid particles of various sizes and compositions, including polycyclic aromatic hydrocarbons (PAH), elemental carbon, organic carbon compounds, transition metals and reactive components. Particulate matter, the air pollution ‘cocktail’, is believed to be responsible for many air pollution-induced adverse health effects. Although the risk to one individual at any single time point is small, given the high prevalence of exposure, particulate matter air pollution has large global public health implications, and ranks as the 13th leading cause of mortality (Brook 2008). Ambient particulate matter accounts for about 95% of the total air pollution-related damage cost (Pervin et al. 2008).
Short-term exposure to high levels of air pollution exacerbates pre-existing illness and increases mortality among those suffering from various serious chronic diseases. However, it is not clear whether reducing exposure has measurable physiological effects on lung function, breath rate and blood pressure in healthy adults. Peak expiratory flow is defined as the maximum flow generated during expiration, performed with maximal force and started after a full inspiration. It has been used as one of the most direct measurements of lung function, especially in the treatment of asthma. Exposure to a high concentration of air pollution has been linked to the changes in peak expiratory flow, especially among asthma patients (Qian et al. 2009) (Hong et al. 2010; Ma et al. 2008; Peters et al. 1996; Pope et al. 1991; Romieu et al. 1996; Wiwatanadate and Liwsrisakun 2011; Wiwatanadate and Trakultivakorn 2010; Yamazaki et al. 2011), chronic obstructive pulmonary disease patients (Dusseldorp et al. 1995), children (Pope and Dockery 1992), (Hoek et al. 1993; Kasamatsu et al. 2006; Mengersen et al. 2011; Nordling et al. 2008; Roemer et al. 1993) and the elderly (Lee et al. 2007). Studies have suggested that air pollution is linked to cardiovascular events, frequent hospitalizations, exacerbation of preexisting cardiac diseases and cardiac related mortality (Franchini and Mannucci 2012; Hoek et al. 2001). However, evidence linking air pollution with pre-clinical perturbations has been limited among healthy adults. Systemic inflammation has been hypothesized as one of the major signaling mediators linking particulate matter exposure with various adverse outcomes (Calderon-Garciduenas et al. 2008; Diaz-Sanchez 2000; Seagrave 2008; Swiston et al. 2008). High levels of particulate matter are related to upregulated inflammatory levels in both in vitro and in vivo studies (Diaz-Sanchez 2000; Watterson et al. 2007). In addition, although most previous research has studied the effect of air pollution among non-smokers, because it has been believed that smoking plays an overwhelming role in the respiratory function, it is important to see if air pollution has any effect on these already effected individuals. It is of scientific and public health interest to understand whether ambient air pollution exposure will equally affect smokers and nonsmokers.
Beijing, China, has high levels of air pollution due to rapid industrial expansion and the increased number of automobiles on the road. Beijing’s annual level of PM10 (particulate matter less than 10 microns in diameter) exceeds 150μg/m3, and is ranked the sixth highest among the monitored cities in China (2008). Studies have also reported high concentrations of polycyclic aromatic hydrocarbons, ranging from 28.53 to 362.15ng/m3, particularly during winter months (Zhao et al. 2010). The Chinese government took steps during the Beijing Olympics and Paralympics to reduce air pollution and particulate matter levels in order to provide all athletes and guests with a cleaner atmospheric environment. Factories were temporarily closed across a large geographic area and vehicle exhaust emissions were reduced by preventing half of Beijing’s 3.3 million cars from being driven on any given day. Consequently, the city’s ambient air quality dramatically improved during the Olympics and particulate matter decreased to half of the pre-Olympic levels. After cessation of the control measures, particulate matter returned to pre-Olympic levels. These circumstances created a natural experiment with bi-directional change in particulate matter levels, allowing us to observe short-term biological responses to both decreases and increases in air pollution, and may be informative regarding the mechanisms potentiating long-term effect of exposure to particulate matter.
2. Materials and Methods
2.1 Study design
Taking advantage of the changes in air pollution levels that occurred during the 2008 Beijing Olympic Games, we designed and conducted a panel study in which we enrolled a cohort of 201 participants residing in Beijing, China. We conducted in-person structured interviews with all subjects during their three clinic visits before (Baseline) and during (1st follow-up) and after (2nd follow-up) the Olympics that coincided with the changes in air pollution levels in Beijing.
2.2. Study population
Subject Recruitment
All subjects enrolled in the study lived in a community in the Haidian district. Using the community’s health registration system, 260 subjects were randomly selected from a roster of potential participants identified by the community health service center. Research personnel contacted potential participants by phone, and described the study including the voluntary nature of participation in the proposed research project. After receiving detailed information, those who agreed to participate were then asked to schedule three clinical visits and interviews. Prior to the initiation of the field investigation, all participants completed a signed consent process that was approved by Institutional Review Boards at the University at Buffalo and Peking University, China.
Participant Selection Criteria
Participants were females and males between the ages of 20 and 65 years. Those older than 65 years were excluded because of potential pre-existing medical conditions. Participants were restricted to Han (ethnic) Chinese residing in the Haidian district for at least 1 year (ie. since August 2007). Participants with a prior medical history of cancer, serious immunological or chronic respiratory diseases were excluded. Participants were not excluded based on their smoking status.
2.3 Clinical visits
The official period for the Olympic and Paralympics was between August 8, 2008, and September 17, 2008. We conducted a series of three clinical visits: one baseline (prior to pollution control measures) and two follow-up visits during and after the Olympics and Paralympics, respectively. Each visit included an in-person interview and physical examination.
2.4 Data collection
2.4.1 General information
Questionnaires were administered by the trained interviewers to all participants during each interview. Two different questionnaires were designed for the baseline and follow-up interviews, respectively. The baseline questionnaire included questions regarding residential address, tobacco use, alcohol use, tea drinking, dietary habits, occupational history, medical history, frequency of stir/deep frying food per day and usage of ventilation in their kitchen, recent incidence of colds and other respiratory diseases, and recent subjective feeling about outdoor air pollution and related symptoms. The follow-up questionnaire focused on questions regarding subjects’ change in residential location, lifestyle, occupation, physical condition, and disease symptoms since the previous interview.
2.4.2 Clinical visits were conducted at the district’s community health service center
All participants arrived at the center before 8 a.m. on the day of their interview. At each visit, a standard physical examination was conducted by study physicians and trained nurses. All results were recorded, and all participants received a copy of the report at the end of the examinations. The physical examination included the following: (1) basic measurements: Height (cm), weight (kg), body temperature (°C) and blood pressure (mmHg). For the blood pressure measurement, a standard protocol was followed by the research staff throughout the three study periods. All participants were required to sit for at least five minutes to rest before the measurements. During the measurements, they were seated in a chair with their backs being supported, feet flat on the floor and their arms being supported at the heart level. (2) pulmonary function: respiration rate (breath/min) and peak expiratory flow (PEF). Peak expiratory flow was measured by a pre-calibrated peak flow meter. To ensure that the maneuvers were performed correctly, a trained nurse supervised each participant. In total, three correct maneuvers were performed. For each maneuver, the subject waited at least 10 seconds between blows. If the two largest values differed by greater than 40 L/min, participants performed two extra maneuvers. The highest value of the three maneuvers was recorded as the peak expiratory flow for this person.
2.4.3 Particulate matter air pollution
Air pollution data were collected during the study period, including measurement of instantaneous and continuous particulate matter concentration in the study area. Particle mass monitor (Met One® 531 AEROCET Particulate Profiler, Met One Instruments, Inc. Grant Pass, Oregon) was used to measure the concentration of particulate matter in the study area, which was located in the center of the community. The monitor was placed in an open space close to a main road. The particulate matter monitor machine was placed 1.5 meters above the ground, the average respiratory height. Particulate matter with the following size ranges was measured: PM1, PM2.5, PM7, PM10, and total suspended particulates (TSP), along with temperature and relative humidity. The concentration range was up to 1mg/m3. Particulate matter was measured four times a day, twice at 10:00 a.m. and twice at 4:00 p.m., every four days over the whole study period.
2.5 Statistical Analyses
Descriptive analysis
Univariate analyses were conducted to characterize air pollution levels, participant demographic information and physical measurements. Means and corresponding 95% confidence intervals were calculated for continuous variables, including peak expiratory flow, breath rate, blood pressure and pulse. The frequencies and percentages were calculated for categorical variables.
Longitudinal Analysis
In the longitudinal analyses, we compared measurements over different time periods within individuals. For continuous variables, linear mixed-effects models were used to compare across the three study time periods to account for the repeated measures and the lack of independence within individuals, estimating parameters and dealing with missing values. In the linear mixed-effects models, we used study time period as an independent variable as well as the variable indicating the time of repeat (coded as 1,2 and 3). The subject ID was used to indicate the within individual comparison. The models controlled for age, sex, smoking status, BMI, in categories and their interaction with time Period. The following categories were created based on underlying biology and distribution: Age><50yrs were categorized by the median age of the participants; BMI><24 was categorized by cut point for overweight among Chinese. Non-smokers were defined as those who had never smoked more than 100 cigarettes in their lifetime. The correlated structure between repeated measures was set as “unstructured”, i.e. no special structure will be imposed and the correlation structure is completely determined by data. The models provided results on the comparisons between any two time points, during- vs pre-, post- vs during-, post- vs pre- Olympics. The current analysis only presented the comparison results on during- vs pre-, and post- vs during-Olympics. Given that categorical analyses may provide more biological and clinical meaning and allow for assessing the non-linear relationship, we further analyzed major outcomes in categories to present direct changes of participants’ distribution through categories over the three study periods. Linear mixed-effects models are only appropriate for outcome variables measured on a continuous scale. Therefore, we used generalized estimating equation (GEE) models to examine changes in categorical variables (e.g., PEF in four categories and breath rate in two categories) over the study periods, simultaneously controlling for age, gender and smoking status and their interaction with time period. Interactions were examined by including interaction terms in linear mixed-effects models and generalized estimating equation models. Finally, given that peak expiratory flow levels differ between males and females, as well as smokers and non-smokers, we conducted stratified analyzes by gender and smoking status, and percentage changes from pre- to during-, from during to post-Olympic was calculated for each subgroup. We also assessed the potential for effect measure modification by age.
Potential confounding factors
The panel study has the advantage of comparing measurements within individual over different time periods. The study design minimizes potential effects of confounding from factors that do not vary over time. The potential for time-dependent confounding from time-varying factors such as smoking is a distinct possibility. However, such factors are unlikely to change substantially in such a short time period. It is known that smoking status, age and gender are determinants of lung function. These factors may modify individual’s PEF response to changes in air pollution levels. Thus, we conducted stratified analysis by gender, smoking status and age and also adjusted those factors and their interactions with air pollution in the models. Further adjustment for lymph nodes swelling and presence of cold symptoms did not alter the association (data not shown). The beta coefficients were very similar between models with and without these two variables.
3. Results
3.1 General characteristics of study participants
We enrolled two hundred and one subjects who completed the baseline interview and clinical exam. However, 21 subjects (about 10%) failed to complete the second and third interviews for various reasons. Participants’ general information is shown in Table 1. No differences were observed between those who completed the two follow-up investigations (180 subjects) and those who were lost in the follow-ups (21 subjects) (data not shown). One hundred and three female participants (57.2%) and seventy-seven male participants (42.8%) completed all three interviews. Forty-six percent of subjects were older than 50 years of age, 33% of subjects have education of primary school or lower. Fifteen subjects (8.3%) had a BMI greater than 28. Of all subjects, 60 (33.3%) were active smokers including 55 males (71.4%) and 5 female (4.9%).
Table 1.
Demographic Information of Study Participants
| Variables | Female (N=103) | Male (N=77) | ||
|---|---|---|---|---|
|
| ||||
| N | % | N | % | |
| Age | ||||
| ≤30 | 5 | 4.8 | 2 | 2.6 |
| 30–40 | 25 | 24.3 | 4 | 5.2 |
| 40–50 | 41 | 39.8 | 21 | 27.3 |
| >50 | 32 | 31.1 | 50 | 64.9 |
| Education | ||||
| Primary school & illiterate | 40 | 39.2 | 19 | 24.7 |
| Middle school | 53 | 52.0 | 43 | 55.8 |
| High school and higher | 9 | 8.8 | 15 | 19.5 |
| BMI | ||||
| <24 | 50 | 62.5 | 47 | 66.2 |
| 24–28 | 20 | 25.0 | 19 | 26.8 |
| ≥28 | 10 | 12.5 | 5 | 7.0 |
| Active smoking | ||||
| Nonsmoker | 98 | 95.1 | 22 | 28.6 |
| Smoker | 5 | 4.9 | 55 | 71.4 |
| Alcohol drinking | ||||
| Nondrinker | 85 | 82.5 | 30 | 39.5 |
| Drinker | 18 | 17.5 | 46 | 60.5 |
3.2 Particulate matter air pollution variation over the study period
Particulate matter pollution levels of different sizes were reduced by half during the mid-Olympics time point and increased again after the Olympics. The trend was more pronounced for particulate matter in larger sizes (Table 2). Total suspended particulates (TSP) and PM10 returned to the pre-Olympic levels more rapidly than the smaller particulate matter sizes.
Table 2.
Changes of Particulate Matters over the Course of Beijing Olympics
| PM (ug/m3) | PM1 | PM2.5 | PM7 | PM10 | TSP |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Before Olympics | 24 (20.0) | 83(92.7) | 116(117.0) | 128(122.0) | 141(126.9) |
| During Olympics | 11(13.3) | 33(48.7) | 49(59.1) | 56(60.4) | 63(61.7) |
| After Olympics | 14(16.9) | 46(57.2) | 106(96.7) | 140(120.7) | 187(157.9) |
3.3 Respiratory function
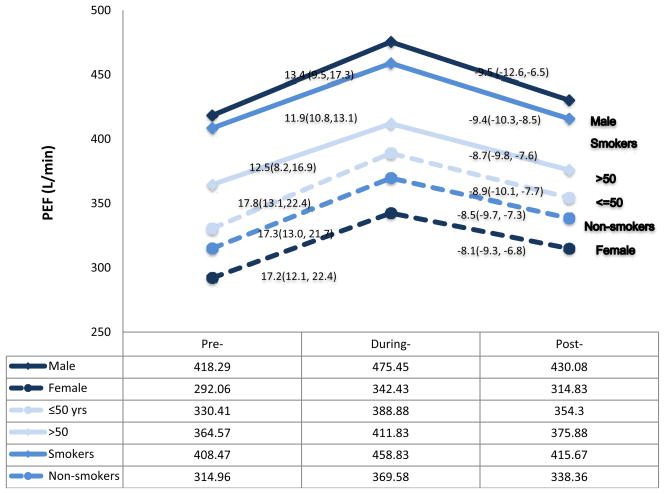
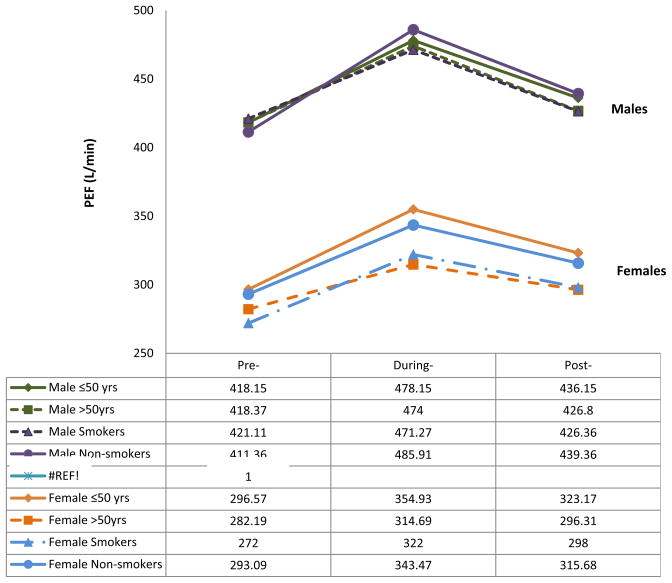
Using either the continuous or the categorical scale, we observed that peak expiratory flow levels varied over the three time periods. The percentage of participants who had peak expiratory flow greater than 400 L/min increased from 30% in the pre-Olympic period to 49.4% in the during-Olympic period and decreased to 33.4% in the post-Olympic period (Table 4). The mean values of peak expiratory flow were 292.1L/min, 342.4L/min, and 314.8 L/min among females, and 418.3L/min, 475.5L/min, 430.1 L/min among males, respectively, over the three study periods (Table 3). The overall mean values for both male and female were 346.0L/min, 399.3 L/min, and 364.1 L/min over the three study periods. The increased peak expiratory flow levels were seen in 78% of participants when comparing the during-Olympic values to the pre-Olympic values. The peak expiratory flow increased by 50 L/min in females (percentage change: 17.2%, 95%CI: 12.1%–22.4%), and 56 L/min in males (percentage change: 13.4%, 95%CI: 9.5%–14.3%) (Table 3 and figure 1). Interestingly, decreased peak expiratory flow levels were observed in a similar proportion of participants (80%) when the post- and during-Olympic values were compared. The peak expiratory flow decreased by 27 L/min in females (percentage change: 8.1%, 95%CI: 6.8%–9.3%) and 45 L/min in males 9.5% (95%CI: 6.5%–12.6%). Peak expiratory flow variation over the three waves of interviews was further analyzed in different participant subgroups (Figure 1 and 2). Older male participants had higher peak expiratory flow than younger female participants, respectively. Female nonsmokers had higher peak expiratory flow than female smokers. Among the subgroup of smokers, peak expiratory flow also varied over the three study periods. However, we found higher percentage changes in peak expiratory flow (around 17%) in female, younger and non-smoking participants than in male, elderly and smokers (12%–13%) when comparing during- to pre- Olympics (Figure 1 and 2).
Table 4.
Respiratory Functions over the Course of Beijing Olympics
| Pre-Olympics | During-Olympics | Post-Olympics | |
|---|---|---|---|
|
| |||
| Changes & 95% CI (During vs Pre) | Changes & 95% CI (Post vs During) | ||
| FEMALES | |||
| PEF (L/min) | N (%) | N (%) | N (%) |
| <300 | 48 (47.0) | 23 (22.3) | 25 (24.3) |
| 300–400 | 47 (46.1) | 56 (54.4) | 68 (66.0) |
| 400–500 | 7 (6.9) | 22 (21.4) | 10 (9.7) |
| ≥500 | 0 (0.0) | 2 (1.9) | 0 (0.0) |
| 1.38 (95CI: 0.89, 1.87) | −0.51 (95CI: −0.74, −0.28) | ||
| Breath Rate | |||
| Slow/Normal(≤20/min) | 93(90.3) | 98(95.1) | 72(69.9) |
| Fast(>20/min) | 10(9.7) | 5(4.9) | 31(30.1) |
| −0.76 (95CI: −1.83, 0.31) | 2.14 (95CI: 1.16, 3.13) | ||
| MALES | |||
| PEF (L/min) | N (%) | N (%) | N (%) |
| <300 | 9 (11.8) | 3 (3.9) | 4 (5.2) |
| 300–400 | 20 (26.3) | 9 (11.7) | 23 (29.9) |
| 400–500 | 31 (40.8) | 32 (41.6) | 29 (37.6) |
| ≥500 | 16 (21.1) | 33 (42.8) | 21 (27.3) |
| 1.11 (95CI: 0.75, 1.48) | −0.82 (95CI: −1.11, −0.53) | ||
| Breath Rate | |||
| Slow/Normal(≤20/min) | 70(92.1) | 75(97.4) | 56(72.7) |
| Fast(>20/min) | 6(7.9) | 2(2.6) | 21(27.3) |
| −0.69 (95CI:−2.64, 1.25) | 2.65 (95CI: 1.07, 4.24) | ||
The GEE models were used in the analysis and models were adjusted for age (≤50, >50), smoking status (never, ever), BMI status (<24, ≥24) and interaction terms for age, smoking, and BMI with time-points.
Table 3.
Comparison among Continuous Measurements over the Course of Beijing Olympics
| Pre-Olympics Mean(95%CI) | During-Olympics Mean(95%CI) | Post-Olympics Mean(95%CI) | |
|---|---|---|---|
|
| |||
| Changes&95%CI* (During vs Pre) | Changes&95%CI* (Post vs During) | ||
| FEMALES | |||
| PEF (L/min) | 292.06(278.28,305.84) | 342.43(329.14, 355.71) | 314.83 (304.37, 325.28) |
| 50.41 (95CI: 36.82, 64.00) | −27.60 (95CI: −32.34, −22.86) | ||
| Breath Rate | 19.33(19.08, 19.58) | 18.59(18.32, 18.86) | 19.50(19.24, 19.75) |
| −0.74 (95CI: −1.12, −0.36) | 0.90 (95CI: 0.69, 1.12) | ||
| Systolic pressure (mmHg) | 124.53(121.61, 127.46) | 124.56(121.71,127.42) | 124.22(121.39, 127.06) |
| 0.03 (95CI: −2.53, 2.59) | −0.34 (95CI: −0.73, 0.05) | ||
| Diastolic pressure (mmHg) | 79.32(77.23, 81.41) | 81.38(79.47, 83.29) | 80.92(78.92, 82.92) |
| 2.06 (95CI: −0.12, 4.24) | −0.46 (95CI: −1.21, 0.29) | ||
| Pulse pressure | 45.21(43.18, 47.25) | 43.18(41.20, 45.17) | 43.30(41.48, 45.12) |
| −2.03 (95CI: −4.54, 0.48) | 0.12 (95CI: −0.58, 0.81) | ||
| Pulse | 78.71(76.92, 80.50) | 77.47(75.34, 79.59) | 76.66(74.79, 78.53) |
| −1.24 (95CI: −3.37, 0.89) | −0.81 (95CI: −1.63, 0.02) | ||
| MALES | |||
| PEF (L/min) | 418.29(394.94, 441.64) | 475.45(452.41, 498.50) | 430.08 (409.90, 450.25) |
| 56.19 (95CI: 41.41, 70.98) | −45.37 (95CI: −51.41, −39.33) | ||
| Breath Rate | 19.04(18.73, 19.35) | 18.52(18.20, 18.84) | 19.55(19.26, 19.83) |
| −0.53 (95CI: −0.92, −0.13) | 1.03 (95CI: 0.80, 1.25) | ||
| Systolic pressure | 128.03(124.97, 131.09) | 129.71(126.55,132.88) | 129.60(126.36, 132.84) |
| 1.69 (95CI: −0.84, 4.22) | −0.12 (95CI: −1.12, 0.88) | ||
| Diastolic pressure | 82.05(79.95, 84.15) | 86.09(83.74, 88.44) | 86.21(83.85, 88.56) |
| 4.04 (95CI: 1.90, 6.18) | 0.12 (95CI: −0.79, 1.03) | ||
| Pulse pressure | 45.97(43.55, 48.40) | 43.62(41.71, 45.54) | 43.39(41.56, 45.22) |
| −2.35 (95CI: −4.58, −0.12) | −0.23 (95CI: −1.33, 0.86) | ||
| Pulse | 75.82(73.73, 77.90) | 73.34(70.62, 76.06) | 73.04(70.56, 75.52) |
| −2.45 (95CI: −5.19, 0.28) | −0.30 (95CI: −1.16, 0.56) | ||
The linear mixed-effects model were used in the analysis and the models were adjusted for age (≤50, >50), smoking status (never, ever), BMI status (<24, ≥24) and interaction terms for age, smoking, and BMI with time-points.
Figure 1. PEF Changes over the Course of the Olympics among Different Groups.
PEF changes in subgroups. The percentage changes of PEF levels over the three time periods were compared among six subgroups in the figure. X-axis: Three time periods. Y-axis: PEF levels (L/min). “lines in different color and styles” represents different subgroups.
Figure 2. PEF changes over the course of Olympics by gender.
PEF changes in subgroups by gender. X-axis: Three time periods. Y-axis: PEF levels (L/min). “lines in different color and styles” represents different subgroups. The upper four lines are PEF levels among males, and the lower four lines are PEF levels among females.
The breath rate significantly decreased during the Olympics (mean values: 18.6/min for females, 18.5/min for males) from the pre-Olympic level (mean values: 19.3/min for females, 19.0/min for males) and returned to previous levels after the Olympics (mean values: 19.5/min for females, 19.6/min for males). The percentage of participants with a fast breath rate (>20/min) varied from 9.7%, to 4.9%, to 30.1% among females, and from 7.9%, to 2.6%, to 27.3% among males over the three time points, respectively (Table 3 and 4),
3.4 Blood pressure
Diastolic pressure among males increased during the games from the pre-Olympic level by 4.04 mmHg (95CI: 1.90, 6.18). The increased diastolic pressure maintained after the games (mean value: 86.21mmHg). The pulse pressure decreased among males during the games from the pre-Olympic level by 2.35 mmHg (95CI: −4.58, −0.12) and remained at the similar level after the games. Overall, the study didn’t observe any clear pattern of blood pressure in response to the air pollution levels.
4. Discussion
In Beijing, the mean PM2.5 (83 μg/m3) was more than three times higher than the WHO’s guideline of 25 μg/m3. The air pollution control measures efficiently reduced all particulate matter levels by 54–60% during the course of the Olympic games. When all control measures were removed after the games, particulate matter levels increased, although for small particulate matter, the increase was much slower compared to larger particulate matter. During the course of the Olympics, some physiological measurements, including peak expiratory flow, breath rate had responses to the changes in air pollution level, but blood pressure didn’t show a clear response.
Peak expiratory flow is a direct measurement of the airway obstruction and lung function, and it has been used to evaluate the effects of different factors, including medical treatment and air pollution exposure, on airway caliber. The peak expiratory flow measurements at baseline in the study population were 418 L/min for men and 292 L/min for women, which were lower than the normal range for both men (500–700L/min) and women (380–500L/min)(Cross and Nelson 1991). Long-term exposure to polluted air may have resulted in the poor status of the airways and explains the relative low baseline peak expiratory flow. The low peak expiratory flow also could be partly due to the early morning measurements in this study, which tended to be the lowest level of an entire day. Compared to a Western population, subjects included in this study might have relatively smaller body size in terms of height and weight, as well as higher smoking prevalence in male participants. This may also contribute to the low baseline levels because the absolute peak expiratory flow is also associated with race, age, height, and smoking status (Gregg and Nunn 1973).
Particulate matter levels declined by 50% during the Olympics, which corresponded to, peak expiratory flow increasing by 17% in females and 13% in males, suggesting an improved airway status after 40 days of lowered exposure to air pollution. Furthermore, peak expiratory flow levels decreased when air pollution levels increased after the games, although the declines in peak expiratory flow were smaller in this period, about 8.1% in females and 9.5% in males. It seems plausible that peak expiratory flow would return to the previous level if given longer time exposure to heavy air pollution and if smaller size particulate matter would have also returned to the original high levels. In addition, less traveling during the games might affect their personal exposure to air pollution because people usually have higher exposure during transportation. Consequently, the true exposure changes during the Olympics may be greater than the observed changes in PM levels. Other than air pollution, the influence of travel on PEF might be limited, however, because all the physical examinations were conducted in the early morning, inside of the community with an area around 0.27 km2. The majority of previous studies have found a association between air pollution and peak expiratory flow level among the most sensitive groups including asthmatic children (Yamazaki et al. 2011), children with respiratory illness (Correia-Deur et al. 2012), healthy children (Jacobson et al. 2012; Linares et al. 2010; Oftedal et al. 2008), chronic obstructive pulmonary disease (COPD) patients (Dusseldorp et al. 1995), and elderly and occupational populations (Chawla A 2008). Very few studies were conducted in healthy adults and these have reported inconsistent results (van der Zee et al. 2000). Generally, environmental exposure in adults is much more complicated, and potential confounding factors can mask the association, such as smoking and respiratory disease. It is also possible that healthy adults have a higher threshold to respond to air pollution exposure. Fast breath rate is also an indicator of poor airway status, and it showed a similar trend to peak expiratory flow in our study, although the change in breath rate over the three time periods was relatively small.
Smoking is one of the major factors that affects lung function. Because more than 60% of Chinese men are active smokers, the current study included both smoking and non-smoking subjects to increase generalizability of the study and allow the assessments of potential effect modification by smoking. It has been reported that peak expiratory flow only changes among smokers, not non-smokers (Linares et al. 2010), However our finding provides evidence that peak expiratory flow responds to air pollution changes in both smokers and non-smokers. Peak expiratory flow was higher in nonsmokers than that in smokers among both males and females (figure 1). Although trends are similar in subgroups by gender, smoking, and age, when air pollution levels decreased during the game, peak expiratory flow level increased more (around 17%) in non-smokers, female and younger individuals than that in smokers, male and older individuals (around 12%). For those smokers, males and older individual, lung function could have been impaired to a certain degree, such that their response to improved air quality was muted and the extent of the improvement in lung function smaller, although only a very slight difference in peak expiratory flow percentage change was identified between two groups (smokers vs non-smokers, males vs females) when air quality went down again.
Particulate matter air pollution, especially fine and ultrafine particulate matter, possesses oxidative capacity. Deposition of particulate matter induces increased oxidative stress that not only results in DNA/lipid/protein damage, but also leads to the inflammatory responses. Pulmonary inflammation is the primary reaction of the lung tissue to eliminate pathogenic agents. Phagocytic alveolar macrophages, polymorphonuclear leukocytes, and eosinophils are integral to the innate defense mechanisms of the lung (Earley et al. 2002). The cellular responses to particulate matter deposition in pulmonary tract might serve to initiate various adverse effects. Air pollution exposure affects the vascular system through the mechanisms of endothelial dysfunction and vasoconstriction. Although there is a well-established relationship between long-term exposure to air pollution and cardiovascular disease, the effect of short-term exposure on blood pressure is not clear (Brook et al. 2002; Chuang et al. 2010; Mar et al. 2005; Urch et al. 2005). We found that diastolic pressure (DBP) increased in males during the Olympics games when the air pollution level was low, which is inconsistent with findings from other studies (Baccarelli et al. 2011; Cosselman et al. 2012; Jacobs et al. 2012; Sorensen et al. 2011). But there are also studies that found that decreased SBP was associated with increased level of elemental carbon and ozone, as well as particulate matter (Chen et al. 2012) (Baccarelli et al. 2011; Hoffmann et al. 2012). Our study was conducted over the course of the Olympics, and participants’ intensive attention to the high competition in the games might have influenced the blood pressure. Also, the policy of taking half of the cars off the road during the period created difficulties in traveling, and local residents might have postponed their hospital visit even though they didn’t feel well. Symptoms related to blood pressure and inflammation may not have been treated immediately as before.
The current study has some noted limitations. First, we did not collect personal air pollution exposure data. Exposure misclassification, undoubtedly, may occur because of the spatial variability of air pollution and the peripatetic nature of people. The study only measured ambient particulate matter to represent the overall air pollution level, although we understand that the outcome is the synergistic effect from various air pollutants and other pollutants may not vary in the same pattern over the study period. For example, another study conducted during the same time periods found that ozone level was high during the Olympics (Rich et al. 2012). In addition, indoor air pollution was not included in this analysis. Second, ambient temperature and humidity might have some influence on the lung function and blood pressure. However, we were not able to adjust these two variables in the model since the model ran out of degrees of freedom. Third, taking medication might affect the results on both blood pressure and peak expiratory flow. But unfortunately, we didn’t collect any information on medication use during the study period. It is possible that people who are on medication might have taken less medicine during the game because of the difficulties to travel. In addition, unblinded bias cannot be excluded in these measurements including peak expiratory flow, since they might potentially be affected by an individual’s attitude and awareness of the study purpose. Although the study personnel never specified the purpose of each interview to participants, the air pollution intervention was fully publicized and discussed by the media before and during the Olympics. In addition, to acquire the signed informed consent form, study staff explained the study to all participants. Their awareness of the study aims for each wave of interview may have resulted in a bias. Last, The current study cannot rule out the possibility in misclassification. Both current and former smokers and drinkers were defined as smoker and drinker in the analysis, which might lead to misclassification. Compared to current smokers, former smokers might have different responses to air pollution level. Although the three physicians involved in the physical examination were very experienced and perform the standard examinations regularly, we recognize that measurement errors by physicians and related misclassification are a possibility given the subjective nature of such measurements. Those doctors might be aware of the study hypothesis and they could have been more likely to report the symptoms before and after the games. However, during each study period, participants were randomly assigned to doctors and very likely each participant saw different doctors at different time points. This might not decrease the misclassification, but might decrease the possibility of misclassification towards to the anticipated direction, namely high inflammation before and after games and low inflammation during the games.
However, the design of with-in person comparison in the current study enabled us to avoid the potential confounding factors caused by inappropriate selection of controls. Inclusion of smoking individual in the study allows us to investigate the influence of air pollution on both smokers and non-smokers, and investigate the potential modifying effects of smoking on the biological responses to dramatic changes in air pollution. The design with a bi-directional intervention strengthens the scientific rigor and provides internal validity of our results. Our finding successfully filled in the gap in understanding related to the short-term effects of particulate matter air pollution on healthy adults.
5. Conclusion
The current study found that short-term exposure to different air pollution levels has significant effects on respiratory function measured by peak expiratory flow and breath rate. The effects of different air pollution exposure on heart rate, blood pressure and other physical examinations were not clear.
Highlights.
Peak expiratory flow levels changed significantly over the three study periods
Peak expiratory flow levels increased during the Olympics among 78% of participants
Percentage changes of peak expiratory flow are different among subgroups
Breath rate varied significantly over the three study periods
Acknowledgments
This work was supported in part by the National Institute of Environmental Health Sciences grant awarded to Dr. Lina Mu (Grant number: R01ES018846) and in part by Department of Social and Preventive Medicine, UB School of Public Health and Health Professions.
Footnotes
IRB approval: Prior to the initiation of the field investigation, IRB approvals were obtained from the University at Buffalo (SPM1080708E). The following is the most updated IRB approval letter.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baccarelli A, Barretta F, Dou C, Zhang X, McCracken JP, et al. Effects of particulate air pollution on blood pressure in a highly exposed population in Beijing, China: a repeated-measure study. Environmental health: a global access science source. 2011;10:108. doi: 10.1186/1476-069X-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat Res. 2006;608:157–162. doi: 10.1016/j.mrgentox.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clinical science. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, et al. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Villarreal-Calderon R, Valencia-Salazar G, Henriquez-Roldan C, Gutierrez-Castrellon P, et al. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhalation toxicology. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- Chen H, Goldberg MS, Villeneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Reviews on environmental health. 2008;23:243–297. doi: 10.1515/reveh.2008.23.4.243. [DOI] [PubMed] [Google Scholar]
- Chen SY, Su TC, Lin YL, Chan CC. Short-term effects of air pollution on pulse pressure among nonsmoking adults. Epidemiology. 2012;23:341–348. doi: 10.1097/EDE.0b013e3182452f1d. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2010;52:258–262. doi: 10.1097/JOM.0b013e3181ceff7a. [DOI] [PubMed] [Google Scholar]
- Correia-Deur JE, Claudio L, Imazawa AT, Eluf-Neto J. Variations in peak expiratory flow measurements associated to air pollution and allergic sensitization in children in Sao Paulo, Brazil. Am J Ind Med. 2012 doi: 10.1002/ajim.22060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Krishnan RM, Oron AP, Jansen K, Peretz A, et al. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension. 2012;59:943–948. doi: 10.1161/HYPERTENSIONAHA.111.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D, Nelson HS. The role of the peak flow meter in the diagnosis and management of asthma. J Allergy Clin Immunol. 1991;87:120–128. doi: 10.1016/0091-6749(91)90223-b. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D. Pollution and the immune response: atopic diseases--are we too dirty or too clean? Immunology. 2000;101:11–18. doi: 10.1046/j.1365-2567.2000.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusseldorp A, Kruize H, Brunekreef B, Hofschreuder P, de Meer G, et al. Associations of PM10 and airborne iron with respiratory health of adults living near a steel factory. American journal of respiratory and critical care medicine. 1995;152:1932–1939. doi: 10.1164/ajrccm.152.6.8520758. [DOI] [PubMed] [Google Scholar]
- Earley MC, Vogt RF, Jr, Shapiro HM, Mandy FF, Kellar KL, et al. Report from a workshop on multianalyte microsphere assays. Cytometry. 2002;50:239–242. doi: 10.1002/cyto.10140. [DOI] [PubMed] [Google Scholar]
- Franchini M, Mannucci PM. Short-term effects of air pollution on cardiovascular diseases: outcomes and mechanisms. J Thromb Haemost. 2007;5:2169–2174. doi: 10.1111/j.1538-7836.2007.02750.x. [DOI] [PubMed] [Google Scholar]
- Franchini M, Mannucci PM. Air pollution and cardiovascular disease. Thrombosis research. 2012;129:230–234. doi: 10.1016/j.thromres.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Gregg I, Nunn AJ. Peak expiratory flow in normal subjects. Br Med J. 1973;3:282–284. doi: 10.1136/bmj.3.5874.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Kosterink P, Van den Berg R, Hofschreuder P. Effect of ambient ozone on peak expiratory flow of exercising children in The Netherlands. Archives of environmental health. 1993;48:27–32. doi: 10.1080/00039896.1993.9938390. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Luttmann-Gibson H, Cohen A, Zanobetti A, de Souza C, et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environmental health perspectives. 2012;120:241–246. doi: 10.1289/ehp.1103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YC, Pan XC, Kim SY, Park K, Park EJ, et al. Asian Dust Storm and pulmonary function of school children in Seoul. The Science of the total environment. 2010;408:754–759. doi: 10.1016/j.scitotenv.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Jacobs L, Buczynska A, Walgraeve C, Delcloo A, Potgieter-Vermaak S, et al. Acute changes in pulse pressure in relation to constituents of particulate air pollution in elderly persons. Environ Res. 2012 doi: 10.1016/j.envres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Jacobson LD, Hacon SD, Castro HA, Ignotti E, Artaxo P, et al. Association between fine particulate matter and the peak expiratory flow of schoolchildren in the Brazilian subequatorial Amazon: A panel study. Environ Res. 2012 doi: 10.1016/j.envres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Kasamatsu J, Shima M, Yamazaki S, Tamura K, Sun G. Effects of winter air pollution on pulmonary function of school children in Shenyang, China. Int J Hyg Environ Health. 2006;209:435–444. doi: 10.1016/j.ijheh.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Lee JT, Son JY, Cho YS. The adverse effects of fine particle air pollution on respiratory function in the elderly. Sci Total Environ. 2007;385:28–36. doi: 10.1016/j.scitotenv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Linares B, Guizar JM, Amador N, Garcia A, Miranda V, et al. Impact of air pollution on pulmonary function and respiratory symptoms in children. Longitudinal repeated-measures study. BMC Pulm Med. 2010;10:62. doi: 10.1186/1471-2466-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Shima M, Yoda Y, Yamamoto H, Nakai S, et al. Effects of airborne particulate matter on respiratory morbidity in asthmatic children. J Epidemiol. 2008;18:97–110. doi: 10.2188/jea.JE2007432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- Mar TF, Koenig JQ, Jansen K, Sullivan J, Kaufman J, et al. Fine particulate air pollution and cardiorespiratory effects in the elderly. Epidemiology. 2005;16:681–687. doi: 10.1097/01.ede.0000173037.83211.d6. [DOI] [PubMed] [Google Scholar]
- Mengersen K, Morawska L, Wang H, Murphy N, Tayphasavanh F, et al. Association between indoor air pollution measurements and respiratory health in women and children in Lao PDR. Indoor Air. 2011;21:25–35. doi: 10.1111/j.1600-0668.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- Nordling E, Berglind N, Melen E, Emenius G, Hallberg J, et al. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology. 2008;19:401–408. doi: 10.1097/EDE.0b013e31816a1ce3. [DOI] [PubMed] [Google Scholar]
- Oftedal B, Brunekreef B, Nystad W, Madsen C, Walker SE, et al. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19:129–137. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- Pervin T, Gerdtham UG, Lyttkens CH. Societal costs of air pollution-related health hazards: A review of methods and results. Cost effectiveness and resource allocation: C/E. 2008;6:19. doi: 10.1186/1478-7547-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, I, Goldstein F, Beyer U, Franke K, Heinrich J, et al. Acute health effects of exposure to high levels of air pollution in eastern Europe. American journal of epidemiology. 1996;144:570–581. doi: 10.1093/oxfordjournals.aje.a008967. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Dockery DW. Acute health effects of PM10 pollution on symptomatic and asymptomatic children. The American review of respiratory disease. 1992;145:1123–1128. doi: 10.1164/ajrccm/145.5.1123. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Dockery DW, Spengler JD, Raizenne ME. Respiratory health and PM10 pollution. A daily time series analysis. The American review of respiratory disease. 1991;144:668–674. doi: 10.1164/ajrccm/144.3_Pt_1.668. [DOI] [PubMed] [Google Scholar]
- Qian Z, Lin HM, Chinchilli VM, Lehman EB, Stewart WF, et al. Associations between air pollution and peak expiratory flow among patients with persistent asthma. Journal of toxicology and environmental health Part A. 2009;72:39–46. doi: 10.1080/15287390802445517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA: the journal of the American Medical Association. 2012;307:2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer W, Hoek G, Brunekreef B. Effect of ambient winter air pollution on respiratory health of children with chronic respiratory symptoms. The American review of respiratory disease. 1993;147:118–124. doi: 10.1164/ajrccm/147.1.118. [DOI] [PubMed] [Google Scholar]
- Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, et al. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. American journal of respiratory and critical care medicine. 1996;154:300–307. doi: 10.1164/ajrccm.154.2.8756798. [DOI] [PubMed] [Google Scholar]
- Seagrave J. Mechanisms and implications of air pollution particle associations with chemokines. Toxicology and applied pharmacology. 2008;232:469–477. doi: 10.1016/j.taap.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen M, Hvidberg M, Hoffmann B, Andersen ZJ, Nordsborg RB, et al. Exposure to road traffic and railway noise and associations with blood pressure and self-reported hypertension: a cohort study. Environmental health: a global access science source. 2011;10:92. doi: 10.1186/1476-069X-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, et al. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2008;32:129–138. doi: 10.1183/09031936.00097707. [DOI] [PubMed] [Google Scholar]
- Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environmental health perspectives. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee SC, Hoek G, Boezen MH, Schouten JP, van Wijnen JH, et al. Acute effects of air pollution on respiratory health of 50–70 yr old adults. Eur Respir J. 2000;15:700–709. doi: 10.1034/j.1399-3003.2000.15d13.x. [DOI] [PubMed] [Google Scholar]
- Watterson TL, Sorensen J, Martin R, Coulombe RA., Jr Effects of PM2.5 collected from Cache Valley Utah on genes associated with the inflammatory response in human lung cells. Journal of toxicology and environmental health Part A. 2007;70:1731–1744. doi: 10.1080/15287390701457746. [DOI] [PubMed] [Google Scholar]
- Wiwatanadate P, Liwsrisakun C. Acute effects of air pollution on peak expiratory flow rates and symptoms among asthmatic patients in Chiang Mai, Thailand. Int J Hyg Environ Health. 2011;214:251–257. doi: 10.1016/j.ijheh.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Wiwatanadate P, Trakultivakorn M. Air pollution-related peak expiratory flow rates among asthmatic children in Chiang Mai, Thailand. Inhal Toxicol. 2010;22:301–308. doi: 10.3109/08958370903300327. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Shima M, Ando M, Nitta H, Watanabe H, et al. Effect of hourly concentration of particulate matter on peak expiratory flow in hospitalized children: a panel study. Environ Health. 2011;10:15. doi: 10.1186/1476-069X-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Gu J, Xu H, Yang B, Han Y, et al. Meta-analysis of the relationship between passive smoking population in China and lung cancer. Zhongguo Fei Ai Za Zhi. 2010;13:617–623. doi: 10.3779/j.issn.1009-3419.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]