Abstract
Context
Smoking cessation clinical trial
Objective
Assess the relative efficacy of bupropion and varenicline on smoking cessation and emotional functioning.
Design
Placebo controlled randomized clinical trial
Setting
University Medical Center
Patients
294 community volunteers who wanted to quit smoking
Interventions
12 weeks of Varenicline, Bupropion-SR, or Placebo plus intensive smoking cessation counseling (10 sessions ~240 minutes).
Main Outcome Measures
Prolonged abstinence from smoking, and weekly measures of depression, negative affect and other symptoms of nicotine withdrawal
Results
Significant differences were found in abstinence at the end of treatment and through the 3-month post-quit follow-up favoring both active medications vs. placebo At the 6 month follow-up only the varenicline vs. placebo comparison remained significant. Varenicline was also associated with a generalized suppression of depression and reduced smoking reward compared to the other treatments, while both medications improved concentration, reduced craving, and decreased negative affect and sadness compared to placebo, while having little impact (increase or decrease) on anxiety and anger. No differences were noted in self-reported rates of neuropsychiatric adverse events.
Conclusions
Varenicline exerts a robust and favorable impact on smoking cessation relative to placebo and may have a favorable (suppressive effect) on symptoms of depression and other affective measures with no clear unfavorable impact on neuropsychiatric adverse events in a community sample
Over 50% of the 45.3 million Americans who still smoke make a serious cessation attempt each year but only 6% of them remain abstinent for at least 6 months 1 The provision of pharmacotherapy, particularly when combined with smoking cessation counseling can substantially improve the success of a cessation attempt 2 Previous meta-analyses of bupropion or varenicline have confirmed that both are effective for smoking cessation 3–5 and both are considered front line therapies in clinical practice guidelines 2.
Bupropion (amfebutamone) is an atypical antidepressant whose mechanism of action is thought to involve modest inhibition of norepinephrine uptake and weaker inhibition of dopamine uptake 6. In addition, bupropion and in particular, one of its metabolites (2S,3S) hydroxybupropion are also thought to have an antagonist effect on the α4β2 nicotine acetylcholine receptor (nAChR) 7 Varenicline is a highly selective partial agonist of the α4β2 (nAChR). Its properties include stimulation of dopamine release in the nucleus accumbens (nAC) but to a much less extent than nicotine itself. Also given its relatively long half-life (24 hrs), it is thought to have antagonist properties as well, which may prevent full stimulation of the receptor when nicotine is co-administered 8 thereby reducing the risk of relapse
Given the actions of both medications on neurobiological targets related to affect and reward, it is thought that the modulation of nicotine withdrawal symptoms may contribute to their effectiveness. A pooled analysis of withdrawal symptoms over the first week of quitting using data from the phase-3 trials 9 showed that vs. placebo both varenicline and bupropion reduced negative affect (overall mean of ratings for anger, depression, anxiety and difficulty concentrating) using the Minnesota Nicotine Withdrawal Scale 10. No differences in negative affect using this composite measure were noted between the active drugs. Moreover in a separate analysis these differences were limited to those abstaining from smoking. Varenicline also reduced craving to a greater extent than bupropion among both abstainers and non abstainers and neither medication affected ratings of restlessness, hunger or insomnia. In addition, both varenicline and bupropion significantly reduced ratings of satisfaction and psychological reward vs. placebo (first cigarette following the quit-date) with varenicline producing a greater reduction than bupropion.
The present study was a double blind placebo controlled randomized clinical trial of varenicline and bupropion-SR, which used a more intensive form of counseling (i.e., 240 minutes of over 10 sessions) than that used in the varenicline phase-3 trials (120 minutes over 12 session)11;12 The study was designed with three goals in-mind: (1) To assess the relative efficacy of bupropion and varenicline on smoking cessation and symptoms of nicotine withdrawal in conjunction with an intensive form of behavioral counseling; and to assess medication compliance and measures of counseling exposure (i.e., duration, frequency) actually received by the participants. (2) To utilize multiple and weekly measures of negative affect to help clarify withdrawal pattern differences associated with these medications, rather than rely on one composite measure of negative affect as previously noted9. This is particularly important given the recent reports associating varenicline with increased risk of neuropsychiatric adverse events 13 and the need to tease apart such effects from the effects of cessation alone. (3) To serve as the parent clinical trial for a series of smaller sub-investigations evaluating psychophysiological and neural predictors of smoking cessation (ERP, acoustic startle, fMRI) along with potential genetic markers mapping onto to these predictors. These studies utilized subsets of those participating in the parent clinical trial and the results of these smaller investigations will be published as separate papers.
Method
Study Design
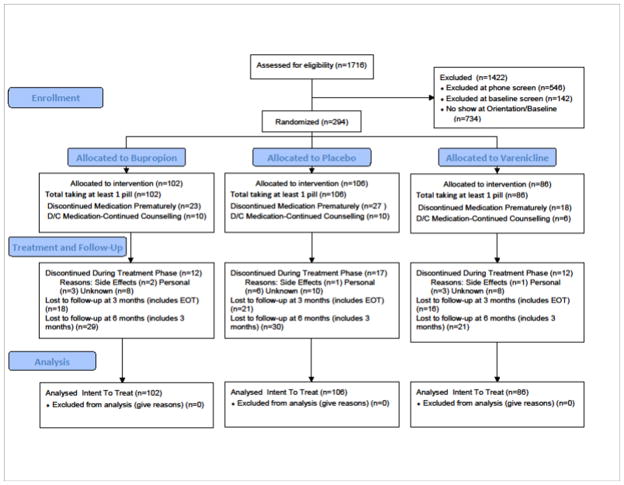
The subject ascertainment is shown in Figure 1 and timeline in Figure 2. All participants were screened for eligibility using a three step process: An initial phone screen, an in-person group orientation visit to more fully explain the study and review the consent form, and a subsequent in-person screening/baseline visit to assess medical and other eligibility criteria and conduct baseline assessments (also see Figure 2) A total of 294 participants were randomized and exposed to treatment as follows: Bupropion (N=102); Placebo (N=106); Varenicline (N=86). Adaptive randomization (minimization)14 was used to stratify the groups on gender, race, history of depression, and baseline smoking rate.
Figure 1.
Consort Table for Patient Allocation
Figure 2.

Study Visit Time Line
Participants
All smokers were volunteers recruited from the Houston, Texas metropolitan area using newspaper, radio, and television advertisements and public service announcements, from August, 2006 through October, 2010. To be included in this trial, smokers were required to provide written consent; be between 18–65 years of age; smoke 5 or more cigarettes per day; have a baseline expired carbon monoxide (CO) level greater than 6 ppm; be fluent in English; have a working telephone; and have no uncontrolled chronic medical illness. Exclusion criteria included: currently taking psychotropic medication; having a current psychiatric disorder including substance abuse (except for smoking), having a psychiatric hospitalization within the last year; having a lifetime history of a psychotic disorder; scoring moderate or higher on the suicidality scale of the Mini International Neuropsychiatric Interview (MINI) 15; being involved in any other concurrent smoking cessation activities, or having contraindications for bupropion (e.g., history of seizures) or varenicline (e.g., severe renal impairment). This research was approved by the University of Texas MD Anderson Cancer Center Internal Review Board.
Treatment
Pharmacotherapy
All participants took both types of study medication on a twice daily basis: either active or matching placebo bupropion tablets (Biomedical Research Institute of New Mexico, Albuquerque, NM); and either active or matching placebo varenicline capsules (Greenpark Pharmacy, Houston Texas).
Pharmacotherapy was initiated the day after the first treatment visit (see Figure 2), 12 19 days prior to the quit date1 and followed the recommended dosing for a total of 12 weeks (i.e., 0.5 mg/day varenicline for days 1–3; 0.5 mg bid; for days 4–7; and 1mg bid thereafter.; 150 mg/day bupropion for days 1–3; 150 mg bid thereafter) Dose adjustments by the blinded study physician were permitted in an effort to control side effects throughout the trial.
Behavioral Counseling
As shown in Figure 2 following randomization all smokers received conducted over 6 in-person visits and 4 phone calls during the 12 week active treatment phase (see previous note regarding cohort 1 and cohort 2). The counseling sessions were approximately 30 and 15 minutes in duration for the in-person and telephone modalities, respectively, yielding a total of 240 minutes of counseling. Counseling involved active effort to prepare for quitting and maintaining abstinence using self monitoring of smoking prior to the quit date, identification of high risk situations for smoking, development of coping skills and direct support before and after the quit date. Additional topics included stress management and relaxation visualization, relapse prevention, managing withdrawal symptoms and medications compliance. Counselors employed motivation enhancement strategies based on techniques of motivational interviewing 16 in response to resistance to keeping or resetting a quit date or to maintaining abstinence following the quit date.
Follow-up
As shown in Figure 2 follow-up sessions of 15 minutes duration were conducted at 3, 4 and 6 month post-quit and involved abstinence and other assessments as noted below.
Assessments
During the baseline screening phase participants were assessed for basic demographics, health, and smoking history; psychiatric disorders using version 5.0 of the M.I.N.I. International Neuropsychiatric Interview15; and nicotine dependence using the Fagerstrom Test for Nicotine Dependence (FTND)17;18
At baseline and at each in-person counseling and follow-up visit participants completed the Positive and Negative Affect Scale (PANAS)19; the Wisconsin Smoking Withdrawal Scale (WSWS) 20 which included subscales of Anger, Anxiety, Concentration, Craving, Hunger, Sadness and Sleep; and The Center for Epidemiologic Studies Depression Scale (CES-D) which has been used to measure depressive symptoms in community samples21;22. The smoking satisfaction and psychological reward subscales of Modified Cigarette Evaluation Questionnaire (mCEQ) 23 was also completed by subjects who reported smoking between visits.
Abstinence data was collected at all contacts using a timeline follow-back (TLFB) procedure24;25 Abstinence outcomes conformed to the Society of Research on Nicotine and Tobacco (SRNT)26 guidelines Seven-day point prevalence abstinence was defined as a self-report of no smoking, not even a puff, in the 7 days prior to the selected time point of interest (e.g., EOT, 3 and 6-months post-quit date). Continuous abstinence was reported using two different starting points for assessment. Continuous Abstinence (2-week grace) was defined as no smoking, not even a puff from 2 weeks past the quit-date (grace period) to a future time point. Continuous Abstinence (FDA) was similarly defined as no smoking but beginning with the last 4 weeks of treatment, or week 8 of medication in this trial. This measure provides comparability to the results of the phase-3 trials for varenicline and other pharmacotherapies, where continuous abstinence over the last four weeks of treatment served as the primary criteria for measuring efficacy and obtaining FDA approval for the pharmacotherapy.
In this study, prolonged abstinence at the end of treatment served as our primary smoking outcome measure. The common starting point for assessing prolonged abstinence was the end of the grace period (i.e., 2 weeks following the quit date). For prolonged abstinence, relapse was defined by 7 or more consecutive days of smoking or smoking at least 1 cigarette over two consecutive weeks from the end of the grace period to a selected future time point (e.g., EOT, 3- and 6-months post-quit date)26. Hence, prolonged abstinence allows a more liberal definition of abstinence than continuous.
In-person reports of abstinence were verified by expired CO < 10 ppm. Abstinent participants at the 3 and 6 month post-quit visits who could not return to the clinic and those reporting abstinence at the 4th (EOT) and 5th phone session were asked to provide a saliva cotinine sample by mail. Values of salivary cotinine of < 15 ng/ml were considered abstinent. Participants unavailable for assessment were considered non-abstinent.
Adverse Event Monitoring
Adverse events were monitored at each contact and were classified and graded using the Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria provided by the National Cancer Institute 27.
Compensation
Participants received compensation for completing assessments for a maximum possible total of $290 across all visits
Statistical Analysis
Analysis of Demographic and Baseline Variables
We used Tukey’s Studentized Range tests (continuous variables) and Fisher’s Exact tests (discrete variables) to examine differences on baseline demographic characteristics by treatment.
Analysis of Abstinence and Treatment of Missing Data
Data collected from the TLFB yielded approximately 64,000 observations (days of data). In the event of missing data (unable to be contacted), all individuals were treated as having smoked during that period (17% of the total observations, predominately at post-treatment) except where data was missing between two acquired data points that were either both coded as abstinent (0.5% of the total observations), or where the first data point was coded as non-abstinent but the second acquired data point was coded as abstinent (0.3% of the total observations). No differences in missing data frequency or number of individuals lost to follow-up (see Figure 1) were noted between the groups. There were also no differences on demographics characteristics shown in Table 1 between those who did or did not attend either of the follow-up sessions, with the exception that those lost to follow up had significantly (F(1,292)=7.45, p=.006) fewer years of education, M=13.4 (SD=1.8) vs. 14.2 (2.06), and higher total FTND scores (F(1,291)=5.53, p=.02), 5.07 (2.0) vs. 4.38 (2.16). In every case, the last known status of these individuals was a smoker. All analyses were carried out on the intent-to-treat (allocated to intervention) sample
Table 1.
Baseline Demographic and Smoking Characteristics
| Variable | Varenicline (n=86) | Bupropion (n=102) | Placebo (N=106) | Total (N=294) |
|---|---|---|---|---|
| Race/Ethnicity | % (N) | % (N) | % (N) | % (N) |
| African-American, Non-Hispanic | 20.9 (18) | 22.6 (23) | 20.8 (22) | 21.4 (63) |
| White, Non-Hispanic | 58.1 (50) | 68.6 (70) | 71.7 (76) | 66.7 (196) |
| Hispanic | 11.6 (10) | 3.9 (4) | 5.7 (6) | 6.8 (20) |
| Other | 9.4 (8) | 4.9 (5) | 1.8 (2) | 5.1 (15) |
| Gender | ||||
| Male | 61.6 (53) | 58.8 (60) | 63.2 (67) | 61.2 (180) |
| Female | 38.4 (33) | 41.2 (42) | 36.8 (39) | 38.8 (114) |
| Marital Status | ||||
| Married or living with partner | 45.4 (39) | 44.1 (45) | 45.3 (48) | 44.9 (132) |
| Other | 54.6 (47) | 55.9 (57) | 54.7 (58) | 55.1 (162) |
| Employment Status | ||||
| Employed/Student | 76.7(66) | 78.4 (76) | 86.8 (92) | 81.0 (234) |
| Unemployed | 23.3 (20) | 21.7 (21) | 13.2 (14) | 19.0 (55) |
| Depression History | ||||
| Positive | 13.9 (12) | 11.7 (12) | 9.4 (10) | 11.6 (34) |
| Negative | 86.1 (74) | 88.2 (90) | 90.6 (96) | 88.4 (260) |
| Smoking & Other | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age (years) | 43.8 (10.79) | 44.0 (9.54) | 45.2 (11.00) | 44.3 (10.43) |
| Years of Education | 14.0 (2.07) | 14.0 (2.08) | 14.1 (2.00) | 14.0 (2.04) |
| Current smoking rate (cigs/day) | 19.2 (8.48) | 20.0 (9.69) | 19.7 (9.77) | 19.7 (9.36) |
| Baseline carbon monoxide (ppm) | 24.5 (10.75) | 25.0 (12.87) | 24.1 (11.85) | 24.5 (11.87) |
| Age started smoking (years) | 18.0 (4.86) | 17.6 (3.97) | 17.9 (5.90) | 17.8 (4.98) |
| Number of previous quit attempts | 4.2 (4.33) | 3.4 (3.29) | 3.5 (3.64) | 3.7 (3.65) |
| FTND Total score | 4.5 (2.24) | 4.7 (2.06) | 4.4 (2.16) | 4.5 (2.15) |
| CES-D | 7.0 (6.40) | 7.0 (5.99) | 8.3 (7.18) | 7.5 (6.57) |
| PANAS Negative | 14.9 (5.55) | 15.8 (4.61) | 16.9 (5.84) | 15.9 (5.40)* |
| PANAS Positive | 35.6 (6.74) | 35.9 (6.07) | 35.7 (6.59) | 35.7 (6.44) |
| WSWS Anger | 3.9 (2.61) | 4.3 (2.65) | 4.3 (2.69) | 4.2 (2.65) |
| WSWS Anxiety | 7.3 (2.70) | 7.5 (3.27) | 8.0 (3.20) | 7.6 (3.09) |
| WSWS Concentration | 3.8 (2.02) | 3.96 (2.12) | 4.11 (2.29) | 4.0 (2.15) |
| WSWS Craving | 9.0 (3.08) | 9.3 (2.83) | 9.1 (2.92) | 9.1 (2.93) |
| WSWS Hunger | 9.9 (3.56) | 9.9 (3.29) | 11.1 (3.75) | 10.3 (3.57)* |
| WSWS Sadness | 4.6 (2.55) | 4.2 (2.53) | 4.7 (2.63) | 4.5 (2.57) |
| WSWS Sleep | 7.9 (4.24) | 9.2 (4.54) | 8.6 (4.62) | 8.6 (4.49) |
Note. All frequencies are calculated within group (column).
Main effect of group, p < .05.
To analyze smoking abstinence, we used SAS PROC LOGISTIC (Version 9.2, SAS Institute Inc, Cary, NC). The effect of treatment on each abstinence outcome was evaluated separately for the EOT (primary outcome point) and 3 and 6 months follow-up time points using models that included Treatment Group (Varenicline, Bupropion, Placebo) as a between-subject fixed effect, and subject as a random effect. All models were tested including Study (Cohort 1, Cohort 2-see previous note1), Gender and Race as covariates. Because no differences were found by including these covariates the results are reported for the unadjusted models. We report overall Wald chi-squares for the effects of treatment as well as corresponding odds ratios and confidence intervals (95% CI’s) for all abstinence analyses.
Effects of Treatment on Affect Other Withdrawal Symptoms and Craving
To analyze the effects of treatment and time on measures of affect, withdrawal, and craving (PANAS, CES-D and WSWS) we used mixed model regression (PROC MIXED, Version 9.2, SAS Institute Inc, Cary, NC) 28;29. The model included fixed effects for Treatment, Time of assessment and their interaction term. Time was expressed as days post-quit and corresponded to each of the post-baseline in-person and phone counseling visits (see Figure 2) on which data was obtained Covariates in the model included the baseline (pre-treatment) value of each of the corresponding scales for the measure being analyzed, as well as abstinence status (1=abstinent/0=non-abstinent) at each of the time points. Significant main effects and interactions were further explored using a least squares mean procedure to contrast pair wise differences among selected means participating in the effect. As described for the analysis of abstinence, we also evaluated an iteration of the basic model that included covariate terms for Study (Cohort), Gender, Race).
Effects of Treatment on Nicotine Reinforcement
To analyze the effects of treatment and time on measures of nicotine reinforcement (mCEQ) we used the same approach as described for the analysis of withdrawal symptoms above but did not include an effect term for abstinence status because this measure was given only to those who indicated smoking in-between assessments.
Adverse events
We used Fisher’s Exact tests to examine differences in frequency of adverse events by treatment
Results
Demographic characteristics of the sample are presented in Table 1. No significant differences between drug groups were noted in any baseline characteristics, with the exception of the PANAS negative affect score and WSWS hunger.
Effects of Treatment on Abstinence
Abstinence rates by drug group are provided in Table 2 for each of the abstinence definitions and for each of three time points: EOT, 3 months and 6 months post-quit day. The results for our primary outcome measure of prolonged abstinence revealed overall group differences at EOT 3-months follow-up and 6-months follow-up. Abstinence rates for varenicline were significantly higher than placebo at all time points while the bupropion vs. placebo comparison was significant at EOT and 3 months follow-up but not at 6 months. Prolonged abstinence rates for varenicline also exceed that of bupropion at each time point, although the differences were not statistically significant. Similar results were noted for the analysis of continuous abstinence rates (2-week grace and FDA) as well as 7-day point prevalence. In all cases abstinence rates for varenicline were significantly greater than placebo at all time points; abstinence rates for bupropion were greater than placebo but the comparisons were not significant at 6 months and were marginal for 7-day point prevalence at 3 months (p=.054). While varenicline outperformed bupropion by a difference of 5%–14% over time, the differences were significant only for 7-day point prevalence at 3-months
Table 2.
Biochemically Verified Abstinence Rates by Treatment Group
| Continuous Abstinence (2-week grace)1 % Abstinent (N) |
Continuous Abstinence (FDA)2 % Abstinent (N) |
Prolonged Abstinence3 % Abstinent (N) |
7-Day Point Prevalence4 % Abstinent (N) |
||
|---|---|---|---|---|---|
| End of Treatment5 | |||||
| Bupropion Group (B) | (N=102) | 36.3 (37) | 39.2 (40) | 46.1 (47) | 49.0 (50) |
| Placebo Group (P) | (N=106) | 17.0 (18) | 18.9 (20) | 26.4 (28) | 28.3 (30) |
| Varenicline Group (V) | (N=86) | 43.0 (37) | 47.7 (41) | 58.1 (50) | 58.1 (50) |
| OR (95% CI): B vs. P | 2.78 (1.46,5.32) | 2.77 (1.48,5.20) | 2.38 (1.33,4.25) | 2.44 (1.37,4.32) | |
| OR (95% CI): V vs. P | 3.69 (1.90,7.16) | 3.92 (2.06,7.47) | 3.87 (2.11,7.11) | 3.52 (1.93,6.42) | |
| OR (95% CI): V vs. B | 1.33 (0.74,2.39) | 1.41 (0.79,2.42) | 1.62 (0.91,2.90) | 1.44 (0.81,2.58) | |
| Wald Chi-Square for Group | χ2(2)= 15.87, p<.0004 | χ2(2)= 18.10, p<.0001 | χ2(2)= 19.55, p<.0001) | χ2(2)= 17.95, p<.0 | |
| 3 month Follow-up6 | |||||
| Bupropion Group | 35.3 (36) | 37.2 (38) | 41.2 (42) | 43.1 (44) | |
| Placebo Group | 16.0 (17) | 17.0 (18) | 25.5 (27) | 31.1 (33) | |
| Varenicline Group | 39.5 (34) | 43.0 (37) | 53.5 (46) | 58.1 (50) | |
| OR (95% CI): B vs. P | 2.86 (1.48,5.52) | 2.90 (1.52,5.54) | 2.05 (1.14,3.69) | 1.68 (0.95,2.96) | |
| OR (95% CI): V vs. P | 3.42 (1.74,6.72) | 3.69 (1.90,7.16) | 3.37 (1.83,6.18) | 3.07 (1.70,5.56) | |
| OR (95% CI): V vs. B | 1.20 (0.66,2.17) | 1.27 (0.71,2.28) | 1.64 (0.92,2.93) | 1.83 (1.02,3.27) | |
| Wald Chi-Square for Group | χ2(2)= 14.21, p<.0008 | χ2(2)= 16.22, p=.0003 | χ2(2)= 15.40, p<.0005 | χ2(2)= 13.73, p<.0 | |
| 6 month Follow-up7 | |||||
| Bupropion Group | 22.5 (23) | 22.5 (23) | 26.5 (27) | 28.4 (29) | |
| Placebo Group | 14.1 (15) | 14.1 (15) | 17.9 (19) | 23.6 (25) | |
| Varenicline Group | 27.9 (24) | 27.9 (24) | 38.4 (33) | 37.2 (32) | |
| OR (95% CI): B vs. P | 1.77 (0.86,3.62) | 1.77 (0.86,3.62) | 1.65 (0.85,3.20) | 1.29 (0.69,2.40) | |
| OR (95% CI): V vs. P | 2.35 (1.14,4.83) | 2.35 (1.14,4.83) | 2.85 (1.47,5.51) | 1.92 (1.03,3.59) | |
| OR (95% CI): V vs. B | 1.33 (0.69, 2.58) | 1.33 (0.68,2.58) | 1.73 (0.93,3.21) | 1.49 (0.81,2.76) | |
| Wald Chi-Square for Group | χ2(2)= 5.45, p<.06 | χ2(2)= 5.45, p<.07 | χ2(2)= 9.80, p<.008 | χ2(2)= 4.26, p<.12 | |
Continuous abstinence (2-week grace)-no smoking on any day beginning with the end of the Grace Period, defined as 2 weeks after Quit Day.
Continuous abstinence (FDA)-no smoking on any day beginning with the last 4 weeks of treatment, or week 8 of medication.
Prolonged abstinence-relapse is defined by smoking for 7 or more consecutive days or by smoking at least 1 cigarette over two consecutive weeks beginning with the end of the 2-week Grace period.
7-day point prevalence is defined as no smoking in the last seven days of the given time period.
Occurs 10 weeks post Quit Date and is the end of pharmacotherapy
Occurs 3 months post Quit Date or 2 weeks following the end of pharmacotherapy
Occurs 6 months post Quit Date or 14 weeks following the end of pharmacotherapy
Effects of Treatment on Affective and Other Withdrawal Symptoms and Nicotine Reinforcement
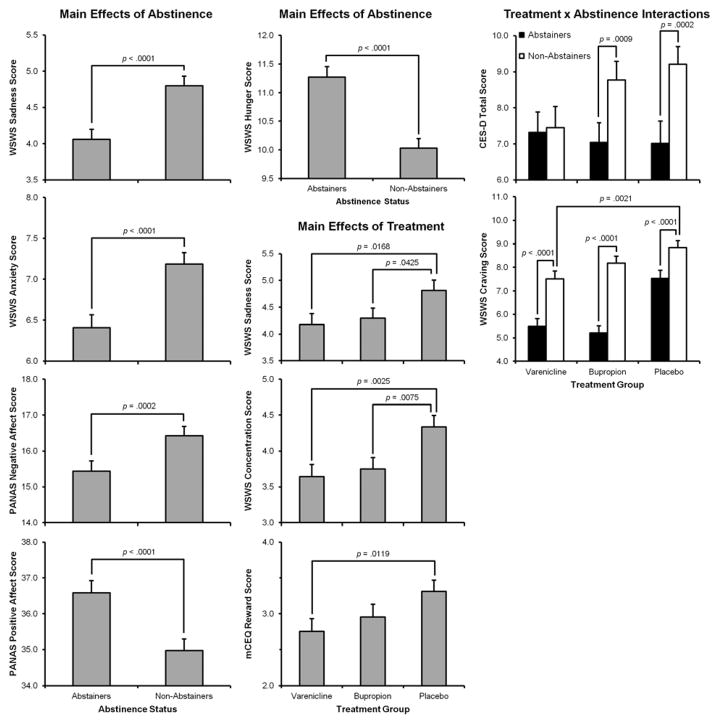
As shown in Figure 3 main effects of Abstinence Status indicated that Abstainers evidenced lower levels of sadness (F(1,203)=34.48, p<.0001) anxiety (F(1,203) = 30.05, p< .0001), and negative affect (F(1,203) = 14.57, p= .0002); and higher levels of positive affect (F(1,203)=31.15, p<.0001) and hunger (F(1,203)=66.03, p<.0001) than Non-Abstainers.
Figure 3.
Main Effects of Abstinence, Treatment, and Treatment by Abstinence Interactions for PANAS Negative and Positive Affect Scores, CES-D, mCEQ Psychological Reward scale (in smokers who relapsed), and WSWS scales of Sadness, Anxiety, Anger Hunger and Craving (p-values are for the actual comparison of individual group means)
Main effects of Treatment (see Figure 3) indicated that those taking varenicline or bupropion experienced less sadness (F(2,284)=3.40, p=.03) and showed better concentration (F(2,284)=5.64, p=.004) than those taking placebo. Relapsers taking varenicline experienced less psychological reward from smoking than those taking placebo (F(2,244)=3.39, p=.04).
Group by Abstinence Status interactions (see Figure 3) indicated that whereas Abstainers experienced significantly lower levels of depression than Non-Abstainers in the Bupropion (F(1,201)=11.36, p=.0009) and Placebo (F(1,201)=14.44, p=.0002) Groups, they did not differ in the Varenicline Group. Depression scores were significantly lower for both Abstainers (F(1,201)=6.86, p=.0094) and Non-Abstainers (F(1,201)=5.62, p=.0189) in the Varenicline Group compared to Non-Abstainers in the Placebo Group. Interestingly, while craving was generally higher for Non-Abstainers vs. Abstainers in both drug groups Non-Abstainers taking varenicline but not bupropion showed significantly less craving than those receiving placebo (F(2,201)=7.89, p=.0005).
No significant main effects or interactions were noted for measures of smoking satisfaction, sleep disturbance or anger
Treatment Compliance
Medication
As shown in Table 3 no differences were noted in the proportion of smokers in each of the groups that were retained for the full 12 week treatment course or in any measure of medication compliance.
Table 3.
Medication Treatment Compliance
| Variable | Varenicline (n=86) | Bupropion (n=102) | Placebo (N=106) | Total (N=294) |
|---|---|---|---|---|
|
| ||||
| % (N) | % (N) | % (N) | % (N) | |
| Retained For 12 Week Treatment | 86 (74) | 87.3 (89) | 84 (89) | 86 (252) |
| Stopped Medication-Total Subjects | 20.9 (18) | 22.5 (23) | 25.5 (27) | 23.1 (68) |
| * Stopped Medication & Continued in Counseling | 33 (6) | 43 (10) | 37 (10) | 38 (26) |
| * Stopped Medication & Dropped | 67 (12) | 57 (13) | 63 (17) | 62 (42) |
| Took ≥ 80% of Medication Dose | 72 (62) | 78 (102) | 75 (79) | 75 (220) |
| Mean Percent of Total Pills Taken1 | ||||
| All Subjects2 | 81 | 83 | 83 | 82 |
| Treatment Completers Only3 | 92 | 94 | 93 | 93 |
| Treatment Non-completers Only4 | 38 | 40 | 49 | 42 |
Percent of those who stopped medication
Total Pills Assigned-Total Pills Returned/Total Pills Assigned. In the case of premature discontinuation of medication, the total pills assigned and returned would be the same and prorated from the time the medication was discontinued through week 12.
Includes those that stopped medication and remained for counseling plus those that withdrew/failed to return during the 12 week treatment.
Includes all smokers who did not prematurely discontinue medication
Includes only those smokers who discontinue medication/withdraw sometime during treatment
Behavioral Counseling
Overall, participants attended an average of 5.67 (SD=1.35) inclinic counseling sessions, resulting in an actual average in-clinic counseling dose of 145.87 (35.42) minutes over the course of the study (based on actual session length). A total of 224 participants (76.19%) completed all 6 in-clinic counseling visits. Additionally, study participants completed an average of 3.43 (1.18) telephone visits for an average telephone counseling dose of 42.56 (14.00) minutes. A total of 200 participants (68.02%) completed all 4 telephone counseling visits. There were no differences by medication group in treatment attendance, for either in-person or telephone visits.
Adverse Events
A total of 80.4%, 79.0% and 86.1% of smokers in the Bupropion, Placebo and Varenicline Groups, respectively, reported at least 1 adverse event with no significant differences in overall frequency noted between the groups. As shown in Table 4, Placebo was unexpectedly associated with increased chest pain, relative to varenicline but as expected varenicline was associated with increased nausea. Bupropion was associated with increased diarrhea and influenza and a trend (p=.06) was observed for increased eczema in the Varenicline group. No significant group differences were noted for any of the psychiatric or neurological adverse events, including anxiety, depression, irritability, disturbances in attention, emotional lability, and sleep disturbances, with the exception of insomnia, which was higher among those receiving bupropion (p=.06). Indeed, though not significant, higher levels of depression, anxiety and attentional disturbances were observed in the Placebo group, relative to the active drug groups.
Table 4.
Adverse Event Frequencies
| Varenicline | Bupropion | Placebo | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body System | AE | Freq | % | Freq | % | Freq | % | Freq | % |
| Cardiovascular | Chest Pain - CardiacV | 1 | 1.16 | 5 | 4.9 | 13 | 12.26 | 19 | 6.46 |
| Cardiovascular | Hypertension | 3 | 3.49 | 3 | 2.94 | 2 | 1.89 | 8 | 2.72 |
| Cardiovascular | Tachycardia/Palpitations | 1 | 1.16 | 4 | 3.92 | 3 | 2.83 | 8 | 2.72 |
| Dermatologic | EczemaV | 8 | 9.3 | 6 | 5.88 | 3 | 2.83 | 17 | 5.78 |
| Gastrointestinal | NauseaV,B, VB | 23 | 26.74 | 17 | 16.67 | 8 | 7.55 | 48 | 16.33 |
| Gastrointestinal | DiarrheaB | 9 | 10.47 | 4 | 3.92 | 12 | 11.32 | 25 | 8.5 |
| Gastrointestinal | Constipation | 7 | 8.14 | 7 | 6.86 | 4 | 3.77 | 18 | 6.12 |
| Gastrointestinal | Gastritis | 6 | 6.98 | 6 | 5.88 | 5 | 4.72 | 17 | 5.78 |
| Gastrointestinal | Flatulence | 2 | 2.33 | 4 | 3.92 | 2 | 1.89 | 8 | 2.72 |
| Gastrointestinal | Vomiting | 4 | 4.65 | 0 | 0 | 2 | 1.89 | 6 | 2.04 |
| Gastrointestinal | Hemorrhoids | 0 | 0 | 1 | 0.98 | 1 | 0.94 | 2 | 0.68 |
| Gastrointestinal | Blood In Stool | 0 | 0 | 1 | 0.98 | 0 | 0 | 1 | 0.34 |
| Gastrointestinal | Dysphagia | 0 | 0 | 1 | 0.98 | 0 | 0 | 1 | 0.34 |
| General Disorders | Appetite Increased | 8 | 9.3 | 9 | 8.82 | 10 | 9.43 | 27 | 9.18 |
| General Disorders | Fatigue | 4 | 4.65 | 6 | 5.88 | 9 | 8.49 | 19 | 6.46 |
| General Disorders | InfluenzaB | 6 | 6.98 | 8 | 7.84 | 2 | 1.89 | 16 | 5.44 |
| Head, Neck And Oral | Taste Perversion | 5 | 5.81 | 4 | 3.92 | 7 | 6.6 | 16 | 5.44 |
| Head, Neck And Oral | Toothache | 3 | 3.49 | 7 | 6.86 | 5 | 4.72 | 15 | 5.1 |
| Musculoskeletal | Musculoskeletal Pain | 12 | 13.95 | 11 | 10.78 | 13 | 12.26 | 36 | 12.24 |
| Neurologic | InsomniaB | 20 | 23.26 | 32 | 31.37 | 21 | 19.81 | 73 | 24.83 |
| Neurologic | Headache | 10 | 11.63 | 15 | 14.71 | 12 | 11.32 | 37 | 12.59 |
| Neurologic | Drowsiness/Hypersomnia | 5 | 5.81 | 4 | 3.92 | 8 | 7.55 | 17 | 5.78 |
| Neurologic | Sensory Disturbance | 1 | 1.16 | 1 | 0.98 | 2 | 1.89 | 4 | 1.36 |
| Neurologic | Paresthesia | 1 | 1.16 | 0 | 0 | 1 | 0.94 | 2 | 0.68 |
| Neurologic | Cerebrospinal Fluid Leakage | 1 | 1.16 | 0 | 0 | 0 | 0 | 1 | 0.34 |
| Psychiatric | Irritability | 12 | 13.95 | 16 | 15.69 | 17 | 16.04 | 45 | 15.31 |
| Psychiatric | Abnormal Dreams | 13 | 15.12 | 6 | 5.88 | 11 | 10.38 | 30 | 10.2 |
| Psychiatric | Anxiety Symptoms | 7 | 8.14 | 8 | 7.84 | 15 | 14.15 | 30 | 10.2 |
| Psychiatric | Depression | 6 | 6.98 | 8 | 7.84 | 14 | 13.21 | 28 | 9.52 |
| Psychiatric | Disturbance In Attention | 3 | 3.49 | 7 | 6.86 | 16 | 15.09 | 26 | 8.84 |
| Psychiatric | Restlessness | 1 | 1.16 | 5 | 4.9 | 6 | 5.66 | 12 | 4.08 |
| Psychiatric | Emotional Lability | 2 | 2.33 | 3 | 2.94 | 4 | 3.77 | 9 | 3.06 |
| Psychiatric | Panic Attack | 1 | 1.16 | 0 | 0 | 2 | 1.89 | 3 | 1.02 |
| Psychiatric | Elevated Mood | 0 | 0 | 0 | 0 | 1 | 0.94 | 1 | 0.34 |
| Psychiatric | Intrusive Thoughts | 0 | 0 | 0 | 0 | 1 | 0.94 | 1 | 0.34 |
| Psychiatric | Reduced Inhibition | 0 | 0 | 1 | 0.98 | 0 | 0 | 1 | 0.34 |
| Psychiatric | Suicidal Ideation | 0 | 0 | 0 | 0 | 1 | 0.94 | 1 | 0.34 |
| Respiratory | Shortness Of Breath | 6 | 6.98 | 7 | 6.86 | 13 | 12.26 | 26 | 8.84 |
| Respiratory | Increased Coughing | 7 | 8.14 | 8 | 7.84 | 5 | 4.72 | 20 | 6.8 |
| Rhinitis And Upper Respiratory Symptoms | Rhinitis | 18 | 20.93 | 28 | 27.45 | 25 | 23.58 | 71 | 24.15 |
| Rhinitis And Upper Respiratory Symptoms | Sore Throat | 4 | 4.65 | 6 | 5.88 | 9 | 8.49 | 19 | 6.46 |
Note: AE=Adverse Event. Contains all adverse events that were reported by at least 5% of the sample, along with all adverse events reported that were classified as Cardiovascular, Gastrointestinal, Neurologic or Psychiatric.
-significant difference between Varenicline and Placebo
-significant difference between Bupropion and Placebo
-significant difference between Varenicline and Bupropion
There were 7 serious adverse events (SAE) reported during the course of this study, all categorized as such due to patient hospitalization. In the Bupropion Group, 3 were noted: Bilateral mammoplasty and facial paralysis, regarded as unrelated (blinded ratings); and syncope, rated as possibly related but could not be verified from hospital records. In the Placebo group, two SAE’s were noted: Diabetes, recorded as unlikely; and chest pain noted as possibly related. In the varenicline group, two were noted: chest pain, regarded as unlikely; and psychiatric hospitalization, noted as possibly related. This patient was found to have a previous psychiatric history which was denied at intake and took an intentional non-study drug overdose which he described as not associated with an intent to die but rather an attempt to gain attention from his girlfriend (A single AE of suicidal ideation was also in the placebo group).
Discussion
The results from this study generally confirm the findings from previous smoking cessation trials in which both varenicline and bupropion have been shown to be more effective than a placebo control, and varenicline to be more effective than bupropion 3;30. Across a variety of definitions of abstinence, varenicline consistently outperformed placebo at every time point; bupropion consistently outperformed placebo through the 3 month follow-up for all but the 7-day point prevalence measure; and while varenicline consistently outperform bupropion the differences were significant only at 3-month for 7-day point prevalence abstinence. The pattern of our results is similar to those observed in the substantially larger phase-3 trials of varenicline 11;12 although, the bupropion vs. placebo and varenicline vs. bupropion comparison remained significant at all time points in those studies.
There were three major differences between this trial and previous trials for varenicline and bupropion including the pivotal trials for varenicline that included a bupropion active control 11;12. First, smokers in this trial were on study medication 12–19 days prior to quitting as opposed to 7 days in the phase-3 trials that also resulted in a corresponding increase in the weekly pre-quit counseling sessions. However, it is unlikely that the small increase in pre-quit medication use would have ultimately contributed to study differences since a varenicline study varying pre-quit medication exposure found very similar results to the phase-3 trials 31.
Second, smokers were also assigned to receive a total of 240 minutes of counseling, of which they received an average of 188.13 minutes based on actual session length, or about 78% of the intended dose This was a longer time allocation than used in the phase III trials for varenicline (120 minutes), although no comparable compliance statistics are available. The abstinence rates and effect sizes from studies using comparable abstinence definitions suggest that continuous abstinence (FDA) rates at 6 months in our study averaged about 4.5% points higher across groups than that observed in previous studies, which resulted in a slightly albeit consistently lower odds ratio than those earlier trials for each of the two-way drug comparisons3;4;11;12;32. While increased counseling may have raised the overall cessation rates slightly, the absolute differences between the drug groups across the trials remained roughly the same.
Thirdly, our sample size provided adequate power for assessing our primary outcome of prolonged abstinence at EOT (i.e., β=.99 for differences relative to placebo for varenicline; β=.84 for bupropion); but modest for detecting drug group differences (β=.74).Prolonged abstinence was selected as the primary endpoint because this study was part of a larger investigation of psychophysiological predictors of smoking cessation, which focused on comparisons with placebo at proximal endpoints. The effects for varenicline vs. placebo were quite robust but our comparisons involving bupropion, while in the expected direction, were limited due to sample size needed to detect relatively smaller treatment effects
An important contribution of this study is a cluster of findings involving measures of depression, negative affect and other symptoms of nicotine withdrawal First, we found that while abstinence alone was associated with improved affect (i.e., increased positive affect and reduced sadness, negative affect and anxiety) relative to non-abstinence, among those taking varenicline, scores for depression did not differ as a function of abstinence as they did for bupropion and placebo and scores on this measure was lower for varenicline non-abstainers than those in the other groups. Smokers taking varenicline and bupropion also reported overall lower levels of sadness (WSWS) relative to those on placebo. In addition, among relapsers, only those in the varenicline group reported decreased psychological reward from smoking. We found no drug related differences in either weekly measures of anxiety or anger (WSWS) nor in spontaneously reported psychiatric adverse events A possible suicide attempt without clear intent was reported in the varenicline group. Although drug effects cannot be completely ruled, there were extenuating circumstances such as a past history of psychiatric disturbance, an emotional precipitating event, and the absence of other prior related affective AE’s (i.e., depression), which reduce this probability.
Taken together, these findings suggest that varenicline might actually be associated with a generalized suppression of some symptoms of negative affect during cessation, particularly those related to depression and have little impact on anxiety and anger. What is particularly noteworthy in this trial is that assessments of affective functioning and nicotine withdrawal were conducted on a weekly basis using standardized instruments. The results using this type of ongoing assessment are informative given the post-marketing reports associating varenicline with increased depression and related affective disturbances such as anxiety and hostility 13;33. Moreover, no differences were noted in self-reported neuropsychiatric adverse events, specifically in anxiety, depression, irritability, disturbances in attention or emotional lability; and in fact while not significant the rates for several of these symptoms were higher in the placebo group. This is most likely due to the effects of “unmedicated” nicotine withdrawal, and points to the difficulty in separating adverse events in this area that are due to drug effects vs. quitting smoking. Relative to placebo both active drugs actually resulted in improved concentration, consistent with the affective data presented above, as well as reduced craving Our analysis of weekly measures of affective and cognitive functioning (concentration) controlled for abstinence and provide an increased degree of confidence that in fact, medication may be attenuating this cluster of neuropsychiatric/nicotine withdrawal symptoms, rather than causing a worsening of these conditions. A limitation in our findings that should be noted is that the inclusion/exclusion criteria used in our community based sample is quite similar to that used in the phase-3 trials of varenicline, which excluded smokers with current psychiatric illness. The presence of a psychiatric disorder could moderate the risk of neuropsychiatric symptoms although one trial with schizophrenic patients have not found this to be the case34
Examination of other voluntarily reported adverse event data showed, as expected, that varenicline was associated with increased reports of nausea and that bupropion was associated with increased reports of insomnia. Neither medication differed from placebo with respect to any other symptom of sleep disturbance. Smokers in the placebo group unexpectedly also reported more chest pain than those in either treatment group, which is inconsistent with a recent meta-analysis of varenicline 35 that suggested an increased risk of cardiovascular sequelae in response to varenicline pharmacotherapy, though our sample size is far too small for any meaningful comparison of cardiovascular events It is unlikely that any treatment related differences in this study are due to differential exposure to pharmacotherapy or counseling as compliance measures did not differ across groups.
In conclusion, the results of this study point to a very robust and consistently favorable effect of varenicline on smoking cessation relative to placebo and while in the expected direction less consistent effects were noted for the comparisons involving bupropion Varenicline also had a suppressive effect on symptoms of depression and little impact on anxiety and anger; and like bupropion, reduced withdrawal related sadness and negative affect. Such findings run counter to current reports of enhanced neuropsychiatric symptoms associated with varenicline therapy. The limitations of our study include the sample size for detecting drug related differences and the fact that this trial used a similar sample to that used in the phase-3 trials which limits the generalizability of our affective findings to the population of smokers at large, particularly among those with current psychiatric disorders.
Acknowledgments
Paul M. Cinciripini, Jason D. Robinson, Jennifer Minnix, Cho Lam, Victoria L. Brown, Francesco Versace and Maher Karam-Hage: Department of Behavioral Science, University of Texas MD Anderson Cancer Center. David W. Wetter: Department of Health Disparities Research University of Texas MD Anderson Cancer Center.
Support for this research was provided by grants from the National Institute on Drug Abuse (DA017073) to Paul M. Cinciripini and the Cancer Center Support Grant from the National Cancer Institute (P50CA70907). Varenicline provided by Pfizer. The PI and co-investigators are solely responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript. All authors had full access to the data from this trial. The PI and co-investigators assume full responsibility for the integrity of the data.
Dr. Cinciripini served on the scientific advisory board of Pfizer Pharmaceuticals and conducted educational talks sponsored by Pfizer on smoking cessation (2006–2008) and has received grant support from Pfizer.
Support for this research was provided by grants from the National Institute on Drug Abuse (DA017073) to Paul M. Cinciripini and the Cancer Center Support Grant from the National Cancer Institute (P50CA70907). Varenicline provided by Pfizer.
Footnotes
This study began recruitment in August 2006. The original trial (cohort 1) involved a comparison of bupropion, nortriptyline and placebo. With the launch of varenicline in late 2006, for both scientific and clinical reasons the research team made a decision to drop the nortriptyline arm of the trial and add a varenicline arm in its place (cohort 2). By that time a total of 37 people had been randomized to bupropion (N=19) and placebo (N=18). Following a delay to obtain IRB and DSMB approval we began recruitment for cohort 2 in January 2008. The only difference between the cohorts was that in cohort 1 in order to accommodate the longer dose titration schedule of nortriptyline, all medication was initiated 19 days prior to quitting and included 3 pre-quit counseling sessions at each of 3 weekly pre-quit visits. In cohort 2, medication and counseling were initiated 12 days prior to quitting and included 2 pre-quit counseling sessions over a 2 week period. We compared cohort 1 and 2 on all measures including demographics, baseline questionnaires, smoking history, dependence, and number of cigarettes per day. We also compared the bupropion vs. placebo abstinence rates across both cohorts for the quit date, 2 weeks following the quit date (grace period), end of treatment and all follow-up points using the same definitions of abstinence described in the text. No differences were found between the cohorts on any of these comparisons. We also conducted all analyses on cohort 2 alone, excluding cohort 1 and the pattern of results were essentially the same. Given these findings cohort 1 and 2 were combined and treated as a single sample throughout this report. For simplicity, all procedures from this point forward are described as for cohort 2.
ClinicalTrials.gov Identifier: NCT00507728
The other authors declare no conflict of interest.
Reference List
- 1.Centers for Disease Control and Prevention. Quitting Smoking Among Adults - United States, 2001–2010. Morbidity and Mortality Weekly Report. 2011;60:1513–1519. [PubMed] [Google Scholar]
- 2.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Orleans CT, Robinson LA, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Treating Tobacco Use and Dependence: 2008 Update, Clinical Practice Guideline. Rockville, MD: U. S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 3.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2011;2:1–87. [Google Scholar]
- 4.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews. 2007;2007:1, CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, Jr, Williams KE. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. American Journal of Health Behavior. 2008;32:664–675. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- 6.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E. Bupropion: A review of its mechanism of antidepressant activity. Journal of Clinical Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 7.Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Molecular Pharmacology. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- 8.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, III, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 9.West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. Information for Healthcare Professionals: Varenicline (marketed as Chantix) and Bupropion (marketed as Zyban, Wellbutrin, and generics) FDA Alert. 2009 [On-line]. Available: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169986.htm.
- 14.Pocock S. Clinical trials. New York: John Wiley & Sons; 1983. [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 16.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. Guilford Publications; 2002. [Google Scholar]
- 17.Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. Journal of Behavioral Medicine. 1982;5:343–351. doi: 10.1007/BF00846161. [DOI] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 19.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 20.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–61. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 21.Ross CE, Mirowsky J. Components of depressed mood in married men and women. The Center for Epidemiologic Studies’ Depression Scale. American Journal of Epidemiology. 1984;119:997–1004. doi: 10.1093/oxfordjournals.aje.a113819. [DOI] [PubMed] [Google Scholar]
- 22.Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: Characteristics of depressed smokers and effects of nicotine replacement. Journal of Consulting and Clinical Psychology. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- 23.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addictive Behaviors. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- 25.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking During Pregnancy and Newborn Neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 26.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- 27.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC) Cancer Therapy Evaluation Program. 2010 [On-line]. Available: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 28.Gibbons R, Hedeker D, Waternaux C. Random regression models: a comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacology. 1988;24:438–443. [PubMed] [Google Scholar]
- 29.Gibbons R, Hedeker D, Elkin I, Waternaux C, Kraemer KH, Greehouse J, Shea T, Imber S, Sotsky S, Watkins J. Some conceptual and statistical issues in the analysis of logitudintal psychiatric data. Archives of General Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 30.Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health. 2006;6:300. doi: 10.1186/1471-2458-6-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, St Aubin L, Russ C. A Randomized Placebo-Controlled Trial of Varenicline for Smoking Cessation Allowing Flexible Quit Dates. Nicotine & Tobacco Research. 2012;14:343–350. doi: 10.1093/ntr/ntr220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, Jr, Williams KE. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. American Journal of Health Behavior. 2008;32:664–675. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- 33.Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal Behavior and Depression in Smoking Cessation Treatments. PloS one. 2011;6:e27016. doi: 10.1371/journal.pone.0027016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, George TP. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73:654–660. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- 35.Sonal S, Yoon K, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. Canadian Medical Association Journal. 2011;183:1359–1366. doi: 10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]