Abstract
Thalamo-cortical feedback loops play a key role in the processing and coordination of processing and integration of perceptual inputs and outputs, and disruption in this connection has long been hypothesized to contribute significantly to neuropsychological disturbances in schizophrenia. To test this hypothesis, we applied diffusion tensor tractography on eighteen patients suffering schizophrenia and 20 control subjects. Fractional anisotropy (FA) was evaluated in the bilateral anterior and posterior limbs of the internal capsule, and correlated with clinical and neurocognitive measures. Patients diagnosed with schizophrenia showed significantly reduced FA bilaterally in the anterior but not the posterior limb of the internal capsule, compared with healthy control subjects. Lower FA correlated with lower scores on tests of declarative episodic memory in the patient group only. These findings suggest that disruptions, bilaterally, in thalamo-cortical connections in schizophrenia may contribute to disease-related impairment in the coordination of mnemonic processes of encoding and retrieval that are vital for efficient learning of new information.
Keywords: Schizophrenia, Disconnection, MRI, Cognitive Neuropsychology
1. INTRODUCTION
Neuropsychiatric symptoms of schizophrenia have been thought by some to reflect a disruption of limbic and sensory feedback loops (e.g.,(Friston & Frith, 1995; Liu, et al., 2008). Thalamo-cortical projections play a key role in this circuitry, as the thalamus filters sensory and higher order inputs, before its further transmission to the cortex (Schlosser, et al., 2003). Disconnectivity in the thalamo-cortical loop might especially be involved in the pathophysiological development of cognitive dysfunctions observed in schizophrenic patients. Of note, the dorso-medial nucleus (DMA) projects to the dorsolateral prefrontal cortex (DLPFC) (Giguere & Goldman-Rakic, 1988) and is involved in the coordination of attentional processes (Hazlett, et al., 2001). Disruptions in attention (Heinrichs & Zakzanis, 1998) and related executive functions of planning, organization and goal-directed action (Weickert, et al., 2000) are among the core cognitive impairments in schizophrenia.
From an anatomical perspective, the internal capsule represents the intercept point in the course of these projection fibers. Further, anatomically, the internal capsule is subdivided into anterior and posterior limbs. The anterior limb (AL) contains fibers reciprocally connecting the thalamus with the frontal lobe (Parent, 1996), also the cortico-pontine fibers, and to a lesser extent caudate/pallidum fibers (Axer & Keyserlingk, 2000). The posterior limb contains cortico-spinal, cortico-bulbal, and cortico-cerebellar fibers. Loss of neurons in the mediodorsal (MD) nucleus of the thalamus (Byne, et al., 2002; Popken, Bunney, Potkin, & Jones, 2000; Young, Manaye, Liang, Hicks, & German, 2000), in the thalamic subnucleus, and in the anterior nuclei (Young, et al., 2000), has been reported in postmortem studies of schizophrenia. In addition, reduced volume of prefrontal cortex has been found (Rajkowska, Selemon, & Goldman-Rakic, 1998), suggesting reduced connectivity in schizophrenic patients in regions connecting the thalamus to the prefrontal cortex through the anterior limb of the internal capsule (AL-IC).
The AL-IC has also been an area of keen interest in understanding the pathophysiology of schizophrenia (see review in (Shenton, Dickey, Frumin, & McCarley, 2001). From a structural anatomical perspective, the anterior and posterior limbs of the internal capsule are not clearly discernible, and fibers from both limbs intercept with the other, complicating dissociation. A functional analysis of the fibers of interest is only possible if fibers of the posterior limb are excluded and separately investigated. This is made possible by fiber tractography, which we use in this study.
Thus far, Magnetic Resonance Imaging (MRI) has been successful in detecting in-vivo structural alterations in patients with schizophrenia in the prefrontal cortex (PFC) (see review in (Shenton, et al., 2001), basal ganglia, and thalamus (e.g., (Young, et al., 2000). These findings suggest that white matter interconnecting these brain regions may be disrupted. Of further note, basal ganglia and thalamo-cortical feedback loops have reciprocal fibers that are projected through the internal capsule from the thalamus to the prefrontal cortex. In recent structural imaging studies, AL-IC volume has also been shown to be decreased in patients with schizophrenia (Brickman, et al., 2006; Lang, et al., 2006; Zhou, et al., 2003). A major concern in the analysis of conventional structural images, however, is that they do not carry information regarding the integrity of the fibers. In this regard, diffusion tensor imaging (DTI) has emerged as an MRI-technique that provides detailed information about fiber integrity. That is, DTI, unlike conventional MRI, contains information about the direction and intensity of water flow in each voxel. One important parameter is fractional anisotropy (FA), which is a scalar value characterizing the deviation from isotropic diffusion. Beaulieu suggested that FA might correlate with high density, diameter, degree of organization, and myelination of axons (Beaulieu, 2002). This information can be useful in characterizing the integrity of white matter (WM) brain tissue, since it predominantly contains myelinated axons. Furthermore, tractography is a relatively new method for analyzing DTI data. Its advantage over ROI methods is that it enables analyzing specific fiber tracts throughout their course until they reach the cortical rim.
Reduced white matter integrity of the AL-IC may also underlie some of the neuropsychological disturbances observed in schizophrenia. (Kubicki, et al., 2005; Zou, et al., 2008). One of the theories linking AL-IC and schizophrenia symptoms involves introduction of cognitive syndrome called “cognitive dysmetria” (Andreasen, et al., 1996). Cognitive dysmetria refers to ‘difficulty in coordinating and monitoring the process of receiving, processing, and expressing logically linked information’ (Andreasen et al, 1996). Moreover, Andreasen postulated that neuropsychological measures of declarative-episodic memory, such as narrative recall ‘not only requires the patient to learn and recall logically linked information, but also to monitor this process while they do so.’ In this model, timing or sequencing the flow of information is required during normal “thought” and speech, and these functions are often disrupted in schizophrenia. As Andreasen et al. (1996) suggested, the ability to coordinate and sequence multiple inputs and outputs may be examined by using the WMS-III Logical Memory subtests, which require the subject to orally recall complex narrative material.
While the notion of “cognitive dysmetria” is speculative, and cognitive deficits postulated by this model can arise from abnormalities within any parts of the network, including AL-IC itself, the focus of the current study is in investigating the integrity of thalamo-cortical and cortico-thalamic fiber tracts running through the AL-IC, their disruptions, and its cognitive consequences (including measures suggested by Andreasen et al., 1996). We chose the cortico-spinal tract passing through the posterior limb of the internal capsule (PL-IC) as a control region, hypothesizing that these fibers, involving mostly sensory and motor axons, would not show differences between patients and controls. In addition, we assessed mnemonic functions requiring monitoring of encoding and retrieval as well as expression of remembered information with story recall tasks of the Wechsler Memory Scale (WMS-III) (measures suggested by Andreasen et al., to be best related to schizophrenia symptomatology and thalamo-cortical connections), where we hypothesized we would find correlations with FA in thalamo-cortical tracts in patients with schizophrenia.
2. METHODS
2.1. Subjects
This study included 18 patients with chronic schizophrenia, and 20 age-matched normal controls. These subjects overlapped with subjects from two prior studies, which addressed different questions (Levitt JJ, 2004; Oh, et al., 2009). Patients were recruited from inpatient, day treatment, outpatient, and foster care programs at the VA Brockton Hospital, Mass. DSM-IV diagnosis was assessed using the Structured Clinical Interview for DSM-IV-patient Version (SCID), and from information in the medical records. The 23 normal comparison subjects completed the non-patient edition of the SCID to rule out any psychiatric illness after having been recruited in response to local advertisement or by word of mouth. Controls were matched to patients by age, gender, handedness, and parental socioeconomic status. The inclusion criteria for all subjects were IQ above 75, negative history of seizures, negative history of head trauma with loss of consciousness or neurological disorder, and no history of alcohol or other drug dependence in the last 5 years and an ability and desire to cooperate with the procedures as evidenced by written informed consent. Comparison subjects also underwent screening to exclude individuals who had a first-degree relative with an axis I disorder. After a complete description of the study, written informed consent was obtained from each subject. The study was approved by the VA Human Subjects Committee, as well as by the Institutional Review Board at Brigham and Women’s Hospital.
All subjects underwent neuropsychological tests, which were carefully selected according to functions that we thought were especially related to the AL-IC function. To investigate concept formation, abstraction, and mental flexibility, subjects completed the Wechsler Memory Scale-3rd Edition (WMS-III) (Wechsler, 1997). This test measures immediate memory, visual immediate memory, auditory delayed memory, visual delayed memory, general-delayed memory, auditory recognition delayed memory, and working memory. The logical memory is a subtest of the WMS-III and assesses verbal memory, demanding recall of two stories consecutively after an oral presentation (Part I) and again after a 30-min delay (Part II).
2.2. MRI Methods
2.2.1. Image Acquisition and Post-Processing
Line-scan diffusion tensor images were acquired for all subjects. Images were obtained using a quadrature head coil on a 1.5-Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, Wisconsin), on which maximum gradient amplitudes of 40 mT/m can be achieved. First, we started off with a set of three orthogonal T1-weighted (T1W) images (sagittal, axial oblique aligned to the anterior commissure-posterior commissure (AC-PC) line and another sagittal oblique aligned to the interhemispheric fissure) which were used as localizers. The LSDI sequence in the coronal orientation from the last sagittal T1-weighted image was then aligned to the ACPC line. Six images with high (1000 sec/mm) diffusion-weighting along six non-collinear directions, and two images for low (5 sec/mm) diffusion-weighting were collected for each line. The following scan parameters were used: 128x128 scan matrix (256x256 image matrix) field of view (FOV); slice thickness 4 mm; inter-slice distance 1 mm; receiver bandwidth 4 kHz; echo time 64 msec; effective repetition time 2592 msec; scan time 60 sec/slice section. We acquired 31–35 coronal slices covering the entire brain, depending on brain size. The total scan time was 31–35 min. After reconstruction, the diffusion-weighted images were transferred to a LINUX workstation, where the label-maps were drawn in slicer (www.slicer.org).
2.2.1. Manual Segmentation: Internal Capsule Anatomical Landmarks
2.2.1.1. ROI (Region of Interest)
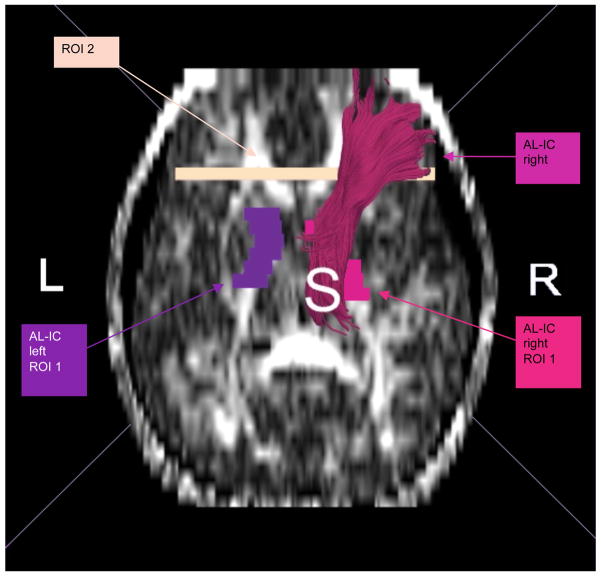
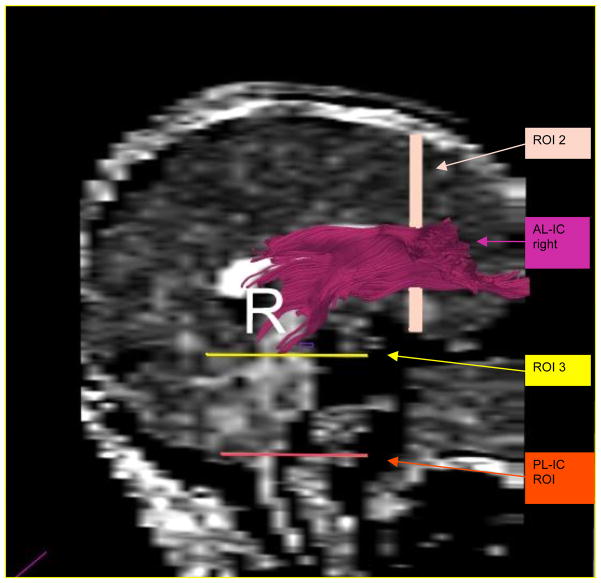
All ROIs were drawn on the FA (fractional anisotropy) map. A single rater (G.R.), blind to diagnosis, gender and age, drew regions of interest (ROIs) for the whole internal capsule (anterior and posterior limb, as well as genu). The borders were placed generously, including tissue surrounding the internal capsule (IC), in order to include all fibers passing through the capsule. This was possible, since second and third ROIs were drawn to eventually exclude extraneous fibers. From the resulting bulky fiber bundle, the fibers connecting the internal capsule and the frontal lobe were extracted. This was done by drawing a second ROI (ROI 2) in the coronal plane anterior to the most anterior slice where the corpus callosum was visible as an area including the frontal lobe. The third ROI was drawn to eliminate extraneous fibers and was placed at the first axial plane beneath the internal capsule ROI 1. Calculation of diverse scalar values, i.e., FA, linear, spherical, and mode within these fibers was performed using MATLAB software (Figures 1 and 2).
Figure 1.
ROIs used to extract the anterior limb of the internal capsule.
Figure 2.
ROIs used for extraction of anterior, as well as posterior limbs of the internal capsule (the pink fiber bundle demonstrates the fronto-thalamic fibers passing through the anterior limb of the internal capsule)
2.2.2. Image Analysis
In the present study, open source in-house software, 3D Slicer (www.slicer.org) was used for diffusion tensor tractography based on whole-white matter seeding. The detailed methodology is described elsewhere (Oh, et al., 2009; Oh, et al., 2007). In brief, once the diffusion tensors were estimated, fiber seeds were placed (one seed per voxel) throughout the entire brain in locations were FA>0.1. Fibers were then traced (using in-house software- slicer3D, that utilizes streamline tractography algorithm using a fourth order Runge-Kutta solver, introduced in Basser et al., 2000) until a curvature angle threshold of 80°/mm was reached to avoid rapid change of direction or the FA was lower than 0.1.
To create fronto-subcortical fiber tracts (i.e., those connecting anterior limb of the internal capsule and frontal lobe, but not extending to the spinal cord) we used predefined ROIs - as described earlier -- ROI 1 representing the AL-IC and ROI 2 representing the posterior most coronal slice of the frontal lobe. To exclude fibers extending to the spinal cord, we used ROI 3 as an exclusion ROI. For assessing spinal cord fiber tracts we used the internal capsule ROI 1 and the spinal cord ROI 3, and we used ROI 2 as exclusion ROI to avoid including frontal fibers (Figure 1 and 2). We then reconstructed a volumetric mask of the fiber tracts and computed averaged across all voxels within this mask to avoid double counting due to several fibers traveling through the same voxel (Figure 1).
2.3. Statistical Analysis
Statistical analysis was done using the Statistical Package for Social Sciences (SPSS v.15.0). To test for specificity in group differences in FA within the anterior and posterior limbs of the internal capsule, analysis of covariance (ANCOVA) was performed, with region (anterior and posterior) and side (left and right) as the within-subject factors, group as the between-subject factor, and age as a covariate. This was followed by separate ANCOVAs for each tract (AL-IC and PL-IC). Finally, protected post-hoc independent sample t-tests were then used to evaluate differences between groups on the left and on the right side.
3. RESULTS
Groups did not differ in age at time of scan (P(1,36)=0.253, t=1.6), in handedness (P(1,33)=0.826, t=0.22) or in gender (all males). Additional demographic data are included in Table 1. Schizophrenics, had fewer years of education and lower SES than controls, but had comparable parental socioeconomic status, i.e., PSES, with controls (P(1,33)=0.12, t=−1.6). A pre-morbid measure of WRAT-3 full-scale IQ, indicated that schizophrenics and controls did not differ significantly from each other on a measure of premorbid IQ (P(1,20)=.593, t=−0.544) Schizophrenics were all chronically ill, with a mean duration of illness at 16.9 years (SD=9.4 years), and all were medicated, with a mean Chlorpromazine Equivalent (CPZ) equivalent of 450 mg per day (SD=345 mg) (Stoll, 2001) (see Table 1 for details). There were no statistically significant correlations between diffusion measures and IQ, PSES, age or handedness for neither group, nor were there any statistically significant correlations between the diffusion measures and age of onset or medication in the schizophrenia group. To make sure that the fiber tractography threshold criteria did not introduce any systematic bias towards one of the groups, we performed t-tests comparing the number of fibers, mean length and mean angle for each of the four fiber tracts that we compared (i.e., left AL-IC, right AL-IC, left PL-IC and right PL-IC). No differences were observed for any of these variables for any fiber tract.
Table 1.
Demographic characteristics for Chronic Schizophrenic and Normal Comparison Subjects
| Characteristic | Schizophrenic Subjects N=18 | Comparison Subjects N=20 | Students’s t-test (two tailed) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Age (years) | 39.2 | 9.0 | 42.1 | 6.5 | 1.2 | 36 | .253 |
| Education (years)a | 13.4 | 1.9 | 15.4 | 2.2 | 2.8 | 33 | .007 |
| WAIS-iii IQb | 94.5 | 13.1 | 107.7 | 12.8 | 2.79 | 28 | .009 |
| WRAT3 RSc | 51.0 | 4.76 | 49.9 | 4.6 | −0.54 | 20 | 0.59 |
| SESa, e | 3.8 | 1.2 | 2.3 | 1.1 | −3.7 | 34 | 0.001 |
| Parental SESd,e | 2.9 | 1.0 | 2.3 | 1.2 | −1.6 | 33 | .35 |
| handednessa | .8 | .17 | .81 | .13 | 0.22 | 33 | .826 |
ANCOVA demonstrated tract by age (F=7.97; df=1,35; p=.008), and tract by diagnosis (F=7.21; df=1,35; p=.011) interactions. Other interactions, including side, side by age, side by group, tract, side by tract, side by tract by age and side by tract by group were all non-significant. Repeated Measures ANCOVA for the AL-IC revealed a main effect for group (F=7.21; df=1,35; p=.011) but not for side by group (F=0.69; df=1,35; p=.41), or side by age interaction (F=1.22; df=1,35; p=.27). Repeated measures ANOVA for the PL-IC revealed no statistically significant group (F=.685; df=1,35; p=.414), side by group (F=.05; df=1,35; p=.823), nor side by age (F=.156; df=1,35; p=.219) interactions.
Post-hoc t-test using independent samples t-statistics revealed that mean FA for schizophrenics was significantly lower in the left (P(1,36) = 0.046), and right (P(1,36) = 0.007) anterior limbs of the internal capsules than was FA for control subjects (Figure 3a). As expected, both left and right posterior limbs of the internal capsules did not show significant FA reductions in schizophrenics vs. controls (Figure 3b).
Figure 3.
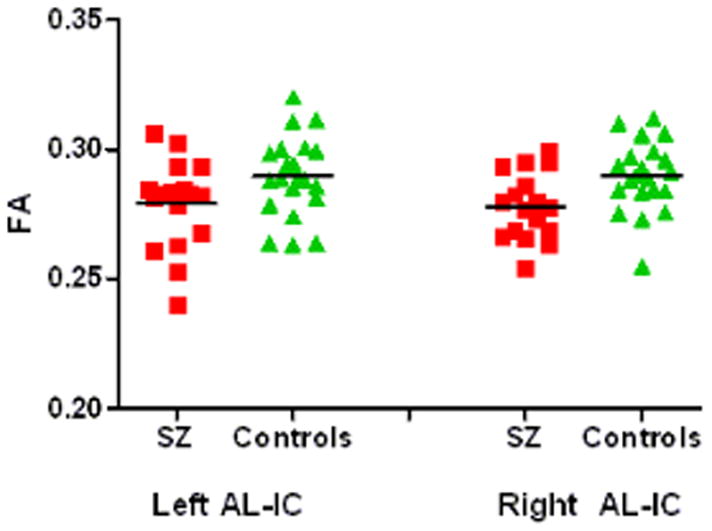
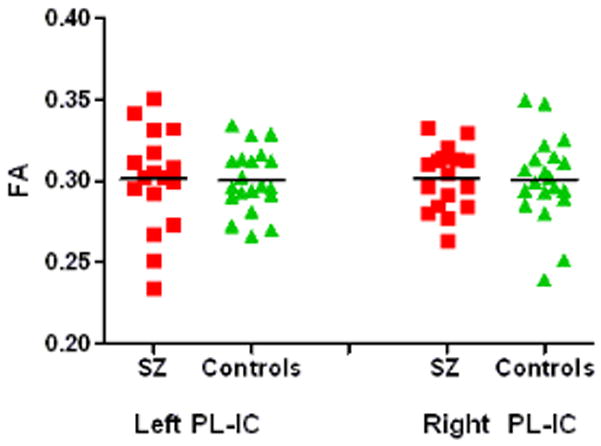
Figure 3a. Mean FA values for thalamic connections passing through the anterior limb of the internal capsule.
Figure 3b. Mean FA values for thalamic connections passing through the posterior limb of the internal capsule.
We next examined Spearman-rank correlation of FA and WMS-III subtests of recall and recognition for the patient and control groups. In the correlational analyses, summarized in Table 3, statistically significant associations were found between averaged FA values for AL-IC in schizophrenics in WMS-III immediate (Logical Memory-I) and delayed (Logical Memory-II) recall of stories (see Table 3) These correlations pointed to statistically significantly correlations of FA AL-IC with narrative recall. In addition, WMS-III LMII delayed recognition total score was positively correlated with left and right AL-IC FA. Also of note, reduced left and right AL-IC was associated with lower values in the WMS-III family pictures I recall unit and also in the family pictures II recall unit – bilaterally. No correlations between WMS scores and AL-IC were found for control subjects.
Table 3.
Correlations between FA measures of AL-IC and neurocognitive measures of recall and recognition.
| Clinical/Cognitive Measures
|
DTI measures
|
SZ Group
|
NC Group
|
||||
|---|---|---|---|---|---|---|---|
| ρ | p | n | ρ | p | n | ||
| WMS-III LM-II Recall total score | Left AL-IC FA Right AL-IC FA |
.65 .73 |
.009 .002 |
15 15 |
−.18 −.3 |
.523 .3 |
15 15 |
| WMS-III LM-II Recognition Total Score | Left AL-IC FA | .69 | .005 | 15 | −.21 | .45 | 15 |
| Right AL-IC FA | .65 | .009 | 15 | −.48 | .07 | 15 | |
| WMS-III LM-II Story A Recall Unit Score | Left AL-IC FA | .65 | .009 | 15 | −.33 | .227 | 15 |
| Right AL-IC FA | .66 | .007 | 15 | −.61 | .015 | 15 | |
| WMS-III LM-II Story B recall unit score | Left AL-IC FA | .54 | .037 | 15 | −.00 | .99 | 15 |
| Right AL-IC FA | .67 | .007 | 15 | .05 | .849 | 15 | |
| WMS-III Family Pictures II Recall total score | Left AL-IC FA | .61 | .015 | 15 | −.17 | .545 | 15 |
| Right AL-IC FA | .54 | .036 | 15 | −.01 | .978 | 15 | |
| WMS-III family pictures I recall total score | Left AL-IC FA | .66 | .007 | 15 | −.33 | .229 | 15 |
| Right AL-IC FA | .53 | .041 | 15 | −.01 | .966 | 15 | |
| WMS-III LM-I recall total score | Left AL-IC FA | .55 | .034 | 15 | −.23 | .404 | 15 |
| Right AL-IC FA | .57 | .025 | 15 | −.1 | .728 | 15 | |
| WMS-III LM-I story B-2nd recall unit score | Left AL-IC FA | .57 | .027 | 15 | −.23 | .412 | 15 |
| Right AL-IC FA | .6 | .017 | 15 | −.23 | .412 | 15 | |
| WMS-III auditory recognition delayed scaled score | Left AL-IC FA | .63 | .011 | 15 | −.283 | .308 | 15 |
| Right AL-IC FA | .56 | .029 | 15 | −.461 | .084 | 15 | |
| WAIS-III matrix reasoning total raw score | Left AL-IC FA | .53 | .036 | 16 | N/A | ||
| Right AL-IC FA | .54 | .03 | 16 | N/A | |||
| CPZ dosage equivalent | Left AL-IC FA | −.12 | .638 | 17 | N/A | ||
| Right AL-IC FA | .03 | .925 | 17 | N/A | |||
| Duration of illness | Left AL-IC FA | −.3 | .227 | 18 | N/A | ||
| Right AL-IC FA | −.33 | .187 | 18 | N/A | |||
3. DISCUSSION
Results from this study strongly suggest bilateral disruption in the integrity of white matter fibers passing through the anterior limb of the internal capsule, but not for those fibers traveling through the posterior limb of the internal capsule, in patients with chronic schizophrenia. Additionally, statistically significant associations were found between disrupted integrity in the anterior limb fiber tract measures and neurocognitive functioning in schizophrenia, which was not observed in control subjects. More specifically, cognitive dysfunctions associated with fiber disruption involved mnemonic functions requiring monitoring of encoding and retrieval as well as expression of remembered information with story recall tasks of the Wechsler Memory Scale (WMS-III) (measures suggested by Andreasen et al., to be best related to schizophrenia symptomatology (Andreasen, Paradiso, & O’Leary, 1998).
Clinical and cognitive correlates of AL-IC in schizophrenia have been found in previous studies (Honey, et al., 2005; Mendrek, et al., 2004). Andreasen et al. (Andreasen, et al., 1998), in fact, have proposed a disease model for schizophrenia called “cognitive dysmetria”. Findings from the current study demonstrate a reduction in white matter integrity in the thalamic-cortical pathway, which correlate with worse performance in the recall of narrative material in patients with schizophrenia but not in normal controls.
In a recent study we observed patients with schizophrenia to show a significant decline in FA with age in the cingulum and uncinate fasciculus (Rosenberger, et al., 2008), but not in the occipito-frontal fasciculus. In this study we did not find a statistically significant correlation between age and AL-IC. Previous studies of normal healthy aging have shown a decrease of FA in white matter correlating with age especially in prefrontal, temporal and parietal areas of the brain (Salat, et al., 2005). In a region of interest study, Schneiderman at al. (2009) found that first episode adolescent patients differed significantly in their pattern of fractional anisotropy in the internal capsule from adults with chronic schizophrenia and that this pattern deviated from the normal pattern of anisotropy change seen with age as demonstrated by age matched controls. These findings, taken together, underscore the importance of understanding local and regional patterns of interactions between age and WM integrity in controls, and abnormalities in such patterns in schizophrenia subjects.
In a previous study by our group, Levitt et al (Levitt JJ, 2004) found no significant FA decrease, but did find a significant volume reduction in AL-IC in schizophrenia. The difference between findings from the current study and those reported by Levitt et al. may be explained by the fact that they used an ROI approach and measured FA only within the region of the AL-IC, whereas we measured FA using tractography measures along the entire course of the fibers passing through the internal capsule. In addition, in the current study fibers running to the prefrontal cortex were examined, whereas in the previous study, other fibers may have been included. The focus exclusively on fibers running to the prefrontal cortex thus eliminated the analysis of “contaminating” fibers running to other areas of the brain (i.e. brainstem). Of note, both studies reported no differences in the PL-IC, which served as a control region. These results thus suggest involvement of AL-IC in schizophrenia pathology, but not PL-IC.
Reduced white matter integrity of the AL-IC, which can be expressed as reduced FA, may also underlie some of the neuropsychological disturbances observed in schizophrenia. Other DTI studies have recently been employed to measure AL-IC integrity in schizophrenia. For example, a reduction in FA in schizophrenia in the AL-IC was reported by Jeong et al (Jeong et al., 2009) using tract-based spatial statistics, by Kubicki et al. (2005) and Sussmann et al. (2009) using voxel-based morphometry, by Mitelman et al. (Mitelman, et al., 2007) using an automated “stereotactic ROI approach”. Zou et al (Zou, et al., 2008) also reported reduced FA in both AL-IC in neuroleptic-naïve schizophrenic patients using an ROI-based DTI approach. Mamah et al. (Mamah et al., 2010) showed reduced FA in the right AL-IC, which also correlated with executive function and working memory. The only other tractography studies focusing on anterior thalamo-frontal projections, but did not focus on the association with neurocognitive functions, have been published by Oh (Oh et al., 2008) and by Buchsbaum et al (2008). Other diffusion tensor imaging studies have also investigated the internal capsule in patients with schizophrenia. One study by An earlier diffusion tensor fiber tractography study (Buchsbaum, et al., 2006) found no FA differences in the AL-IC. However, these investigators did find significantly shorter tracts in this fiber bundle. Because Buchsbaum et al. (Buchsbaum 2006) chose a different approach defining the stopping criteria; they may have effectively excluded voxels that represent parts of the fiber tract potentially causing FA decrease in our study. In Buchsbaum’s paper, the investigators introduced a measure of “relative FA”, or a certain percentage drop of FA along the tract, as a stopping criterion. This method makes tractography extremely sensitive to even small artifact, where even a few noisy voxels, not necessarily related to crossing fibers, can terminate tracts prematurely. Our tractography method, on the other hand, takes into account small artifacts by incorporating tract regularization (smoothing along the bundle). Here, tracts are measured until FA falls and stays below a certain value, which makes tractography less sensitive to noise. Regardless of the method, however, both findings, that is, shorter tracts when more stringent stopping criteria are used, or lower FA when stopping criteria are set lower, may suggest the same underlying abnormality, that is, decreased FA within frontal WM in schizophrenia.
Suggestions about the possible significance of these findings comes from a previous publication from our group (Oh, Kubicki et al. 2009) where a different method was used for tract selection and termination (i.e., the internal capsule was used as the initial ROI, but it was excluded from the analysis, and tracts were terminated at the boundary of gray matter), as well as parameterization along the white matter connections between the thalamus and frontal Brodmann areas (BA 9, 10, 11, 32, 44, 45, 46, and 47). Using this method Oh et al. showed FA abnormalities in chronic schizophrenics in several projections (DLPFC, ACC, BA 44/45, frontal pole, OFC and IPFC), but they did not include the entire bundle trajectories, as was done in the current study.
There are few methodological issues, which need to be mentioned with respect to limitations of the study. First, we used anisotropic, relatively thick diffusion data, characterized by partial volume effects. These artifacts were, however, somewhat minimized by the fact that the images were acquired perpendicular (coronal) to the main fiber tract direction, thus enabling relatively good tracking results. We used relatively anisotropic, thick diffusion data, which are characterized by significant partial volume effects. Fortunately, partial volume effects were somewhat limited in our investigation due to the fact that the acquisition plane (coronal) was perpendicular to the main fiber tract direction, thus giving us relatively good tracking results. In addition, tensors were calculated along the tract at very small steps (1 mm), and the tracking algorithm uses a regularization method along the tract, which limits the influence of thick slices and gaps between slices on data results. Second, the patients tested suffered from chronic schizophrenia and had been medicated for many years. Although CPZ dosage did not correlate with FA measures, cumulative medication effects cannot be entirely ruled out. Third, we note that the cohort size was relatively small, but comparable to studies to date, although we acknowledge the problem of small sample sizes. Fourth, and also of note, possible differences in gender could not be accounted for, since we only had male subjects in our cohorts. Small sample sizes are related to another limitation of our study- that is, even though AL-IC disruptions in patients with schizophrenia were quite strongly correlated with deterioration in their cognitive functions, especially those related to immediate and delayed recognition, and those functions were not significantly correlated with PL-IC integrity, we were not able to demonstrate significant differences between those correlations (using Fisher exact tests). We thus acknowledge these results as exploratory, and suggest to treat them with caution.
We also note a limitation of tract-tracing, which necessitates setting FA at a certain threshold for stopping criterion. This limits the possibility of measuring voxels below a certain FA value. It has, however, been shown by Kanaan and colleagues (Kanaan, et al., 2006) that FA differences between schizophrenia patients and controls fall above 0.2 FA values. We chose to set the threshold at 0.1, thus likely allowing voxels of significant change in schizophrenia to be included in our investigation.
In conclusion, we found DTI tractography to be a useful tool to investigate disturbances in fronto-thalamic circuitry in schizophrenia. Our results show bilateral white matter integrity disruption within the anterior but not posterior limb of the internal capsule in schizophrenia, as well as a statistically significant relationship between these abnormalities and poor memory performance. This study broadens our understanding of cognitive malfunctioning in schizophrenia and its association with cortico-thalamic circuitry abnormalities.
Table 2.
Values of Mean FA of Internal Capsule Regions of Interest In Chronic Schizophrenic and Normal Comparison Subjects
| Schizophrenic N=18 | Comparison N=20 | Students’s t-test Significance (two tailed) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Anterior limb of IC left (FA) | .28 | .019 | .29 | .015 | 2.06 | 36 | .046 |
| Anterior limb of IC right (FA) | .28 | .015 | .29 | .014 | 2.86 | 36 | .007 |
| Posterior limb of IC left (FA) | .30 | .030 | .30 | .020 | −.25 | 36 | .806 |
| Posterior limb of IC right (FA) | .30 | .019 | .30 | .026 | −.25 | 36 | .806 |
Acknowledgments
The authors would like to thank Nancy Maxwell and Jennifer Goodrich for administrative support; Marek Kubicki, M.D., Ph.D., and Martha Shenton, Ph.D. for personal support; and Georgia Bushell, B.A., Kate Smith, B.A., Jorge Alvarado, B.A., and Usman Khan, B.A. for their support as research assistants. Additionally, we gratefully acknowledge the support of the National Institute of Health (K05 MH070047 and R01 MH 50740 to MES, R01 MH 40799 to RWM and R01MH 074794 to CFW, P50MH 080272 to RWM, MES), the Department of Veterans Affairs Merit Awards (MES, RWM), and the VA Schizophrenia Center Grant (RWM/MES), Neuroimage Analysis Center, NAC (NIH P41RR013218) to CFW and RK. This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (MK, RK, MES).
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Journal of Neurology, Neurosurgery & Psychiatry and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
Competing Interest: None declared.
References
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93(18):9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andreason NC, editor. Scale for the Assessment of Negative Symptoms. Iowa City, IA: University of Iowa College of Medicine; 1981. [Google Scholar]
- Andreason NC, editor. Scale for the Assessment of Negative Symptoms. Iowa City, IA: 1984. [Google Scholar]
- Axer H, Keyserlingk DG. Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Methods. 2000;94(2):165–175. doi: 10.1016/s0165-0270(99)00132-6. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, et al. Internal capsule size in good-outcome and poor-outcome schizophrenia. J Neuropsychiatry Clin Neurosci. 2006;18(3):364–376. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, Chu KW, Mitelman S, et al. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann Gen Psychiatry. 2006;5:19. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, et al. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159(1):59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277(2):195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Tang CY, Fleischman MB, Wei TC, Byne W, et al. Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol Psychiatry. 2001;50(4):281–291. doi: 10.1016/s0006-3223(01)01094-0. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128(Pt 11):2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, et al. Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr Res. 2006;87(1–3):89–99. doi: 10.1016/j.schres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Ersner-Hershfield H, Kubicki M, Westin CF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. An MR-DTI study of thalamo-cortical connections in schizophrenia; Paper presented at the Society of Biological Psychiatry Annual Meeting.2004. [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131(Pt 4):945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Laurens KR, Kiehl KA, Ngan ET, Stip E, Liddle PF. Changes in distributed neural circuitry function in patients with first-episode schizophrenia. Br J Psychiatry. 2004;185:205–214. doi: 10.1192/bjp.185.3.205. [DOI] [PubMed] [Google Scholar]
- Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, et al. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp. 2009;30(11):3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Song IC, Lee JS, Kang H, Park KS, Kang E, et al. Tractography-guided statistics (TGIS) in diffusion tensor imaging for the detection of gender difference of fiber integrity in the midsagittal and parasagittal corpora callosa. Neuroimage. 2007;36(3):606–616. doi: 10.1016/j.neuroimage.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Parent A, editor. 9. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A. 2000;97(16):9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55(3):215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, et al. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2008;102(1–3):181–188. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, et al. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19(3):751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll AL. The psychopharmacology reference card 2001 [Google Scholar]
- Wechsler D, editor. The Wechsler Memory Scale. 3 1997. [Google Scholar]
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry. 2000;47(11):944–953. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, et al. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol Psychiatry. 2003;54(4):427–436. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Zou LQ, Xie JX, Yuan HS, Pei XL, Dong WT, Liu PC. Diffusion tensor imaging study of the anterior limb of internal capsules in neuroleptic-naive schizophrenia. Acad Radiol. 2008;15(3):285–289. doi: 10.1016/j.acra.2007.09.026. [DOI] [PubMed] [Google Scholar]