Abstract
The mammalian target of rapamycin (mTOR) plays an important role in the regulation of protein translation, cell growth and metabolism. The mTOR protein forms two distinct multi-subunit complexes: mTORC1 and mTORC2. The mTORC1 complex is activated by diverse stimuli, such as growth factors, nutrients, energy and stress signals; and essential signalling pathways, such as PI3K and MAPK, in order to control cell growth, proliferation and survival. mTORC1 also activates S6K1 and 4EBP1, which are involved in mRNA translation. The mTORC2 complex is resistant to rapamycin inhibitory activity and is generally insensitive to nutrient- and energy-dependent signals. It activates PKC-α and Akt and regulates the actin cytoskeleton. Deregulation of the mTOR-signalling pathway (PI3K amplification/mutation, PTEN loss of function, Akt overexpression, and S6K1, 4EBP1 and eIF4E overexpression) is common in cancer, and alterations in components of the mTOR pathway have a major role in tumour progression. Therefore, mTOR is an appealing therapeutic target in many tumours. Here we summarize the upstream regulators and downstream effectors of the mTORC1 and mTORC2 pathways, the role of mTOR in cancer, and the potential therapeutic values and issues related to the novel agents targeting the mTOR-signalling pathway.
Introduction
The mammalian target of rapamycin (mTOR) signalling pathway integrates both intracellular and extracellular signals, and operates as a central regulator of cell metabolism, growth, proliferation and survival. In particular, mTOR is a key PK that coordinates and transduces, to the level of mRNA and ribosomes, signals originating from various growth factors and upstream proteins; consequently, mTOR activation has profound regulatory effects on cell proliferation and cell cycle progression (Laplante and Sabatini, 2009). In normal cells, mTOR controls the load of signals from its effectors, maintaining a normal cell function and homeostasis. Conversely, in various diseases, and mainly in cancer, this capacity is lost; in this context, mutations or overactivation of mTOR-upstream pathways lead to a persistent proliferation and tumour growth (Dann et al., 2007; Strimpakos et al., 2009; Willems et al., 2012; Zaytseva et al., 2012; Cheng et al., 2013). mTOR is an attractive target for therapeutic intervention because of its key role in the crosstalk of various signalling pathways, such as PI3K/Akt, TSC, Ras, NF-kB) controlling mRNA, ribosome, protein synthesis and translation of significant molecules, which may induce uncontrolled cell proliferation and growth when deregulated. Therefore, the mTOR-signalling pathway represents a key growth and survival pathway involved in the pathogenesis of several human tumours (Guertin and Sabatini, 2007), and it represents a valuable target for the development of innovative anti-tumour treatments. This has indeed fuelled the development of new molecular-targeted therapies, many of which have progressed to clinical trials with some significant success (Don and Zheng, 2011). Combinational strategies are also under investigation in an effort to overcome resistance to mTOR-targeting drugs and enhance their efficacy (Meric-Bernstam and Gonzalez-Angulo, 2009; Zhang et al., 2011; Zaytseva et al., 2012; De et al., 2013). In fact, although rapamycin analogues (‘rapalogs’) have shown clinical efficacy in a subset of cancer types (i.e. clear cell renal carcinoma and pancreatic neuroendocrine tumours), they do not fully exploit the potential anti-tumour activity of the mTOR-targeting drugs, to some extent because of their pharmacodynamics. Because the mTOR kinase domain is important for rapamycin-sensitive and -insensitive functions, mTOR catalytic inhibitors have been developed as a second generation of anti-mTOR agents, with marked improvement of anti-tumour activity both in vitro and in animal models.
The mTORC1 and mTORC2 pathways
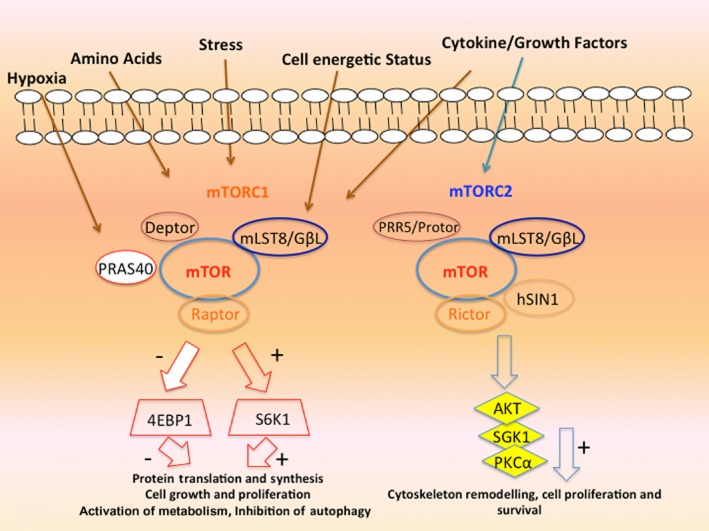
mTOR is a serine/threonine kinase that belongs to the phosphoinositide 3-kinase (PI3K)-related kinase family. The mTOR protein forms at least two distinct multi-protein complexes, mTOR complex 1 (mTORC1) and 2 (mTORC2) (Guertin and Sabatini, 2007; Laplante and Sabatini, 2009). In general, mTORC1 controls cell autonomous growth in response to nutrient availability and growth factors, whereas mTORC2 mediates cell proliferation and survival (Figure 1). mTORC1 is a multi-protein complex containing at least five components: mTOR, which is the catalytic subunit of the complex; regulatory-associated protein of mTOR (Raptor); mammalian lethal with Sec13 protein8 (mLST8, also known as GβL); proline-rich Akt substrate 40 kDa (PRAS40); and DEP-domain-containing mTOR-interacting protein (Deptor) (Loewith et al., 2002). However, the exact components of the mTORC1 complex may differ, depending on cell type and cell localization (Laplante and Sabatini, 2012). Raptor positively regulates mTOR's activity and functions as a scaffold for recruiting mTORC1 substrates (Hara et al., 2002; Kim et al., 2002; Schalm et al., 2003), whereas PRAS40 and Deptor have been characterized as distinct negative regulators of the mTORC1 (Sancak et al., 2007; Peterson et al., 2009). Recent studies suggest that mTORC1 activity can be regulated by the phosphorylation status of Raptor (Gwinn et al., 2008). The mLST8 binds to the kinase domain of mTOR, and regulates positively the mTOR kinase activity; it seems to be able to maintain a rapamycin-sensitive interaction between Raptor and mTOR (Kim et al., 2003). Other studies indicate that mLST8 is also necessary, in the mTORC2 complex, to maintain the Raptor-independent companion of mTOR (Rictor)–mTOR interaction (Guertin et al., 2006a,b), leading to the hypothesis that mLST8 might be important for shuttling mTOR between the two mTOR complexes. Such a function would be compatible with the dynamic equilibrium of these complexes that is known to exist in mammalian cells (Zeng et al., 2007). As already stated above, PRAS40 and Deptor have been characterized as distinct negative regulators of mTORC1 (Sancak et al., 2007; Vander Haar et al., 2007). When the activity of mTORC1 is reduced, PRAS40 and Deptor are recruited to the complex, where they promote the inhibition of mTORC1 (Wang et al., 2007). It has been proposed that PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding (Wang et al., 2007). Upon activation, the mTORC1 directly phosphorylates PRAS40 and Deptor, which reduces their physical interaction with mTORC1 and further activates mTORC1 signalling (Wang et al., 2007). Deptor interacts with both mTORC1 and mTORC2, negatively regulating their activities (Peterson et al., 2009).
Figure 1.
The mTOR pathway is a central regulator of cellular processes. mTORC1 and mTORC2 complexes have different cellular functions but together allow cells to integrate and react to external stimuli such as hypoxia, cellular stress, cytokine and growth factors. mTOR is a central regulator of cell growth and division, integrating cellular cues in response to nutritional and environmental conditions.
The mTORC2 complex encloses mTOR, Rictor, mammalian stress-activated PK-interacting protein 1 (mSIN1), mLST8, the newly identified component protein observed with Rictor-1 (Protor-1), heat shock protein 70-α (Hsp70) and Deptor (Jacinto et al., 2004; Sarbassov et al., 2004; Frias et al., 2006; Pearce et al., 2007). Rictor is a core protein for the mTORC2 catalytic activity, and it recruits substrates to mTORC2 as Raptor does in mTORC1 (Sarbassov et al., 2004; 2005). The mSIN1 is an essential subunit of the mTORC2 complex because it is crucial for the complex integrity and mTOR activity toward Akt Ser473 phosphorylation (Yang et al., 2006). There is evidence that Rictor and mSIN1 stabilize each other, constituting the structural base of mTORC2 complex (Frias et al., 2006; Jacinto et al., 2006). The mLST8 is a stable component of both mTOR complexes (Guertin et al., 2006a,b). Protor-1 interacts with Rictor, although it is not essential for the assembly of other mTORC2 subunits into the complex (Pearce et al., 2007). The Hsp70 is required for the proper formation and kinase activity of mTORC2 under basal conditions and following heat shock (Martin et al., 2008).
Upstream regulators and downstream effectors of the mTORC1 and mTORC2 pathways
The mTOR pathway is regulated by a wide variety of cellular signals, including mitogenic growth factors, hormones such as insulin, nutrients, such as amino acids and glucose, cellular energy levels, and stress conditions such as hypoxia (Figure 1) (Wullschleger et al., 2006). The mTOR pathway responds to growth factors via the PI3K/Akt pathway, which is critically involved in transducing stimuli promoting cell survival and proliferation (Zhang et al., 2007). Signalling through the PI3K/Akt pathway is mainly initiated by mitogenic signals from growth factors, which bind to the cell membrane receptors. These receptors include the insulin-like growth factor receptor (IGFR), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR) and the Her family. Growth factors and hormones, such as insulin, regulate mTORC1 signalling by the activation of class I PI3K and its downstream effector Akt, which reverses the inhibitory effect of TSC1/TSC2 complex and PRAS40 on mTORC1 signalling (Vander Haar et al., 2007). The binding of insulin to its cell surface receptor promotes insulin receptor activation, the recruitment of insulin receptor substrate 1, and the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) through the activation of PI3K (Wullschleger et al., 2006). PIP3 binds to the pleckstrin-homology (PH) domain of target proteins, including Akt and PDK1; the binding of PIP3 to the PH domain of Akt then engages this kinase to the cell membrane, where it is activated by phosphorylation at Thr308 by PDK1 (Stokoe et al., 1997), and at Ser473 by mTORC2 (Sarbassov et al., 2005): both phosphorylations are required for the full activation of Akt kinase activity (Alessi et al., 1997). The phosphatase and tensin homologue (PTEN) is a negative regulator of Akt activation: it converts PIP3 into PIP2, leading to a reduced recruitment of Akt to the cell membrane (Stambolic et al., 1998). Activated Akt has several downstream substrates, including GSK3, FOXO transcription factors and TSC2 (Inoki et al., 2002). TSC2 phosphorylation prevents TSC1/TSC2 complex formation driving the small GTPase Rheb into the GTP-bound active state (Um et al., 2006): this leads to the activation of mTORC1 at Ser2448 (Inoki et al., 2003). The exact mechanism by which Rheb activates mTORC1 is still unknown, but it seems to involve the interaction of GTP-bound Rheb with the amino-terminal lobe of the mTOR kinase domain (Long et al., 2005) together with the farnesylation and subsequent localization of Rheb in the Golgi and endomembranes (Takahashi et al., 2005; Buerger et al., 2006). Akt also phosphorylates and inhibits PRAS40, which negatively regulates mTORC1 antagonizing its activation by Rheb (Kovacina et al., 2003; Sancak et al., 2007).
Activated mTORC1 phosphorylates downstream effectors, including serine/threonine kinase p70S6K1 (S6K1) and 4EBP1, via an interaction between Raptor and a TOR signalling (TOS) motif in S6K and 4EBP1 (Lim et al., 2003; Nojima et al., 2003; Schalm et al., 2003). The TOS motif is a conserved five amino-acid segment found in the N terminus of S6K1 (Phe-Asp-Ile-Asp-Leu) and in the C terminus of 4E-BP1 (Phe-Glu-Met-Asp-Ile), which is necessary for the phosphorylation of these proteins by mTORC1 (Schalm and Blenis, 2002). The S6K1 is one of the best known downstream targets of mTORC1 and this can also be activated by TOR-insensitive signalling pathways, such as PDK1, MAPK and stress-activated PK (Pópulo et al., 2012). Contrarily, the phosphorylation of S6K1 at Thr389 by mTORC1 is required for its activation; the identified three phosphorylation sites of S6K1 can all be blocked by mTOR inhibitors (Dennis et al., 1996). Activated mTORC1 phosphorylates S6K1, which phosphorylates S6 (40S ribosomal protein S6), enhancing the translation of mRNAs with a 5′-terminal oligopolypyrimidine (5′-TOP). Nevertheless, the exact mechanism of control exerted by mTORC1 on TOP mRNA translation is still not well defined (Pópulo et al., 2012; Thoreen et al., 2012). The targets of S6K1 include ribosomal proteins, elongation factors and insulin growth factor 2 (Faivre et al., 2006).
Another well-characterized target of mTORC1 is 4EBP1 which inhibits the initiation of protein translation by binding and inactivating the eukaryotic translation initiation factor 4E (eIF4E) (Sonenberg and Gingras, 1998). The mTORC1 phosphorylates 4EBP1 at multiple sites and this promotes the dissociation of eIF4E from 4EBP1, reducing the inhibitory effect of 4EBP1 on eIF4E-dependent translation initiation (Pause et al., 1994). Free eIF4E can form the multi-subunit eIF4F complex binding to eIF4G (a large scaffolding protein), eIF4A (an ATP-dependent RNA helicase) and eIF4B, enabling cap-dependent protein translation, and inducing increased translation of mRNAs, determining G1-to-S phase transition (Faivre et al., 2006). In quiescent cells, or under low growth factor levels, non-phosphorylated 4EBP1 binds to eIF4E, inhibiting the initiation of protein translation (Sonenberg and Gingras, 1998). The inhibition of mTOR by rapamycin also causes 4EBP1 dephosphorylation, which prevents protein translation (Jastrzebski et al., 2007).
Several studies suggest the existence of a negative feedback loop from the mTOR-S6K1 pathway to the upstream IRS pathway (Harrington et al., 2004; Shah et al., 2004). Activation of mTORC1 and S6K1 regulates IRS-1, both at the transcriptional level and through direct phosphorylation on specific residues, which together prevents its recruitment and binding to receptor tyrosine kinases (RTKs), leading to a negative feedback regulation of both PI3K (Manning and Cantley, 2003) and MAPK signalling pathways (Carracedo et al., 2008). Although the upstream regulators of mTORC2 are less known, there is evidence that a direct association with ribosome is required for mTORC2 activation; this event directly depends on PI3K activity (Zinzalla et al., 2011). This mTORC2 upstream regulatory mechanism suggests a possible link between mTOR complexes, in which mTORC2 promotes mTORC1 activity via the Akt-TSC1/TSC2 pathway and in return, mTORC1 controls mTORC2 activity through the regulation of ribosomal biogenesis (Willems et al., 2012). Moreover, the existence of this link, between PI3K and mTORC2, suggests that mTORC2 is frequently activated in cancer as a result of upstream mutations in the PI3K/Akt pathway (Willems et al., 2012). Thus, the PI3K/Akt and mTOR-signalling pathways are closely interconnected. PI3K/Akt controls mTORC1 activation through Akt-dependent TSC1/TSC2 inhibition, and also activates mTORC2 by promoting its association with ribosomes in normal and tumour cells (Willems et al., 2012). The interaction between mTORC2 and ribosomes correlates with mTORC2 activation in melanoma and colon cancer cells (Zinzalla et al., 2011), while mechanisms by which PI3K promotes this association are still unknown. Although the activation of mTORC1 may be PI3K independent in some tumours, both signalling networks remain subject to complex crosstalk and feedback interactions in normal and tumour cells: the phosphorylated P70S6K protein downstream to mTORC1 exerts a negative feedback on insulin and insulin-like growth factor 1 (IGF1) signalling through proteasomal degradation of IRS-1 and IRS-2, thus leading to PI3K/Akt down-regulation (Um et al., 2004). More recently, GRB10 has been identified as a novel mTORC1 substrate suggesting that the mTORC1 activates GRB10, which is a negative regulator of IGF1 signalling (Hsu et al., 2011; Yu et al., 2011).
Roles of the mTOR signalling pathway in physiology and pathology
The mTOR signalling pathway regulates important activities in eukaryotic cells, such as protein translation, transcription, turnover, cell growth, proliferation, differentiation, survival, metabolism, energy homeostasis, autophagy and response to stress (Watanabe et al., 2011). It represents a central cellular hub where information about the cellular and extracellular status converges, and where molecular responses to cell conditions are finely regulated. Consequently, deregulation of the mTOR pathway is closely associated with several metabolic and degenerative human diseases other than cancer.
The mTOR-signalling pathway has been shown to be implicated in the pathogenesis of diabetes, obesity and cardiovascular diseases, such as cardiomyopathy, both in humans and in preclinical models (Zoncu et al., 2011). This pathway also plays an important role in aging; decreased mTOR activity has been found to extend aging in invertebrates and mice (Kapahi et al., 2004; Wu et al., 2013), and the mTOR inhibitor rapamycin increased lifespan in mice (Harrison et al., 2009). Age-related diseases, such as Alzheimer's disease, are also associated with mTOR pathway deregulation (Li et al., 2005; Zoncu et al., 2011). Although the exact molecular mechanisms by which mTOR deregulation contributes to the development of these conditions are still not well defined, it is possible to envisage mTOR targeting as a future therapeutic option for the treatment of these diseases.
The role of mTOR in tumorigenesis
Tumour growth requires amplification and overactivation of proto-oncogenes and silencing or loss of function of tumour suppressor genes. Proto-oncogenes involved in the mTOR transduction pathway are Ras, PI3K, Akt, Rheb, S6K1, eIF4E and cyclin D1 (Strimpakos et al., 2009). Tumour suppressor genes involved normally in the mTOR pathway include PTEN, tuberous sclerosis 1 and 2 complex (TSC1 and TSC2), LKB1, REDD1, p53 and Beclin1 (Shaw et al., 2004; Levine et al., 2006; Zeng et al., 2006). Other important genes participating in the mTOR pathway are the EGFR, IGFR and IRS. Downstream effectors of EGFR include the Src/STAT pathway, Ras/Raf/MEK/MAPK/ERK pathway, the Ras/PI3K/Akt/mTOR pathway and also the PKC pathway (Strimpakos et al., 2009). In the last few years, significant advances have been made in understanding the role of mTOR in tumour development and progression. Increased mTOR signalling often occurs in tumours as a result of mutations in pathways closely related to mTOR. For instance, up-regulation of the PI3K/Akt pathway through mutations or amplification of the PIK3CA gene can constitutively activate mTOR signalling (Alvarado et al., 2011). Furthermore, loss or inactivation of PTEN, which inhibits activity of PI3K, and mutations of negative regulators of mTOR such as TSC1 and TSC2, p53, and LKB1, can also result in mTOR activation (Wander et al., 2011; Zoncu et al., 2011). Moreover, activating mutations of mTOR itself have been identified through mining of human cancer genome database (Hardt et al., 2011). Through these mechanisms, activation of PI3K/Akt/mTOR pathway has been shown to correlate with tumour progression and reduced survival in patients across a variety of tumour types (Chiang and Abraham, 2007; Gulhati et al., 2009).
The PI3K oncogene plays an important role in the mTOR-signalling pathway. The involvement of this oncogene in the mTOR signalling and in tumour development has been extensively reviewed by Strimpakos et al. (2009). There are three classes of PI3Ks, the most studied being the class IA, which is implicated in cancers and activated by signals via the RTKs and Ras. PI3K class IA is a heterodimer composed of the regulatory subunit p85 and the catalytic subunit p110 (Liu et al., 2009a,b). Mutation and overexpression of PI3K or of one of its components may lead to constitutive activation of the mTOR pathway and potentially to tumorigenesis. Although somatic point mutations of PI3K are often seen in cancers (Seront et al., 2013), the most common cause of its overactivity remains the absence/loss of tumour suppressor gene PTEN (deleted on chromosome 10), which encodes for the phosphatase PTEN (Seront et al., 2013). In the presence of a non-mutated phosphatase PTEN, the PI3K-mediated PIP2 to PIP3 reaction is reversed and this maintains Akt in the inactive form and homeostasis is then achieved.
AKT is a proto-oncogene and encodes for Akt kinase which up-regulates mTOR pathway by phosphorylating TSC2, destabilizing the TSC1/2 complex and leaving Rheb GTPase free to turn on the mTORC1 complex and protein synthesis. However, Akt exerts actually a much broader role in cellular function by controlling cell cycle progression and survival, as well as cell growth and metabolism (Strimpakos et al., 2009). This critical task is achieved by Akt phosphorylation and up- or down-regulation of other key proteins (Shaw and Cantley, 2006). The most important factor regulating Akt activity remains the negative feedback from the tumour suppressor phosphatase PTEN.
The absence or dysfunction of PTEN, secondary to germ line or somatic mutations, allows Akt as well as the mTOR complexes to remain activated, leading to angiogenesis and tumorigenesis (Briest and Grabowski, 2014). Moreover, loss of PTEN results in enhanced Akt activity, which is now independent of the RTK inputs; for example, this can temporarily dissociate the inhibition of the EGFR induced by EGFR inhibitors from that of Akt, representing a mechanism of resistance to these drugs (Seshacharyulu et al., 2012). Indeed, his loss seems to be partially responsible for resistance to gefitinib in prostate cancer cells, glioblastomas and other tumour cells (She et al., 2003; Festuccia et al., 2005). Germ line-inherited mutations of PTEN lead to benign hamartomatous syndromes (Liaw et al., 1997; Murata et al., 1999). Apart from these inherited disorders, random somatic PTEN mutations or loss of heterozygosity have been identified in various solid and haematological tumours (see Strimpakos et al., 2009). Of interest, myeloma cells containing PTEN mutations are more sensitive to the treatment with the mTOR inhibitor CCI-779 (Shi et al., 2002). Similarly, PTEN mutations or loss of function in breast cancer and endometrial hyperplastic cells are associated with sensitization to mTOR inhibitors (DeGraffenried et al., 2004; Milam et al., 2007). Inhibition of mTOR may also be able to improve outcomes in advanced and metastatic disease by enhancing responsiveness to chemotherapeutic agents (Zaytseva et al., 2012), although the molecular mechanisms of this observation are not fully understood. The mTOR involvement in tumour metastasis has been proposed to occur through control of dynamics of the actin cytoskeleton and cell motility via modulation of the expression and activity of small GTPases such as RhoA, Rac1 and Cdc42 (Liu et al., 2010; Gulhati et al., 2011). Furthermore, both mTORC1-mediated S6K1 and 4EBP1 pathways may play a role through phosphorylation of focal adhesion proteins and reorganization of F-actin (Patel et al., 2011). The mTOR may also promote metastasis by altering the tumour microenvironment, given that its inhibition in head and neck cancer is able to prevent tumour cell dissemination to lymph nodes, possibly through inhibition of lymphangiogenesis (Patel et al., 2011).
Activation of mTOR pathway components has been identified in neuroendocrine tumours (Capdevila et al., 2011). In general, these rare neoplasms exhibit limited susceptibility to traditional cytotoxic agents (Li et al., 2011). Neuroendocrine tumours are also unique in their tendency to secrete bioactive products. Relevant to this, mTORC1 is a key regulator in nutrient trafficking and amino acid transport, suggesting its involvement in the transport of vesicles containing peptide hormones (Li et al., 2011). For example, mTORC1 negatively regulates secretion of neurotensin, an intestinal hormone that plays a physiological role in the gastrointestinal tract and is among secreted products of the neuroendocrine tumours (Li et al., 2011). This suggests that the mTOR pathway could have a central role in the pathogenesis of these tumours and, accordingly, recent clinical trials have shown significant therapeutic efficacy of mTOR inhibitors in patients affected by neuroendocrine tumours [Everolimus (RAD001) in Advanced Neuroendocrine Tumours (NETs) – The RADIANT & COOPERATE Programs].
Finally, mTOR activation is able to inhibit autophagy (Meijer and Codogno, 2004; Wendel et al., 2004). Autophagy is a self-degradative process that is important for balancing sources of energy in response to nutrient stress (Glick et al., 2010). In addition to elimination of intracellular aggregates and damaged organelles, autophagy also promotes cellular senescence and cell surface antigen presentation. Moreover, it protects cells against genome instability and prevents necrosis (Glick et al., 2010; Laplante and Sabatini, 2012). At the molecular level, the complex autophagy machinery is orchestrated in several stages; one well-characterized regulatory event is the interaction between the tumour suppressors Beclin-1 and Bcl-2, which disrupts the interaction of Beclin-1 with vesicular protein sorting 34 (Vps34) (Pattingre et al., 2005; Maiuri et al., 2007). Autophagy has been postulated to prevent tumorigenesis by limiting necrosis and inflammation, inducing cell cycle arrest and preventing genome instability (Degenhardt et al., 2006; Karantza-Wadsworth et al., 2007). Consistently, Beclin-1 is deleted in human breast, ovarian and prostate cancers, and while the deletion of Beclin-1 in mice is embryonically lethal (Qu et al., 2007), Beclin-1 heterozygous mice are susceptible to lymphoma, hepatocellular carcinoma and other cancers (Liang et al., 2006). Thus, hyperactivation of the mTOR pathway could lead to the suppression of the cell-autophagy machinery, and this could in turn lead to cancer development. In summary, mTOR is closely related to many of the molecular and biological aspects of cancer and it plays a central role in driving cancer disease development.
Therapeutic targeting: first generation of mTOR inhibitors
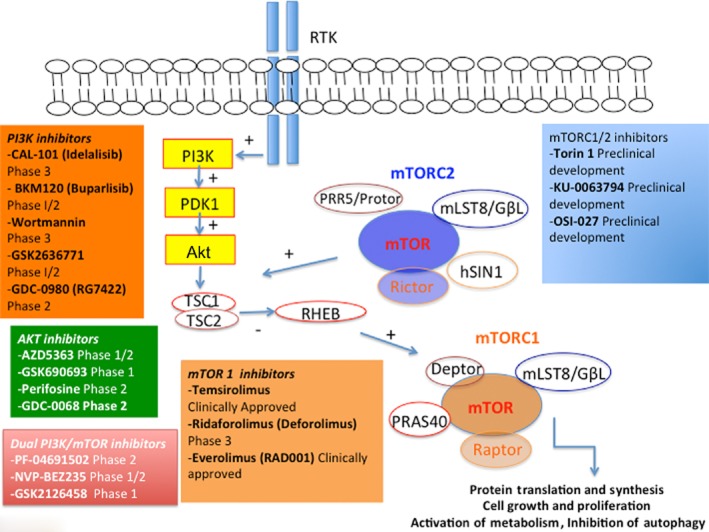
Rapamycin, also named sirolimus, is a natural compound isolated from the soil bacterium Streptomyces hygroscopicus. It was originally used as an anti-fungal and immunosuppressive agent (Sehgal, 2003). Later, the discovery of mTOR as the target of rapamycin, and the demonstration of the inherent antiproliferative properties of this compound, led to the assessment of this compound as a possible anti-tumour agent (Dobbelstein and Moll, 2014). However, limitations in the solubility and pharmacokinetic properties of rapamycin have driven efforts to improve these characteristics, resulting in the first generation of rapamycin analogues (‘rapalogs’), including temsirolimus, everolimus and ridaforolimus (formerly known as deforolimus) (Figure 2).
Figure 2.
The mTOR pathway as a therapeutic target. mTOR represents one of the key growth and survival pathways involved in the pathogenesis of several tumours and thus represents an appealing therapeutic target. Several novel direct or indirect mTOR targeting agents have been developed and assessed in clinical trials.
Rapamycin forms a complex with the small intracellular 12 kDa FK506-binding protein (FKBP12), and irreversibly binds to the FKBP12-rapamycin domain of mTORC1, thus inhibiting its kinase activity (Liu et al., 2009a,b). The chemical modification of the rapalog compounds preserves their interaction with FKBP12 and mTOR and, consequently, rapalog pharmacodynamics remain comparable to that of rapamycin (Liu et al., 2009a,b). Although mTORC2 is less responsive to rapalogs, prolonged exposure to these compounds blocked mTORC2 assembly, with consequent inhibition of Akt signalling (Zeng et al., 2007). The sensitivity of mTORC2 to rapalogs has been demonstrated in patients with acute myeloid leukaemia treated with temsirolimus or everolimus (Zeng et al., 2007). Rapalogs ultimately exert their effects by inducing changes in many downstream signalling pathways involved in cell proliferation and survival, as well as in angiogenesis (Alvarado et al., 2011). One potential mechanism that explains their antiproliferative effects is the prevention of phosphorylation of 4EBP1 and S6K1 by mTORC1, which in turn inhibits the initiation of cap-dependent mRNA translation (Guertin and Sabatini, 2009). The effect of rapalogs on induction of cell apoptosis varies depending on the cell line tested, and appears to correlate with Akt activity (Sabatini, 2006). For instance, in cancer cells in which rapalogs do not inhibit mTORC2, the inhibition of mTORC1 leads to activation of Akt by these drugs; this, in turn, protects the cells from apoptosis (Sabatini, 2006). Because mTOR signalling is closely linked to the PI3K pathway, the level of Akt activation may play a role in determining sensitivity to rapamycin (Gulhati et al., 2009). For instance, cancers dependent on PI3K/Akt signalling are also dependent on downstream activation of mTORC1, thus displaying particular sensitivity to both rapamycin and rapalogs (Guertin et al., 2006a,b). Indeed, rapalogs can block both prostate intraepithelial tumour and lymphoproliferative diseases that are driven by the expression of an activated allele of Akt (Sabatini, 2006). As already discussed, rapamycin and its analogues target specifically the FKBP12-rapamycin-binding domain of the mTORC1 complex, while prolonged treatment may have also an effect on mTORC2 and Akt (Seto, 2012). However, due to their upstream position to mTOR, genetic and signalling abnormalities are subjected to control by rapamycin and its analogues, whereas downstream molecules will remain unaffected by the treatment with mTOR inhibitors. It seems that even in tumours with aberrant activation of the mTOR pathway, the response to rapalogs is limited or peculiar; this phenomenon has been attributed to the complexity and crosstalk of the various signalling transduction pathways (Seto, 2012).
Several clinical trials are testing mTOR inhibitors in solid tumours (including locally advanced breast cancer, advanced pancreatic neuroendocrine tumours, metastatic renal cell cancer, colon-rectal cancer, small and non-small cell lung cancer, recurrent endometrial carcinoma) and in haematological malignancies (Strimpakos et al., 2009; Zaytseva et al., 2012). In particular, as a result of the known interactions between mTOR and other signalling pathways, the synergistic anti-tumour effect of mTOR inhibitors and other signalling inhibitor like anti-angiogenic agents is under evaluation (Faivre et al., 2006). Moreover, recent data suggest that these drugs are more likely to be effective when combined with traditional chemotherapy. Overall, rapalogs are relatively well tolerated in cancer patients, although significant stomatitis, rash and deaths due to bowel perforation and mucositis have been reported in patients with advanced solid tumours receiving temsirolimus in combination with 5-fluorouracil/leucovorin (Punt et al., 2003). Furthermore, because mTOR critically regulates glucose metabolism, hyperglycaemia represents another side effects of rapalogs (Dann et al., 2007), together with elevation of triglyceride and cholesterol levels in blood (Morrisett et al., 2002).
Second generation of mTOR inhibitors
The limitations of rapamycin-based therapies in the clinical setting have led to development of a second generation of mTOR inhibitors known as ATP-competitive mTOR kinase inhibitors (TKIs). TKIs target the mTOR kinase domain and inhibit its catalytic activity (Zaytseva et al., 2012). The mechanistic advantage of these drugs is that they inhibit the kinase activity of both mTOR complexes, resulting in down-regulation of mTOR signalling globally, with the advantage of minimizing the feedback activation of PI3K/Akt (Yu et al., 2009; Zoncu et al., 2011). Numerous TKIs have been developed, and several of them have entered in early clinical trial testing (Zaytseva et al., 2012) (Figure 2). The rationale of using TKIs as anticancer agents is derived from the aberrantly hyperactive PI3K/Akt/mTOR signalling that is a prominent feature of a broad spectrum of human cancers (LoPiccolo et al., 2008). Rapalogs cause activation of Akt through a negative feedback loop on the mTORC2 complex, which is also involved in cancer cell growth and survival. Interestingly, inhibition of mTORC2 and/or PI3K simultaneously with mTORC1 appears to inhibit more robustly the signalling cascades and halt activation of this feedback loop (LoPiccolo et al., 2008). There is strong evidence that mTORC2 plays a critical role in tumour growth and survival; this emphasizes the additional benefit of TKIs over rapalogs. TKIs are indeed able to block the mTORC2-dependent phosphorylation of Akt (Roper et al., 2011). Moreover, inhibition of the mTORC2 protein, Rictor, results in significant reduction of Akt phosphorylation in both rapamycin-sensitive and rapamycin-resistant colon-rectal cancers (Gulhati et al., 2009), thus underlining the critical role of mTORC2 inhibition. Like rapalogs, TKIs have been shown to decrease protein synthesis, attenuate cell cycle progression, and inhibit angiogenesis in multiple cancer cell lines as well as in human cancers (Guertin and Sabatini, 2009). The TKI-mediated inhibition of cell growth and proliferation has been proven to be more potent than that by the rapalogs. For example, global protein synthesis is suppressed by nearly 50% when breast cancer cells are treated with TKIs, compared with a negligible effect observed with rapamycin treatment. This is probably due to more effective targeting of translation initiation by TKIs than rapamycin (Yu et al., 2009). Additionally, TKIs reduce more effectively aerobic glycolysis in human tumour cells with consequent starvation of cells and enhanced anti-tumour effect (Wander et al., 2011). In a subset of Akt-hyperactive tumour cells, treatment with TKIs leads to a higher reduction of lactate production and higher expression of Glut1, HIF-1α and HIF-2α, compared with those induced by rapamycin. All these effects normalize the cell metabolism and the response of cells to the deprivation of oxygen, with a subsequent reduced production of VEGF and angiogenesis (Yu et al., 2009).
Despite the many advantages of TKIs, some drawbacks still remain. For instance, even though TKIs are more effective in rapamycin-resistant cell lines (Zask et al., 2009), they are less effective in KRAS-driven tumours (Engelman et al., 2008), suggesting that combination therapies with multiple kinase inhibitors are needed to combat resistance in these cancers. Another possible disadvantage is related to their safety. Although their broader activity improves anti-tumour efficacy, global inhibition of mTOR is expected to be associated with greater toxicity in normal cells (Liu et al., 2009a,b). Of note, preclinical studies have shown that full inhibition of mTORC1 and mTORC2 could be well tolerated (Janes et al., 2010). Moreover, it has been shown that chronic inhibition of mTORC2 may induce Thr308 phosphorylation of Akt through alternative pathways, even in the absence of the priming Ser473 phosphorylation (Zoncu et al., 2011), thus suggesting a potential mechanism of resistance to these agents.
Immunosuppression is another concern. The immunosuppressive effect of rapamycin derives from the inhibition of T- and B-cell proliferation, through mechanisms similar to those that block cancer cell proliferation (Law, 2005). In a clinical context, a more potent suppression of mTOR signalling by the ATP-competitive TKIs also raises concerns regarding the consequent higher level of immunosuppression. In this regard, however, the dual PI3K/mTOR inhibitor PI-103, although exhibiting immunosuppressive activity, was still able to inhibit in vivo tumour growth, and increased survival of immunocompetent mice bearing sorafenib-treated melanoma (López-Fauqued et al., 2010).
Finally, there are data suggesting that sensitivity or resistance of tumour cells to TKIs is genetically determined (Mitsudomi and Yatabe, 2007). Unfortunately, biomarkers to predict which cancer patient will benefit more from these inhibitors are not yet available. Some of the rapamycin-insensitive mTOR functions can be also profoundly inhibited by TKIs in some but not other cancer cells, such as colon cancer cells (Raynaud et al., 2009; Shor et al., 2009). Therefore, identification of genetic markers associated with response to TKIs is likely to be crucial in determining the clinical success of these drugs. As a general principle, tumours strongly dependent on the PI3K/mTOR pathway should respond favourably to these inhibitors, but it is still unclear if the compounds are similarly efficacious in cancers with distinct genetic lesions in this pathway, such as PIK3A, PTEN and KRAS. For instance, breast cancers with HER2 or PIK3CA mutations, but not those with PTEN mutations, have a favourable response to the treatment with the mTOR/PI3K dual inhibitor NVPBEZ235 (Brachmann et al., 2009). Therefore, this remains an area of intensive research that will eventually determine the successful translation of these compounds from the preclinical to the clinical setting.
The close interaction of mTOR with the PI3K pathway, as well as concerns regarding TKI resistance via feedback activation of PI3K/Akt, prompted the development of mTOR/PI3K dual inhibitors (TPdIs). These agents target the p110α, β- and γ-isoforms of PI3K, as well as the ATP-binding sites of both mTORC1 and mTORC2 complexes, completely suppressing PI3K/Akt signalling pathway (Zaytseva et al., 2012). Inhibition of the biological functions of PI3K/mTOR pathway components has been shown to block proliferation in a broad panel of tumour cell lines by inducing G1 arrest (Garcia-Echeverria, 2011), apoptosis and autophagy (Wander et al., 2011). New data indicate that the TPdIs are more effective than their rapalog predecessors. In a study comparing treatment with NVP-BEZ235 to everolimus in a panel of 21 cell lines of different origins and mutational status, NVP-BEZ235 was found to have greater antiproliferative activity (Serra et al., 2008). Of interest, the utility of these inhibitors may extend beyond cancers with a PI3K pathway mutation. For example, a study showed that NVP-BEZ235 has comparable efficacy in both the wild-type and mutant PIK3CA-carrying tumours (Roper et al., 2011), suggesting its potential use in a broader spectrum of tumours.
Clinical trials of TPdIs are currently underway (Zhang et al., 2011; Zaytseva et al., 2012) in patients with recurrent glioblastoma, advanced or metastatic endometrial carcinoma and other advanced solid tumours (Zaytseva et al., 2012). Despite encouraging results, there is preclinical evidence that some type of cancers may be insensitive to TPdIs. For example, mouse lung cancer driven by mutant KRAS did not respond to monotherapy with NVP-BEZ235, although a combination of NVP-BEZ235 with a MAPK inhibitor, ARRY-142886, resulted in a marked and synergistic inhibitory activity on these KRAS-mutated cancers (Engelman et al., 2008). Similarly, NVP-BEZ235 failed to promote substantial apoptosis in EGFR-mutant lung cancer (Garcia-Echeverria, 2011), further suggesting the need to combine the TPdIs with other TKIs in the clinical setting.
PI3K has several roles in cell survival, differentiation, metabolism and migration, some of which are Akt and mTOR independent (Engelman et al., 2006; Fruman and Bismuth, 2009). Of note, metabolic disturbances are likely to arise as a result of inhibition of the PI3Kα isoform (Foukas et al., 2006), which is the target of several PI3K inhibitors in a broad range of tumours. There is also evidence that PI3K is involved in the regulation of haematopoietic stem cells, suggesting that chronic inhibition of the PI3K pathway may lead to haematological toxicity (Zhang et al., 2006). However, detailed safety profiles are not available so far. The importance of PI3K and mTOR in regulating a wide range of normal biological processes suggests that the dual mTOR/PI3K inhibitors are likely to be associated with increased toxicity (Zoncu et al., 2011). Indeed, in the comparison between the mTOR kinase inhibitor PP242 and the mTOR/PI3K dual inhibitor PI-103, the latter was found to have a substantial narrower therapeutic range (Janes et al., 2010).
Finally, another class of ATP-competitive inhibitors of mTOR, named Torins (Torin-1 and Torin-2), has been shown to affect protein synthesis and proliferation much more than rapamycin, largely due to their ability to inhibit rapamycin-resistant functions of mTORC1 (Thoreen et al., 2012). Torin-1 affects cell growth, motility, invasion and survival in vitro, and inhibits tumour growth in vivo with a concomitant anti-angiogenic effect. It also affects the expression of markers for cell proliferation, angio- and lymphogenesis, and stemness, with no effects on normal stem cells, suggesting its selectivity towards cancer cells (Thoreen et al., 2012; Francipane and Lagasse, 2013). Torin-2, a potent orally bioavailable mTOR kinase inhibitor with significant selectivity over other PKs, has shown potent anti-tumour effects in preclinical studies (Liu et al., 2011; 2013).
Conclusions
In the last few years, significant advances have been made in understanding the role of mTOR pathway in cancer development and progression. The evidence that the mTOR-signalling pathway is overactivated in several types of cancer has led to the development of many new mTOR inhibitors. Encouraging data from preclinical studies have offered new opportunities to fully exploit the therapeutic potential of mTOR targeting in cancer. At present, many clinical trials of mTOR inhibitors and their combination with other novel drugs and traditional treatments are underway to examine the therapeutic activity of these new agents in a variety of solid and haematological tumours (http://www.ClinicalTrials.gov). However, several factors limit the therapeutic potential of these drugs. Most importantly, many of these molecules do not inhibit both mTORC1 and mTORC2, and consequently they may activate feedback regulatory loops involving the mTORC2 complex. This may explain the lack of success obtained so far in many clinical trials, as well as disclosing an important role of mTORC2 in promoting cancer cell activities. On the other hand, a strong inhibition of the mTOR pathway (both mTORC1 and mTORC2 complexes) could also lead to severe adverse effects, thus limiting the potential clinical utility of this approach. Because of the many limitations of currently available inhibitors, new approaches for mTOR targeting are under investigation, such as selective inhibition of the mTORC2 complex (Zoncu et al., 2011). In addition, a key future target should be the identification of predictive biomarkers of response in order to better select cancer patients likely to respond to mTOR pathway inhibitors. The discovery of such markers will allow the benefits and safety for treated patients to be maximized.
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan, Italy) – Investigator Grant (Grant No. 10099) and 5 per thousand Molecular Clinical Oncology Special Program (Grant No. 9965); by the European Commission Seventh Framework Programme (EU FP7 OVER-MyR, Grant No. 278206), Brussels, Belgium; and by the Ministry of Health (Project No. PRIN 2012), Rome, Italy. The sponsors of this study are public or non-profit organizations that support science in general. They had no role in gathering, analysing or interpreting the data. The authors are fully responsible for the content and editorial decisions for this manuscript.
Glossary
- Deptor
DEP-domain-containing mTOR-interacting protein
- EGFR
epidermal growth factor receptor
- eIF4E
translation initiation factor 4E
- FKBP12
FK506-binding protein
- GSK3
glycogen synthase kinase 3
- HIF-1α
hypoxia inducible factor-1α
- Hsp70
heat shock protein 70-α
- IGFR
insulin-like growth factor receptor
- mLST8
mammalian lethal with Sec13 protein8 (also known as GβL)
- mSIN1
mammalian stress-activated protein kinase-interacting protein 1
- mTOR
mammalian target of rapamycin
- mTORC1 and mTORC2
mTOR complex 1 and 2
- PDK1
3-phosphoinositide-dependent protein kinase-1
- PI3K
phosphoinositide 3-kinase
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PRAS40
proline-rich Akt substrate 40 kDa
- PTEN
phosphatase and tensin homologue
- Raptor
regulatory-associated protein of mTOR
- Rictor
Raptor-independent companion of mTOR
- RTK
receptor tyrosine kinase
- S6K1
S6 kinase 1
- TPdIs
mTOR/PI3K dual inhibitors
- TSC1 and TSC2
tuberous sclerosis 1 and 2 complex
Conflict of interest
All the authors declared no conflicts of interest.
References
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Alvarado Y, Mita MM, Vemulapalli S, Mahalingam D, Mita AC. Clinical activity of mammalian target of rapamycin inhibitors in solid tumors. Target Oncol. 2011;6:69–94. doi: 10.1007/s11523-011-0178-5. [DOI] [PubMed] [Google Scholar]
- Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briest F, Grabowski P. PI3K-AKT-mTOR-Signaling and beyond: the Complex Network in Gastroenteropancreatic Neuroendocrine. Theranostics. 2014;4:336–365. doi: 10.7150/thno.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger C, DeVries B, Stambolic V. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun. 2006;344:869–880. doi: 10.1016/j.bbrc.2006.03.220. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Salazar R, Halperín I, Abad A, Yao JC. Innovations therapy: mammalian target of rapamycin (mTOR) inhibitors for the treatment of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30:27–34. doi: 10.1007/s10555-011-9290-3. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Walls M, Baxi SM, Yin MJ. Targeting the mTOR pathway in tumor malignancy. Curr Cancer Drug Targets. 2013;13:267–277. doi: 10.2174/1568009611313030005. [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–442. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- De P, Miskimins K, Dey N, Leyland-Jones B. Promise of rapalogues versus mTOR kinase inhibitors in subset specific breast cancer: old targets new hope. Cancer Treat Rev. 2013;39:403–412. doi: 10.1016/j.ctrv.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraffenried LA, Fulcher L, Friedrichs WE, Grünwald V, Ray RB, Hidalgo M. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol. 2004;15:1510–1516. doi: 10.1093/annonc/mdh388. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16:6242–6251. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelstein M, Moll U. Targeting tumour-supportive cellular machineries in anticancer drug development. Nat Rev Drug Discov. 2014;13:179–196. doi: 10.1038/nrd4201. [DOI] [PubMed] [Google Scholar]
- Don AS, Zheng XF. Recent clinical trials of mTOR-targeted cancer therapies. Rev Recent Clin Trials. 2011;6:24–35. doi: 10.2174/157488711793980147. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Festuccia C, Muzi P, Millimaggi D, Biordi L, Gravina GL, Speca S, et al. Molecular aspects of gefitinib antiproliferative and pro-apoptotic effects in PTEN-positive and PTEN-negative prostate cancer cell lines. Endocr Relat Cancer. 2005;12:983–998. doi: 10.1677/erc.1.00986. [DOI] [PubMed] [Google Scholar]
- Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, et al. Critical role for the p110-alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- Francipane MG, Lagasse E. Selective targeting of human colon cancer stem-like cells by the mTOR inhibitor Torin-1. Oncotarget. 2013;4:1948–1962. doi: 10.18632/oncotarget.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Echeverria C. Blocking the mTOR pathway: a drug discovery perspective. Biochem Soc Trans. 2011;39:451–455. doi: 10.1042/BST0390451. [DOI] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Guntur KV, Bell GW, Thoreen CC, Sabatini DM. Functional genomics identifies TOR-regulated genes that control growth and division. Curr Biol. 2006a;16:958–970. doi: 10.1016/j.cub.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006b;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Gulhati P, Cai Q, Li J, Liu J, Rychahou PG, Qiu S, et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res. 2009;15:7207–7216. doi: 10.1158/1078-0432.CCR-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- Hardt M, Chantaravisoot N, Tamanoi F. Activating mutations of TOR (target of rapamycin) Genes Cells. 2011;16:141–151. doi: 10.1111/j.1365-2443.2010.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;16:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, et al. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, et al. Identification of a proline-rich Akt substrate as a 14–3-3 binding partner. J Biol Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law BK. Rapamycin: an anti-cancer immunosuppressant? Crit Rev Oncol Hematol. 2005;56:47–60. doi: 10.1016/j.critrevonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- Li J, Liu J, Song J, Wang X, Weiss HL, Townsend CM, Jr, et al. mTORC1 inhibition increases neurotensin secretion and gene expression through activation of the MEK/ERK/c-Jun pathway in the human endocrine cell line BON. Am J Physiol Cell Physiol. 2011;301:C213–C226. doi: 10.1152/ajpcell.00067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Lim HK, Choi YA, Park W, Lee T, Ryu SH, Kim SY, et al. Phosphatidic acid regulates systemic inflammatory responses by modulating the Akt-mammalian target of rapamycin-p70 S6 kinase 1 pathway. J Biol Chem. 2003;278:45117–45127. doi: 10.1074/jbc.M303789200. [DOI] [PubMed] [Google Scholar]
- Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, et al. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J Biol Chem. 2010;285:38362–38373. doi: 10.1074/jbc.M110.141168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009a;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR Mediated Anti-Cancer Drug Discovery. Drug Discov Today Ther Strateg. 2009b;6:47–55. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wang J, Kang SA, Thoreen CC, Hur W, Ahmed T, et al. Discovery of 9-(6-aminopyridin-3-yl)-1-(3- (trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective and orally available mTOR inhibitor for treatment of cancer. J Med Chem. 2011;54:1473–1480. doi: 10.1021/jm101520v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Xu C, Kirubakaran S, Zhang X, Hur W, Liu Y, et al. Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM and ATR. Cancer Res. 2013;73:2574–2586. doi: 10.1158/0008-5472.CAN-12-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Fauqued M, Gil R, Grueso J, Hernandez-Losa J, Pujol A, Moliné T, et al. The dual PI3K/mTOR inhibitor PI-103 promotes immunosuppression, in vivo tumor growth and increases survival of sorafenib-treated melanoma cells. Int J Cancer. 2010;126:1549–1561. doi: 10.1002/ijc.24926. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Martin J, Masri J, Bernath A, Nishimura R, Gera J. Hsp70 associates with Rictor and is required for mTORC2 formation and activity. Biochem Biophys Res Commun. 2008;372:578–583. doi: 10.1016/j.bbrc.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam MR, Celestino J, Wu W, Broaddus RR, Schmeler KM, Slomovitz BM, et al. Reduced progression of endometrial hyperplasia with oral mTOR inhibition in the Pten heterozygote murine model. Am J Obstet Gynecol. 2007;196:247.e1–2475. doi: 10.1016/j.ajog.2006.10.872. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, et al. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170–1180. [PubMed] [Google Scholar]
- Murata J, Tada M, Sawamura Y, Mitsumori K, Abe H, Nagashima K. Dysplastic gangliocytoma (Lhermitte-Duclos disease) associated with Cowden disease: report of a case and review of the literature for the genetic relationship between the two diseases. J Neurooncol. 1999;41:129–136. doi: 10.1023/a:1006167421100. [DOI] [PubMed] [Google Scholar]
- Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 2011;71:7103–7112. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt CJ, Boni J, Bruntsch U, Peters M, Thielert C. Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol. 2003;14:931–937. doi: 10.1093/annonc/mdg248. [DOI] [PubMed] [Google Scholar]
- Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther. 2009;8:1725–1738. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper J, Richardson MP, Wang WV, Richard LG, Chen W, Coffee EM, et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 induces tumor regression in a genetically engineered mouse model of PIK3CA wild-type colorectal cancer. PLoS ONE. 2011;6:e25132. doi: 10.1371/journal.pone.0025132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol. 2003;13:797–806. doi: 10.1016/s0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- Seront E, Pinto A, Bouzin C, Bertrand L, Machiels J-P, Feron British O. PTEN deficiency is associated with reduced sensitivity to mTOR inhibitor in human bladder cancer through the unhampered feedback loop driving PI3K/Akt activation. Br J Cancer. 2013;109:1586–1592. doi: 10.1038/bjc.2013.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti A, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto B. Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clin Transl Med. 2012;1:29. doi: 10.1186/2001-1326-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signaling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin Cancer Res. 2003;9:4340–4346. [PubMed] [Google Scholar]
- Shi Y, Gera J, Hu L, Hsu JH, Bookstein R, Li W, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev. 2009;35:148–159. doi: 10.1016/j.ctrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nakagawa M, Young SG, Yamanaka S. Differential membrane localization of ERas and Rheb, two Ras-related proteins involved in the phosphatidylinositol 3-kinase/mTOR pathway. J Biol Chem. 2005;280:32768–32774. doi: 10.1074/jbc.M506280200. [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signaling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Wei L, Huang J. mTOR signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med. 2011;52:497–500. doi: 10.2967/jnumed.111.089623. [DOI] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S. Survival signaling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr Oncol Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;5:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villén J, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zask A, Verheijen JC, Curran K, Kaplan J, Richard DJ, Nowak P, et al. ATP-competitive inhibitors of the mammalian target of rapamycin: design and synthesis of highly potent and selective pyrazolopyrimidines. J Med Chem. 2009;52:5013–5016. doi: 10.1021/jm900851f. [DOI] [PubMed] [Google Scholar]
- Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319:1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011;16:325–331. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]