Abstract
BACKGROUND AND PURPOSE
Although serine proteases and agonists of protease-activated receptor 2 (PAR2) cause inflammation and pain, the spectrum of proteases that are activated by proinflammatory and algesic stimuli and their contribution to inflammatory pain are uncertain.
EXPERIMENTAL APPROACH
Enzymic assays and selective inhibitors were used to characterize protease activity in mice after intraplantar injections of formalin, bradykinin, PAR2 activating peptide (AP) or vehicle. The capacity of these proteases and of recombinant mouse trypsin 4 to cleave fragments of PAR2 and to activate PAR2 in cell lines was determined. Protease inhibitors and par2−/− mice were used to assess the contributions of proteases and PAR2 to pain and inflammation.
KEY RESULTS
Intraplantar injection of formalin, bradykinin or PAR2-AP led to the activation of proteases that were susceptible to the serine protease inhibitor melagatran but resistant to soybean trypsin inhibitor (SBTI). Melagatran inhibited mouse trypsin 4, which degraded SBTI. Proteases generated in inflamed tissues cleaved PAR2-derived peptides. These proteases and trypsin 4 increased [Ca2+]i in PAR2-transfected but not in untransfected cells, and melagatran suppressed this activity. Melagatran or PAR2 deletion suppressed oedema and mechanical hypersensitivity induced by intraplantar formalin, bradykinin and PAR2-AP, but had no effect on capsaicin-induced pain.
CONCLUSIONS AND IMPLICATIONS
Diverse proinflammatory and algesic agents activate melagatran-sensitive serine proteases that cause inflammation and pain by a PAR2-mediated mechanism. By inducing self-activating proteases, PAR2 amplifies and sustains inflammation and pain. Serine protease inhibitors can attenuate the inflammatory and algesic effects of diverse stimuli, representing a useful therapeutic strategy.
Introduction
Damaging and inflammatory stimuli activate proteases in the circulation and in immune, epithelial and neuronal tissues that cleave protease-activated receptors (PARs), a family of four GPCRs (Ossovskaya and Bunnett, 2004; Ramachandran et al., 2012; nomenclature follows Alexander et al., 2013a). Activation of PAR2 on primary spinal afferent neurons stimulates the release of neuropeptides that cause neurogenic inflammation and pain (Steinhoff et al., 2000; Vergnolle et al., 2001; Cenac et al., 2003; Nguyen et al., 2003). PAR2 also sensitizes transient receptor potential (TRP) ion channels, which amplify inflammation and pain (Amadesi et al., 2004; 2006; Dai et al., 2007; Grant et al., 2007; Poole et al., 2013; channel nomenclature follows Alexander et al., 2013b). Several serine proteases that are generated during injury can activate PAR2, including trypsins (Nystedt et al., 1994; Bohm et al., 1996; Knecht et al., 2007), mast cell tryptase (Corvera et al., 1997), coagulation factors VIIa and Xa (Camerer et al., 2000) and kallikreins (Oikonomopoulou et al., 2006). Although trypsins are the most potent PAR2 activators, their contributions to inflammation and pain are not understood. The human trypsinogen genes PRSS1, PRSS2 and PRSS3 encode trypsinogen I, trypsinogen II and mesotrypsinogen, which are secreted from the pancreas into the intestine, where enterokinase cleaves these zymogens to generate active proteases that degrade dietary proteins (Emi et al., 1986; Nyaruhucha et al., 1997). Trypsinogen IV is a splice variant of mesotrypsinogen, although the active proteases mesotrypsin and trypsin IV are identical (Wiegand et al., 1993; Szmola et al., 2003). Trypsinogen IV is expressed by neurons, astrocytes and extrapancreatic epithelial cells (Wiegand et al., 1993; Cottrell et al., 2004; Gallatz et al., 2007; Toth et al., 2007), but the physiological function of extrapancreatic trypsins is unclear. Whereas endogenous polypeptide inhibitors control the activities of trypsin I/II, trypsin IV (mesotrypsin) is resistant to and degrades many polypeptide inhibitors (Nyaruhucha et al., 1997; Szmola et al., 2003; Cottrell et al., 2004; Sahin-Toth, 2005; Knecht et al., 2007), with the exception of nexin-1, which can inhibit trypsin IV (Koistinen et al., 2009). Thus, trypsin IV may remain active for prolonged periods. Although trypsin-related serine proteases cause PAR2-dependent neurogenic inflammation and pain in the skin (Steinhoff et al., 2000; Vergnolle et al., 2001), pancreas (Hoogerwerf et al., 2004), colon (Cenac et al., 2002; Cattaruzza et al., 2011) and joints (Ferrell et al., 2003), and may contribute to the pain of irritable bowel syndrome (Cenac et al., 2007) and cancer (Lam and Schmidt, 2010), the spectrum of serine proteases that are activated during inflammation and their contribution to inflammation and pain remain uncertain.
We examined the activation of serine proteases in the mouse during inflammation and investigated the contributions of proteases and PAR2 to inflammation and pain. As proteases are regulated by post-translational control of activity (e.g. by zymogen processing and endogenous inhibitors) rather than by gene or protein expression, we studied protease activity in paw tissues of mice after the intraplantar injection of agents that induce inflammation and pain by activating different pathways. These agents included formalin, which can activate the TRPA1 ion channel on sensory nerves (McNamara et al., 2007), and bradykinin and a PAR2-selective activating peptide (PAR2-AP), which can activate GPCRs on sensory nerves (Vergnolle et al., 2001). We found that formalin, bradykinin and PAR2-AP activated proteases in paw tissues that were inhibited by the serine protease inhibitor, melagatran (Gustafsson et al., 1998), but not by soybean trypsin inhibitor (SBTI), consistent with activation of trypsin IV-like protease (Ceppa et al., 2011). In common with human trypsin IV, mouse trypsin 4 was inhibited by melagatran, degraded SBTI, and activated PAR2. Proteases that were activated in the inflamed mouse paw also cleaved and activated PAR2. Notably, melagatran or PAR2 deletion suppressed hypersensitivity and oedema induced by formalin, bradykinin and PAR2-AP. Our results indicate that diverse inflammatory and painful stimuli may activate melagatran-sensitive serine proteases that cleave and activate PAR2 to cause pain and inflammation. Importantly, PAR2 agonists stimulate the activation of inhibitor-resistant proteases that can degrade endogenous polypeptide inhibitors and activate PAR2. This novel mechanism of positive feedback may amplify and sustain PAR2-mediated pain and inflammation.
Methods
Mice
All animal care and experimental procedures were approved by the Institutional Animal Care Use Committees. All studies involving animals are in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 468 animals were used in the experiments described here.
C57BL/6 mice (Charles River Labs, Hollister, CA, USA) and par2−/− and par2+/+ littermates (C57BL/6 background; Lindner et al., 2000) (8–12 weeks, male and female) were studied. Mice were maintained under temperature- (22 ± 4°C) and light- (12 h light/dark cycle) controlled conditions with free access to food and water.
Proteases
Recombinant human trypsin I was from Polymun Scientific (Vienna, Austria). Expression, activation and characterization of recombinant human trypsin IV and rat P23 has been described (Knecht et al., 2007). P23 resembles trypsin IV in terms of its susceptibility to inhibitors and its capacity to activate PAR2 (Knecht et al., 2007). Mouse trypsin 4 was selected for study because of its sequence homology with rat P23. Mouse trypsin 4 was expressed with an artificial pro-peptide sequence comprising a histidine tag and an enterokinase cleavage site (MHHHHHHHHV↓PFDDDDK) (↓ enterokinase site). After the enterokinase cleavage site, the amino acid sequence is identical to the sequence of Acc. No. NM_023707. Mouse trypsinogen 4 was expressed, purified and activated to yield mouse trypsin 4 as described for human trypsin IV and rat P23 (Knecht et al., 2007). The Km and Vmax for human trypsin I (25 ng·mL−1), human trypsin IV (25 ng·mL−1) and mouse trypsin 4 (50 ng·mL−1) were determined by incubation with graded concentrations of Bz-Val-Gly-Arg-pNA (Bz-VGR-pNA, Bachem) and N-(p-Tosyl)-Gly-Pro-Lys 4-nitroanilide acetate salt (tosyl-GPR-pNA, Sigma-Aldrich) in 100 mM Tris HCl, pH 8.0, 1 mM CaCl2 at 25°C.
Inhibitor characterization
Inhibition constants (IC50) for melagatran and SBTI with human trypsin I and IV and mouse trypsin 4 were determined using Bz-VGR-pNA as a substrate ([S]) in 100 mM Tris HCl, pH 8, 1 mM CaCl2 at 25°C. Substrate concentrations were selected to be close to Km values for each protease (human trypsin I, 360 μM; human trypsin IV, 55 μM; mouse trypsin 4, 215 μM). The IC50 values were converted into binding affinity Kic using the Cheng-Prusoff equation: Kic = IC50/(1 + [S]/Km) and the respective Km values as described (Knecht et al., 2007; Ceppa et al., 2011). Inhibitors were incubated with recombinant human trypsin I, recombinant human trypsin IV and recombinant mouse trypsin 4 for different times before determining the IC50.
Generation of PAR2 transfected cell lines
cDNA encoding mouse (m) PAR2 was subcloned into pcDNA5/FRT (Invitrogen Life Technologies, Carlsbad, CA, USA). A C-terminal HSV epitope tag was added by PCR and sequenced to confirm identity. Sarcoma virus transformed rat kidney epithelial cells (KNRK) were from American Tissue Type Collection (Rockville, MD, USA). KNRK-FLP cells were created with the Flp-In™ system (Invitrogen; Jacob et al., 2005). Stable cell lines were created by cotransfection with pcDNA5/FRT and pOG44, a vector that transiently expresses the Flp recombinase. Cells were placed in medium containing 300 μg·mL−1 hygromycin B. After 7–10 days, viable clones were picked and PAR2 expression confirmed by immunofluorescence and Ca2+ signalling (Jacob et al., 2005). These KNRK-mPAR2 cells were maintained in 150 μg·mL−1 hygromycin B. As a control, KNRK cells were transfected with empty vector (KNRK-vector control, KNRK-VC cells).
Activation of proteases in inflamed tissues
Mice were anaesthetized with isoflurane and received intraplantar injections of formalin (2.5%), bradykinin (0.5–5 μg), PAR2-AP (10–50 μg) or NaCl (0.9%, vehicle control) (all 5 or 10 μL). After 60 min, mice were killed and paws were homogenized and sonicated in HBSS, 10 mM HEPES, pH 7.4, and centrifuged (20 000 g, 30 min, 4°C). Supernatants were assayed for serine protease activity by fluorogenic assay, or were incubated with synthetic fragments of PARs to assess their capacity to cleave these receptors, or were assayed for their ability to activate PAR2 in transfected cell lines.
Protease activity assays
Paw extracts (10–20 μg protein) obtained from mice 60 min after intraplantar formalin, bradykinin or PAR2-AP were diluted in 10 mM HEPES pH 7.4 (100–200 μL) and incubated with 30 mM of the rhodamine 110-based proteinase substrate BZiPAR (Molecular Probes, Eugene, OR, USA). Activity was monitored by measuring the fluorescence intensity at 498 nm excitation and 521 nm emission, from 15 to 180 min at 25°C. Enzymic activity is expressed as relative fluorescence unit ·μg−1 protein. In some experiments, samples were pre-incubated on ice for 30 min with protease inhibitors (melagatran, SBTI, 1 μM) or vehicle (control) before addition of substrate.
Cleavage of PAR-derived peptides
Peptides containing the cleavage and activation sites (↓) of PAR1 (LDPR↓SFLL), PAR2 (SKGR↓SLIG) and PAR4 (PAPR↓GYPG) (P4 to P4' positions) flanked by a N-terminal DABCYL fluorophore and a C-terminal Glu(EDANS)-amide quencher moiety were obtained from JPT Peptide Technologies GmbH (Berlin, Germany). Cleavage of any peptide bond between the fluorophore and the quencher moiety would generate a fluorescence signal. Paw extracts obtained from mice 60 min after intraplantar PAR2-AP or saline were tested for their ability to cleave PAR-derived peptides. PAR-derived peptides (3.75 μM) were incubated with extracts (diluted 1:160 in 100 mM Tris HCl pH 8, 1 mM CaCl2 in 20 μL total volume, room temperature). Fluorescence was continuously monitored every minute for 100 min at 350 nm excitation and 490 nm emission. LDH activity in the extracts was measured with the Cytotoxicity Detection Kit (Roche Applied Science, Indianapolis, IN, USA). The rate of cleavage determined with the PAR-derived peptides was normalized against LDH activity measured in each sample.
PAR2 activation assays
KNRK-mPAR2 or KNRK-VC cells were loaded with Fura-2AM (Molecular Probes, Eugene, OR, USA) for measurement of [Ca2+]i in individual cells at 37°C as described (Knecht et al., 2007). Mouse trypsin IV (1–100 nM, final concentration) or paw extracts (30–40 μg protein, 50 μL) were added directly to the chamber (350 μL). The extracts were incubated at 37°C for 30 min prior to assay, which was necessary to reveal PAR2-activating enzymic activity. Results are expressed as the 340/380 nm emission ratio which is proportional to [Ca2+]i. In some experiments, samples were pre-incubated on ice for 30 min with protease inhibitors (melagatran, 1 μM) or vehicle (control) before assay.
Measurement of paw oedema and mechanical hypersensitivity
Formalin (2.5%), PAR2-AP (0.3 or 5 μg), bradykinin (0.3 or 3 μg), capsaicin (5 μg) or vehicle (0.9% NaCl or 10% Tween 80, 10% EtOH, 80% NaCl for capsaicin) were injected into the plantar surface of left hindpaw (10 μL). The lower doses were used for measuring mechanical hypersensitivity with von Frey filaments and the higher doses were used for measuring oedema; lower doses of bradykinin and PAR2-AP did not cause detectable oedema. In some experiments, mice were pretreated with melagatran either locally (430 ng, 10 μL, intraplantar injection 15 min before injection of inflammatory and algesic agents) or systemically (0.2 mg·kg−1, intraperitoneal injection, 45 min before the injection of inflammatory and algesic agents). The nociceptive responses to mechanical stimuli were determined by measuring the withdrawal threshold (g) of the hindpaw as described (Eilers et al., 2010). Inflammatory oedema was assessed by measuring paw thickness with a digital calliper (Mitutoyo Corporation, Aurora, IL, USA) before and after the injections (Vergnolle et al., 2010). Results are expressed as increase in paw thickness normalized to basal values. In assays of oedema and hypersensitivity, we used a balanced proportion of male and female mice in each group and ensured that basal withdrawal responses were similar (0.7–0.9 g) for each group.
Data analysis
Results are reported as mean ± SEM (unless otherwise stated) and were compared using unpaired t-test analyses (for comparison between two groups) and one-way and two-way anova (for comparisons among multiple groups), followed by Bonferroni's test. P < 0.05 was considered significant.
Materials
PAR2-AP (SLIGRL-NH2) was from SynPep Corp. (Dublin, CA, USA). Bradykinin was from Bachem (Torrance, CA, USA). Melagatran was from AstraZeneca (Mölndal, Sweden) and the use of melagatran as a trypsin IV inhibitor has been described (Ceppa et al., 2011). Unless otherwise indicated, other reagents were from Sigma-Aldrich (St Louis, MO, USA).
Results
Proinflammatory and algesic stimuli activate melagatran-sensitive trypsin-like proteases
To determine whether stimuli that cause inflammation and pain by diverse mechanisms activate serine proteases, we made intraplantar injections of formalin (2.5%), bradykinin (0.5 or 5 μg), PAR2-AP (10 or 50 μg) or NaCl (0.9%, control) to mice. After 60 min, the paws were removed, homogenized and extracts were incubated with BZiPAR, a fluorogenic substrate for serine proteases. Compared with control tissues, formalin induced an ∼8-fold increase in trypsin-like activity (Figure 1A,B). Similarly, both bradykinin (Figure 1A,C) and PAR2-AP (Figure 1A,D) induced a dose-dependent increase in trypsin-like activity in mouse paw when compared with controls. We pre-incubated tissue extracts with protease inhibitors to further characterize the enzymic activity. Melagatran, but not SBTI, inhibited activity induced by formalin, bradykinin and PAR2-AP (Figure 1B–D). Thus, diverse proinflammatory and algesic agents activate serine proteases in the mouse paw that were inhibited by melagatran but resistant to a polypeptide inhibitor. These activities resemble those of human trypsin IV or rat P23 (Knecht et al., 2007; Ceppa et al., 2011). The reason for the differences in protease activity of control tissues is unknown, but it may reflect day-to-day variability between the different experimental groups.
Figure 1.

Detection of trypsin-like activity in the inflamed paw. Formalin (2.5%) (A,B), bradykinin (BK, 0.5 or 5 μg) (A,C), PAR2-AP (10 μg or 50 μg) (A,D) or NaCl (0.9%) were injected into mouse paws. After 1 h, paws were collected, homogenized and extracts (30 μg protein) were assayed for trypsin-like activity using the fluorogenic substrate BZiPAR. (A) Time course of substrate cleavage, indicating that all proinflammatory and algesic agents increased trypsin-like activity. (B–D) Effects of inhibitors on enzymatic activity normalized to control (inhibitor vehicle). Melagatran (MGT, 1 μM) but not SBTI (1 μM) abolished activity. n ≥ 3 experiments, *P < 0.05, significantly different from saline-treated tissues, #P < 0.05, significantly different from inhibitor vehicle.
Mouse trypsin 4 is susceptible to melagatran and activates PAR2
The isoforms of human trypsins differ in their sensitivity to inhibitors and their capacity to activate PARs. Whereas human trypsin I and II are sensitive to polypeptide trypsin inhibitors, such as SBTI, human trypsin IV is resistant to and degrades these inhibitors (Nyaruhucha et al., 1997; Szmola et al., 2003; Cottrell et al., 2004; Sahin-Toth, 2005; Knecht et al., 2007). The serine protease inhibitor melagatran can inhibit SBTI-resistant isoforms of trypsin, such as human trypsin IV and rat P23 (Ceppa et al., 2011). Human trypsin I/II potently activate PAR2 (Nystedt et al., 1994; Bohm et al., 1996), and human trypsin IV and rat P23 activate PAR2 and PAR1 (Knecht et al., 2007; Ceppa et al., 2011). However, nothing is known about the inhibitor selectivity of extrapancreatic isoforms of mouse trypsins or about their ability to activate PARs.
To examine the inhibitor selectivity of mouse trypsin 4 and to determine whether mouse trypsin 4 can activate PAR2, we expressed and purified mouse trypsin 4. Mouse trypsin 4 was selected for study because of its sequence homology to rat P23. Mouse trypsinogen 4 was expressed in Escherichia coli, purified by immobilized metal ion chromatography, and was converted to trypsin 4 using enterokinase, as we have described for human trypsin IV and rat P23 (Knecht et al., 2007) (Figure 2A). Mouse trypsin 4 degraded Bz-VGR-pNA with a Km 215 ± 20 μM and Vmax 114 ± 19 U·mg−1 (mean ± SD, n = 3 experiments). Human trypsin IV also degraded Bz-VGR-pNA with a Km 56 ± 3 μM and Vmax 44 ± 28 U·mg−1 (mean ± SD, n = 3 experiments). For mouse trypsin 4, the Kic was 195 nM for melagatran and 170 nM for SBTI. For human trypsin I, the Kic was 4.5 nM for melagatran and 3.7 nM for SBTI, and for rat P23 the Kic was 17 nM for melagatran and 230 nM for SBTI (Ceppa et al., 2011). Thus, melagatran inhibits mouse trypsin 4, human trypsin IV and rat P23. Mouse trypsin 4, like rat P23, is less susceptible to the polypeptide inhibitor SBTI than human trypsin I. Moreover, we found that the inhibitory effect of SBTI on human trypsin IV and mouse trypsin 4 was decreased by 2.8- and 4.2-fold, respectively, when SBTI was pre-incubated with either protease for 60 min before activity assay. In contrast, the inhibitory effect of SBTI on human trypsin I increased by twofold after pre-incubation with protease. The IC50 values (μM) for SBTI were: human trypsin I – initial 0.0005, after 60 min 0.00025; human trypsin IV – initial 1.6, after 1 h 4.4; mouse trypsin 4 – initial 0.34, after 60 min 1.4 (n = 2). These results are consistent with the proposal that mouse trypsin 4 and human trypsin IV are resistant to polypeptide inhibitors, such as SBTI, and that these proteases can also degrade these inhibitors.
Figure 2.

Purification and activity of mouse trypsin 4. (A) Purification and activation of mouse trypsinogen 4. Lane 1: Mouse trypsinogen 4 purified by immobilized metal ion chromatography. Lane 2: Enterokinase-activated mouse trypsin 4. Lane 3: Purified mouse trypsin 4 with degradation products. Products were identified by mass spectrometry. (B,C) The effects of graded concentrations of mouse trypsin 4 on [Ca2+]i measured in KNRK-mPAR2 (B) or KNRK-VC (C) cells. n = 3 experiments.
To determine whether mouse trypsin 4 can cleave and activate PAR2, we examined Ca2+ signalling in KNRK-mPAR2 and KNRK-VC cells. In KNRK-mPAR2 cells, mouse trypsin 4 caused a concentration-dependent increase in [Ca2+]i from 1 to 100 nM, whereas the same concentrations had no effect on [Ca2+]i in KNRK-VC cells (Figure 2B). Thus, mouse trypsin 4 can activate mouse PAR2.
Melagatran-sensitive serine proteases mediate the algesic actions of diverse stimuli
To determine if proinflammatory and algesic stimuli induce pain by activating serine proteases, we gave intraplantar injections of formalin (2.5%), bradykinin (0.3 μg), PAR2-AP (0.3 μg) or NaCl (0.9%, vehicle control) to mice pretreated with melagatran or vehicle. Melagatran was administered locally (430 ng intraplantar, 15 min pretreatment) or systemically (0.2 mg·kg−1, i.p., 45 min pretreatment). Mechanical hypersensitivity was assessed by measuring the nociceptive threshold for paw withdrawal from calibrated von Frey filaments. Formalin significantly reduced nociceptive threshold, measured 30 to 240 min after injection, indicating sustained mechanical hypersensitivity (Figures 3A and 4A). The local administration of melagatran prevented formalin-induced hypersensitivity at all times except 30 min (Figure 3A). Similarly, systemic administration of melagatran prevented formalin-induced hypersensitivity at all times except 30 min (Figure 4A). Bradykinin (Figures 3B and 4B) and PAR2-AP (Figures 3C and 4C) also caused mechanical hypersensitivity. Locally and systemically administered melagatran prevented these effects at all time points. Systemic melagatran was also analgesic in mice treated with formalin and PAR2-AP, but by unknown mechanisms (Figure 4).
Figure 3.
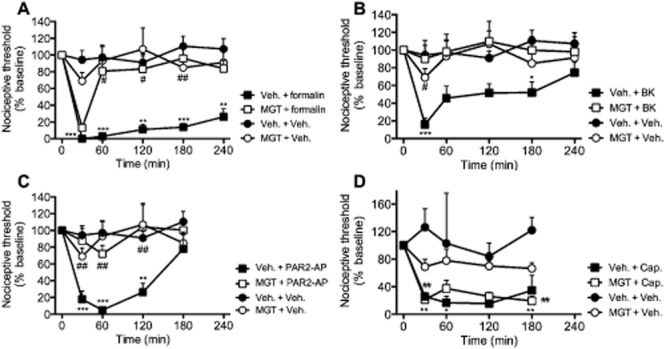
Effects of local administration of melagatran on mechanical hypersensitivity. Mice received intraplantar injections of formalin (2.5%) (A), bradykinin (BK, 0.3 μg) (B), PAR2-AP (0.3 μg) (C), capsaicin (Cap., 5 μg) (D) or vehicle (Veh.). Melagatran (MGT) or vehicle was administered locally (430 ng, 10 μL, intraplantar, 15 min pretreatment). Formalin, bradykinin, PAR2-AP and capsaicin all caused a robust decrease in the nociceptive threshold for stimulation of the injected paws with von Frey filaments, consistent with mechanical hypersensitivity. Locally administered melagatran significantly inhibited mechanical hypersensitivity induced by formalin, bradykinin and PAR2-AP, but not to capsaicin. n = 6–16 mice per group. *P < 0.05, **P < 0.005, ***P < 0.001, proinflammatory and algesic agents significantly different from vehicle controls. #P < 0.05, ##P < 0.005, melagatran-treated mice receiving proinflammatory and algesic agents significantly different from vehicle controls.
Figure 4.

Effects of systemic administration of melagatran on mechanical hypersensitivity. Mice received intraplantar injections of formalin (2.5%) (A), bradykinin (BK, 0.3 μg) (B), PAR2-AP (0.3 μg) (C) or vehicle (Veh.). Melagatran (MGT) or vehicle was administered systemically (0.2 mg·kg−1 i.p., 45 min pretreatment). Systemically administered melagatran significantly inhibited mechanical hypersensitivity induced by formalin, bradykinin and PAR2-AP. n = 6–16 mice per group. *P < 0.05, **P < 0.005, ***P < 0.001 proinflammatory and algesic agents significantly different from vehicle controls. #P < 0.05, ##P < 0.005, ###P < 0.001 melagatran-treated mice receiving proinflammatory and algesic agents significantly different from vehicle controls.
To assess the specificity of the analgesic properties of melagatran, we examined the effects of melagatran on the algesic actions of capsaicin, which causes hyperalgesia by directly activating TRPV1 channels on nociceptive neurons (Caterina et al., 1997). Intraplantar capsaicin (5 μg) significantly reduced the nociceptive threshold at all time points, consistent with sustained mechanical hypersensitivity (Figure 3D). Local administration of melagatran did not affect capsaicin-induced mechanical hypersensitivity.
These findings suggest that formalin, bradykinin and a PAR2 agonist activate melagatran-sensitive serine proteases that cause mechanical hyperalgesia. The results are consistent with formalin-, bradykinin- and PAR2-AP-induced activation of melagatran-sensitive proteases in the paw (Figure 1). However, proteases do not contribute to the algesic actions of capsaicin, which directly activates TRPV1 on nociceptive neurons.
Melagatran-sensitive serine proteases mediate the proinflammatory actions of diverse stimuli
We similarly determined if proinflammatory agents induce local inflammation by generating serine proteases. We made intraplantar injections of formalin (2.5%), bradykinin (3 μg), PAR2-AP (50 μg),or NaCl (vehicle control, 0.9%) to mice pretreated with melagatran or vehicle. Paw thickness was measured as an index of oedema formation. Formalin caused a robust increase in paw thickness for up to 4 h and intraplantar injection of melagatran reduced the oedema formation at all time points (Figure 5A). Bradykinin and PAR2-AP caused a smaller oedema, which was also significantly inhibited by melagatran (Figure 5B,C). Thus, melagatran-sensitive serine proteases mediate the effects of formalin, bradykinin and PAR2-AP on oedema formation in the mouse paw.
Figure 5.

Effects of melagatran on inflammation. Mice received intraplantar injections of formalin (2.5%) (A), bradykinin (BK, 3 μg) (B), PAR2-AP (50 μg) (C) or vehicle (Veh). Melagatran (MGT) or vehicle was administered locally (430 ng, 10 μL, intraplantar, 15 min pretreatment). Paw thickness was measured with callipers. All treatments significantly increased paw thickness over baseline, indicating oedema formation and inflammation. Melagatran significantly decreased oedema formation, indicating inhibition of inflammation. n = 10–12 mice per group. ***P < 0.001 proinflammatory and algesic agents compared with vehicle controls. #P < 0.05, ###P < 0.001 melagatran-treated mice receiving proinflammatory and algesic agents compared with vehicle controls.
Proinflammatory and algesic stimuli generate melagatran-sensitive serine proteases that cleave and activate PAR2 to induce mechanical hypersensitivity
To determine whether the proteases that are generated by proinflammatory and algesic stimuli can activate PAR2, we bioassayed tissue extracts for PAR2-dependent Ca2+ signalling in KNRK-mPAR2 and KNRK-VC cells. These cells are useful for studying proteolytic activation of PARs, as Ca2+ responses of KNRK cells to many serine proteases require PAR expression (Knecht et al., 2007). Extracts were prepared from paw tissue collected 60 min after intraplantar injection of PAR2-AP (50 μg), bradykinin (5 μg) or NaCl (0.9%). Extracts (30 or 40 μg protein) from PAR2-AP-treated tissues caused a prompt and transient increase in [Ca2+]i in KNRK-mPAR2 cells, followed by a gradual increase that reached a plateau within ∼3 min (Figure 6A,E). In contrast, these extracts did not cause the prompt and transient increase in [Ca2+]i in KNRK-VC cells, but only caused a gradual and delayed response (Figure 6B,E). These results suggest that the rapid increase in [Ca2+]i is PAR2-dependent. The minor delayed response observed in KNRK-mPAR2 and KNRK-VC cells may be a non-specific effect of the injection of high protein content solutions or attributable to other substances that can stimulate KNRK cells. Extracts from tissues treated with bradykinin also caused a prompt and transient increase in [Ca2+]i in KNRK-mPAR2 cells (Figure 6C), which was markedly diminished in KNRK-VC cells (Figure 6E). The residual responses from bradykinin-treated extracts in KNRK-VC cells are presumably because of a PAR2-independent process that remains to be identified. In contrast, extracts from control tissues (0.9% NaCl) had no effect on [Ca2+]i in KNRK-mPAR2 cells (Figure 6D,E). Importantly, treatment of extracts from PAR2-AP- or bradykinin-treated tissues with melagatran (1 μM) significantly reduced or abolished the rapid response in KNRK-mPAR2 cells (Figure 6E). These results indicate that PAR2-AP and bradykinin generate melagatran-sensitive serine proteases that can activate PAR2. The observation that melagatran prevented the actions of extracts on KNRK-PAR2 cells suggests a requirement of protease activity for activation of PAR2, and thus excludes the possibility that residual PAR2-AP within the paw mediates these effects. Whether melagatran has non-selective actions in this assay that are independent of protease inhibition remains to be determined.
Figure 6.

Generation of PAR2-activating proteases. Extracts (30 μg protein) from tissues treated with PAR2-AP (50 μg), bradykinin (5 μg) or NaCl (0.9%) were bioassayed for effects on [Ca2+]i measured in single cells. (A–D) show representative changes in [Ca2+]i in KNRK-mPAR2 and KNRK-VC cells, with each line a response of a single cell. Extracts from tissues pretreated with PAR2-AP (A) or bradykinin (BK) (C) rapidly increased [Ca2+]i in KNRK-mPAR2 cells. This effect was not detected in KNRK-VC cells (B). Extracts from tissues treated with NaCl did not rapidly raise [Ca2+]i in KNRK-mPAR2 cells (D). (E) shows the magnitude of the peak increase in [Ca2+]i in KNRK-mPAR2 or KNRK-VC cells. Melagatran reduced or prevented the increase in [Ca2+]i of KNRK-mPAR2 cells challenged with extracts of tissue from PAR2-AP- and bradykinin-treated mice. n = 2–5 coverslips per group, with 16–36 cells analysed. Extracts were assayed from n = 4 mice per experimental treatment. *P < 0.05 compared with NaCl-treated tissues in KNRK-PAR2 cells. #P < 0.05 vehicle compared with melagatran, bradykinin-treated tissues in KNRK-PAR2 cells. **P < 0.05 responses in KNRK-VC compared with KNRK-PAR2 cells.
We have previously reported that PAR2 deletion attenuates the algesic effects of formalin in the mouse paw, suggesting that the effect of formalin includes generation of proteases that can activate PAR2 (Vergnolle et al., 2001). PAR2 deletion also prevents the algesic actions of PAR2-AP, which require direct activation of this receptor (Vergnolle et al., 2001). To determine whether protease generation and PAR2 activation mediates the algesic effects of bradykinin, we compared mechanical hypersensitivity in par2+/+ and par2−/− mice. Bradykinin (300 ng intraplantar) induced the expected mechanical hypersensitivity in par2+/+ mice that was sustained for 240 min (Figure 7). This effect was significantly reduced in par2−/− mice at all times, with the exception of 120 min. Thus, bradykinin-induced mechanical hypersensitivity depends in part on the generation of PAR2-activating proteases.
Figure 7.

Bradykinin-induced mechanical hypersensitivity. Bradykinin (0.3 μg) was administered by intraplantar injection to par2+/+ and par2−/− mice. In par2+/+ mice, bradykinin caused a robust decrease in the nociceptive threshold for stimulation of the injected paws with von Frey filaments, consistent with mechanical hypersensitivity. This effect was significantly reduced in par2−/− mice. n = 7–8 mice per group. *P < 0.05, ***P < 0.01 par2−/− significantly different from par2+/+ mice.
Activation of PAR2 generates proteases that can cleave peptides derived from PAR2, PAR4 and PAR1
To determine whether a PAR2 agonist could induce the activation of proteases that cleave multiple PARs, we incubated tissue extracts from mice treated with PAR2-AP (50 μg, intraplantar) or NaCl (0.9%) with fluorogenic peptides derived from the canonical cleavage and activation sites (↓) of PAR2 (SKGR↓SLIG), PAR4 (PAPR↓GYPG) and PAR1 (LDPR↓SFLL). Cleavage at any site within these sequences would generate a fluorescence signal. Extracts from tissues treated with PAR2-AP more rapidly degraded all PAR-derived peptides than extracts of NaCl-treated tissues (Table 2013a). This difference was most pronounced for cleavage of PAR2 and PAR4, where the cleavage by PAR2-AP-treated extracts was 2.6-fold (PAR2) and 3.9-fold (PAR4) of the cleavage by NaCl-treated extracts. These results support the concept that PAR2 agonists stimulate the generation of proteases that can cleave and activate multiple PARs. In this manner, PAR2 agonists may lead to the generation of proteases that cleave and activate PAR2. This novel mechanism of positive feedback control may amplify and sustain PAR2-stimulated inflammation and pain.
Table 1.
Activity of tissue extracts for cleavage of PAR-derived peptides
| PAR-derived substrate | Intraplantar injection | Activity (%) | SD | Fold change |
|---|---|---|---|---|
| PAR2 | NaCl | 100.0 | 44.4 | 2.6 (P = 0.0251 vs. NaCl) |
| PAR2-AP | 264.0 | 68.2 | ||
| PAR4 | NaCl | 46.0 | 48.4 | 3.9 (P = 0.0231 vs NaCl) |
| PAR2-AP | 179.4 | 43.1 | ||
| PAR1 | NaCl | 57.3 | 6.4 | 1.7 (P = 0.0851 vs. NaCl) |
| PAR2-AP | 94.8 | 27.8 |
Extracts of paw prepared 60 min after intraplantar injection of PAR2-AP (50 μg) or NaCl (0.9%) were incubated with fluorogenic peptides derived from the canonical cleavage and activation sites for PAR2, PAR4 and PAR1. Activities were normalized against LDH activity of the extracts. The activity of NaCl-treated extracts for cleavage of PAR2-derived peptide was arbitrarily set to 100%. The change in activity between NaCl- (control) and PAR2-AP-treated animals, expressed in fold difference, is shown in the last column. Results are the mean ± SD of three independent experiments.
Discussion and conclusions
Our results show that injection of formalin, bradykinin and PAR2-AP into the mouse paw results in the local formation of active proteases that, in common with mouse trypsin 4, are susceptible to the serine protease inhibitor melagatran yet are relatively resistant to the polypeptide inhibitor SBTI. These proteases cleave PAR-derived peptides and signal to cells by a PAR2-dependent mechanism, which indicates that they can activate PAR2. By studying mice treated with protease inhibitors or lacking PAR2, we conclude that formalin, bradykinin and PAR2-AP can cause inflammation and hyperalgesia by generating melagatran-sensitive serine proteases that activate PAR2. Our finding that PAR2 agonists activate proteases that cause inflammation and pain supports the existence of a novel mechanism of positive feedback that could amplify and sustain PAR2-dependent inflammation and algesia.
The contributions of serine proteases to the proinflammatory and algesic actions of diverse stimulants
Serine and cysteine proteases can cause neurogenic inflammation and pain by activating PAR2 on nociceptive neurons (Steinhoff et al., 2000; Vergnolle et al., 2001; Cenac et al., 2002; 2007; Ferrell et al., 2003; Lam and Schmidt, 2010; Cattaruzza et al., 2011). However, the proteases that are generated by specific proinflammatory and algesic stimuli remain to be identified and their contributions to inflammation and pain are not fully understood. To determine whether proteases are activated in pain states, we assayed protease activity in tissue extracts using a fluorogenic substrate. This analysis revealed that intraplantar formalin, bradykinin and PAR2-AP induced the activation of proteases.
We have yet to identify the proteases that are activated by intraplantar injections of formalin, bradykinin and PAR2-AP. However, each stimulus generated proteases that were susceptible to the serine protease inhibitor melagatran yet were resistant to SBTI. Melagatran potently inhibits several serine proteases, including thrombin, trypsin and kallikrein that can activate PAR2 and other PARs (Gustafsson et al., 1998). However, the sensitivity of activated proteases to melagatran, but not to SBTI, is consistent with activation of polypeptide inhibitor-resistant isoforms of trypsin, which include trypsin IV in human and P23 in rat (Nyaruhucha et al., 1997; Szmola et al., 2003; Cottrell et al., 2004; Sahin-Toth, 2005; Knecht et al., 2007). In view of the interspecies differences in trypsinogen genes, and as the mouse equivalent of trypsin IV and P23 was unknown, we expressed mouse trypsinogen 4 and examined the susceptibility of trypsin 4 to inhibitors and its ability to activate PAR2. Mouse trypsin 4, like human trypsin IV and rat P23, was inhibited by melagatran. SBTI was a less effective inhibitor of trypsin 4 than human trypsin I. Notably, incubation with trypsin 4 reduced the potency of SBTI, which suggests that trypsin 4 can degrade SBTI, as is the case with trypsin IV. A glutamine to aspartate substitution at residue 198 of mouse trypsin 4, equivalent to that of P23, probably explains its resistance to SBTI. Mouse trypsin 4 increased [Ca2+]i in KNRK cells expressing mouse PAR2 but not empty vector, which indicates that trypsin 4, like trypsin IV and P23, can activate PAR2. However, whether trypsin 4 is the target of the algesic and anti-inflammatory effects of melagatran remains to be determined.
The main finding of our study was that melagatran inhibited the proinflammatory and algesic actions of formalin, bradykinin and PAR2-AP. Although melagatran had a larger inhibitory effect on hypersensitivity than inflammatory oedema, lower doses of formalin, bradykinin and PAR2-AP were used to induce hypersensitivity than oedema, which may account for this apparent difference. Whereas melagatran inhibited bradykinin- and PAR2-AP-evoked hypersensitivity at all times, it did not prevent the immediate (30 min) hypersensitivity to formalin, which presumably occurs initially by a protease-independent mechanism involving the direct activation of TRPA1 channels (McNamara et al., 2007). The anti-inflammatory and analgesic actions of melagatran are likely to be related to inhibition of proteases rather than to a non-specific effect on neurotransmission, as melagatran had no effect on the actions of capsaicin, which induces hyperalgesia by directly activating TRPV1 channels (Caterina et al., 1997). We also observed that systemically but not locally administered melagatran induced analgesia in mice treated with formalin and PAR2-AP. Whether this analgesia is also due to inhibition of systemic protease activity or is due to a non-selective effect of melagatran is unknown and requires further investigation.
A potentially confounding aspect of our study is that we examined nociceptive behaviour in both male and female mice, as there are gender-related differences in pain perception in humans and experimental animals (Wiesenfeld-Hallin, 2005). Female mice have a lower paw withdrawal threshold to mechanical stimulation than male mice, and carrageenan-induced allodynia, but not paw oedema, is more profound in female than male mice (Li et al., 2009). Although the use of mice of both genders could have introduced variability into our studies, we used similar proportions of male and female mice in each experimental group. Whether there are gender-related differences in protease activation during inflammation remains to be investigated.
Minor trypsin isoforms, such as trypsin IV, trypsin 4 and P23, may contribute to diverse pathophysiological processes. Human trypsinogen IV is expressed by neurons and glial cells, mainly astrocytes, of human brain and spinal cord, as well as by epithelial cells of the human intestine, airway and prostate (Wiegand et al., 1993; Cottrell et al., 2004; Gallatz et al., 2007; Toth et al., 2007). Although the physiological functions of this widely expressed protease are unknown, trypsin IV can activate PAR2 and PAR1, and induces inflammation and hyperalgesia by PAR-dependent mechanisms (Cottrell et al., 2004; Grishina et al., 2005; Wang et al., 2006; Knecht et al., 2007). Trypsin IV/mesotrypsin may be preferentially activated during pancreatitis (Szmola et al., 2003), and P23 is also up-regulated in the inflamed rat pancreas (Schick et al., 1984). The mesotrypsinogen/trypsinogen IV gene PRSS3 is up-regulated in metastatic non-small cell lung cancers (Diederichs et al., 2004). Trypsinogen IV overexpression in neurons of the mouse brain results in generation of glial fibrillary acidic protein in astrocytes, suggesting a role for trypsin IV in astrocyte proliferation (Minn et al., 1998). Trypsin IV can degrade myelin basic protein and thus may contribute to multiple sclerosis (Medveczky et al., 2006). Thus, these trypsin isoforms are induced and activated during inflammation and in tumours, where the sustained, inhibitor-resistant activity of these proteases could contribute to inflammation and pain, possibly by activating PARs.
The contributions of PAR2 to the proinflammatory and algesic actions of diverse stimulants
PAR2 is expressed by primary spinal afferent neurons and has been implicated in neurogenic inflammation and pain in several systems (Steinhoff et al., 2000; Vergnolle et al., 2001; Cenac et al., 2003; 2007; Ferrell et al., 2003; Hoogerwerf et al., 2004; Lam and Schmidt, 2010; Cattaruzza et al., 2011). PAR2 deletion prevents the proinflammatory and hyperalgesic actions of PAR2-AP, trypsin and tryptase in the skin and intestine, and attenuates the hyperalgesia associated with inflammation in these tissues (Vergnolle et al., 2001; Cenac et al., 2002; Hansen et al., 2005). Several observations indicate that PAR2 also contributes to the proinflammatory and hyperalgesic actions of formalin, bradykinin and PAR2-AP. First, extracts of tissues treated with these stimulants induced Ca2+ signals in KNKR-mPAR2 cells, but not in KNRK-VC cells, consistent with PAR2 activation. Melagatran attenuated these signals, which thus require protease activity. Second, extracts cleaved peptide fragments of PAR1, PAR2 and PAR4, with high activity towards PAR2-derived peptides. Although this analysis did not identify the site of hydrolysis, cleavage at the canonical activation site is to be expected given the stimulatory actions of extracts on Ca2+ signalling. Third, bradykinin-induced mechanical hypersensitivity was attenuated in PAR2 deficient mice, in line with previous reports that PAR2 deletion attenuated the algesic actions of formalin and PAR2-AP (Vergnolle et al., 2001). Thus, in addition to the direct activation of nociceptive neurons, formalin, bradykinin and PAR2-AP may also generate proteases that can induce inflammation and pain by activating PAR2.
The identification of a novel mechanism of PAR2- and protease-dependent positive feedback
The two unexpected findings of our investigation were that PAR2-AP stimulated the generation of melagatran-sensitive proteases, and that melagatran suppressed PAR2-AP-stimulated inflammation and pain. These observations suggest that PAR2 generates self-activating proteases that can further activate this receptor and thereby amplify and sustain inflammation and pain, which suggests the existence of a novel mechanism of positive feedback control of PAR2 signalling. The cellular origin of these proteases is unknown. However, PAR2 is expressed by many cell types in the skin, including nociceptive neurons, keratinocytes and cells of the immune system, all of which may release proteases when stimulated by PAR2 agonists (Steinhoff et al., 1999). Although human trypsin IV/mesotrypsin is expressed by many types of epithelial cells and is capable of activating PAR2 and PAR1 (Cottrell et al., 2004; Grishina et al., 2005; Wang et al., 2006; Knecht et al., 2007), little is known about the mechanisms of secretion and activation of trypsinogen IV/mesotrypsinogen. To our knowledge, the control of trypsinogen 4 secretion and activation in mice has not been investigated. However, our results suggest that PAR2 agonists may cause neurogenic inflammation and pain both by the direct activation of PAR2 on nociceptive neurons, and by generating proteases from other cell types that in turn activate neuronal PAR2.
In summary, we report that agents that cause inflammation and pain by diverse pathways activate serine proteases in the skin, where proteolytic activation of PAR2 makes a substantial contribution to the inflammatory and hyperalgesic responses. Although these proteases resemble inhibitor-resistant isoforms of trypsin, further studies are required to identify and localize activated proteases. Our results suggest that inhibitors of serine proteases may be valuable treatments for inflammatory pain. The observation that melagatran inhibits pancreatic pain supports this suggestion (Ceppa et al., 2011).
Acknowledgments
Supported by NHMRC grants 63303, 1049682, 1031886 and Monash University (N. W. B.), and NIH grant DK46385 (N. W. B., K. K.).
Glossary
- PAR
protease-activated receptor
- PAR2-AP
protease-activated receptor 2 activating peptide
- SBTI
soybean trypsin inhibitor
- tosyl-GPR-pNA
N-(p-Tosyl)-Gly-Pro-Lys 4-nitroanilide acetate salt
- TRP
transient receptor potential
Author contributions
F Cattaruzza, S Amadesi, J F Carlsson, J E Murphy, V Lyo and G S Cottrell completed the experiments. K Kirkwood designed the experiments. M Bogyo provided the reagents and evaluated the results. W Knecht completed the experiments and wrote the manuscript. N W Bunnett designed the experiments and wrote the manuscript.
Conflict of interest
Wolfgang Knecht and Johan Carlsson were employed by Astra-Zeneca. The other authors have no conflicts of interest to declare.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, et al. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J. 1996;314(Pt 3):1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cattaruzza F, Lyo V, Jones E, Pham D, Hawkins J, Kirkwood K, et al. Cathepsin S is activated during colitis and causes visceral hyperalgesia by a PAR2-dependent mechanism in mice. Gastroenterology. 2011;141:1864–1874. doi: 10.1053/j.gastro.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, et al. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceppa EP, Lyo V, Grady EF, Knecht W, Grahn S, Peterson A, et al. Serine proteases mediate inflammatory pain in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1033–G1042. doi: 10.1152/ajpgi.00305.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, et al. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Grady EF, Bunnett NW. Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J Biol Chem. 2004;279:13532–13539. doi: 10.1074/jbc.M312090200. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs S, Bulk E, Steffen B, Ji P, Tickenbrock L, Lang K, et al. S100 family members and trypsinogens are predictors of distant metastasis and survival in early-stage non-small cell lung cancer. Cancer Res. 2004;64:5564–5569. doi: 10.1158/0008-5472.CAN-04-2004. [DOI] [PubMed] [Google Scholar]
- Eilers H, Cattaruzza F, Nassini R, Materazzi S, Andre E, Chu C, et al. Pungent general anesthetics activate transient receptor potential-A1 to produce hyperalgesia and neurogenic bronchoconstriction. Anesthesiology. 2010;112:1452–1463. doi: 10.1097/ALN.0b013e3181d94e00. [DOI] [PubMed] [Google Scholar]
- Emi M, Nakamura Y, Ogawa M, Yamamoto T, Nishide T, Mori T, et al. Cloning, characterization and nucleotide sequences of two cDNAs encoding human pancreatic trypsinogens. Gene. 1986;41:305–310. doi: 10.1016/0378-1119(86)90111-3. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatz K, Medveczky P, Nemeth P, Szilagyi L, Graf L, Palkovits M. Human trypsin(ogen) 4-like immunoreactivity in the white matter of the cerebral cortex and the spinal cord. Ideggyogy Sz. 2007;60:118–123. [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishina Z, Ostrowska E, Halangk W, Sahin-Toth M, Reiser G. Activity of recombinant trypsin isoforms on human proteinase-activated receptors (PAR): mesotrypsin cannot activate epithelial PAR-1, -2, but weakly activates brain PAR-1. Br J Pharmacol. 2005;146:990–999. doi: 10.1038/sj.bjp.0706410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson D, Antonsson T, Bylund R, Eriksson U, Gyzander E, Nilsson I, et al. Effects of melagatran, a new low-molecular-weight thrombin inhibitor, on thrombin and fibrinolytic enzymes. Thromb Haemost. 1998;79:110–118. [PubMed] [Google Scholar]
- Hansen KK, Sherman PM, Cellars L, Andrade-Gordon P, Pan Z, Baruch A, et al. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci U S A. 2005;102:8363–8368. doi: 10.1073/pnas.0409535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf WA, Shenoy M, Winston JH, Xiao SY, He Z, Pasricha PJ. Trypsin mediates nociception via the proteinase-activated receptor 2: a potentially novel role in pancreatic pain. Gastroenterology. 2004;127:883–891. doi: 10.1053/j.gastro.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, Bunnett NW. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280:16076–16087. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht W, Cottrell GS, Amadesi S, Mohlin J, Skaregarde A, Gedda K, et al. Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia. J Biol Chem. 2007;282:26089–26100. doi: 10.1074/jbc.M703840200. [DOI] [PubMed] [Google Scholar]
- Koistinen H, Koistinen R, Zhang WM, Valmu L, Stenman UH. Nexin-1 inhibits the activity of human brain trypsin. Neuroscience. 2009;160:97–102. doi: 10.1016/j.neuroscience.2009.02.042. [DOI] [PubMed] [Google Scholar]
- Lam DK, Schmidt BL. Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway. Pain. 2010;149:263–272. doi: 10.1016/j.pain.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fan X, Warner M, Xu XJ, Gustafsson JA, Wiesenfeld-Hallin Z. Ablation of estrogen receptor alpha or beta eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain. 2009;143:37–40. doi: 10.1016/j.pain.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, et al. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medveczky P, Antal J, Patthy A, Kekesi K, Juhasz G, Szilagyi L, et al. Myelin basic protein, an autoantigen in multiple sclerosis, is selectively processed by human trypsin 4. FEBS Lett. 2006;580:545–552. doi: 10.1016/j.febslet.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Minn A, Schubert M, Neiss WF, Muller-Hill B. Enhanced GFAP expression in astrocytes of transgenic mice expressing the human brain-specific trypsinogen IV. Glia. 1998;22:338–347. doi: 10.1002/(sici)1098-1136(199804)22:4<338::aid-glia3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Coelho AM, Grady E, Compton SJ, Wallace JL, Hollenberg MD, et al. Colitis induced by proteinase-activated receptor-2 agonists is mediated by a neurogenic mechanism. Can J Physiol Pharmacol. 2003;81:920–927. doi: 10.1139/y03-080. [DOI] [PubMed] [Google Scholar]
- Nyaruhucha CN, Kito M, Fukuoka SI. Identification and expression of the cDNA-encoding human mesotrypsin(ogen), an isoform of trypsin with inhibitor resistance. J Biol Chem. 1997;272:10573–10578. doi: 10.1074/jbc.272.16.10573. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, et al. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. 2006;281:32095–32112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Poole DP, Amadesi S, Veldhuis NA, Abogadie FC, Lieu T, Darby W, et al. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem. 2013;288:5790–5802. doi: 10.1074/jbc.M112.438184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov. 2012;11:69–86. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- Sahin-Toth M. Human mesotrypsin defies natural trypsin inhibitors: from passive resistance to active destruction. Protein Pept Lett. 2005;12:457–464. doi: 10.2174/0929866054395356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick J, Kern H, Scheele G. Hormonal stimulation in the exocrine pancreas results in coordinate and anticoordinate regulation of protein synthesis. J Cell Biol. 1984;99:1569–1574. doi: 10.1083/jcb.99.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Szmola R, Kukor Z, Sahin-Toth M. Human mesotrypsin is a unique digestive protease specialized for the degradation of trypsin inhibitors. J Biol Chem. 2003;278:48580–48589. doi: 10.1074/jbc.M310301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth J, Siklodi E, Medveczky P, Gallatz K, Nemeth P, Szilagyi L, et al. Regional distribution of human trypsinogen 4 in human brain at mRNA and protein level. Neurochem Res. 2007;32:1423–1433. doi: 10.1007/s11064-007-9327-8. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, et al. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Cenac N, Altier C, Cellars L, Chapman K, Zamponi GW, et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br J Pharmacol. 2010;159:1161–1173. doi: 10.1111/j.1476-5381.2009.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Luo W, Wartmann T, Halangk W, Sahin-Toth M, Reiser G. Mesotrypsin, a brain trypsin, activates selectively proteinase-activated receptor-1, but not proteinase-activated receptor-2, in rat astrocytes. J Neurochem. 2006;99:759–769. doi: 10.1111/j.1471-4159.2006.04105.x. [DOI] [PubMed] [Google Scholar]
- Wiegand U, Corbach S, Minn A, Kang J, Muller-Hill B. Cloning of the cDNA encoding human brain trypsinogen and characterization of its product. Gene. 1993;136:167–175. doi: 10.1016/0378-1119(93)90460-k. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gender Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]


