Abstract
A myogenic cell suspension was isolated from the breast muscles of 10-day-old chicken embryos by trypsin digestion. The cell preparation was subjected to Percoll density centrifugation to reduce the number of fibroblast like cells present. The Percoll-isolated, predominantly myogenic cell population was then fractionated by flow cytometry using 90° light scattering as the parameter for sorting. Cells exhibiting lower scatter, with a peak of 45 units, produced cultures containing myotubes and gave rise only to myogenic clones. Cells exhibiting higher scatter (120–200 units) produced nonmyogenic cultures and gave rise to nonmyogenic clones. Cells with intermediate light scatter were also detected. The latter produced both myogenic and nonmyogenic clones. The differences in light scatter presumably reflect higher cytoplasmic complexity of the nonmyogenic cells compared with the myogenic cells. Moreover, the differences in light scattering properties of the different cell types offer a means for the isolation of pure populations of myogenic cells directly from the intact muscle.
Key terms: Myoblasts, fibroblasts, cell sorting, tissue culture
Both myogenic and nonmyogenic cells have been identified in embryonic muscles (3,23). Whereas the myogenic cells are confined to developing muscle bundles, the nonmyogenic cells (primarily fibroblast-like cells) reside in regions of connective tissue. Muscle fibroblasts probably contribute to the formation of the basal lamina during myogenesis (9,17). When preparing myogenic cultures, these so-called fibroblasts are usually coisolated with the myogenic cells and contribute, depending on the species and the organismal age, up to 50% of the cultured cells (1,5,8,20,23,26). The identification of the nonmyogenic cells in cultures has depended largely on their morphology; i.e., because they are flattened and stellate, with no other distinguishing characteristics, they are often called fibroblasts. It has been suggested that the cultured muscle fibroblasts represent both “true” fibroblasts and mesenchymal-like muscle precursors, which may express muscle differentiation only under certain conditions (e.g., the addition of conditioned medium) (5,11,21). Other investigators have proposed the existence of precursor cells that are ancestral to both myogenic and fibrogenic lineages (1). The latter derived from the observation that large myogenic clones, consisting of hundreds of cells, usually contain fibroblast-like cells in addition to myoblasts and myotubes. It has also been suggested that some fibroblasts in tissue culture may represent phenotypic modulations or alternate states of a variety of cell types (4). Removal of fibroblast-like cells from primary myogenic cultures has most often been attempted by differential attachment (9,10,26). However, such approaches are not always successful, and often the myogenic cell preparations thus obtained still contain nonmyogenic cells.
Recently, we began an investigation into the isolation and characterization of fibroblast-like cells from embryonic muscle (23,24). The approach, which employs Percoll density centrifugation, allows the separation of myogenic cell populations from embryonic and adult muscle (23–25). Studying the embryonic muscle, we have demonstrated that the Percoll-isolated fibroblasts represent an homogeneous nonmyogenic cell population. The Percoll-isolated, myogenic cell population, which was composed, predominantly, of myoblasts, also contain some cells that do not express myogenic properties (23,24). To investigate further the heterogeneity of embryonic muscle cell populations and to develop a means for the isolation of pure myogenic cells from embryonic muscle, we studied the light scattering properties of the Percoll-isolated myogenic cell population.
Light scattering by cells is a complex function of their size, shape, composition, and internal structure. The relative contributions to light scattering by the various cell parameters differ significantly with the angle of scatter. Generally, forward scatter (or narrow scatter angle) depends primarily on cell size, whereas structure or cytoplasmic complexity (i.e., number of organelles) becomes more important at wider scatter angles (7,12,15,18,19). Using 90° light scattering, we found that the Percoll-isolated myogenic cell population can be further sorted into subpopulations exhibiting different ranges of light scatter. The subpopulation exhibiting the lowest scatter values is composed only of myogenic cells, whereas the subpopulation with the highest scatter is composed primarily of nonmyogenic, fibroblast-like cells. Hence, the difference in light scattering properties of the myogenic and nonmyogenic cells can now be used for the complete isolation of myogenic cells from the developing muscle.
MATERIALS AND METHODS
Source of Cells and Medium
Ten-day-old chicken embryos (White Leghorn) were used throughout this study. Standard medium consisted of 85 parts Eagle’s minimal essential medium (MEM; Gibco, Grand Island, NY), 10 parts horse serum (Gibco), 5 parts embryo extract, penicillin and streptomycin at 105 units per liter each, and fungizone and gentamicin at 2.5 and 5 mg per liter, respectively. Single cell suspensions were obtained by enzymatic digestion of the breast muscles (23). Briefly, muscles were excised, finely minced, incubated in trypsin (Gibco; adjusted to 0.1% final concentration) for 30–45 mm at 37°C, and centrifuged at approximately 300g for 5 mm. The trypsin solution was decanted and the pellet resuspended in 5–10 ml of standard medium. The cell suspension was recentrifuged as above and the final pellet resuspended in 2.5–3 ml of standard medium. Cells were mechanically dissociated by five passages through a Pasteur pipette followed by five passages through an 18-gauge needle. The resulting cell suspension was filtered through a double layer of lens tissue to eliminate myotubes, counted in a hemocytometer, and immediately subjected to density centrifugation as described below.
Percoll Density Centrifugation
Percoll density centrifugation was performed as recently described (23) to reduce the number of nonmyogenic cells present in myogenic cell preparations. Percoll (Pharmacia) was made iso-osmotic by adding 1 part of 10× concentrated MEM to 9 parts of Percoll. Further dilutions of the 90% Percoll were made with 1 x MEM. Centrifugation was performed using 15 ml Corex tubes, which were first treated for 2 h with horse serum to minimize adherence of cells to the glass walls. About 11.5 ml of 20% Percoll in MEM was layered over a cushion of 60% Percoll (1.5 ml). The cell suspension (5 × 107 cells in 2 ml standard medium per tube) was then layered over the 20% Percoll, and the sample was centrifuged for 5 mm at 15,000g at 8°C with brakes off in a fixed angle rotor (Sorvall SS-34). Following centrifugation, a cell population that contained skeletal myogenic precursor cells was recovered from the 20/60% Percoll interface (fibroblast-like cells can be recovered from the 20% Percoll region). The recovery of cells from the Percoll solutions was performed by centrifugation (300g, 10 mm) following the withdrawal of the cells at the interface and dilution with 5 vol of MEM. Cell pellets were resuspended in 2 ml of standard medium and counted in a hemocytometer. Cells were then subjected to flow cytometry as described below.
Flow Cytometry
Cells were sorted with an Ortho 50 H cytofluorograph. Cells were illuminated with 200 mW of 488 nm light. Forward angle scatter was first gated to remove debris and dead cells. Regions were then established on the histogram of 90° scatter. The Percoll-isolated myogenic fraction was sorted on the basis of variation in this parameter using a 100 μm orifice and sorting rates of 1,500 cells/s. The cell suspension was sorted using phosphate-buffered saline, and cells were collected into standard medium in a ratio of 1 ml sorted cell suspension per 1 ml medium. Cells were recovered by centrifugation (300g, 10 mm), resuspended in 1 ml of standard medium, counted in a hemocytometer, and cultured. Bivariate data were displayed using the program CONTOUR kindly provided by Dr. Peter S. Rabinovitch (University of Washington, Seattle).
Cell Culture
Cells were plated onto 35 mm tissue culture dishes coated with 2% gelatin and preincubated for 3 hr with 25% horse serum in MEM to promote cellular adherence. Cultures were maintained at 37.5°C in a water-saturated atmosphere containing 5% CO2 in air. Cells were plated at 4 × 104 cells in 1.5 ml of standard medium, and the medium was changed 24 h after plating and every other day thereafter. Photographs of live cells were obtained with a Zeiss inverted microscope and Kodak Tri-X film. Clonal cultures were prepared as decribed previously (13,22,23,25). Briefly, cultures were initiated with 100 cells per 60 mm dish and maintained for 4–10 days. They were then fixed for antibody staining as described below.
Antibody Staining
Cultures were fixed with 70% ethanol:formalin:acetic acid (20:2:1), and immunolabeling of cells in culture was performed with the indirect immunofluorescence technique using antiskeletal myosin or antimuscle type creatine phosphokinase as previously described (14,22, 23,25).
RESULTS
When the Percoll-isolated myogenic fraction was compared with a myogenic cell suspension that was not fractionated by Percoll, we observed more cells with greater 90° light scatter in the unfractionated sample. This led us to adapt further the 90° light scatter for sorting the Percoll-isolated myogenic fraction. A typical 90° scatter analysis of the cells is shown in Figure 1. A sharp peak of light scatter was observed at about 45 units (regions A and B), followed by a broad shoulder at 65–120 units (region C) and a trailing region at 120–200 units (region D). Cells were then sorted into four populations (A–D) according to the light scatter regions. The first peak was subdivided into two regions (A and B) to obtain a representative peak population (i.e., region A) in which the presence of cells from the shoulder region was minimal.
Fig. 1.
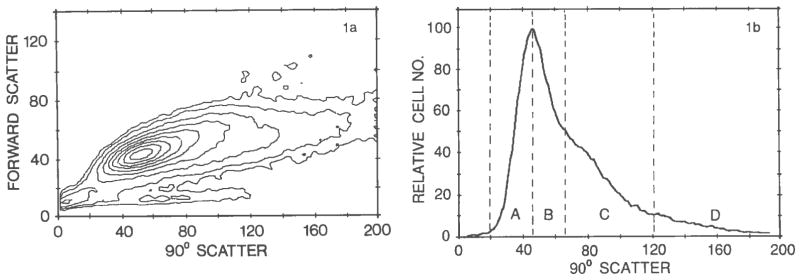
Light scatter analysis of a representative myogenic cell fraction isolated by Percoll density centrifugation. a: A contour plot of the bivariate forward angle scatter versus 90° scatter data. b: A 90° light scatter histogram from the same experiment as in a. Regions A–D show the four regions used for sorting subpopulations. Forward and 90° scatter units are channel numbers in arbitrary units.
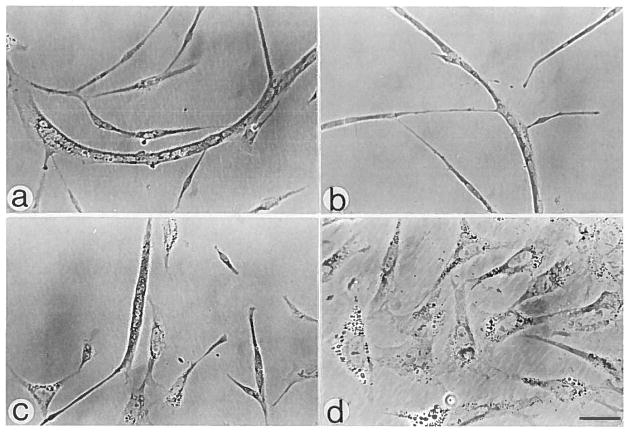
Following sorting, the cells were cultured at a mass culture density to verify the nature of the cells in the different regions. Figure 2 demonstrates the presence of myogenic cells in regions A, B, and C (Fig. 1) as determined by the appearance of myotubes. Region D contained primarily nonmyogenic cells. Less then 5% of the cells in region D were myogenic as determined by a positive reaction to skeletal muscle specific antibodies. Additional myogenic cells were not observed in cells from region D even after prolonged culturing (up to 10 days). Figure 2 also demonstrates the presence of punctate material in the cytoplasm of the nonmyogenic cells. This material was not detected in the cytoplasm of the myogenic cells under the conditions described in this study.
Fig. 2.
Phase micrographs of cultures prepared from the cells in the different 90° light scatter regions. a–d: Representative fields from Figure 1, regions A–D, respectively. Cultures were initiated with 4 × 104 cells per 35 mm dish and maintained for 3 days. a–c bar = 48 μm; d, 30 μm.
Less than 2% of the cells in the original Percoll-isolated cell fraction expressed properties of terminally differentiated myoblasts (i.e., reacted positively with skeletal muscle specific antibodies such as antiskeletal myosin heavy chain or antimuscle-type creatine phosphokinase). Hence, the vast majority of the myogenic cells in the cell suspension applied to the cell sorter could not be identified by muscle specific characteristics. Therefore, a clonal analysis of the cells in regions A–D was performed to determine the proportion of cells in each group that can eventually give rise to myogenic clones (i.e., clones with myotubes and/or cells expressing muscle specific proteins). The cells were cultured at a low density, and descendants of single cells were studied. It has been established that, in the chicken embryo, some myogenic precursors divide a limited number of times and give rise only to small clones (up to 16 cells per clone). Other cells give rise to much larger clones (13). To detect both kinds of clones, cultures were fixed after 4 days for analysis of small clones and after 7–10 days for the analysis of large clones. All clones were then reacted with an antibody to muscle specific myosin or to muscle-type creatine phosphokinase. Such an approach has enabled the detection of differentiated cells that have not fused (13). Representative cultures from the different sorted regions were scored for the total number of myogenic and nonmyogenic clones (small and large). The results of this study are summarized in Table 1. It is clear that there were no nonmyogenic cells in region A, and the number of nonmyogenic clones in cultures from region B was reduced compared with the Percoll-isolated myogenic cell population, which had not been subjected to light-scattering fractionation. The number of nonmyogenic clones in cultures from region C was higher than the number in region B. This was probably due to large, overlapping regions of the myogenie and nonmyogenic cells. Obviously, although based on preliminary experiments, the exact borders between the two cell types on the light scatter histogram are somewhat arbitrary. Clonal cultures of region D contained very few myogenic clones.
Table 1.
Distribution of Myogenic and Nonmyogenic Clones in Cells Recovered From the Different Scatter Regions
| Regions | % Myogenic clones | % Nonmyogenic clones | No. of clones analyzed |
|---|---|---|---|
| A | 100 | 0 | 55 |
| B | 88 | 12 | 35 |
| C | 70 | 30 | 72 |
| D | 3 | 97 | 59 |
| Percoll-isolated cells | 70 | 30 | 43 |
DISCUSSION
This study demonstrates that the predominantly myogenic cell population prepared by Percoll density centrifugation from embryonic muscle can be further fractionated into myogenic and nonmyogenic cell populations based on the light scattering properties of the cells. At small angles, the light scattering of cells is dominated by the cell size. In the 90° direction, scatter from cytoplasmic structures such as mitochondria, microvilli, lysosomes, vacuoles, and other organelles as well as cytoskeletal fibers becomes important (7,15,16,18). Hence, the nonmyogenic cells are probably enriched in such entities compared with the myogenic cells. The punctate material observed in the cytoplasm of the cultured nonmyogenic cells may reflect the presence of such entities (Fig. 2d) (23). Moreover, studying suspensions of the freshly isolated cells with electron microscopy suggests that the nonmyogenic cells, at the time of the isolation, are highly enriched with membrane bound vesicles, whereas the myogenic cells have only a paucity of such vesicles and their cytoplasm is more condensed (24). This property could lead to the higher light scatter exhibited by the nonmyogenic cells.
Earlier studies have suggested that the latter difference between fibroblasts and myoblasts might also be true for cultured cells (11). Obviously, it is still possible that the cytoplasm of some myogenic as well as nonmyogenic cells is of an intermediate type in respect to light scatter properties (e.g., number of vesicles is higher than for cells in region A but lower than for cells in region D). Such cells could give rise to region C in the light scatter histogram. It is noteworthy that 90° light scatter has proved efficient in distinguishing among cells of comparable size, such as the various classes of leukocytes (15). The granularity of the cytoplasm led to the complete separation of lymphocytes, monocytes, and granulocytes (15). More recent studies regarding the separation of different blood cells followed similar approaches (18,19).
A separation of human myoblasts from fibroblasts by fluorescence-activated cell sorting (FACS) has been reported (6). However, this approach requires the availability of an antibody against a cell type-specific surface molecule. The separation of myoblasts by light scattering does not require the availability of such antibodies and can be performed on cells soon after their enzymatic dissociation from the intact tissue.
We previously prepared a fibroblast-like cell population from embryonic chicken muscle using Percoll density centrifugation (23,24). The Percoll-isolated fibroblasts and the nonmyogenic cells isolated by light scatter in the current study exhibit very similar morphology in culture (i.e., spread out, stellate cells with punctate material). Also, our studies using electron microscopy suggest that during the isolation procedure the cytoplasm of both kinds of nonmyogenic cells is highly enriched with membrane bound vesicles. However, the fibroblasts isolated by Percoll density centrifugation are less dense than the nonmyogenic cells, which were coisolated with the myogenic cells from the 20/60% Percoll interface. The latter density difference could derive from factors such as the stage in the cell cycle or the cell diameter (23). It is also possible that muscle fibroblasts show different properties depending on their localization in the embryonic muscle. This possibility is supported by the findings of a spatial distribution of the different collagen types in the connective tissue regions of chick muscle; type I was mainly present in epimysium and perimysium, type III in the perimysium, and type V in the endomysium (2).
In summary, the data presented in this report demonstrate differences in cytoplasmic properties of myogenic and nonmyogenic cells from embyronic muscle. Moreover, these differences, which can be physically monitored by 90° light scatter, can be used to obtain pure populations of myogenic precursor cells from embryonic chicken and possibly from other species.
Acknowledgments
This investigation was supported by a grant from the American Heart Association, Washington Affiliate, to the author and by a grant from the National Institutes of Health (AR-28154) to Dr. M. Nameroff.
I am grateful to Dr. Mark Nameroff for his continuing support and interest during the course of this study and to Dr. Peter S. Rabinovitch for advice on the cell sorting analyses. The helpful suggestions on the manuscript by the above investigators and by Dr. J.W. Prothero are highly appreciated. The sorting analyses were performed at the cell sorting facilities of the Department of Pathology, University of Washington.
Footnotes
This investigation was supported by a grant from the American Heart Association, Washington Affiliate, to the author and by a grant from the National Institutes of Health (AR-28154) to Dr. M. Nameroff.
LITERATURE CITED
- 1.Abbott J, Schiltz J, Dienstman S, Holtzer H. The phenotypic complexity of myogenic clones. Proc Natl Acad Sci USA. 1974;7(1):1506–1510. doi: 10.1073/pnas.71.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey AJ, Shellswell GB, Duance VC. Identification and change of collagen types in differentiating myoblasts and developing chick muscle. Nature. 1979;278:67–69. doi: 10.1038/278067a0. [DOI] [PubMed] [Google Scholar]
- 3.Fischman DA. An electron microscopy study of myofibril formation in embryonic chick skeletal muscle. J Cell Biol. 1967;32:557–575. doi: 10.1083/jcb.32.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrett DM, Conrad GW. Fibroblast-like cells from embryonic chick cornea, heart and skin are antigenically distinct. Dev Biol. 1979;70:50–70. doi: 10.1016/0012-1606(79)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Hauschka SD. Clonal analysis of vertebrate myogenesis. III. Developmental changes in the muscle-colony-forming cells of the human fetal limb. Dev Biol. 1974;37:345–368. doi: 10.1016/0012-1606(74)90154-7. [DOI] [PubMed] [Google Scholar]
- 6.Hurko O, Mckee L, Zuurveld JGEM, Walsh FS, Swick HM. Proliferative capacity of Duchenne and wild-type myoblasts derived from a DMD-G6PD double heterozygote. In: Emerson C, Fischman D, Nadal-Ginard B, Siddiqui MAQ, editors. Molecular Biology of Muscle Development. Alan R. Liss, Inc; New York: 1986. pp. 921–928. [Google Scholar]
- 7.Kerker M. Elastic and inelastic light scattering in flow cytometry. Cytometry. 1983;4:1–10. doi: 10.1002/cyto.990040102. [DOI] [PubMed] [Google Scholar]
- 8.Konigsberg IR. Clonal analysis of myogenesis. Science. 1963;140:1273–1284. doi: 10.1126/science.140.3573.1273. [DOI] [PubMed] [Google Scholar]
- 9.Kühl U, Öcalan M, Timpl R, Mayne R, Hay E, von der Mark K. Role of muscle fibroblasts in the deposition of type TV collagen in the basal lamina of myotubes. Differentiation. 1984;28:164–172. doi: 10.1111/j.1432-0436.1984.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 10.Kühl U, Öcalan M, Timpl R, von der Mark K. Role of laminin and fibronectin in selecting myogenic versus fibrogenic cells from skeletal muscle cells in vitro. Dev Biol. 1986;117:628–635. doi: 10.1016/0012-1606(86)90331-3. [DOI] [PubMed] [Google Scholar]
- 11.Lipton BH. A fine-structural analysis of normal and modulated cells in myogenic cultures. Dev Biol. 1977;60:26–47. doi: 10.1016/0012-1606(77)90108-7. [DOI] [PubMed] [Google Scholar]
- 12.Loken MR, Sweet RG, Herzenberg LA. Cell discrimination by multiangle light scattering. J Histochem Cytochem. 1976;24:284–291. doi: 10.1177/24.1.1254923. [DOI] [PubMed] [Google Scholar]
- 13.Quinn LS, Holtzer H, Nameroff M. Generation of chick skeletal muscle cells in groups of 16 from stem cells. Nature. 1985;313:692–694. doi: 10.1038/313692a0. [DOI] [PubMed] [Google Scholar]
- 14.Robinson MM, Quinn LS, Nameroff M. BB creatine kinase and myogenic differentiation. Immunocytochemical identification of a distinct precursor compartment in the chicken skeletal myogenic lineage. Differentiation. 1984;26:112–120. doi: 10.1111/j.1432-0436.1984.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 15.Salzman GC, Crowell JM, Martin JC, Trujillo PT, Romero A, Mullaney PF, LaBauve PM. Cell classification by laser light scattering: Identification and separation of unstained leukocytes. Acta Cytol. 1975;19:374–377. [PubMed] [Google Scholar]
- 16.Salzman GC, Mullaney PF, Price BJ. Light-scattering approaches to cell characterization. In: Melamed MR, Mullaney PF, Mendelsohn ML, editors. Flow Cytometry and Sorting. John Wiley & Sons; New York: 1979. pp. 105–124. [Google Scholar]
- 17.Sanderson RD, Fitch JM, Linsenmayer TR, Mayne R. Fibroblasts promote the formation of a continuous basal lamina during myogenesis in vitro. J Cell Biol. 1986;102:740–747. doi: 10.1083/jcb.102.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steen HB, Lindmo T. Differential of light-scattering detection in an arc-lamp-based epi-illumination flow cytometer. Cytometry. 1985;6:281–285. doi: 10.1002/cyto.990060402. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JM, Gralow JR, Levy R, Miller RA. The optimal application of forward and ninety-degree light scatter in flow cytometry for the gating of mononuclear cells. Cytometry. 1985;6:401–406. doi: 10.1002/cyto.990060503. [DOI] [PubMed] [Google Scholar]
- 20.Turner DC. Differentiation in cultures derived from embryonic chicken muscle, The postmitotic, fusion-capable myoblast as a distinct cell type. Differentiation. 1978;10:81–93. doi: 10.1111/j.1432-0436.1978.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 21.White NK, Bonner PH, Nelson OR, Hauschka SD. Clonal analysis of vertebrate myogenesis: TV. Medium-dependent classification of colony-forming cells. Dev Biol. 1975;44:346–361. doi: 10.1016/0012-1606(75)90405-4. [DOI] [PubMed] [Google Scholar]
- 22.Yablonka-Reuveni Z, Nameroff M. Immunocytochemical studies on the expression of desmin by dividing cells from skeletal muscle. In: Emerson C, Fischman D, Nadal-Ginard B, Siddiqui MAQ, editors. Molecular Biology of Muscle Development. Alan R. Liss, Inc; New York: 1986. pp. 47–60. [Google Scholar]
- 23.Yablonka-Reuveni Z, Nameroff M. Skeletal muscle cell populations: Separation and partial characterization of fibroblast-like cells from embryonic tissue using density centrifugation. Histochemistry. 1987;87:27–38. doi: 10.1007/BF00518721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yablonka-Reuveni Z, Anderson SK, Bowen-Pope DF, Nameroff M. Biochemical and morphological differences between fibroblasts and myoblasts from chicken embryonic skeletal muscle. Cell Tissue Res. 1988 doi: 10.1007/BF00214376. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Yablonka-Reuveni Z, Quinn LS, Nameroff M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev Biol. 1987;119:252–259. doi: 10.1016/0012-1606(87)90226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 1969;4:37–77. doi: 10.1016/s0070-2153(08)60480-9. [DOI] [PubMed] [Google Scholar]