Abstract
Despite high sequence similarity between NOP (nociceptin/orphanin FQ opioid peptide) and opioid receptors, marked differences in endogenous ligand selectivity, signal transduction, phosphorylation, desensitization, internalization and trafficking have been identified; underscoring the evolutionary difference between NOP and opioid receptors. Activation of NOP receptors affects nociceptive transmission in a site-specific manner, with antinociceptive effects prevailing after peripheral and spinal activation, and pronociceptive effects after supraspinal activation in rodents. The net effect of systemically administered NOP receptor agonists on nociception is proposed to depend on the relative contribution of peripheral, spinal and supraspinal activation, and this may depend on experimental conditions. Functional expression and regulation of NOP receptors at peripheral and central sites of the nociceptive pathway exhibits a high degree of plasticity under conditions of neuropathic and inflammatory pain. In rodents, systemically administered NOP receptor agonists exerted antihypersensitive effects in models of neuropathic and inflammatory pain. However, they were largely ineffective in acute pain while concomitantly evoking severe motor side effects. In contrast, systemic administration of NOP receptor agonists to non-human primates (NHPs) exerted potent and efficacious antinociception in the absence of motor and sedative side effects. The reason for this species difference with respect to antinociceptive efficacy and tolerability is not clear. Moreover, co-activation of NOP and μ-opioid peptide (MOP) receptors synergistically produced antinociception in NHPs. Hence, both selective NOP receptor as well as NOP/MOP receptor agonists may hold potential for clinical use as analgesics effective in conditions of acute and chronic pain.
Introduction
In 1994, soon after the cloning of μ-, δ- and κ-opioid receptors (MOP, DOP and KOP, respectively), several groups identified a GPCR with high homology to opioid receptors (Bunzow et al., 1994; Fukuda et al., 1994; Mollereau et al., 1994; Nishi et al., 1994; Wang et al., 1994; for receptor nomenclature see Alexander et al., 2013a), but very low affinity for opioid ligands. Thus, this receptor was named opioid receptor like 1 (ORL1). In 1995, two groups independently identified the endogenous ORL1-ligand, a heptadecapeptide that was named nociceptin (Meunier et al., 1995) for its ability to elicit hyperalgesia after supraspinal administration in mice and orphanin FQ (Reinscheid et al., 1995) for its ability to recognize a previous orphan receptor and for its first and last amino acid residues [F (Phe) and Q (Gln)]. Following identification of nociceptin/orphanin FQ (N/OFQ) as the endogenous agonist of ORL1, the receptor was renamed nociceptin opioid peptide receptor and abbreviated as NOP receptor, considered a subcategory of the opioid peptide receptor family by IUPHAR (Cox et al., 2014). However, N/OFQ acts at the molecular and cellular level in very much the same way as opioids to produce pharmacological effects that sometimes differ from, and even oppose, those of opioids. In fact, activation of NOP receptors translates into a very complex pharmacology of pain modulation leading to either pronociceptive or antinociceptive activity, depending on the route of administration, pain model and species employed. Furthermore, functional expression of the NOP receptor system has been shown to display a high degree of plasticity and is up-regulated under conditions of chronic pain (Briscini et al., 2002; Chen and Sommer, 2006). Importantly, systemic administration of selective NOP receptor agonists exerted potent and efficacious analgesia in non-human primate (NHP) models of acute and inflammatory pain in the absence of side effects (Ko et al., 2009; Podlesnik et al., 2011; Cremeans et al., 2012; Sukhtankar et al., 2014). Activation of NOP receptors has been demonstrated to be devoid of reinforcing effects, but to inhibit opioid-mediated reward in rodents and NHPs (Ciccocioppo et al., 2000; Rutten et al., 2010; Podlesnik et al., 2011). Strong opioids acting at MOP receptors continue to be widely used to treat moderate to severe acute and chronic pain. However, their therapeutic window is limited by severe side effects such as nausea and vomiting, constipation, dizziness, somnolence, respiratory depression, physical dependence and abuse (Zollner and Stein, 2007). Reduced effectiveness of MOP receptor agonists under conditions of chronic and neuropathic pain further narrows their therapeutic window (Rosenblum et al., 2008; Labianca et al., 2012). Hence, strong analgesics for the treatment of moderate to severe chronic nociceptive and neuropathic pain are urgently needed (Kissin, 2010). Both NOP receptor selective and bifunctional NOP/MOP receptor agonists have been proposed to have clinical value as analgesics with reduced abuse liability as compared with opioids (Lin and Ko, 2013; Toll, 2013). This review will focus on the functional expression and plasticity of the N/OFQ-NOP receptor system and its interaction with opioid receptors in relation to analgesia.
NOP receptor structure
Despite high sequence similarity of the NOP receptor to opioid receptor subtypes (63–65%), opioid peptides have very low affinity for the NOP receptor. However, the endogenous NOP receptor ligand N/OFQ shares sequence homology with other opioid peptides such as the endogenous κ-ligand dynorphin A, but does not interact with opioid receptors. Previous biochemical studies attributed this distinct selectivity profile to three residue positions in the binding pocket of the NOP receptor that differs from all other opioid receptors: Ala216 (Lys in others), Gln280 (His in others) and Thr305 (Ile in others). Recently, the three-dimensional crystal structures of the NOP receptor and of the opioid receptors MOP, DOP and KOP have been identified (Granier et al., 2012; Manglik et al., 2012; Thompson et al., 2012; Wu et al., 2012), revealing atomic details of ligand–receptor recognition and selectivity. In fact, the crystal structure of the human NOP receptor, solved in complex with the peptide mimetic antagonist compound-24 (C-24) revealed substantial conformational differences in the binding pocket regions between the NOP receptor and opioid receptors (Thompson et al., 2012). The crystal structure of the NOP receptor provides evidence that the three residues Ala216, Gln280 and Thr305 point towards the interior of the binding pocket and that Gln280 and Thr305 are involved in C-24 interaction. Remarkably, the NOP receptor binding pocket with Gln280 does not have any hydrogen-bonding water molecules, whereas in opioid receptors, this glutamine is replaced by histidine forming a hydrogen bond network with two water molecules. This has consequences for the terminal structural moieties of binding peptide ligands, as the hydrophobic and hydrophilic parts of the binding pockets of opioid receptors and NOP receptor are different. In the case of opioid peptides with a Tyr–Gly–Gly–Phe sequence, the hydroxy group of the phenols are involved in the hydrogen bond network, whereas in nociceptin with Phe–Gly–Gly–Phe sequence that has no hydroxy group, hydrophobic interactions are preferred as in C-24. Furthermore, it has been shown that the three NOP receptor-specific residue changes are involved in a large-scale reshaping of the binding pocket. In opioid receptors, Lys227 is located at the entrance of the ligand binding pocket and involved in salt bridges with the side chains of Asp223 and Glu297. In the NOP receptor, Lys is replaced by alanine, preventing these stabilizing ionic interactions and leading to an outward shift of the extracellular half of helix V in the NOP crystal structure, and an inward shift of helix VI, reshaping the entrance to the pocket. Thus, despite high sequence homology between the NOP receptor and opioid receptors, marked differences in endogenous ligand selectivity between these receptors go in hand with substantial changes in the structure of their binding pockets, underscoring the evolutionary differences between NOP and opioid receptors.
N/OFQ precursor
N/OFQ is produced from a larger precursor pre-pro-N/OFQ (ppN/OFQ) composed of 176 amino acids that is located on chromosome 8p21 in humans (Mollereau et al., 1996). ppN/OFQ encodes two additional peptides, N/OFQ-II (17 amino acids) and nocistatin (NST; 30 amino acids in human) (Calo’ et al., 2000). PCR-based evidence for N/OFQ mRNA turnover is in fact measuring the precursor and hence these additional cleavage products. The target site(s) for N/OFQ-II and NST are the subject of intense debate. N/OFQ-II has antinociceptive actions (Rossi et al., 1998; 2002) and increases locomotor activity in rodents (Florin et al., 1997). The precise cellular target site is unknown. There is more interest and data for NST (Okuda-Ashitaka et al., 1998), which is described, in general, as an anti-N/OFQ peptide where reversal of N/OFQ effects on glutamate release (Nicol et al., 1998), the antimorphine effect in the brain (Zhao et al., 1999) and impairment of learning and memory (Hiramatsu et al., 2008) have been reported. There are a range of direct actions that are beyond the scope of this paper. What is clear is that NST does not interact with the NOP receptor (Neal et al., 2003), but the cellular target again remains unknown (Johnson and Connor, 2007). One study from Okuda-Ashitaka et al. (1998) attempts to address this using NST-conjugated affinity latex beads to uncover NST binding partners. One target of interest was NIPSNAP1 (4-nitrophenylphosphate domain and non-neuronal SNAP25-like protein homologue 1), a protein involved in vesicle trafficking, and it was shown that NST inhibition of N/OFQ-induced tactile allodynia is absent in NIPSNAP1 knockout mice (Okuda-Ashitaka et al., 2012). What is clear from this discussion is that tissues capable of producing and releasing N/OFQ are also producing a potential antagonist of these actions; the relative amounts and actions at different sites are at present unclear.
Signal transduction of the NOP receptor
Similar to opioid receptors, the NOP receptor has been shown to inhibit adenylate cyclase, to activate inwardly rectifying potassium channels, and to close calcium channels via coupling to Pertussis toxin (PTX)-sensitive Gi/o proteins (Ma et al., 1997; Margas et al., 2008). However, in contrast to opioid receptors, the NOP receptor has also been shown to couple to PTX-insensitive G-proteins, such as Gz, G16 or Gs (Chan et al., 1998; Klukovits et al., 2010).
According to cell type or tissue, numerous studies revealed that the activated NOP receptor triggers a variety of intracellular signalling events, including modulation of adenylate cyclase activity (Ma et al., 1997; Klukovits et al., 2010) and activation of PKC (Lou et al., 1997), PLA2 (Fukuda et al., 1998) and PLC (Lou et al., 1997), ERK1/2 (Fukuda et al., 1997; Lou et al., 1997), p38 MAPK (Zhang et al., 1999), JNK (Chan and Wong, 2000), and NF-κB (Donica et al., 2011). Moreover, it has been reported that STAT3 may be involved in the transduction of NOP receptor signalling (Wu et al., 2003).
NOP receptor activation reduces neuronal excitability and neurotransmitter release by inhibition of presynaptic, voltage-gated calcium channels (Connor et al., 1996b; Knoflach et al., 1996) and activation of inwardly rectifying potassium channels (Connor et al., 1996a; Vaughan et al., 1997). NOP receptor activation has been shown to inhibit the release of a wide range of neurotransmitters including noradrenaline, dopamine, 5-HT, ACh and glutamate (Nicol et al., 1996; 1998,2002; Schlicker and Morari, 2000). This NOP receptor-mediated reduction in neurotransmitter release is the basis for its modulation of many biological functions that rely on synaptic transmission, including nociception, anxiety and reward.
Indeed, activation of Gi-coupled NOP receptors has been shown to inhibit Cav2.2 N-type calcium channels (for nomenclature see Alexander et al., 2013b) to attenuate nociception. However, it has been demonstrated that the sensitivity of N-type channels to G–protein-mediated inhibition is regulated by alternative splicing of the exons 37a and 37b in Cav2.2 pre-mRNAs (Raingo et al., 2007). The most common form of G–protein-mediated inhibition of N-type currents is voltage-dependent and requires Gβγ, which binds directly to Cav2.2 (both channel variants 37a and 37b) and is independent of Src tyrosine kinase. Voltage-independent inhibition is unique to the Cav2.2-e37a isoform, requires Src tyrosine kinase-mediated phosphorylation of the C-terminal Y1747 and is independent of Gβγ-binding. Activation of Gi-coupled NOP receptor produces free Gβγ-subunit mediating a signalling pathway involving PI-3K and Src-kinase (Hawes et al., 1998). Activated Src-kinase then plays a role in the phosphorylation and inhibition of the nociceptor-specific exon 37a splice isoform of Cav2.2. The 37b splice isoform of Cav2.2 lacks the Src-specific tyrosine phosphorylation site and therefore can only be inhibited via direct Gβγ-binding. The e37a splice variant of Cav2.2 is highly enriched in nociceptors of dorsal root ganglia (Bell et al., 2004) leading to an increased cellular sensitivity to inhibition by activated MOP receptors and behavioural sensitivity to spinal morphine-induced analgesia (Andrade et al., 2010).
Interaction of NOP receptors with N-type calcium channels
Recent data support the concept that NOP receptors and N-type calcium channels can form signalling complexes, which are internalized into vesicular compartments after prolonged NOP receptor activation, effectively inhibiting calcium influx into the cell (Altier et al., 2006; Evans et al., 2010). However, a recent study examining the effect of NOP receptor activation on N-type calcium channels in a highly N/OFQ-sensitive subpopulation of rat dorsal root ganglion (DRG) and spinal cord neurons found that, although N/OFQ treatment inhibited primary afferent excitatory postsynaptic currents on dorsal horn neurons, it did not induce internalization of N-type calcium channels in the cell body or nerve terminals of DRG neurons (Murali et al., 2012). Other studies revealed that the NOP receptor associates with and inhibits N-type calcium channels, even in the absence of N/OFQ (Beedle et al., 2004). Thus, although there is agreement over the ability of the N/OFQ-NOP receptor system to inhibit N-type calcium channels, the precise mechanism by which the NOP receptor regulates calcium channel activity, and whether this involves NOP receptor-mediated internalization of these channels is still under debate.
Cellular NOP receptor expression and function in tissues
The NOP receptor and its ligand N/OFQ are widely expressed in the CNS and in the peripheral nervous system as well as in many peripheral organs and the immune system in rodents, NHPs and humans (reviewed in Mollereau and Mouledous, 2000; Civelli, 2008). In particular, the NOP receptor is expressed in DRG, in the dorsal and ventral horns of the spinal cord, in the forebrain, including cortical areas, olfactory regions, the thalamus, and a variety of limbic structures, such as the hippocampus, septum, the bed nucleus of the stria terminalis, the diagonal band of Broca, the habenula, the amygdaloid complex, and in several nuclei of the hypothalamus, that are involved in the processing of emotional stimuli. The NOP receptor is also localized in 5-hydroxytryptaminergic, noradrenergic, and dopaminergic nuclei, such as the raphe complex, the locus coeruleus, the nucleus of the solitary tract, the ventral tegmental area, and the substantia nigra (Neal et al., 1999a; Mollereau and Mouledous, 2000). A similar pattern of N/OFQ and NOP receptor expression in human and rodent CNS has been observed (Peluso et al., 1998; Berthele et al., 2003; Witta et al., 2004).
NOP receptor expression has also been found in other peripheral tissues, namely in rodent and human intestines (Menzies et al., 1999; Agostini et al., 2009; Li et al., 2013), in human blood lymphocytes (Wick et al., 1995), in several peripheral sensory and sympathetic ganglia from guinea-pigs (Kummer and Fischer, 1997) and rats (Xie et al., 1999), in rabbit retina (Neal et al., 1997) and in rat heart (Giuliani et al., 1997; Dumont and Lemaire, 1998). In line with this wide distribution of the N/OFQ-NOP receptor system, N/OFQ modulates many physiological responses/systems, including anxiety (Jenck et al., 1997), food intake (Pomonis et al., 1996), learning and memory (Sandin et al., 1997), locomotor activity (Reinscheid et al., 1995; Florin et al., 1996), respiratory (Corboz et al., 2000; 2001; Takita et al., 2003; Takita and Morimoto, 2008; Singh et al., 2013), immune (Peluso et al., 2001; Serhan et al., 2001), urinary bladder (Lecci et al., 2000; Lazzeri et al., 2001; 2006), cardiovascular and renal functions (Kapusta et al., 1997). NOP receptors identified on pre- and/or postganglionic sympathetic and parasympathetic nerve fibres and innervating blood vessels and heart are involved in the cardiovascular effects of N/OFQ, and might play a role in the pathophysiology of inflammation, arterial hypertension and cardiac or brain circulatory ischaemia (Malinowska et al., 2002; Serrano-Gomez et al., 2011; Brookes et al., 2013; Thompson et al., 2013).
Splice variants of the NOP receptor
There are several publications describing NOP receptor splice variants. For example, RT-PCR and RNAse protection studies of NOP receptor transcripts in the human CNS as well as human immune cells identified a splice variant of the human NOP receptor lacking 15 nucleotides at the junction between exons 1 and 2 (Halford et al., 1995; Peluso et al., 1998). This splice variant (Δ15hNOP receptor) encodes a receptor that lacks a -YVILR- motif in the N-terminal portion of the first intracellular loop. Analysis of the distribution of this shorter receptor isoform demonstrated no significant difference from that of the full-length human NOP receptor (Peluso et al., 1998). However, the short (truncated) form displays a marked reduction in binding affinity for a range of NOP receptor ligands and this is coupled with loss of function (Pan et al., 1998; Xie et al., 2000; Curro et al., 2001).
Remarkably, four additional NOP receptor splice variants have been identified, including a rat variant that contains a 81 bp insertion between the second and third coding exons (Wang et al., 1994), and three splice variants isolated from mouse brain with insertions of 34 bp (KOR-3a), 98 bp (KOR-3b), and 139 bp (KOR-3c) between exons 1 and 2 (Pan et al., 1998). The expression of the three variants in the mouse brain varies markedly among brain regions with a distribution quite distinct from the NOP receptor itself, indicating region-specific NOP receptor splicing. However, whether these splice variants have a functional relevance beyond ligand decoy awaits further investigation.
NOP receptor internalization and trafficking
For many GPCRs, it is widely known that sustained agonist activation can lead to receptor phosphorylation and internalization (Pierce and Lefkowitz, 2001). Activation of NOP receptors with N/OFQ produces rapid and robust receptor internalization over time (Spampinato et al., 2001; 2002; 2007; Corbani et al., 2004; Baiula et al., 2013). Recently, it was demonstrated that NOP receptor phosphorylation at carboxyl terminal serine 363 by GPCR kinase 3 plays an important role in N/OFQ-induced NOP receptor desensitization, β-arrestin2-recruitment, internalization and arrestin-dependent JNK MAPK signalling (Zhang et al., 2012). This finding is consistent with previous data suggesting that arrestin binding to GPCRs may enable MAPK activation, and acts to regulate receptor function (Bohn et al., 2004; Bruchas et al., 2006; Shenoy and Lefkowitz, 2011). Similar to opioid receptors, the NOP receptor has been shown to efficiently recycle to the plasma membrane after agonist-induced internalization (Spampinato et al., 2007). Together, these findings indicate, that NOP receptors are regulated via similar, but unique mechanisms as compared with opioid receptors.
Heterodimerization of NOP receptors with opioid receptors
NOP receptors have also been shown to form heterodimers with MOP, DOP or KOP receptors (Pan et al., 2002; Wang et al., 2005; Evans et al., 2010). The NOP/opioid receptor heterodimers were co-internalized after N/OFQ or opioid agonist treatment indicating that the heterodimers can be trafficked together as a complex (Evans et al., 2010). The formation of MOP/NOP receptor heterodimers was found to impair MOP receptor-activated signalling pathways (Mandyam et al., 2003; Wang et al., 2005). Therefore, it seems reasonable to suggest, that MOP/NOP receptor heterodimerization may lead to the impairment of MOP receptor-mediated biological effects in the brain contributing to NOP receptor-mediated antiopioid effects. However, a recent study revealed that the internalization of NOP receptor/Cav2.2 complexes following prolonged exposure to N/OFQ may be dependent on the formation of NOP/MOP receptor heterodimers (Evans et al., 2010). Moreover, the NOP receptor was shown to function as a molecular link that allows MOP receptors to trigger N-type channel internalization. These findings indicate that formation of NOP/MOP receptor heterodimers affects receptor function with consequences for NOP receptor- and MOP receptor-mediated N-type calcium channel regulation. Indeed, the search for drug molecules that target MOP/NOP receptor heterodimers has already met with some success. Recently, iodobenzoylnaltrexamide (IBNtxA) has been shown to target MOR1G/NOP receptor heterodimers, displaying a full analgesic response without the side effects of opioids (Majumdar et al., 2011). MOR1G is a truncated, six-transmembrane variant of the MOP receptor, which lacks exon 1, and is generated from a second, upstream promoter associated with exon 11. The exon 11-associated MOR-1G splice variant alone is insufficient to generate the IBNtxA binding site and requires heterodimerization with NOP receptors. Collectively, these findings strongly indicate that pharmacological and signalling properties of NOP/MOP receptor heterodimers are different from those of the individual receptors.
Functional expression of the N/OFQ-NOP receptor system in acute pain
In rodents, N/OFQ and NOP receptor expression has been extensively studied at both transcript and protein levels using a combination of in situ hybridization, radioligand binding and immunohistochemical approaches. Their constitutive expression pattern has been described and reviewed elsewhere (Anton et al., 1996; Neal et al., 1999a,b; 2001; Mollereau and Mouledous, 2000). Because of the widespread distribution of NOP receptors and the pleiotropic effects of N/OFQ, the NOP receptor holds promise for numerous therapeutic applications (Lambert, 2008; Calo and Guerrini, 2013). In this review, we specifically focus on the functional expression of the N/OFQ-NOP receptor system in relation to nociception and its regulation under conditions of chronic neuropathic and inflammatory pain. Functional NOP receptors are expressed at peripheral, spinal and supraspinal sites of the ascending and descending pain pathways (Table 1, Figure 1). N/OFQ reduced capsaicin-induced nociception after peripheral administration in mice (Sakurada et al., 2005) and exerted spinal antinociceptive effects in the tail flick test in rats (Xu et al., 1996; Tian et al., 1997a) and mice (King et al., 1997). Moreover, spinal N/OFQ potentiated systemic morphine antinociception (Tian et al., 1997a). However, when administered intracerebroventricularly (i.c.v.), N/OFQ was pronociceptive in the hot plate test (Meunier et al., 1995) and tail flick test (Reinscheid et al., 1995) in mice. Other authors found that i.c.v. N/OFQ was not effective alone, but reversed antinociception induced by systemic and i.c.v. morphine in the tail flick test in rats (Tian et al., 1997a) and mice (King et al., 1998) respectively. The potent truncated N/OFQ analogue [Phe1psi(CH2-NH)Gly2]N/OFQ(1-13)-NH2 was antinociceptive after intrathecal (i.t.) administration whereas it exerted pronociceptive effects when administered i.c.v. in the rat tail flick test (YQ Wang et al., 1999b). In the mouse tail withdrawal assay, i.c.v. N/OFQ and [Phe1psi(CH2-NH)Gly2]N/OFQ(1-13)-NH2 were pronociceptive per se and inhibited i.c.v. morphine antinociception (Calo et al., 1998). In addition, evidence was provided showing that low doses of N/OFQ induce nociceptive behaviour after peripheral and i.t. administration in naïve mice (Inoue et al., 1999; Sakurada et al., 1999). In summary, when comparing different routes of local administration, NOP receptor agonists elicited either antinociceptive or pronociceptive effects as well as potentiated or counteracted opioid-mediated antinociception depending on the site of action in rodent models of acute pain.
Table 1.
Functional expression of the NOP receptor in rodent nociceptive system and effects of N/OFQ and NOP receptor agonists in rodent models of acute pain
| NOP receptor expression | Function of N/OFQ and NOP receptor agonists | |||||
|---|---|---|---|---|---|---|
| Region | mRNA | Protein | In vitro | In vivo | ||
| Supraspinal | Thalamus | Many thalamic nuclei [ISH] (Neal et al., 1999a) | Many thalamic nuclei [RLB] (Neal et al., 1999a) | ↓ firing rate of thalamic neurons [in vivo electrophysiology rat] (Albrecht et al., 2001) | i.c.v.: no effect alone, but reduced i.c.v. DAMGO-induced antinociception [tail withdrawal mouse] (Mogil et al., 1996b) | |
| Amygdala | Many amygdaloid nuclei [ISH] (Neal et al., 1999a) | Many amygdaloid nuclei [RLB] (Neal et al., 1999a), [IHC] (Anton et al., 1996) | ↑ GIRK current in CeA neurons [patch clamp] (Meis and Pape, 1998) | m.i.: antinociceptive [tail flick rat] (Shane et al., 2001) | i.c.v.: no effect alone, but reversed systemic and i.c.v. morphine-induced antinociception in rats [tail flick] (Tian et al., 1997a) and mice [tail flick] (King et al., 1998), [tail withdrawal] (Mogil et al., 1996a) | |
| ↓ IPSC in CeA neurons [patch clamp] (Roberto and Siggins, 2006) | ||||||
| ↑ K+ current, hyperpolarized CeA-PAG projection neurons [patch clamp] (Chen et al., 2009) (Chieng and Christie, 2010) | ||||||
| PAG | High expression through entire extent [ISH] (Neal et al., 1999a) | Dense N/OFQ binding [RLB] (Neal et al., 1999a) | ↑ K+ current, ↓ IPSCs and EPSCs [patch clamp] (Vaughan et al., 1997) | m.i.: reduced intra-PAG morphine-induced antinociception [tail flick rat] (Morgan et al., 1997) | i.c.v.: pronociceptive alone, inhibited i.c.v. morphine-induced antinociception [tail withdrawal mouse] (Calo et al., 1998) | |
| ↓ N-, P/Q-type Ca2+ current [patch clamp] (Connor and Christie, 1998) | m.i.: antinociceptive [tail flick rat] (Shane et al., 2003) | i.c.v. N/OFQ(1-13): pronociceptive [tail flick rat] (YQ Wang et al., 1999b) | ||||
| m.i.: pronociceptive [tail flick rat] (Lu et al., 2010) | i.c.v.: pronociceptive [hot plate mouse] (Liu et al., 2006) | |||||
| i.c.v.: pronociceptive [tail flick mouse] (Wang et al., 2006) | ||||||
| RVM | High expression in C1, A1 cell group [ISH] (Neal et al., 1999a) | Moderate N/OFQ binding in C1, A1 cell group [RLB] (Neal et al., 1999a) | ↑ K+ current, ↓ Ca2+ current in primary and secondary RVM neurons [patch clamp] (Vaughan et al., 2001) | m.i.: no effect alone, but reduced intra-RVM DAMGO-induced antinociception [tail flick rat] (Heinricher et al., 1997) | ||
| m.i.: no effect alone, but reduced intra-PAG DAMGO-induced antinociception [tail flick rat] (Pan et al., 2000) | ||||||
| Spinal | Intrinsic neurons | Lamina I, II, X, ventral horn [ISH] (Pettersson et al., 2002) | Dorsal horn [RLB] (Pettersson et al., 2002) | ↓ A- and C-fibre responses [hemisected spinal cord] (Faber et al., 1996) | i.t.: ↓ C-fibre evoked wind-up of DH neurons [in vivo electrophysiology rat] (Stanfa et al., 1996) | |
| Dorsal, ventral horn [ISH] (Mika et al., 2003) | Majority of N/OFQ binding sites spared by rhizotomy (Le Cudennec et al., 2002) | Hyperpolarized lamina II neurons by ↑ K+ conductance [patch clamp] (Lai et al., 1997) | i.t.: antinociceptive, potentiated s.c. morphine-induced antinociception [tail flick rat] (Tian et al., 1997a) | |||
| ↓ glutamate-, kainate-, quisqualate-induced currents in DH neurons [patch clamp] (Shu et al., 1998) | i.t. N/OFQ(1-13): antinociceptive [tail flick rat] (YQ Wang et al., 1999b) | |||||
| ↑ GIRK current in lamina II neurons [patch clamp] (Luo et al., 2001) | i.t. N/OFQ(1-13): antinociceptive [tail flick rat] (YQ Wang et al., 1999b) | |||||
| Proximal PAF (presynaptic) | Rhizotomy reduced N/OFQ binding sites by 18% [RLB] (Le Cudennec et al., 2002) | ↓ Aδ-, C-fibre EPSCs of lamina I and lamina II neurons [patch clamp] (Lai et al., 1997; Liebel et al., 1997; Luo et al., 2002; Murali et al., 2012) | ||||
| Peripheral | DRG neuron (cell body) | Large neurons [ISH] (Pettersson et al., 2002) | Medium and large neurons [RLB] (Pettersson et al., 2002) | ↓ T-type Ca2+ currents in medium-sized neurons [patch clamp] (Abdulla and Smith, 1997) | ||
| Co-localization in majority of SP- and CGRP-positive neurons [ISH, RT-PCR] (Mika et al., 2003) | Small and medium neurons [IHC] (Chen and Sommer, 2006) | ↓ N-type Ca2+ currents (Abdulla and Smith, 1998) in small IB4 negative neurons [patch clamp] (Murali et al., 2012) | ||||
| Distal PAF (dendritic) | Not in skin [ISH] (Pettersson et al., 2002) | s.c. in the tail: antinociceptive [tail flick mouse] (Kolesnikov and Pasternak, 1999) | ||||
| i.pl.: ↓ i.pl. capsaicin-induced nociception in mice (Sakurada et al., 2005) | ||||||
| i.pl. Ro64-6198: no effect on mechanical and thermal nociception [PPT, PWL rat] (Obara et al., 2005) | ||||||
No data are published for fields that are left blank. ↑, activation/increase; ↓, inhibition/decrease; CeA, central nucleus of amygdala; CGRP, calcitonin gene-related peptide; DAMGO, (D-Ala2, N-Me-Phe4, Gly-ol)-enkephalin; DH, dorsal horn; EPSC, excitatory postsynaptic current; GIRK, G–protein-activated inward rectifier K+; IB4, isolectin B4; IHC, immunohistochemistry; IPSC, inhibitory postsynaptic current; ISH, in situ hybridization; m.i., microinjected; PAF, primary afferent fibre; PPT, paw pressure test; PWL, paw withdrawal latency; RLB, radioligand binding; Ro64-6198, selective NOP receptor agonist; SP, substance P.
Figure 1.
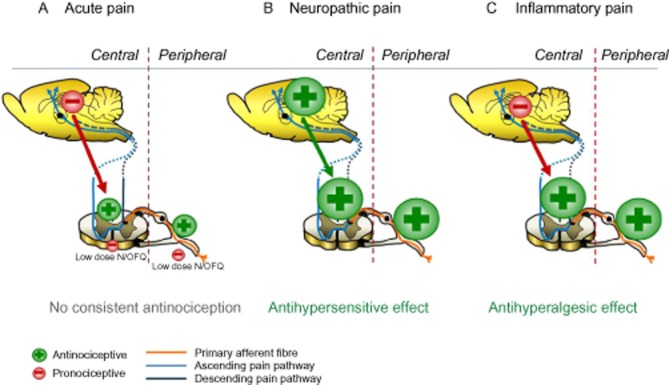
Schematic presentation summarizing the effects of NOP receptor activation on nociceptive processing at peripheral, spinal and supraspinal sites, and resulting analgesic effects of systemically administered NOP receptor agonists under conditions of acute, neuropathic and inflammatory pain in rodents. (A) NOP receptor agonists were largely ineffective in acute pain after systemic administration as activation of supraspinal NOP receptors counteracted spinally and peripherally mediated antinociception. Pronociceptive effects were also elicited by low concentrations of N/OFQ at peripheral and spinal sites. (B) In contrast, systemic administration of NOP receptor agonists elicited antihypersensitive effects in neuropathic pain as, here, activation of supraspinal NOP receptors did not counteract, but contributed to analgesic efficacy. In addition, peripheral, spinal and supraspinal NOP receptors were up-regulated and functionally sensitized. (C) Inhibition of nociceptive processing elicited by activation of functionally sensitized peripheral and spinal NOP receptors is hypothesized to overcome pronociceptive effects of supraspinal NOP receptor activation, thus leading to antihyperalgesic efficacy after systemic administration of NOP receptor agonists in inflammatory pain. Larger symbols indicate up-regulation/functional sensitization of NOP receptors.
The effects of N/OFQ on nociceptive processing in rodents after peripheral, spinal and supraspinal administration as well as the underlying cellular and molecular mechanisms have been reviewed in detail elsewhere (Grisel and Mogil, 2000; Moran et al., 2000; Mogil and Pasternak, 2001; Zeilhofer and Calo, 2003). In the peripheral nervous system, N/OFQ was shown to inhibit neurotransmitter release (Giuliani et al., 2000), which might explain its inhibitory effect on substance P-mediated nociceptive flexor reflex in mice (Inoue et al., 1999).
In the spinal cord, N/OFQ analgesia is mediated by inhibiting excitatory glutamatergic nociceptive transmission via activation of pre- and postsynaptic NOP receptors. The majority of NOP receptors in rat spinal cord seems to be expressed on intrinsic neurons (Le Cudennec et al., 2002). Presynaptic NOP receptors are located in primary afferents arising from small- and medium-sized DRG neurons, corresponding to C- and Aδ-fibres respectively (Chen and Sommer, 2006). Activation of presynaptic NOP receptors inhibited N-type Ca2+ channels leading to reduced transmitter release from central terminals of primary afferent fibres (Helyes et al., 1997), thereby attenuating excitatory nociceptive input to the rat spinal cord dorsal horn (Lai et al., 1997; Liebel et al., 1997; Luo et al., 2002; Murali et al., 2012). Dorsal horn spino-thalamic tract neurons convey nociceptive signals to supraspinal sites. N/OFQ hyperpolarized and hence inhibited electrical activity of these neurons by increasing K+ currents (Lai et al., 1997; Luo et al., 2001) secondary to postsynaptic NOP receptor activation. Consequently, N/OFQ inhibited A– and C–fibre-mediated compound action potentials in an ex vivo rat hemisected spinal cord preparation (Faber et al., 1996) as well as C-fibre-evoked wind-up of rat spinal dorsal horn neurons in vivo (Stanfa et al., 1996). Interestingly, N/OFQ inhibited C–fibre-evoked responses more potently than A–fibre-evoked responses (Faber et al., 1996; Luo et al., 2002). Furthermore, N/OFQ inhibited excitatory amino acid-evoked responses of rat trigeminal dorsal horn neurons by a postsynaptic mechanism (Wang et al., 1996).
We have already demonstrated that spinal NOP receptor activation elicits an antinociceptive response via mechanisms similar to those described for opioids; pre- and post-synaptic inhibition at the first synapse in the pain pathway. Moreover, that supraspinal NOP receptor activation produces an anti-opioid action resulting in a hyperalgesic response. Anti-opioid actions cover opiates produced by stress in the original description of N/OFQ by Meunier et al. (1995), several peptide and non-peptide opioids selective for opioid receptors (Mogil et al., 1996b; Chen et al., 2007) and electro-acupuncture (Tian et al., 1997b). As an anti-opioid N/OFQ is not unique as there is evidence of similar actions for other peptides such as cholecystokinin (Heinricher et al., 2001). This apparent hyperalgesia results from an interaction with descending inhibitory control machinery from the rostral ventromedial medulla (RVM) back to the spinal dorsal horn. In its simplest form, this can be explained based on the activity of two types of cells located in the RVM; OFF (or primary) cells and ON (or secondary) cells. OFF cells project from the RVM back to the spinal cord and increased activity reduces afferent inflow – resulting in ‘descending inhibition’. In the RVM, OFF cells are tonically inhibited by ON cells via GABAergic mechanisms (Fields, 2004). MOP receptors are located on the ON cells where activation with drugs like morphine (or endogenous opioids) inhibits their activity (Heinricher et al., 1994). This effectively takes the brakes from the system (disinhibits) and allows the OFF cells to fire leading to a supraspinal MOP receptor-mediated antinociceptive response (Heinricher et al., 1994). NOP receptors are located in both the ON and OFF cells, but in terms of explaining their anti-opioid actions their location on, and inhibition of OFF cells takes primacy (Heinricher et al., 1997). Direct inhibition of OFF cell firing would effectively reverse any disinhibition of this subset of cells produced by either endogenous or exogenous MOP receptor agonists (Heinricher et al., 1997). The resulting anti-opioid action would produce hyperalgesia. This is described schematically in several publications (Pan et al., 2000; Zeilhofer and Calo, 2003; Lambert, 2008). The effect of chronic pain conditions on the functionality and interaction between the ON and OFF cells has not been investigated in rodents. In addition, it is not clear whether this circuitry also exists in the RVM of NHPs and humans.
The net effect of systemically administered NOP receptor agonists on nociception is proposed to depend on the relative contribution of peripheral, spinal and supraspinal sites of action, which in turn may depend on experimental conditions (Figure 1). While systemic administration of the potent and selective non-peptide NOP receptor agonist Ro64-6198 exerted antinociceptive effects in the mouse hot plate test, it increased pain sensitivity in the mouse tail flick test, an effect attributed to a supraspinally mediated inhibition of stress-induced analgesia (Reiss et al., 2008). Ro64-6198 was not antinociceptive in the rat tail flick test (Jenck et al., 2000; Dautzenberg et al., 2001), or in the tail immersion test in mice after systemic administration (Kotlinska et al., 2003). In agreement with these latter studies, Ro64-6198 did not change mechanical and thermal nociceptive thresholds of naïve rats after i.t. and intraplantar (i.pl.) administration in the paw pressure and paw withdrawal latency test respectively (Obara et al., 2005). While systemic Ro64-6198 was not convincingly antinociceptive across a range of rodent models of acute pain, it consistently produced severe motor side effects over the same dose range (Higgins et al., 2001; Varty et al., 2005; Reiss et al., 2008).
As in rodents, peripherally and spinally delivered peptide NOP receptor agonists inhibited thermal nociception in NHPs (Ko et al., 2002; 2006; Ko and Naughton, 2009; Hu et al., 2010). Interestingly, pronociceptive effects after peripheral and spinal administration of low doses of N/OFQ were not evident in NHPs (Ko et al., 2002; Ko and Naughton, 2009). The effect of supraspinally delivered NOP receptor agonists on nociception has not yet been investigated in NHPs. However, in sharp contrast to results obtained in rodents, systemic administration of Ro64-6198 to NHPs exerted potent and fully efficacious thermal antinociception in the absence of motor and sedative side effects (Ko et al., 2009; Podlesnik et al., 2011; Cremeans et al., 2012). Hence, a profound species difference exists between rodents and NHPs with respect to antinociceptive efficacy and tolerability of systemically administered selective NOP receptor agonists (Table 2). The reason for this functional difference is not yet clear. Although radioligand binding autoradiography also demonstrated widespread expression of NOP receptors in the brain and spinal cord of NHPs, subtle differences in areas relevant for nociception were observed as compared with the expression pattern in rodents. Specifically, lower NOP receptor expression was observed in raphe nuclei and in the spinal cord dorsal horn in NHPs compared with rodents (Bridge et al., 2003). Therefore, it is tempting to speculate that the functional species difference described earlier might result from differences in NOP receptor expression and from a different functional effect of NOP receptors on nociceptive processing particularly in supraspinal pain circuits. Elucidating the consequences of NOP receptor activation on functionally characterized cells of such neuronal networks in the NHP and human RVM should be one focus of future research. Moreover, investigating the effect of supraspinally delivered NOP receptor agonists on nociception in NHPs will further contribute to our understanding of species differences in antinociceptive efficacy of systemically administered NOP receptor agonists and is, therefore, highly warranted.
Table 2.
Comparison of analgesic properties of N/OFQ and NOP receptor agonists between rodents and NHPs
| Administration route | Pain modality | Pharmacological action | Rodents | NHPs |
|---|---|---|---|---|
| Peripheral | Acute pain | Antinociceptive effects | s.c. N/OFQ in the mouse tail Local antinociception inhibited by naloxone (Kolesnikov and Pasternak, 1999) | |
| No antinociceptive effects | i.pl. Ro64-6198 in naïve rats (Obara et al., 2005) | s.c. N/OFQ in the NHP tail (Ko et al., 2002) | ||
| Biphasic (low dose: nociceptive vs. high dose: antinociceptive) | i.pl. N/OFQ (0.01 fmol–1 nmol) in the nociceptive flexor test in mice (Inoue et al., 1999) | |||
| No biphasic effects | s.c. N/OFQ (1 pg–30 µg) in the NHP tail withdrawal assay (Ko et al., 2002) | |||
| Capsaicin allodynia | Antiallodynic effects | i.pl. N/OFQ in naïve mice (Sakurada et al., 2005) | s.c. N/OFQ in the NHP tail | |
| Local antinociception inhibited by J-113397 (Ko et al., 2002) | ||||
| Neuropathic pain | Antihypersensitive effects | i.pl. N/OFQ and Ro64-6198 in CCI rats (Obara et al., 2005) | ||
| Local antihypersensitive effect inhibited by Nphe (Obara et al., 2005) | ||||
| Spinal | Acute pain | Antinociceptive effects | i.t. N/OFQ in the rat and mouse tail flick assay (Xu et al., 1996; King et al., 1997; Tian et al., 1997a) | i.t. N/OFQ in the NHP tail withdrawal assay (Ko et al., 2006; Ko and Naughton, 2009) |
| Spinal antinociception inhibited by naltrexone (King et al., 1997) and UFP-101 (Nazzaro et al., 2007) in mice, by naloxone in rats (Jhamandas et al., 1998), by MOP and DOP receptor antagonists in rats (Yu et al., 2002) | Spinal antinociception inhibited by J-113397, not naltrexone (Ko et al., 2006) | |||
| No antinociceptive effects | i.t. Ro64-6198 in naïve rats (Obara et al., 2005) | |||
| Biphasic (low dose: nociceptive vs. high dose: antinociceptive) | i.t. N/OFQ (fmol) in mice (Inoue et al., 1999; Sakurada et al., 1999) | |||
| No biphasic effects | i.t. N/OFQ (1 fmol–1 µmol) in the NHP tail withdrawal assay (Ko and Naughton, 2009) | |||
| Capsaicin allodynia | Antiallodynic effects | i.t. N/OFQ in rats with mechanical allodynia (Nozaki-Taguchi and Yamamoto, 2005) | i.t. UFP-112 in NHPs with thermal allodynia (Hu et al., 2010) | |
| Neuropathic pain | Antihypersensitive effects | See details in Table 3 | ||
| Systemic | Acute pain | Antinociceptive effects | i.p. Ro64-6198 in the mouse hot plate test (Reiss et al., 2008) | s.c. Ro64-6198 in the NHP tail withdrawal assay (Ko et al., 2009) |
| Systemic antinociception was absent in NOP receptor knockout mice (Reiss et al., 2008) | i.v. Ro64-6198 in the NHP tail withdrawal assay (Ko, 2004; Podlesnik et al., 2011) | |||
| i.m. Ro64-6198 and SCH221510 in the NHP tail withdrawal assay (Cremeans et al., 2012) | ||||
| Systemic antinociception inhibited by J-113397 (Ko et al., 2009) | ||||
| No antinociceptive effects | i.p. Ro64-6198 in the rat tail flick test (Jenck et al., 2000; Dautzenberg et al., 2001) | |||
| i.p. Ro64-6198 in the mouse tail immersion test (Kotlinska et al., 2003) | ||||
| Pronociceptive effects | i.p. Ro64-6198 in the mouse tail flick assay (Reiss et al., 2008) | |||
| Potentiation of MOP receptor agonist-induced antinociception | Ro64-6198 potentiated morphine antinociception in an additive manner (Reiss et al., 2008) | Ro64-6198 potentiated buprenorphine antinociception in a synergistic manner (Cremeans et al., 2012) | ||
| Capsaicin allodynia | Antiallodynic effects | s.c. Ro64-6198 in the NHP thermal allodynia assay (Ko et al., 2009) | ||
| Neuropathic pain | Antihypersensitive effects | i.v. GRT-TA2210 and Ro65-6570 in CCI mice (Linz et al., 2013) | ||
| i.v. and i.p. Ro65-6570 in neuropathic mice and rats (Schiene et al., 2013) | ||||
| Inflammatory pain | Antihyperalgesic effects | i.v. GRT-TA2210 in the mouse formalin test (Linz et al., 2013) | s.c. Ro64-6198 in the NHP carrageenan assay (Sukhtankar et al., 2014) | |
| i.v. Ro65-6570 in the rat formalin and CFA assays (Schiene et al., 2013) |
No data are published for fields that are left blank. GRT-TA2210, selective NOP receptor agonist; J-113397, selective NOP receptor antagonist; Nphe, [Nphe1]N/OFQ(1-13)NH2, a selective NOP receptor antagonist; Ro64-6198, Ro65-6570, selective NOP receptor agonists; SCH-221510, selective NOP receptor agonist; UFP-101, selective NOP receptor antagonist; UFP-112, selective NOP receptor agonist.
Also in humans, both N/OFQ and NOP receptor mRNA and protein has been detected in nociceptive structures of the CNS and peripheral nervous system (Peluso et al., 1998; Mollereau and Mouledous, 2000; Berthele et al., 2003; Witta et al., 2004). The pattern of NOP receptor expression in humans was in general agreement with that seen in NHPs (Peluso et al., 1998; Bridge et al., 2003). It has been suggested that NHP models of nociception can provide a translational bridge for research and development of NOP receptor and opioid receptor-related ligands (Lin and Ko, 2013). However, clinical proof-of-concept trials testing for analgesic properties of NOP receptor agonists after systemic administration to assess their therapeutic potential for diverse pain indications are still urgently awaited.
Functional expression and regulation of the N/OFQ-NOP receptor system in neuropathic pain
N/OFQ elicited potent and efficacious antihypersensitive effects in rodent models of neuropathic pain (Table 3). For example, spinally delivered N/OFQ inhibited thermal hyperalgesia as well as mechanical allodynia and hyperalgesia in the rat chronic constriction injury (CCI) model (Yamamoto et al., 1997c; Corradini et al., 2001; Courteix et al., 2004) and reduced mechanical allodynia in the rat spinal nerve ligation (SNL) model (Ju et al., 2013). N/OFQ selectively inhibited mechanical hyperalgesia in CCI rats while it had no effect on mechanical pain thresholds in naïve rats (Courteix et al., 2004). Of more interest, pre-emptive i.t. administration of N/OFQ delayed the development of thermal hyperalgesia and decreased expression of c-Fos in the rat CCI model (Yamamoto et al., 2000). Similar to N/OFQ, Ro64-6198 inhibited mechanical and cold allodynia after peripheral and spinal administration in rats subjected to CCI, whereas Ro64-6198 had no effect on mechanical and thermal pain thresholds in naïve animals (Obara et al., 2005). This is mirrored by the fact that microiontophoretically applied N/OFQ inhibited spontaneous and noxious mechanically evoked activity of spinal wide dynamic range (WDR) neurons in CCI, but not in sham and intact rats (Sotgiu et al., 2004). This result is in line with the observation of increased inhibition of N-type Ca2+ channel currents by N/OFQ in DRG neurons after sciatic nerve section (Abdulla and Smith, 1998). Furthermore, the antinociceptive potency of spinal N/OFQ in the tail flick test was greater in mice with diabetic polyneuropathy than in non-diabetic mice (Kamei et al., 1999). This neuropathy-related functional sensitization of the NOP receptor system might be explained, at least in part, by an up-regulation of the NOP receptor. Indeed, NOP receptor mRNA was up-regulated in ipsilateral lumbar (L)5-L6 DRG and lumbar spinal cord of rats displaying mechanical allodynia 7 days after induction of CCI with transcript levels returning to baseline as allodynia resolved at day 15 (Briscini et al., 2002). Moreover, the number of NOP receptor mRNA positive cells increased also in the rat periaqueductal grey (PAG) and RVM 7–14 days after CCI (Ma et al., 2005). N/OFQ immunoreactivity was found to be increased in rat cingulate cortex, but not in the PAG and RVM, 14 days after CCI (Rosen et al., 2000) and in rat amygdala and PAG 36 days after SNL (Sun et al., 2001). Both NOP receptor protein and N/OFQ immunoreactivity seemed to be up-regulated in small- and medium-sized L4 DRG neurons in rats 7 and 14 days after partial sciatic nerve transection (Chen and Sommer, 2006). Therefore, the peripheral, spinal and supraspinal N/OFQ-NOP receptor system seems to respond to nerve injury with a reversible and temporally coordinated up-regulation at discrete sites of the nociceptive pathways in rodents.
Table 3.
Functional expression of the NOP receptor in rodent nociceptive system and effects of N/OFQ and non-peptide NOP receptor agonists under conditions of neuropathic pain
| NOP receptor expression | Function of N/OFQ and NOP receptor agonists | |||||
|---|---|---|---|---|---|---|
| Region | mRNA | Protein | In vitro | In vivo | ||
| Supraspinal | Thalamus | |||||
| Amygdala | ||||||
| PAG | Up-regulated 7–14 days after CCI [ISH] (Ma et al., 2005) | Endogenous N/OFQ tone maintained, since m.i. UFP-101, inhibited mechanical allodynia [CCI rat] (Scoto et al., 2009) | i.c.v. GRT-TA2210, Ro65-6570: ↓ cold allodynia [CCI mouse] (Linz et al., 2013) | |||
| RVM | Up-regulated 7–14 days after CCI [ISH] (Ma et al., 2005) | |||||
| Spinal | Intrinsic neurons | Up-regulated in lumbar enlargement 7 days after CCI [RT-PCR] (Briscini et al., 2002) | i.t.: ↓ thermal hyperalgesia [partial sciatic nerve injury rat, CCI rat] (Yamamoto and Nozaki-Taguchi, 1997; Yamamoto et al., 1997c) | |||
| i.t.: ↓ mechanical and cold allodynia [sciatic nerve injury rat] (Hao et al., 1998) | ||||||
| i.t.: ↓ flexor reflex [sciatic nerve transection rat] (Xu et al., 1999) | ||||||
| i.t.: antinociceptive in diabetic poly-neuropathy [tail flick mouse] (Kamei et al., 1999) | ||||||
| i.t.: ↓ mechanical allodynia and hyperalgesia [CCI rat] (Corradini et al., 2001; Briscini et al., 2002; Courteix et al., 2004) | ||||||
| i.t.: synergistic interaction with i.t. morphine to suppress mechanical hyperalgesia [CCI rat] (Courteix et al., 2004) | ||||||
| Proximal PAF (presynaptic) | i.t.: ↓ spontaneous and mechanically evoked activity of lamina V WDR neurons [CCI rat] (Sotgiu et al., 2004) | |||||
| i.t. Ro64-6198, N/OFQ: ↓ mechanical and cold allodynia [CCI rat] (Obara et al., 2005) | ||||||
| i.t.: ↓ mechanical allodynia [SNL rat] (Ju et al., 2013) i.t. GRT-TA2210, Ro65-6570: ↓ cold allodynia [CCI mouse] (Linz et al., 2013) | ||||||
| Peripheral | DRG neuron (cell body) | Up-regulated in L5-L6 7 days after CCI [RT-PCR] (Briscini et al., 2002) | Up-regulated 7 days after sciatic nerve injury [IHC] (Chen and Sommer, 2006) | ↑ inhibition of N-type Ca2+ current after sciatic nerve section [patch clamp] (Abdulla and Smith, 1998) | ||
| Distal PAF (dendritic) | i.pl. Ro64-6198, N/OFQ: ↓ mechanical and cold allodynia [CCI rat] (Obara et al., 2005) | |||||
No data are published for fields that are left blank. ↑, activation/increase; ↓, inhibition/decrease; GRT-TA2210, selective NOP receptor agonist; IHC, immunohistochemistry; ISH, in situ hybridization; m.i., microinjected; PAF, primary afferent fibre; Ro64-6198, Ro65-6570, selective NOP receptor agonists; UFP-101, selective NOP receptor antagonist.
The moderately selective non-peptide NOP receptor agonists SR14150 and SR16835 displayed NOP, but not MOP receptor-dependent antiallodynic efficacy in SNL mice at rather high systemic doses (Khroyan et al., 2011). In addition, the selective non-peptide NOP receptor agonists GRT-TA2210 and Ro65-6570 also exerted potent antiallodynic effects in the mouse CCI model after spinal, supraspinal and systemic administration (Linz et al., 2013). It is especially noteworthy that, after supraspinal administration, NOP receptor agonists showed antiallodynic efficacy in a model of neuropathic pain. This is in sharp contrast to their lacking or even pronociceptive efficacy under conditions of acute pain (see earlier) and inflammatory pain (see later). It might be hypothesized that this qualitative change in supraspinal NOP receptor functionality in rodent models of neuropathic pain translates into antihypersensitive efficacy after systemic administration as activation of both spinal and supraspinal NOP receptors sustain efficacy (Figure 1B). In line with this notion, systemic administration of Ro65-6570 exerted potent and efficacious antihyperalgesic and antiallodynic effects in mouse and rat models of mono- and poly-neuropathic pain without confounding locomotor side effects (Schiene et al., 2013). Exploring the influence of chronic neuropathic pain conditions on the expression and functional interaction of NOP receptors with the ON–OFF cell circuitry in the rodent RVM is needed to further substantiate this concept.
For ethical reasons, models of neuropathic pain are only sparsely available in NHPs. While analgesics like pregabalin (α2δ Ca2+ channel subunit modulator) and duloxetine (serotonin-noradrenaline re-uptake inhibitor) have been shown to exert antiallodynic efficacy in a cynomolgus monkey L7 SNL model (Hygate et al., 2012a,b), no published data are available on effects of NOP receptor agonists in NHP models of neuropathic pain or on the regulation of N/OFQ or NOP receptor expression in NHPs under neuropathic conditions.
Functional expression and regulation of the N/OFQ-NOP receptor system in inflammatory pain
N/OFQ also elicited potent and efficacious antihypersensitive effects in rodent models of inflammatory pain (Table 4). N/OFQ exerted antihyperalgesic effects in a rat model of trinitrobenzene sulfonic acid (TNBS)-induced colonic hyperalgesia after peripheral administration. Interestingly, peripheral injection of the NOP receptor-selective peptide antagonist UFP-101 not only inhibited the effect of N/OFQ, but exacerbated visceral hyperalgesia when administered alone (Agostini et al., 2009).
Table 4.
Functional expression of the NOP receptor in rodent nociceptive system and effects of N/OFQ under conditions of inflammatory pain
| NOP receptor expression | N/OFQ function | ||||
|---|---|---|---|---|---|
| Region | mRNA | Protein | In vitro | In vivo | |
| Supraspinal | Thalamus | i.c.v.: ↑ pain response, antagonized opioid analgesia [formalin test rat] (Zhu et al., 1997; JL Wang et al., 1999a) | |||
| Amygdala | |||||
| PAG | i.c.v.: antagonized morphine analgesia [CFA inflammation rat] (Bertorelli et al., 1999) | ||||
| RVM | i.c.v.: no inhibition of colonic hyperalgesia [colorectal distension in TNBS-treated rats] (Agostini et al., 2009) | ||||
| Spinal | Intrinsic neurons | Up-regulation in L4 dorsal horn (laminae I, II) 4 days after CFA [RLB] (Jia et al., 1998) | i.t.: analgesic [formalin test rat] (Erb et al., 1997; Yamamoto et al., 1997a; Hao and Ogawa, 1998; YQ Wang et al., 1999a) | ||
| i.t.: ↓ thermal hyperalgesia, ↓ flexor reflex [carrageenan inflammation rat] (Yamamoto et al., 1997b; Hao et al., 1998; Xu et al., 1999) | |||||
| Proximal PAF (presynaptic) | ↑ inhibition of C-fibre evoked responses of spinal neurons 4h after carrageenan [in vivo electrophysiology rat] (Carpenter et al., 2000) | ||||
| Peripheral | DRG neuron (cell body) | Up-regulation at day 1 and 7 after CFA [IHC] (Chen and Sommer, 2006; 2007) | |||
| Distal PAF (dendritic) | i.v.: inhibition of articular mechanosensitivity [carrageenan-induced knee joint inflammation, rat] (McDougall et al., 2000) | ||||
| i.p.: inhibition of colonic hyperalgesia [colorectal distension in TNBS-treated rats] (Agostini et al., 2009) | |||||
No data are published for fields that are left blank. ↑, activation/increase; ↓, inhibition/decrease; IHC, immunohistochemistry; PAF, primary afferent fibre; RLB, radioligand binding.
In the rat formalin test, N/OFQ was antinociceptive after i.t. administration, whereas it exerted pronociceptive effects and antagonized opioid analgesia when administered i.c.v. (Erb et al., 1997; Yamamoto et al., 1997a; Zhu et al., 1997; Hao and Ogawa, 1998; JL Wang et al., 1999a). The ability of NOP receptors to bidirectionally modulate nociception in a site-specific manner was also corroborated in the mouse formalin test where UFP-101 exerted antinociceptive and pronociceptive effects after i.c.v. and i.t. administration respectively (Rizzi et al., 2006). This supports the notion of endogenous N/OFQ tone mediating spinal antinociception and supraspinal pronociception. Likewise, in the rat complete Freund's adjuvant (CFA)-induced arthritis model, [Phe1psi(CH2-NH)Gly2]N/OFQ(1-13)-NH2 induced hyperalgesia, and similar to N/OFQ, inhibited systemic morphine antinociception after i.c.v. administration (Bertorelli et al., 1999). In the rat model of carrageenan-induced inflammation, i.t. N/OFQ inhibited thermal hyperalgesia (Yamamoto et al., 1997b; Hao et al., 1998) and the nociceptive flexor reflex (Xu et al., 1999). Hence, in rodent models of inflammatory pain, NOP receptor agonists also elicited antinociceptive or pronociceptive effects depending on spinal or supraspinal sites of action, respectively (Figure 1C).
N/OFQ-mediated inhibition of C-fibre evoked responses of rat spinal dorsal horn neurons was increased 4 h after carrageenan treatment, which is indicative of a functional sensitization of spinal NOP receptors under inflammatory conditions (Carpenter et al., 2000). Indeed, several studies reported increased expression of N/OFQ and NOP receptors after inflammatory challenges in rodents. As rapidly as 0.5–3 h after carrageenan-induced inflammation, a transient increase in ppN/OFQ mRNA expression was observed in transient receptor potential cation channel, subfamily V 1-positive rat DRG neurons (Andoh et al., 1997; Itoh et al., 2001). N/OFQ immunoreactivity was increased in rat dorsal spinal cord, cingulate cortex and hypothalamus 14 days after carrageenan inflammation (Rosen et al., 2000). NOP receptor protein was found to be up-regulated in rat superficial dorsal spinal cord 4 days after CFA-induced inflammation using radioligand binding (Jia et al., 1998). Both NOP receptor protein and N/OFQ immunoreactivity seemed to be up-regulated in rat DRG neurons 7 days after CFA inflammation (Chen and Sommer, 2006).
Systemic administration of GRT-TA2210 displayed full antihyperalgesic efficacy in the mouse formalin test (Linz et al., 2013), whereas Ro65-6570 exerted moderate antihyperalgesic efficacy in the rat models of formalin- and CFA-induced inflammatory pain without confounding locomotor side effects (Schiene et al., 2013). The selective non-peptide NOP receptor agonist SCH-221510 showed anti-inflammatory and analgesic activity in a mouse model of TNBS-induced inflammatory bowel disease after systemic administration (Sobczak et al., 2013; 2014). Systemically administered selective NOP receptor agonists displayed antihyperalgesic efficacy in inflammatory pain, but lacked antinociceptive efficacy in acute pain in rodents. This pain state-dependent functionality of the N/OFQ-NOP receptor system is mirrored in mice carrying a global knockout of either ppN/OFQ or the NOP receptor, or both. These mice displayed normal sensitivity to acute pain, but showed increased inflammatory hyperalgesia compared with their wild-type littermates (Depner et al., 2003).
In rhesus monkeys, systemic Ro64-6198 attenuated carrageenan-induced thermal hyperalgesia an order of magnitude more potently than it blocked acute thermal nociception (Sukhtankar et al., 2014) reminiscent of the increased potency of spinal N/OFQ in carrageenan-treated rats (Carpenter et al., 2000). It would be interesting to investigate whether functional sensitization of the N/OFQ-NOP receptor system observed in NHPs under inflammatory conditions is accompanied by altered expression as described for rodents.
Interaction of NOP and MOP receptors in relation to analgesia
Early immunohistochemical studies in rodents demonstrated an overlapping distribution, but no co-localization of N/OFQ and the NOP receptor with opioid peptides and the MOP receptor, respectively, in areas involved in nociceptive processing (Schulz et al., 1996; Monteillet-Agius et al., 1998). However, doubts were raised with respect to the specificity of the NOP receptor antibody used (Neal et al., 1999a). Indeed, a patch clamp study analysing N/OFQ- and morphine-induced suppression of N-type Ca2+ channels in rat DRG neurons demonstrated that NOP receptors may be functionally co-expressed with MOP receptors on the very same neuron (Abdulla and Smith, 1998). Cellular co-expression and heterodimerization of NOP and MOP receptors was also found in human neuroblastoma cells (Mandyam et al., 2003). In the rat RVM, NOP and MOP receptors are functionally co-expressed in ON cells, whereas only NOP, but no MOP receptors are expressed in OFF cells (Pan et al., 2000; Vaughan et al., 2001). The functional consequences of this site-specific pattern of NOP and MOP receptors being either co-expressed or showing segregated cellular expression are believed to underlie spinal antinociceptive and supraspinally mediated pronociceptive actions of N/OFQ. Hence, NOP and MOP receptors interact directly at the level of individual cells as well as indirectly at the neuronal circuitry level. Effects of N/OFQ were not inhibited by the opioid receptor antagonist naloxone in in vitro (Faber et al., 1996; Abdulla and Smith, 1997; 1998; Lai et al., 1997; Liebel et al., 1997; Shu et al., 1998) and in vivo (Xu et al., 1996; Sotgiu et al., 2004) electrophysiological studies. However, behavioural studies reported discrepant results. Although naltrexone, a MOP receptor antagonist, inhibited spinal N/OFQ analgesia in the tail flick and formalin test (King et al., 1997; Hao and Ogawa, 1998), the MOP receptor antagonists naloxone (Erb et al., 1997; Yamamoto et al., 1997a) and β-funaltrexamine (Kamei et al., 1999) did not. I.t. N/OFQ increased withdrawal latencies to thermal and mechanical stimuli in naïve rats, antinociceptive effects that were attenuated by i.t. NOP, MOP and DOP, but not KOP receptor antagonists (Jhamandas et al., 1998; Yu et al., 2002). In the rat SNL model, spinal MOP as well as DOP and KOP receptors contributed to the antiallodynic effects of i.t. N/OFQ (Ju et al., 2013). Another line of evidence elucidating complex interactions between NOP and opioid receptors comes from studies with knockout mice in acute heat nociception and diabetic heat hyperalgesia (Christoph et al., 2013). From the above, it seems clear that MOP as well as other opioid receptors interact with NOP receptors in a complex manner and may contribute to NOP receptor-mediated analgesia depending on experimental conditions.
Indeed, initial studies demonstrated that spinal N/OFQ increased systemic and spinal morphine analgesia in rodent models of acute and neuropathic pain (Tian et al., 1997a; Courteix et al., 2004). Isobolographic analysis indicated that spinal NOP and MOP receptors interact synergistically to inhibit mechanical hyperalgesia in the rat CCI model (Courteix et al., 2004), whereas combining systemic subthreshold doses of Ro64-6198 and morphine reduced pain sensitivity in an additive manner in the mouse hot plate test (Reiss et al., 2008). Subsequent studies in NHPs reported that spinal N/OFQ potentiated spinal morphine-induced antinociception (Ko and Naughton, 2009), and that a combination of inactive spinal doses of the selective peptide NOP receptor agonist University of Ferrara Peptides [(pF)Phe4Aib7Arg14Lys15]N/OFQ-NH2 (UFP)-112 and morphine produced antihyperalgesia (Hu et al., 2010). Interestingly, this antihyperalgesic effect was completely antagonized only by a combination of the NOP receptor antagonist J-113397 with the MOP receptor antagonist naltrexone whereas either antagonist alone tended to partially inhibit antihyperalgesic efficacy without reaching statistical significance (Hu et al., 2010). Most importantly, co-activation of NOP and MOP receptors after systemic administration of respective agonists synergistically produced antinociception in NHPs as revealed by isobolographic analysis (Cremeans et al., 2012). These findings suggest that NOP/MOP receptor agonists may hold potential for clinical use as analgesics with efficacy in acute and chronic pain. Furthermore, as both modes of action contribute to analgesia, the relative dose of each component may be reduced, thus potentially leading to an improved side effect profile of NOP/MOP receptor agonists over selective MOP receptor agonists (Lin and Ko, 2013; Toll, 2013). Recently, the bifunctional NOP/MOP receptor agonists BU08028 and SR16435 were shown to exert antihyperalgesic and anti-allodynic effects after spinal administration in mouse models of inflammatory and neuropathic pain with potencies higher than those of selective NOP and MOP receptor agonists. Anti-allodynic efficacy of both BU08028 and SR16435 was partially inhibited by either spinal J-113397 or naltrexone, but only the combined administration of both antagonists completely inhibited this response (Sukhtankar et al., 2013). The increased potencies of bifunctional NOP/MOP receptor agonists as compared with selective NOP and MOP receptor agonists in inflammatory and neuropathic pain is hypothesized to result from a (supra)additive interaction between both mechanisms of action at the spinal level. Under inflammatory conditions both spinal NOP (see earlier) as well as MOP receptors (Maekawa et al., 1996) are up-regulated, whereas under conditions of neuropathic pain, functional sensitization of the NOP component (see earlier) might compensate for reduced contribution of the MOP component to spinal analgesia (Ossipov et al., 1995; Porreca et al., 1998; Rashid et al., 2004; Kohno et al., 2005), thus retaining good efficacy and potency for bifunctional NOP/MOP receptor agonists also in neuropathic pain. In addition, spinal SR16435 showed delayed development of analgesic tolerance to anti-allodynic efficacy as compared with a MOP receptor agonist, an advantageous effect also attributed to co-activation of NOP and MOP receptors (Sukhtankar et al., 2013). Whereas SR16435 was equally rewarding as morphine (Khroyan et al., 2007), another bifunctional NOP/MOP receptor agonist, SR14150, did not display rewarding properties in the rat conditioned place preference (CPP) paradigm (Toll et al., 2009). This latter observation is in line with previous reports demonstrating that i.c.v. N/OFQ (Ciccocioppo et al., 2000) and systemic Ro65-6570 (Rutten et al., 2010) reduced, whereas pharmacological blockade or genetic ablation of the NOP receptor (Rutten et al., 2011) increased the rewarding properties of systemic morphine in the rat CPP test. Clearly, the rewarding properties of bifunctional NOP/MOP agonists are determined by the relative affinities and activities at NOP and MOP receptors (Toll, 2013). In summary, these findings indicate that combined NOP/MOP receptor agonists may prove to be effective analgesics in acute and chronic pain with reduced tolerance and abuse liability as compared with selective MOP receptor agonists.
Translational approaches to the N/OFQ-NOP receptor system
In addition to a demonstration of sufficient compound exposure to the site of action allowing target engagement, another important aspect of translational research is the demonstration of functional target modulation across species (Morgan et al., 2012).
Functional NOP receptor modulation might be monitored by suitable blood-borne biomarkers; for example, i.v. administration of N/OFQ to naïve rats increased the expression of CD11b on circulating neutrophils (Brookes et al., 2007). Moreover, i.c.v. N/OFQ has been reported to transiently increase plasma prolactin levels in rats (Bryant et al., 1998), an effect related to the NOP receptor-mediated inhibition of tuberoinfundibular dopaminergic neurons of the hypothalamic–pituitary circuitry (Chesterfield et al., 2006). Interestingly, systemic administration of the KOP receptor agonist spiradoline has been shown to elevate plasma prolactin levels both in rats and humans (Chang et al., 2011). Whether this also holds true after systemic administration of NOP receptor agonists should be addressed in future research.
Central NOP receptor modulation has also been reported as an inhibition of spontaneous bursting in the rat electroencephalogram (EEG) after i.v. administration of the selective NOP receptor agonist Ro65-6570 (Byford et al., 2007). Assessment of EEG activity changes induced by dosing of investigational compounds (pharmaco-EEG) is an established assessment in a number of species, including humans. A prerequisite for recording pharmaco-EEG in NHPs and humans is that the target needs to be expressed cortically to allow signal detection by external electrodes. Cortical expression of NOP receptors in NHPs and humans has recently been demonstrated using a PET approach (Kimura et al., 2011; Lohith et al., 2012; Hostetler et al., 2013). In a double-blind, placebo-controlled study in visceral pain patients suffering from chronic pancreatitis, the analgesic pregabalin induced EEG changes, with individual changes correlating with brief pain inventory composite scores. In contrast, placebo treatment was devoid of EEG effects (Graversen et al., 2012). Thus, for pregabalin, pharmaco-EEG has been successfully used as a biomarker for functional target modulation that was also related to analgesic efficacy. Whether pharmaco-EEG may be suited to detect functional activation of NOP receptors in humans requires detailed investigation.
Finally, identification of biomarkers for efficacy of NOP receptor activation with utility across species and allowing prediction of treatment outcomes in humans is the ultimate aim of translational research in this area. In a recent elegant study, it was demonstrated that responses of spinal cord WDR neurons of naïve rats evoked by suprathreshold thermal stimulation translate to intensities of perceived heat pain in healthy volunteers (Sikandar et al., 2013). Spinal N/OFQ inhibited evoked responses of WDR neurons both in naïve rats (Stanfa et al., 1996) and in rats with carrageenan-induced inflammation and CCI-induced mononeuropathic pain (Carpenter et al., 2000; Sotgiu et al., 2004). Interestingly, these studies showed that the N/OFQ-mediated inhibition of spinal nociceptive processing was enhanced after induction of inflammatory and neuropathic pain states. Therefore, it is tempting to speculate that NOP receptor agonists might be effective analgesics also in human conditions of acute and chronic pain. Indeed, several analgesics like morphine and fentanyl (MOP receptor agonists), and pregabalin and tapentadol (MOP receptor agonist/noradrenaline re-uptake inhibitor) attenuated evoked WDR neuron responses in naïve animals as well as in rat models of chronic pain after spinal, and more importantly, from a clinical perspective, also after systemic administration (Homma et al., 1983; Suzukawa et al., 1983; Urch et al., 2005; Bee and Dickenson, 2008; Bee et al., 2011). As described earlier, systemically administered NOP receptor agonists exert analgesic effects in models of acute and inflammatory pain in NHPs, whereas in rodents, they are largely ineffective in models of acute pain, but effective in inflammatory and neuropathic pain. Therefore, elucidating the ability of systemically administered NOP receptor agonists to inhibit evoked activity of rat WDR neurons, depending on pain condition and stimulus modality, will be a next step to define whether this rodent approach holds translational predictability for the analgesic activity of NOP receptor agonists in NHPs and, eventually, in humans.
The property of NOP receptor agonists to modulate sensory processing of nociceptive input at various neuraxial sites in rodent and NHP models of evoked pain responses is well documented. Demonstrating the ability of an analgesic to affect spontaneous pain as well as the affective component of pain perception has been suggested to be more predictive for analgesic efficacy and improvement of quality of life in pain patients (Rice et al., 2008; Mao, 2012). Indeed, spinal N/OFQ was reported to inhibit spontaneous activity of WDR neurons (Sotgiu et al., 2004) as well as spontaneous pain behaviour in rats (Sun et al., 2004). With respect to the affective dimension of pain, the central nucleus of the amygdala is believed to represent one neuroanatomical substrate where an emotional connotation is added to the pain experience (Neugebauer et al., 2004). Effective analgesic treatments that also reduce comorbidities of chronic pain like anxiety are urgently needed. Most interestingly, negative emotion may even lead to or exacerbate pain, a relationship, which is also encoded in the amygdala (Wiech and Tracey, 2009). N/OFQ and its receptor were detected in the amygdala of rodents (Neal et al., 1999a,b), NHPs (Bridge et al., 2003) and humans (Peluso et al., 1998; Witta et al., 2004). In rats, N/OFQ has been reported to increase inwardly rectifying K+ currents in central amygdala neurons (Meis and Pape, 1998), and to exert anxiolytic-like effects when administered i.c.v. or microinjected in the central amygdala (Jenck et al., 1997; Vitale et al., 2006; Uchiyama et al., 2008). Furthermore, selective non-peptide NOP receptor agonists were also reported to induce anxiolytic-like effects in rodents after systemic administration (Jenck et al., 2000; Varty et al., 2005; 2008; Hayashi et al., 2009; Lu et al., 2011; Goeldner et al., 2012). Likewise, an anxiogenic-like phenotype was demonstrated in NOP receptor-knockout mice (Gavioli et al., 2007) and rats (Rizzi et al., 2011), further supporting a role for the N/OFQ-NOP receptor system in modulating anxiety-related behaviour. Hence, it is possible that NOP receptor agonists may also exert anxiolytic effects in chronic pain patients.
Conclusions
Although the N/OFQ-NOP receptor system shares similarities with opioid receptor systems, pronounced differences exist at the molecular, cellular and behavioural level. The N/OFQ-NOP receptor system modulates nociceptive processing in a site-dependent manner with antinociceptive effects dominating at peripheral and spinal sites and pronociceptive effects at supraspinal sites in rodents. In addition, the system is subject to functional regulation under conditions of chronic pain and interacts with opioid receptor systems to produce powerful analgesia. Whereas systemic administration of NOP receptor agonists exerts potent and efficacious antinociception in NHPs, they largely lack efficacy in rodent models of acute pain. The intriguing plasticity of the N/OFQ-NOP receptor system in pain states and the interaction with MOP receptors offers new avenues of investigation in the field of opioid research. Although there are still many specific gaps in our understanding of rodent N/OFQ-NOP receptor pain pharmacology, focusing future research on investigations of antinociceptive efficacy and tolerability in NHPs is probably more compelling. Specifically, elucidating effects of supraspinally administered NOP receptor agonists on NHP pain processing and exploring mechanisms of functional NOP receptor sensitization in inflammatory pain states clearly deserves further attention. Finally, establishing a robust translational trajectory, especially for measures of target modulation and analgesic efficacy from rodents to NHPs and eventually to humans will be the key to successfully moving NOP receptor agonists into appropriate clinical proof-of-concept trials to investigate their potential as innovative analgesics in diverse pain indications.
Acknowledgments
The authors would like to thank Drs Pradeep Banerjee, Thomas Christoph, Marielle Eerdekens, Julie Frisolone, Stefanie Frosch, Peter Hein and Kris Rutten for comments on the paper and Dr Klaus Schiene for artworks. The author M. C. K. is thankful for all NHP studies supported by the U.S. Department of Defense, PPMRP, Grant No. W81XWH-13-2-0045; and the National Institutes of Health, Grant No. AR059193, DA032568 and DA035359.
Glossary
- CCI
chronic constriction injury
- CFA
complete Freund's adjuvant
- CPP
conditioned place preference
- DOP
δ-opioid peptide
- DRG
dorsal root ganglion
- i.c.v.
intracerebroventricular
- i.pl
intraplantar
- i.t
intrathecal
- KOP
κ-opioid peptide
- MOP
μ-opioid peptide
- N/OFQ
nociceptin/orphaninFQ
- NHP
non-human primate
- NOP
nociceptin/orphaninFQ opioid peptide
- NST
nocistatin
- PAG
periaqueductal grey
- RVM
rostral ventromedial medulla
- SNL
spinal nerve ligation
- WDR
wide dynamic range
Conflict of interest
D. G. L. held consultancies with Grünenthal GmbH. M. C. K. holds consultancies with Grünenthal GmbH. W. S. and T. K. are employees of Grünenthal GmbH.
References
- Abdulla FA, Smith PA. Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J Neurosci. 1997;17:8721–8728. doi: 10.1523/JNEUROSCI.17-22-08721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulla FA, Smith PA. Axotomy reduces the effect of analgesic opioids yet increases the effect of nociceptin on dorsal root ganglion neurons. J Neurosci. 1998;18:9685–9694. doi: 10.1523/JNEUROSCI.18-23-09685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini S, Eutamene H, Broccardo M, Improta G, Petrella C, Theodorou V, et al. Peripheral anti-nociceptive effect of nociceptin/orphanin FQ in inflammation and stress-induced colonic hyperalgesia in rats. Pain. 2009;141:292–299. doi: 10.1016/j.pain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Albrecht D, Bluhdorn R, Siegmund H, Berger H, Calo’ G. Inhibitory action of nociceptin/orphanin FQ on functionally different thalamic neurons in urethane-anaesthetized rats. Br J Pharmacol. 2001;134:333–342. doi: 10.1038/sj.bjp.0704264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- Andoh T, Itoh M, Kuraishi Y. Nociceptin gene expression in rat dorsal root ganglia induced by peripheral inflammation. Neuroreport. 1997;8:2793–2796. doi: 10.1097/00001756-199708180-00028. [DOI] [PubMed] [Google Scholar]
- Andrade A, Denome S, Jiang YQ, Marangoudakis S, Lipscombe D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat Neurosci. 2010;13:1249–1256. doi: 10.1038/nn.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Baiula M, Bedini A, Carbonari G. Molecular mechanisms mediating nociceptin/orphanin FQ receptor signaling, desensitization and internalization. Curr Mol Pharmacol. 2013;5:372–381. [PubMed] [Google Scholar]
- Bee LA, Dickenson AH. Descending facilitation from the brainstem determines behavioural and neuronal hypersensitivity following nerve injury and efficacy of pregabalin. Pain. 2008;140:209–223. doi: 10.1016/j.pain.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Bee LA, Bannister K, Rahman W, Dickenson AH. Mu-opioid and noradrenergic alpha(2)-adrenoceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve-injured rats. Pain. 2011;152:131–139. doi: 10.1016/j.pain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, et al. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci. 2004;7:118–125. doi: 10.1038/nn1180. [DOI] [PubMed] [Google Scholar]
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Buttner A, et al. [3H]-nociceptin ligand-binding and nociceptin opioid receptor mRNA expression in the human brain. Neuroscience. 2003;121:629–640. doi: 10.1016/s0306-4522(03)00484-6. [DOI] [PubMed] [Google Scholar]
- Bertorelli R, Corradini L, Rafiq K, Tupper J, Calo G, Ongini E. Nociceptin and the ORL-1 ligand [Phe1psi(CH2-NH)Gly2]nociceptin(1-13)NH2 exert anti-opioid effects in the Freund's adjuvant-induced arthritic rat model of chronic pain. Br J Pharmacol. 1999;128:1252–1258. doi: 10.1038/sj.bjp.0702884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Caron MG. G protein-coupled receptor kinase/beta-arrestin systems and drugs of abuse: psychostimulant and opiate studies in knockout mice. Neuromolecular Med. 2004;5:41–50. doi: 10.1385/NMM:5:1:041. [DOI] [PubMed] [Google Scholar]
- Bridge KE, Wainwright A, Reilly K, Oliver KR. Autoradiographic localization of (125)i[Tyr(14)] nociceptin/orphanin FQ binding sites in macaque primate CNS. Neuroscience. 2003;118:513–523. doi: 10.1016/s0306-4522(02)00927-2. [DOI] [PubMed] [Google Scholar]
- Briscini L, Corradini L, Ongini E, Bertorelli R. Up-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol. 2002;447:59–65. doi: 10.1016/s0014-2999(02)01833-2. [DOI] [PubMed] [Google Scholar]
- Brookes ZL, Stedman EN, Guerrini R, Lawton BK, Calo G, Lambert DG. Proinflammatory and vasodilator effects of nociceptin/orphanin FQ in the rat mesenteric microcirculation are mediated by histamine. Am J Physiol Heart Circ Physiol. 2007;293:H2977–H2985. doi: 10.1152/ajpheart.00448.2007. [DOI] [PubMed] [Google Scholar]
- Brookes ZL, Stedman EN, Brown NJ, Hebbes CP, Guerrini R, Calo G, et al. The nociceptin/orphanin FQ receptor antagonist UFP-101 reduces microvascular inflammation to lipopolysaccharide in vivo. PLoS ONE. 2013;8:e74943. doi: 10.1371/journal.pone.0074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone release in male and female rats. Brain Res. 1998;807:228–233. doi: 10.1016/s0006-8993(98)00802-6. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, et al. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Byford AJ, Anderson A, Jones PS, Palin R, Houghton AK. The hypnotic, electroencephalographic, and antinociceptive properties of nonpeptide ORL1 receptor agonists after intravenous injection in rodents. Anesth Analg. 2007;104:174–179. doi: 10.1213/01.ane.0000250403.88649.51. [DOI] [PubMed] [Google Scholar]
- Calo G, Guerrini R. Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for the nociceptin/orphanin FQ receptor. In: Ko MC, editor. Research and Development of Opioid-Related Ligands. Washington, DC: American Chemical Society; 2013. pp. 275–325. [Google Scholar]
- Calo G, Rizzi A, Marzola G, Guerrini R, Salvadori S, Beani L, et al. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br J Pharmacol. 1998;125:373–378. doi: 10.1038/sj.bjp.0702087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo’ G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KJ, Vithlani M, Dickenson AH. Unaltered peripheral excitatory actions of nociceptin contrast with enhanced spinal inhibitory effects after carrageenan inflammation: an electrophysiological study in the rat. Pain. 2000;85:433–441. doi: 10.1016/S0304-3959(99)00301-2. [DOI] [PubMed] [Google Scholar]
- Chan AS, Wong YH. Regulation of c-Jun N-terminal kinase by the ORL(1) receptor through multiple G proteins. J Pharmacol Exp Ther. 2000;295:1094–1100. [PubMed] [Google Scholar]
- Chan JS, Yung LY, Lee JW, Wu YL, Pei G, Wong YH. Pertussis toxin-insensitive signaling of the ORL1 receptor: coupling to Gz and G16 proteins. J Neurochem. 1998;71:2203–2210. doi: 10.1046/j.1471-4159.1998.71052203.x. [DOI] [PubMed] [Google Scholar]
- Chang C, Byon W, Lu Y, Jacobsen LK, Badura LL, Sawant-Basak A, et al. Quantitative PK-PD model-based translational pharmacology of a novel kappa opioid receptor antagonist between rats and humans. AAPS J. 2011;13:565–575. doi: 10.1208/s12248-011-9296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Geller EB, Adler MW. Nociceptin/orphanin FQ blocks the antinociception induced by mu, kappa and delta opioid agonists on the cold water tail-flick test. Eur J Pharmacol. 2007;557:32–36. doi: 10.1016/j.ejphar.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sommer C. Nociceptin and its receptor in rat dorsal root ganglion neurons in neuropathic and inflammatory pain models: implications on pain processing. J Peripher Nerv Syst. 2006;11:232–240. doi: 10.1111/j.1529-8027.2006.0093.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. Activation of the nociceptin opioid system in rat sensory neurons produces antinociceptive effects in inflammatory pain: involvement of inflammatory mediators. J Neurosci Res. 2007;85:1478–1488. doi: 10.1002/jnr.21272. [DOI] [PubMed] [Google Scholar]
- Chen YL, Li AH, Yeh TH, Chou AH, Wang HL. Nocistatin and nociceptin exert opposite effects on the excitability of central amygdala nucleus-periaqueductal gray projection neurons. Mol Cell Neurosci. 2009;40:76–88. doi: 10.1016/j.mcn.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Chesterfield M, Janik J, Murphree E, Lynn C, Schmidt E, Callahan P. Orphanin FQ/nociceptin is a physiological regulator of prolactin secretion in female rats. Endocrinology. 2006;147:5087–5093. doi: 10.1210/en.2006-0707. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Somatostatin and nociceptin inhibit neurons in the central nucleus of amygdala that project to the periaqueductal grey. Neuropharmacology. 2010;59:425–430. doi: 10.1016/j.neuropharm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Christoph T, Koch T, Tzschentke TM, Kögel B, Schiene K. 2013. Interactions of opioid and nociceptin/orphaninFQ (NOP) receptors in acute nociception and diabetic heat hyperalgesia as revealed in opioid/NOP receptor knockout mice. In: EFIC – 8th ‘Pain in Europe’ Congress; 9–12 October 2013; Florence, Italy. 2013. p. Abs 600.
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Civelli O. The orphanin FQ/nociceptin (OFQ/N) system. Results Probl Cell Differ. 2008;46:1–25. doi: 10.1007/400_2007_057. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MJ. Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J Physiol. 1998;509:47–58. doi: 10.1111/j.1469-7793.1998.047bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol. 1996a;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Yeo A, Henderson G. The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol. 1996b;118:205–207. doi: 10.1111/j.1476-5381.1996.tb15387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbani M, Gonindard C, Meunier JC. Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology. 2004;145:2876–2885. doi: 10.1210/en.2004-0062. [DOI] [PubMed] [Google Scholar]
- Corboz MR, Rivelli MA, Egan RW, Tulshian D, Matasi J, Fawzi AB, et al. Nociceptin inhibits capsaicin-induced bronchoconstriction in isolated guinea pig lung. Eur J Pharmacol. 2000;402:171–179. doi: 10.1016/s0014-2999(00)00505-7. [DOI] [PubMed] [Google Scholar]
- Corboz MR, Fernandez X, Egan RW, Hey JA. Inhibitory activity of nociceptin/orphanin FQ on capsaicin-induced bronchoconstriction in the guinea-pig. Life Sci. 2001;69:1203–1211. doi: 10.1016/s0024-3205(01)01197-3. [DOI] [PubMed] [Google Scholar]
- Corradini L, Briscini L, Ongini E, Bertorelli R. The putative OP(4) antagonist, [Nphe(1)]nociceptin(1-13)NH(2), prevents the effects of nociceptin in neuropathic rats. Brain Res. 2001;905:127–133. doi: 10.1016/s0006-8993(01)02520-3. [DOI] [PubMed] [Google Scholar]
- Courteix C, Coudore-Civiale MA, Privat AM, Pelissier T, Eschalier A, Fialip J. Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain. 2004;110:236–245. doi: 10.1016/j.pain.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR. Challenges for opioid receptor nomenclature. Br J Pharmacol. 2014 doi: 10.1111/bph.12612. doi: 10.1111/bph.12612; [Epub: 15/2/2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko MC. Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther. 2012;343:72–81. doi: 10.1124/jpet.112.194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curro D, Yoo JH, Anderson M, Song I, Del Valle J, Owyang C. Molecular cloning of the orphanin FQ receptor gene and differential tissue expression of splice variants in rat. Gene. 2001;266:139–145. doi: 10.1016/s0378-1119(00)00553-9. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wichmann J, Higelin J, Py-Lang G, Kratzeisen C, Malherbe P, et al. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther. 2001;298:812–819. [PubMed] [Google Scholar]
- Depner UB, Reinscheid RK, Takeshima H, Brune K, Zeilhofer HU. Normal sensitivity to acute pain, but increased inflammatory hyperalgesia in mice lacking the nociceptin precursor polypeptide or the nociceptin receptor. Eur J Neurosci. 2003;17:2381–2387. doi: 10.1046/j.1460-9568.2003.02676.x. [DOI] [PubMed] [Google Scholar]
- Donica CL, Ramirez VI, Awwad HO, Zaveri NT, Toll L, Standifer KM. Orphanin FQ/nociceptin activates nuclear factor kappa B. J Neuroimmune Pharmacol. 2011;6:617–625. doi: 10.1007/s11481-011-9279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Lemaire S. Characterization of the high affinity [3H]nociceptin binding site in membrane preparations of rat heart: correlations with the non-opioid dynorphin binding site. J Mol Cell Cardiol. 1998;30:2751–2760. doi: 10.1006/jmcc.1998.0838. [DOI] [PubMed] [Google Scholar]
- Erb K, Liebel JT, Tegeder I, Zeilhofer HU, Brune K, Geisslinger G. Spinally delivered nociceptin/orphanin FQ reduces flinching behaviour in the rat formalin test. Neuroreport. 1997;8:1967–1970. doi: 10.1097/00001756-199705260-00034. [DOI] [PubMed] [Google Scholar]
- Evans RM, You H, Hameed S, Altier C, Mezghrani A, Bourinet E, et al. Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem. 2010;285:1032–1040. doi: 10.1074/jbc.M109.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Chambers JP, Evans RH, Henderson G. Depression of glutamatergic transmission by nociceptin in the neonatal rat hemisected spinal cord preparation in vitro. Br J Pharmacol. 1996;119:189–190. doi: 10.1111/j.1476-5381.1996.tb15969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier JC, Costentin J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur J Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier JC, Costentin J. Orphan neuropeptide NocII, a putative pronociceptin maturation product, stimulates locomotion in mice. Neuroreport. 1997;8:705–707. doi: 10.1097/00001756-199702100-00025. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, et al. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Shoda T, Morikawa H, Kato S, Mori K. Activation of mitogen-activated protein kinase by the nociceptin receptor expressed in Chinese hamster ovary cells. FEBS Lett. 1997;412:290–294. doi: 10.1016/s0014-5793(97)00815-6. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Shoda T, Morikawa H, Kato S, Mima H, Mori K. Activation of phospholipase A2 by the nociceptin receptor expressed in Chinese hamster ovary cells. J Neurochem. 1998;71:2186–2192. doi: 10.1046/j.1471-4159.1998.71052186.x. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Rizzi A, Marzola G, Zucchini S, Regoli D, Calo’ G. Altered anxiety-related behavior in nociceptin/orphanin FQ receptor gene knockout mice. Peptides. 2007;28:1229–1239. doi: 10.1016/j.peptides.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Giuliani S, Tramontana M, Lecci A, Maggi CA. Effect of nociceptin on heart rate and blood pressure in anaesthetized rats. Eur J Pharmacol. 1997;333:177–179. doi: 10.1016/s0014-2999(97)01128-x. [DOI] [PubMed] [Google Scholar]
- Giuliani S, Lecci A, Maggi CA. Nociceptin and neurotransmitter release in the periphery. Peptides. 2000;21:977–984. doi: 10.1016/s0196-9781(00)00237-0. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Spooren W, Wichmann J, Prinssen EP. Further characterization of the prototypical nociceptin/orphanin FQ peptide receptor agonist Ro 64-6198 in rodent models of conflict anxiety and despair. Psychopharmacology (Berl) 2012;222:203–214. doi: 10.1007/s00213-012-2636-x. [DOI] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, et al. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graversen C, Olesen SS, Olesen AE, Steimle K, Farina D, Wilder-Smith OH, et al. The analgesic effect of pregabalin in patients with chronic pain is reflected by changes in pharmaco-EEG spectral indices. Br J Clin Pharmacol. 2012;73:363–372. doi: 10.1111/j.1365-2125.2011.04104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Mogil JS. Effects of supraspinal orphanin FQ/nociceptin. Peptides. 2000;21:1037–1045. doi: 10.1016/s0196-9781(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJ. Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol. 1995;59:91–101. doi: 10.1016/0165-5728(95)00030-6. [DOI] [PubMed] [Google Scholar]
- Hao JX, Xu IS, Wiesenfeld-Hallin Z, Xu XJ. Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain. 1998;76:385–393. doi: 10.1016/S0304-3959(98)00071-2. [DOI] [PubMed] [Google Scholar]
- Hao S, Ogawa H. Naltrexone, but not atropine or yohimbine, antagonizes suppression of formalin-induced spinal sensitization by intrathecal nociceptin. Life Sci. 1998;63:PL163–PL173. doi: 10.1016/s0024-3205(98)00359-2. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Fried S, Yao X, Weig B, Graziano MP. Nociceptin (ORL-1) and mu-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem. 1998;71:1024–1033. doi: 10.1046/j.1471-4159.1998.71031024.x. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Hirao A, Nakamura H, Yamamura K, Mizuno K, Yamashita H. Discovery of 1-[1-(1-methylcyclooctyl)-4-piperidinyl]-2-[(3R)-3-piperidinyl]-1H-benzimidazole: integrated drug-design and structure-activity relationships for orally potent, metabolically stable and potential-risk reduced novel non-peptide nociceptin/orphanin FQ receptor agonist as antianxiety drug. Chem Biol Drug Des. 2009;74:369–381. doi: 10.1111/j.1747-0285.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Grandy DK. Circuitry underlying antiopioid actions of orphanin FQ in the rostral ventromedial medulla. J Neurophysiol. 1997;78:3351–3358. doi: 10.1152/jn.1997.78.6.3351. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol. 2001;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Nemeth J, Pinter E, Szolcsanyi J. Inhibition by nociceptin of neurogenic inflammation and the release of SP and CGRP from sensory nerve terminals. Br J Pharmacol. 1997;121:613–615. doi: 10.1038/sj.bjp.0701209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshima H, et al. Influence of the selective ORL1 receptor agonist, Ro64-6198, on rodent neurological function. Neuropharmacology. 2001;41:97–107. doi: 10.1016/s0028-3908(01)00048-x. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Miwa M, Hashimoto K, Kawai S, Nomura N. Nociceptin/orphanin FQ reverses mecamylamine-induced learning and memory impairment as well as decrease in hippocampal acetylcholine release in the rat. Brain Res. 2008;1195:96–103. doi: 10.1016/j.brainres.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Homma E, Collins JG, Kitahata LM, Matsumoto M, Kawahara M. Suppression of noxiously evoked WDR dorsal horn neuronal activity by spinally administered morphine. Anesthesiology. 1983;58:232–236. doi: 10.1097/00000542-198303000-00005. [DOI] [PubMed] [Google Scholar]
- Hostetler ED, Sanabria-Bohorquez S, Eng W, Joshi AD, Patel S, Gibson RE, et al. Evaluation of [(1)(8)F]MK-0911, a positron emission tomography (PET) tracer for opioid receptor-like 1 (ORL1), in rhesus monkey and human. Neuroimage. 2013;68:1–10. doi: 10.1016/j.neuroimage.2012.11.053. [DOI] [PubMed] [Google Scholar]
- Hu E, Calo G, Guerrini R, Ko MC. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148:107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygate DA, Wong WJ, Manguiat R, Collins D, Jani M, Wilson A, et al. 2012a. Evaluation of pregabalin analgesic effects in a non-human primate (NHP) model of neuropathic pain induced by L7 spinal nerve ligation (SNL). In: 14th World Congress on Pain; 27–31 August 2012; Milano, Italy. 2012. p. Abs PF261.
- Hygate DA, Wong WJ, Manguiat R, Collins D, Jani M, Wilson A, et al. 2012b. Validation of a non-human primate (NHP) model of neuropathic pain induced by spinal nerve ligation (SNL). In: 14th World Congress on Pain; 27–31 August 2012; Milano, Italy. 2012. p. Abs PF260.
- Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, et al. Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther. 1999;291:308–313. [PubMed] [Google Scholar]
- Itoh M, Takasaki I, Andoh T, Nojima H, Tominaga M, Kuraishi Y. Induction by carrageenan inflammation of prepronociceptin mRNA in VR1-immunoreactive neurons in rat dorsal root ganglia. Neurosci Res. 2001;40:227–233. doi: 10.1016/s0168-0102(01)00230-9. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, et al. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci U S A. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, et al. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas KH, Sutak M, Henderson G. Antinociceptive and morphine modulatory actions of spinal orphanin FQ. Can J Physiol Pharmacol. 1998;76:314–324. [PubMed] [Google Scholar]
- Jia Y, Linden DR, Serie JR, Seybold VS. Nociceptin/orphanin FQ binding increases in superficial laminae of the rat spinal cord during persistent peripheral inflammation. Neurosci Lett. 1998;250:21–24. doi: 10.1016/s0304-3940(98)00430-3. [DOI] [PubMed] [Google Scholar]
- Johnson EE, Connor M. Towards a receptor for nocistatin? Br J Pharmacol. 2007;152:415–416. doi: 10.1038/sj.bjp.0707384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J, Shin DJ, Na YC, Yoon MH. Role of spinal opioid receptor on the antiallodynic effect of intrathecal nociceptin in neuropathic rat. Neurosci Lett. 2013;542:118–122. doi: 10.1016/j.neulet.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Kamei J, Ohsawa M, Kashiwazaki T, Nagase H. Antinociceptive effects of the ORL1 receptor agonist nociceptin/orphanin FQ in diabetic mice. Eur J Pharmacol. 1999;370:109–116. doi: 10.1016/s0014-2999(99)00112-0. [DOI] [PubMed] [Google Scholar]
- Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ) Life Sci. 1997;60:L15–L21. doi: 10.1016/s0024-3205(96)00593-0. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, et al. SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther. 2007;320:934–943. doi: 10.1124/jpet.106.111997. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Orduna J, Montenegro J, Jiang F, Zaveri NT, et al. Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: studies with bifunctional NOP/mu receptor agonists in the sciatic nerve ligation chronic pain model in mice. J Pharmacol Exp Ther. 2011;339:687–693. doi: 10.1124/jpet.111.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Fujita M, Hong J, Lohith TG, Gladding RL, Zoghbi SS, et al. Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med. 2011;52:1638–1645. doi: 10.2967/jnumed.111.091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Chang A, Pasternak GW. Functional blockade of opioid analgesia by orphanin FQ/nociceptin. Biochem Pharmacol. 1998;55:1537–1540. doi: 10.1016/s0006-2952(98)00023-9. [DOI] [PubMed] [Google Scholar]
- King MA, Rossi GC, Chang AH, Williams L, Pasternak GW. Spinal analgesic activity of orphanin FQ/nociceptin and its fragments. Neurosci Lett. 1997;223:113–116. doi: 10.1016/s0304-3940(97)13414-0. [DOI] [PubMed] [Google Scholar]
- Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg. 2010;110:780–789. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- Klukovits A, Tekes K, Gunduz CO, Benyhe S, Borsodi A, Deak BH, et al. Nociceptin inhibits uterine contractions in term-pregnant rats by signaling through multiple pathways. Biol Reprod. 2010;83:36–41. doi: 10.1095/biolreprod.109.082222. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Reinscheid RK, Civelli O, Kemp JA. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J Neurosci. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC. Antinociceptive effects of ORL1 agonists in monkeys. FASEB J. 2004;18:A961. [Google Scholar]
- Ko MC, Naughton NN. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain. 2009;10:509–516. doi: 10.1016/j.jpain.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN, Traynor JR, Song MS, Woods JH, Rice KC, et al. Orphanin FQ inhibits capsaicin-induced thermal nociception in monkeys by activation of peripheral ORL1 receptors. Br J Pharmacol. 2002;135:943–950. doi: 10.1038/sj.bjp.0704535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006;318:1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, et al. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Kolesnikov YA, Pasternak GW. Peripheral orphanin FQ/nociceptin analgesia in the mouse. Life Sci. 1999;64:2021–2028. doi: 10.1016/s0024-3205(99)00149-6. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Wichmann J, Rafalski P, Talarek S, Dylag T, Silberring J. Non-peptidergic OP4 receptor agonist inhibits morphine antinociception but does not influence morphine dependence. Neuroreport. 2003;14:601–604. doi: 10.1097/00001756-200303240-00015. [DOI] [PubMed] [Google Scholar]
- Kummer W, Fischer A. Nociceptin and its receptor in guinea-pig sympathetic ganglia. Neurosci Lett. 1997;234:35–38. doi: 10.1016/s0304-3940(97)00662-9. [DOI] [PubMed] [Google Scholar]
- Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32(Suppl. 1):53–63. doi: 10.2165/11630080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lai CC, Wu SY, Dun SL, Dun NJ. Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience. 1997;81:887–891. doi: 10.1016/s0306-4522(97)00251-0. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Lazzeri M, Calo G, Spinelli M, Guerrini R, Beneforti P, Sandri S, et al. Urodynamic and clinical evidence of acute inhibitory effects of intravesical nociceptin/orphanin FQ on detrusor overactivity in humans: a pilot study. J Urol. 2001;166:2237–2240. [PubMed] [Google Scholar]
- Lazzeri M, Calo G, Spinelli M, Malaguti S, Guerrini R, Salvadori S, et al. Daily intravesical instillation of 1 mg nociceptin/orphanin FQ for the control of neurogenic detrusor overactivity: a multicenter, placebo controlled, randomized exploratory study. J Urol. 2006;176:2098–2102. doi: 10.1016/j.juro.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Le Cudennec C, Suaudeau C, Costentin J. Evidence for a localization of [(3)H]nociceptin binding sites on medullar primary afferent fibers. J Neurosci Res. 2002;68:496–500. doi: 10.1002/jnr.10218. [DOI] [PubMed] [Google Scholar]
- Lecci A, Giuliani S, Meini S, Maggi CA. Nociceptin and the micturition reflex. Peptides. 2000;21:1007–1021. doi: 10.1016/s0196-9781(00)00241-2. [DOI] [PubMed] [Google Scholar]
- Li L, Dong L, Wang S. Expression of the nociceptin/orphanin FQ receptor in the intestinal mucosa of IBS patients. Exp Ther Med. 2013;6:679–683. doi: 10.3892/etm.2013.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebel JT, Swandulla D, Zeilhofer HU. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br J Pharmacol. 1997;121:425–432. doi: 10.1038/sj.bjp.0701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Ko MC. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci. 2013;4:214–224. doi: 10.1021/cn300124f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz K, Christoph T, Schiene K, Koch T, Englberger W. 2013. GRT-TA2210, a selective NOP receptor agonist, is active in mouse models of inflammatory and neuropathic pain. In: EFIC – 8th ‘Pain in Europe’ Congress; 9–12 October 2013; Florence, Italy. 2013. p. Abs 599.
- Liu EH, Nishiuchi Y, Kimura T, Tachibana S. Supraspinal nocistatin and its amide derivative antagonize the hyperalgesic effects of nociceptin in mice. Neurosci Lett. 2006;397:59–63. doi: 10.1016/j.neulet.2005.11.060. [DOI] [PubMed] [Google Scholar]
- Lohith TG, Zoghbi SS, Morse CL, Araneta MF, Barth VN, Goebl NA, et al. Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J Nucl Med. 2012;53:385–392. doi: 10.2967/jnumed.111.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou LG, Ma L, Pei G. Nociceptin/orphanin FQ activates protein kinase C, and this effect is mediated through phospholipase C/Ca2+ pathway. Biochem Biophys Res Commun. 1997;240:304–308. doi: 10.1006/bbrc.1997.7654. [DOI] [PubMed] [Google Scholar]
- Lu N, Han M, Yang ZL, Wang YQ, Wu GC, Zhang YQ. Nociceptin/orphanin FQ in PAG modulates the release of amino acids, serotonin and norepinephrine in the rostral ventromedial medulla and spinal cord in rats. Pain. 2010;148:414–425. doi: 10.1016/j.pain.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Lu SX, Higgins GA, Hodgson RA, Hyde LA, Del Vecchio RA, Guthrie DH, et al. The anxiolytic-like profile of the nociceptin receptor agonist, endo-8-[bis(2-chlorophenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octane-3-carboxami de (SCH 655842): comparison of efficacy and side effects across rodent species. Eur J Pharmacol. 2011;661:63–71. doi: 10.1016/j.ejphar.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Luo C, Kumamoto E, Furue H, Yoshimura M. Nociceptin-induced outward current in substantia gelatinosa neurones of the adult rat spinal cord. Neuroscience. 2001;108:323–330. doi: 10.1016/s0306-4522(01)00398-0. [DOI] [PubMed] [Google Scholar]
- Luo C, Kumamoto E, Furue H, Chen J, Yoshimura M. Nociceptin inhibits excitatory but not inhibitory transmission to substantia gelatinosa neurones of adult rat spinal cord. Neuroscience. 2002;109:349–358. doi: 10.1016/s0306-4522(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Ma F, Xie H, Dong ZQ, Wang YQ, Wu GC. Expression of ORL1 mRNA in some brain nuclei in neuropathic pain rats. Brain Res. 2005;1043:214–217. doi: 10.1016/j.brainres.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Ma L, Cheng ZJ, Fan GH, Cai YC, Jiang LZ, Pei G. Functional expression, activation and desensitization of opioid receptor-like receptor ORL1 in neuroblastoma x glioma NG108-15 hybrid cells. FEBS Lett. 1997;403:91–94. doi: 10.1016/s0014-5793(97)00031-8. [DOI] [PubMed] [Google Scholar]
- Maekawa K, Minami M, Masuda T, Satoh M. Expression of mu- and kappa-, but not delta-, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic rats. Pain. 1996;64:365–371. doi: 10.1016/0304-3959(95)00132-8. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, et al. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci U S A. 2011;108:19778–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B, Godlewski G, Schlicker E. Function of nociceptin and opioid OP4 receptors in the regulation of the cardiovascular system. J Physiol Pharmacol. 2002;53:301–324. [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Standifer KM. Mu-opioid-induced desensitization of opioid receptor-like 1 and mu-opioid receptors: differential intracellular signaling determines receptor sensitivity. J Pharmacol Exp Ther. 2003;306:965–972. doi: 10.1124/jpet.103.051599. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. Current challenges in translational pain research. Trends Pharmacol Sci. 2012;33:568–573. doi: 10.1016/j.tips.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margas W, Sedeek K, Ruiz-Velasco V. Coupling specificity of NOP opioid receptors to pertussis-toxin-sensitive G alpha proteins in adult rat stellate ganglion neurons using small interference RNA. J Neurophysiol. 2008;100:1420–1432. doi: 10.1152/jn.90405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall JJ, Pawlak M, Hanesch U, Schmidt RF. Peripheral modulation of rat knee joint afferent mechanosensitivity by nociceptin/orphanin FQ. Neurosci Lett. 2000;288:123–126. doi: 10.1016/s0304-3940(00)01211-8. [DOI] [PubMed] [Google Scholar]
- Meis S, Pape HC. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to nociceptin/orphanin FQ. J Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies JR, Glen T, Davies MR, Paterson SJ, Corbett AD. In vitro agonist effects of nociceptin and [Phe(1)psi(CH(2)-NH)Gly(2)]nociceptin(1-13)NH(2) in the mouse and rat colon and the mouse vas deferens. Eur J Pharmacol. 1999;385:217–223. doi: 10.1016/s0014-2999(99)00700-1. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mika J, Li Y, Weihe E, Schafer MK. Relationship of pronociceptin/orphanin FQ and the nociceptin receptor ORL1 with substance P and calcitonin gene-related peptide expression in dorsal root ganglion of the rat. Neurosci Lett. 2003;348:190–194. doi: 10.1016/s0304-3940(03)00786-9. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996a;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett. 1996b;214:131–134. doi: 10.1016/0304-3940(96)12917-7. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mouledous L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides. 2000;21:907–917. doi: 10.1016/s0196-9781(00)00227-8. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, et al. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Simons MJ, Soularue P, Liners F, Vassart G, Meunier JC, et al. Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene. Proc Natl Acad Sci U S A. 1996;93:8666–8670. doi: 10.1073/pnas.93.16.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteillet-Agius G, Fein J, Anton B, Evans CJ. ORL-1 and mu opioid receptor antisera label different fibers in areas involved in pain processing. J Comp Neurol. 1998;399:373–383. doi: 10.1002/(sici)1096-9861(19980928)399:3<373::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Moran TD, Abdulla FA, Smith PA. Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides. 2000;21:969–976. doi: 10.1016/s0196-9781(00)00235-7. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Grisel JE, Robbins CS, Grandy DK. Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport. 1997;8:3431–3434. doi: 10.1097/00001756-199711100-00003. [DOI] [PubMed] [Google Scholar]
- Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today. 2012;17:419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Murali SS, Napier IA, Rycroft BK, Christie MJ. Opioid-related (ORL1) receptors are enriched in a subpopulation of sensory neurons and prolonged activation produces no functional loss of surface N-type calcium channels. J Physiol. 2012;590:1655–1667. doi: 10.1113/jphysiol.2012.228429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Rizzi A, Salvadori S, Guerrini R, Regoli D, Zeilhofer HU, et al. UFP-101 antagonizes the spinal antinociceptive effects of nociceptin/orphanin FQ: behavioral and electrophysiological studies in mice. Peptides. 2007;28:663–669. doi: 10.1016/j.peptides.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999a;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- Neal CR, Jr, Akil H, Watson SJ., Jr Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J Chem Neuroanat. 2001;22:219–249. doi: 10.1016/s0891-0618(01)00135-1. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Owens CE, Taylor LP, Hoversten MT, Akil H, Watson SJ., Jr Binding and GTPgammaS autoradiographic analysis of preproorphanin precursor peptide products at the ORL1 and opioid receptors. J Chem Neuroanat. 2003;25:233–247. doi: 10.1016/s0891-0618(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Neal MJ, Cunningham JR, Paterson SJ, McKnight AT. Inhibition by nociceptin of the light-evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol. 1997;120:1399–1400. doi: 10.1038/sj.bjp.0701135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Nicol B, Lambert DG, Rowbotham DJ, Smart D, McKnight AT. Nociceptin induced inhibition of K+ evoked glutamate release from rat cerebrocortical slices. Br J Pharmacol. 1996;119:1081–1083. doi: 10.1111/j.1476-5381.1996.tb16007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol B, Lambert DG, Rowbotham DJ, Okuda-Ashitaka E, Ito S, Smart D, et al. Nocistatin reverses nociceptin inhibition of glutamate release from rat brain slices. Eur J Pharmacol. 1998;356:R1–R3. doi: 10.1016/s0014-2999(98)00545-7. [DOI] [PubMed] [Google Scholar]
- Nicol B, Rowbotham DJ, Lambert DG. Nociceptin/orphanin FQ inhibits glutamate release from rat cerebellar and brain stem slices. Neurosci Lett. 2002;326:85–88. doi: 10.1016/s0304-3940(02)00317-8. [DOI] [PubMed] [Google Scholar]
- Nishi M, Takeshima H, Mori M, Nakagawara K, Takeuchi T. Structure and chromosomal mapping of genes for the mouse kappa-opioid receptor and an opioid receptor homologue (MOR-C) Biochem Biophys Res Commun. 1994;205:1353–1357. doi: 10.1006/bbrc.1994.2814. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yamamoto T. Spinal opioid receptor like1 receptor agonist, but not N-methyl-D-aspartic acid antagonist, reverses the secondary mechanical allodynia induced by intradermal injection of capsaicin in rats. Anesth Analg. 2005;100:1087–1092. doi: 10.1213/01.ANE.0000147509.01309.EB. [DOI] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain. 2005;116:17–25. doi: 10.1016/j.pain.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Minami T, Tachibana S, Yoshihara Y, Nishiuchi Y, Kimura T, et al. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Minami T, Tsubouchi S, Kiyonari H, Iwamatsu A, Noda T, et al. Identification of NIPSNAP1 as a nocistatin-interacting protein involving pain transmission. J Biol Chem. 2012;287:10403–10413. doi: 10.1074/jbc.M111.271866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. Inhibition by spinal morphine of the tail-flick response is attenuated in rats with nerve ligation injury. Neurosci Lett. 1995;199:83–86. doi: 10.1016/0304-3940(95)12026-z. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Wan BL, Zuckerman A, Pasternak GW. Identification and differential regional expression of KOR-3/ORL-1 gene splice variants in mouse brain. FEBS Lett. 1998;435:65–68. doi: 10.1016/s0014-5793(98)01039-4. [DOI] [PubMed] [Google Scholar]
- Pan YX, Bolan E, Pasternak GW. Dimerization of morphine and orphanin FQ/nociceptin receptors: generation of a novel opioid receptor subtype. Biochem Biophys Res Commun. 2002;297:659–663. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]
- Pan Z, Hirakawa N, Fields HL. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron. 2000;26:515–522. doi: 10.1016/s0896-6273(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gaveriaux-Ruff C. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol. 1998;81:184–192. doi: 10.1016/s0165-5728(97)00178-1. [DOI] [PubMed] [Google Scholar]
- Peluso J, Gaveriaux-Ruff C, Matthes HW, Filliol D, Kieffer BL. Orphanin FQ/nociceptin binds to functionally coupled ORL1 receptors on human immune cell lines and alters peripheral blood mononuclear cell proliferation. Brain Res Bull. 2001;54:655–660. doi: 10.1016/s0361-9230(01)00482-8. [DOI] [PubMed] [Google Scholar]
- Pettersson LM, Sundler F, Danielsen N. Expression of orphanin FQ/nociceptin and its receptor in rat peripheral ganglia and spinal cord. Brain Res. 2002;945:266–275. doi: 10.1016/s0006-8993(02)02817-2. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP, Woods JH. The effects of nociceptin/orphanin FQ receptor agonist Ro 64-6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology (Berl) 2011;213:53–60. doi: 10.1007/s00213-010-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport. 1996;8:369–371. doi: 10.1097/00001756-199612200-00072. [DOI] [PubMed] [Google Scholar]
- Porreca F, Tang QB, Bian D, Riedl M, Elde R, Lai J. Spinal opioid mu receptor expression in lumbar spinal cord of rats following nerve injury. Brain Res. 1998;795:197–203. doi: 10.1016/s0006-8993(98)00292-3. [DOI] [PubMed] [Google Scholar]
- Raingo J, Castiglioni AJ, Lipscombe D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat Neurosci. 2007;10:285–292. doi: 10.1038/nn1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: comparison to morphine. Eur J Pharmacol. 2008;579:141–148. doi: 10.1016/j.ejphar.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, et al. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, et al. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain. 2006;124:100–108. doi: 10.1016/j.pain.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Molinari S, Marti M, Marzola G, Calo’ G. Nociceptin/orphanin FQ receptor knockout rats: in vitro and in vivo studies. Neuropharmacology. 2011;60:572–579. doi: 10.1016/j.neuropharm.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A, Lundeberg T, Bytner B, Nylander I. Central changes in nociceptin dynorphin B and Met-enkephalin-Arg-Phe in different models of nociception. Brain Res. 2000;857:212–218. doi: 10.1016/s0006-8993(99)02432-4. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi GC, Mathis JP, Pasternak GW. Analgesic activity of orphanin FQ2, murine prepro-orphanin FQ141-157 in mice. Neuroreport. 1998;9:1165–1168. [PubMed] [Google Scholar]
- Rossi GC, Pellegrino M, Shane R, Abbadie CA, Dustman J, Jimenez C, et al. Characterization of rat prepro-orphanin FQ/nociceptin( (154-181) ): nociceptive processing in supraspinal sites. J Pharmacol Exp Ther. 2002;300:257–264. doi: 10.1124/jpet.300.1.257. [DOI] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. Effects of the NOP receptor agonist Ro65-6570 on the acquisition of opiate- and psychostimulant-induced conditioned place preference in rats. Eur J Pharmacol. 2010;645:119–126. doi: 10.1016/j.ejphar.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug Alcohol Depend. 2011;114:253–256. doi: 10.1016/j.drugalcdep.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Sakurada T, Katsuyama S, Sakurada S, Inoue M, Tan-No K, Kisara K, et al. Nociceptin-induced scratching, biting and licking in mice: involvement of spinal NK1 receptors. Br J Pharmacol. 1999;127:1712–1718. doi: 10.1038/sj.bjp.0702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada T, Komatsu T, Moriyama T, Sasaki M, Sanai K, Orito T, et al. Effects of intraplantar injections of nociceptin and its N-terminal fragments on nociceptive and desensitized responses induced by capsaicin in mice. Peptides. 2005;26:2505–2512. doi: 10.1016/j.peptides.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Schiene K, Christoph T, Kögel B, Tzschentke TM. 2013. Antinociceptive, antihyperalgesic and antiallodynic activity of the NOP receptor agonist Ro65-6570 in rodent models of pain. In: EFIC – 8th ‘Pain in Europe’ Congress; 9–12 October 2013; Florence, Italy. 2013. p. Abs 606.
- Schlicker E, Morari M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- Schulz S, Schreff M, Nuss D, Gramsch C, Hollt V. Nociceptin/orphanin FQ and opioid peptides show overlapping distribution but not co-localization in pain-modulatory brain regions. Neuroreport. 1996;7:3021–3025. doi: 10.1097/00001756-199611250-00045. [DOI] [PubMed] [Google Scholar]
- Scoto GM, Arico G, Iemolo A, Ronsisvalle S, Parenti C. Involvement of the Nociceptin/Orphanin FQ-NOP receptor system in the ventrolateral periaqueductal gray following mechanical allodynia in chronic pain. Life Sci. 2009;85:206–210. doi: 10.1016/j.lfs.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Fierro IM, Chiang N, Pouliot M. Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epi-lipoxin A4. J Immunol. 2001;166:3650–3654. doi: 10.4049/jimmunol.166.6.3650. [DOI] [PubMed] [Google Scholar]
- Serrano-Gomez A, Thompson JP, Lambert DG. Nociceptin/orphanin FQ in inflammation and sepsis. Br J Anaesth. 2011;106:6–12. doi: 10.1093/bja/aeq337. [DOI] [PubMed] [Google Scholar]
- Shane R, Lazar DA, Rossi GC, Pasternak GW, Bodnar RJ. Analgesia elicited by OFQ/nociceptin and its fragments from the amygdala in rats. Brain Res. 2001;907:109–116. doi: 10.1016/s0006-8993(01)02612-9. [DOI] [PubMed] [Google Scholar]
- Shane R, Acosta J, Rossi GC, Bodnar RJ. Reciprocal interactions between the amygdala and ventrolateral periaqueductal gray in mediating of Q/N(1-17)-induced analgesia in the rat. Brain Res. 2003;980:57–70. doi: 10.1016/s0006-8993(03)02887-7. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu YS, Zhao ZQ, Li MY, Zhou GM. Orphanin FQ/nociceptin modulates glutamate- and kainic acid-induced currents in acutely isolated rat spinal dorsal horn neurons. Neuropeptides. 1998;32:567–571. doi: 10.1016/s0143-4179(98)90087-7. [DOI] [PubMed] [Google Scholar]
- Sikandar S, Ronga I, Iannetti GD, Dickenson AH. Neural coding of nociceptive stimuli-from rat spinal neurones to human perception. Pain. 2013;154:1263–1273. doi: 10.1016/j.pain.2013.03.041. [DOI] [PubMed] [Google Scholar]
- Singh SR, Sullo N, D'Agostino B, Brightling CE, Lambert DG. The effects of nociceptin peptide (N/OFQ)-receptor (NOP) system activation in the airways. Peptides. 2013;39:36–46. doi: 10.1016/j.peptides.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Sobczak M, Salaga M, Storr M, Fichna J. Nociceptin/orphanin FQ (NOP) receptors as novel potential target in the treatment of gastrointestinal diseases. Curr Drug Targets. 2013;14:1203–1209. doi: 10.2174/13894501113149990174. [DOI] [PubMed] [Google Scholar]
- Sobczak M, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Salaga M, Storr M, et al. Anti-inflammatory and antinociceptive action of an orally available nociceptin receptor agonist SCH 221510 in a mouse model of inflammatory bowel diseases. J Pharmacol Exp Ther. 2014;348:401–409. doi: 10.1124/jpet.113.209825. [DOI] [PubMed] [Google Scholar]
- Sotgiu ML, Bellomi P, Biella GE. Efficacy of nociceptin inhibition on WDR neuron activity is enhanced in mononeuropathic rats. Brain Res. 2004;998:251–254. doi: 10.1016/j.brainres.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Spampinato S, Di TR, Qasem AR. Nociceptin-induced internalization of the ORL1 receptor in human neuroblastoma cells. Neuroreport. 2001;12:3159–3163. doi: 10.1097/00001756-200110080-00035. [DOI] [PubMed] [Google Scholar]
- Spampinato S, Di TR, Alessandri M, Murari G. Agonist-induced internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Cell Mol Life Sci. 2002;59:2172–2183. doi: 10.1007/s000180200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Baiula M, Calienni M. Agonist-regulated internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Curr Drug Targets. 2007;8:137–146. doi: 10.2174/138945007779315641. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Chapman V, Kerr N, Dickenson AH. Inhibitory action of nociceptin on spinal dorsal horn neurones of the rat, in vivo. Br J Pharmacol. 1996;118:1875–1877. doi: 10.1111/j.1476-5381.1996.tb15618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC. Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/mu-opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J Pharmacol Exp Ther. 2013;346:11–22. doi: 10.1124/jpet.113.203984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC. Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 2014;231:1377–1387. doi: 10.1007/s00213-013-3341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RQ, Wang Y, Zhao CS, Chang JK, Han JS. Changes in brain content of nociceptin/orphanin FQ and endomorphin 2 in a rat model of neuropathic pain. Neurosci Lett. 2001;311:13–16. doi: 10.1016/s0304-3940(01)02095-x. [DOI] [PubMed] [Google Scholar]
- Sun YY, Luo C, Li Z, Chen J. Differential actions of intrathecal nociceptin on persistent spontaneous nociception, hyperalgesia and inflammation produced by subcutaneous bee venom injection in conscious rats. Sheng Li Xue Bao. 2004;56:321–327. [PubMed] [Google Scholar]
- Suzukawa M, Matsumoto M, Collins JG, Kitahata LM, Yuge O. Dose-response suppression of noxiously evoked activity of WDR neurons by spinally administered fentanyl. Anesthesiology. 1983;58:510–513. doi: 10.1097/00000542-198306000-00005. [DOI] [PubMed] [Google Scholar]
- Takita K, Morimoto Y. Nociceptin/orphanin FQ causes non-quantal slowing of respiratory rhythm in brainstem-spinal cord preparation from newborn rat. Neurosci Lett. 2008;443:129–133. doi: 10.1016/j.neulet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Takita K, Morimoto Y, Kemmotsu O. Roles of nociceptin/orphanin FQ and nociceptin/orphanin FQ peptide receptor in respiratory rhythm generation in the medulla oblongata: an in vitro study. Br J Anaesth. 2003;91:385–389. doi: 10.1093/bja/aeg191. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature. 2012;485:395–399. doi: 10.1038/nature11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Serrano-Gomez A, McDonald J, Ladak N, Bowrey S, Lambert DG. The nociceptin/orphanin FQ system is modulated in patients admitted to ICU with sepsis and after cardiopulmonary bypass. PLoS ONE. 2013;8:e76682. doi: 10.1371/journal.pone.0076682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, et al. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol. 1997a;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JH, Xu W, Zhang W, Fang Y, Grisel JE, Mogil JS, et al. Involvement of endogenous orphanin FQ in electroacupuncture-induced analgesia. Neuroreport. 1997b;8:497–500. doi: 10.1097/00001756-199701200-00024. [DOI] [PubMed] [Google Scholar]
- Toll L. The use of bifunctional NOP/mu and NOP receptor selective compounds for the treatment of pain, drug abuse, and psychiatric disorders. Curr Pharm Des. 2013;19:7451–7460. [PubMed] [Google Scholar]
- Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther. 2009;331:954–964. doi: 10.1124/jpet.109.157446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama H, Toda A, Hiranita T, Watanabe S, Eyanagi R. Role of amygdaloid nuclei in the anxiolytic-like effect of nociceptin/orphanin FQ in rats. Neurosci Lett. 2008;431:66–70. doi: 10.1016/j.neulet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Urch CE, Donovan-Rodriguez T, Gordon-Williams R, Bee LA, Dickenson AH. Efficacy of chronic morphine in a rat model of cancer-induced bone pain: behavior and in dorsal horn pathophysiology. J Pain. 2005;6:837–845. doi: 10.1016/j.jpain.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, et al. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology (Berl) 2005;182:132–143. doi: 10.1007/s00213-005-0041-4. [DOI] [PubMed] [Google Scholar]
- Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, et al. The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510) J Pharmacol Exp Ther. 2008;326:672–682. doi: 10.1124/jpet.108.136937. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Christie MJ. Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J Neurosci. 1997;17:996–1003. doi: 10.1523/JNEUROSCI.17-03-00996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Jennings EA, Marinelli S, Allen RG, Christie MJ. Actions of nociceptin/orphanin FQ and other prepronociceptin products on rat rostral ventromedial medulla neurons in vitro. J Physiol. 2001;534:849–859. doi: 10.1111/j.1469-7793.2001.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Arletti R, Ruggieri V, Cifani C, Massi M. Anxiolytic-like effects of nociceptin/orphanin FQ in the elevated plus maze and in the conditioned defensive burying test in rats. Peptides. 2006;27:2193–2200. doi: 10.1016/j.peptides.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wang HL, Hsu CY, Huang PC, Kuo YL, Li AH, Yeh TH, et al. Heterodimerization of opioid receptor-like 1 and mu-opioid receptors impairs the potency of micro receptor agonist. J Neurochem. 2005;92:1285–1294. doi: 10.1111/j.1471-4159.2004.02921.x. [DOI] [PubMed] [Google Scholar]
- Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, et al. cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett. 1994;348:75–79. doi: 10.1016/0014-5793(94)00557-5. [DOI] [PubMed] [Google Scholar]
- Wang JL, Zhu CB, Cao XD, Wu GC. Distinct effect of intracerebroventricular and intrathecal injections of nociceptin/orphanin FQ in the rat formalin test. Regul Pept. 1999a;79:159–163. doi: 10.1016/s0167-0115(98)00161-x. [DOI] [PubMed] [Google Scholar]
- Wang T, Li SR, Dai X, Peng YL, Chen Q, Wang R. Effects of melatonin on orphanin FQ/nociceptin-induced hyperalgesia in mice. Brain Res. 2006;1085:43–48. doi: 10.1016/j.brainres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Wang XM, Zhang KM, Mokha SS. Nociceptin (orphanin FQ), an endogenous ligand for the QRL1 (opioid-receptor-like1) receptor; modulates responses of trigeminal neurons evoked by excitatory amino acids and somatosensory stimuli. J Neurophysiol. 1996;76:3568–3572. doi: 10.1152/jn.1996.76.5.3568. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Zhu CB, Cao XD, Wu GC. Supraspinal hyperalgesia and spinal analgesia by [Phe1psi(CH2-NH)Gly2]nociceptin-(1-13)-NH2 in rat. Eur J Pharmacol. 1999b;376:R1–R3. doi: 10.1016/s0014-2999(99)00399-4. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH. Expression of alternate forms of brain opioid ‘orphan’ receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Brain Res Mol Brain Res. 1995;32:342–347. doi: 10.1016/0169-328x(95)00096-b. [DOI] [PubMed] [Google Scholar]
- Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- Witta J, Palkovits M, Rosenberger J, Cox BM. Distribution of nociceptin/orphanin FQ in adult human brain. Brain Res. 2004;997:24–29. doi: 10.1016/j.brainres.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Wu EH, Lo RK, Wong YH. Regulation of STAT3 activity by G16-coupled receptors. Biochem Biophys Res Commun. 2003;303:920–925. doi: 10.1016/s0006-291x(03)00451-0. [DOI] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Ito E, Maruyama K, Pietruck C, Sharma M, Yu L, et al. An alternatively spliced transcript of the rat nociceptin receptor ORL1 gene encodes a truncated receptor. Brain Res Mol Brain Res. 2000;77:1–9. doi: 10.1016/s0169-328x(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Xie GX, Meuser T, Pietruck C, Sharma M, Palmer PP. Presence of opioid receptor-like (ORL1) receptor mRNA splice variants in peripheral sensory and sympathetic neuronal ganglia. Life Sci. 1999;64:2029–2037. doi: 10.1016/s0024-3205(99)00150-2. [DOI] [PubMed] [Google Scholar]
- Xu IS, Grass S, Wiesenfeld-Hallin Z, Xu XJ. Effects of intrathecal orphanin FQ on a flexor reflex in the rat after inflammation or peripheral nerve section. Eur J Pharmacol. 1999;370:17–22. doi: 10.1016/s0014-2999(99)00111-9. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Wiesenfeld-Hallin Z. Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport. 1996;7:2092–2094. [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N. Effects of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, and N-methyl-D-aspartate receptor antagonists on the thermal hyperalgesia induced by partial sciatic nerve injury in the rat. Anesthesiology. 1997;87:1145–1152. doi: 10.1097/00000542-199711000-00019. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience. 1997a;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by carageenan injection into the rat paw. Brain Res. 1997b;754:329–332. doi: 10.1016/s0006-8993(97)00186-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by unilateral constriction injury to the sciatic nerve in the rat. Neurosci Lett. 1997c;224:107–110. doi: 10.1016/s0304-3940(97)13475-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ohtori S, Chiba T. Effects of pre-emptively administered nociceptin on the development of thermal hyperalgesia induced by two models of experimental mononeuropathy in the rat. Brain Res. 2000;871:192–200. doi: 10.1016/s0006-8993(00)02480-x. [DOI] [PubMed] [Google Scholar]
- Yu LC, Lu JT, Huang YH, Meuser T, Pietruck C, Gabriel A, et al. Involvement of endogenous opioid systems in nociceptin-induced spinal antinociception in rats. Brain Res. 2002;945:88–96. doi: 10.1016/s0006-8993(02)02743-9. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Calo G. Nociceptin/orphanin FQ and its receptor – potential targets for pain therapy? J Pharmacol Exp Ther. 2003;306:423–429. doi: 10.1124/jpet.102.046979. [DOI] [PubMed] [Google Scholar]
- Zhang J, Salojin KV, Gao JX, Cameron MJ, Bergerot I, Delovitch TL. p38 mitogen-activated protein kinase mediates signal integration of TCR/CD28 costimulation in primary murine T cells. J Immunol. 1999;162:3819–3829. [PubMed] [Google Scholar]
- Zhang NR, Planer W, Siuda ER, Zhao HC, Stickler L, Chang SD, et al. Serine 363 is required for nociceptin/orphanin FQ opioid receptor (NOPR) desensitization, internalization, and arrestin signaling. J Biol Chem. 2012;287:42019–42030. doi: 10.1074/jbc.M112.405696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CS, Li BS, Zhao GY, Liu HX, Luo F, Wang Y, et al. Nocistatin reverses the effect of orphanin FQ/nociceptin in antagonizing morphine analgesia. Neuroreport. 1999;10:297–299. doi: 10.1097/00001756-199902050-00017. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Cao XD, Xu SF, Wu GC. Orphanin FQ potentiates formalin-induced pain behavior and antagonizes morphine analgesia in rats. Neurosci Lett. 1997;235:37–40. doi: 10.1016/s0304-3940(97)00704-0. [DOI] [PubMed] [Google Scholar]
- Zollner C, Stein C. Opioids. Handb Exp Pharmacol. 2007;177:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]


