Abstract
BACKGROUND AND PURPOSE
Pulmonary arteries (PAs) are innervated, but little is known about the role of neuronal axis in pulmonary hypertension (PH). Here, we have examined the role of the neuropeptide Y (NPY) and its Y1 receptor in PH pathogenesis.
EXPERIMENTAL APPROACH
NPY was localized by immunofluorescence. Expression of NPY and Y1 receptor were determined by quantitative PCR. Cellular response to NPY stimulation was assessed by Western blotting, thymidine incorporation and calcium imaging. Wire myography and isolated perfused mouse lung were applied to study pulmonary vasoactive effects of NPY. Selective receptor antagonists were used to assess the contribution of receptor subtypes in mediating NPY effects.
KEY RESULTS
Samples from PH patients showed increased NPYergic innervation within the PA wall and higher Y1 receptor expression, compared with donors. However, NPY levels were unchanged in both PA and serum. In the chronic hypoxic mouse model, Y1 receptor were up-regulated, while expression of both NPY and Y1 receptor was increased in the lungs of monocrotaline and SU5416-hypoxia rats. On a functional level, NPY acutely increased intracellular calcium levels and enhanced vasoconstriction of lung vessels preconstricted with adrenaline. Furthermore, NPY stimulated proliferation of human pulmonary arterial smooth muscle cells and activated p38 and PKD pathways. Correspondingly, higher phosphorylation of PKD was observed in remodelled vessels from PH patients. The selective Y1 receptor antagonist, BIBO 3304, concentration-dependently inhibited vasoconstrictive and proliferative effects of NPY.
CONCLUSIONS AND IMPLICATIONS
NPY and Y1 receptor are possible mediators of both vasoconstriction and pulmonary vascular remodelling in PH.
Introduction
Pulmonary hypertension (PH) is a serious and progressive condition characterized by increased pulmonary artery pressure (PAP) as a consequence of vasoconstriction and pulmonary vascular remodelling (Morrell et al., 2009; Stacher et al., 2012). Despite intensive research, the exact pathomechanisms underlying these processes are still not completely understood. Increased knowledge of molecular pathways involved in the development and progression of PH could help to define new therapeutic targets.
The majority of research and current treatment options for PH have focused on imbalance of vasodilating and vasoconstricting substances, such as NO, prostacylin and endothelin, as well as their concomitant effects on the proliferation of vascular cells (Morrell et al., 2009). Early studies have shown increased sympathetic innervation of resistance arteries of neonates with PH (Allen et al., 1989). Despite this, the role of vascular innervation and neurohormonal activation has largely been neglected (de Man et al., 2013). A recent review from de Man et al. (2013) suggested that sympathetic activation is present in PH and strongly influences survival of PH patients. Furthermore, it has been recently reported that denervation of the pulmonary arteries (PAs) could have beneficial effects for those patients with idiopathic pulmonary arterial hypertension (IPAH) who do not respond to standard therapy (Chen et al., 2013).
Pulmonary vessels possess both sympathetic and parasympathetic innervation (Kummer, 2009). Neuropeptide Y (NPY) is a 36-amino acid peptide neurotransmitter, released from postganglionic sympathetic perivascular neurons (Lobaugh and Blackshear, 2008) and is the predominant neuropeptide associated with pulmonary vascular nerves (Allen et al., 1989). NPY augments vasoconstrictor effects of noradrenergic neurons and the release of NPY into the circulation occurs during sympathetic activation (Ekblad et al., 1984; Painsipp et al., 2010). It acts through several GPCRs, four of which are expressed in humans and elicit a range of effects in the cardiovascular, immune, central and peripheral nervous systems (Pons et al., 2010; receptor nomenclature follows Alexander et al., 2013). Detrimental vasoconstrictive and proliferative effects of NPY in the cardiovascular system seem to be mainly mediated by Y1 receptors and, potentially, by the Y5 receptor (Abe et al., 2007).
Here, we report increased expression of NPY Y1 receptors in experimental PH models and IPAH lung samples. Functional relevance and the role of Y1 receptor downstream signalling was confirmed in vitro and in vivo, further supporting the concept of pharmacological synergism and neurohormonal activation in pathology of PH (MacLean and Morecroft, 2001). Taken together, these results suggest a possible role of the NPY/ Y1 receptor axis in the processes underlying pathogenesis of PH.
Material and methods
Human material
The protocol and tissue usage were approved by the institutional ethics committees (Medical University of Vienna, Austria (976/2010) and Justus-Liebig-University School of Medicine (AZ 31/93), Giessen, Germany). Full informed consent was obtained before lung transplantation. The patients' characteristics are given in Supporting Information Table S1. The study protocol for blood donation was approved by the Institutional Review Board of the Medical University of Graz in accordance with national law, and patient characteristics are included in Supporting Information Table S2. Human lung tissue samples were obtained from IPAH patients who were undergoing lung transplantation at the Department of Cardiothoracic Surgery, Medical University of Vienna, Austria. Downsized non-transplanted, non-tumour bearing donor lungs served as healthy controls.
Animals
All animal care and experimental procedures complied with German Austrian and international guidelines and were approved by the Regierungspraesidium Giessen. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All measures were taken to keep animal suffering to a minimum.
All animal materials derived from chronic hypoxia, monocrotaline and SU5416 models (details given below) were obtained from previously published studies (Schermuly et al., 2005; Dumitrascu et al., 2006; Kwapiszewska et al., 2009; Lang et al., 2011; Weisel et al., 2012). Adult male C57BL/6J mice (20–22 g) and adult male Sprague-Dawley rats (250–350 g) were purchased from Charles River Laboratories and randomly distributed to groups. All animals were housed under controlled temperature and lighting and allowed food and water ad libitum.
Exposure to chronic hypoxia
Lung samples (n = 4–8 animals per group) from mice exposed to normobaric normoxia [fraction of inspired oxygen (FiO2) of 0.21], normobaric hypoxia (FiO2 of 0.10) for 21 days, or hypoxia with subsequent re-exposure to normoxia for 1, 7 or 21 days were obtained as described previously (Weisel et al., 2012).
Rat monocrotaline and SU5416 models of PAH
Lung samples (n = 4–10 animals per group) from the monocrotaline [28 days after single subcutaneous injection, 60 mg·kg−1] as well as the SU5416 rat model [single subcutaneous injection of VEGFR inhibitor SU5416 (20 mg·kg−1) followed by 3 weeks exposure to normobaric hypoxia (FiO2 of 0.10)] were obtained as described previously (Schermuly et al., 2005; Dumitrascu et al., 2006; Kwapiszewska et al., 2009; Lang et al., 2011).
Immunofluorescence and immunohistochemistry staining
Localization of the protein gene product 9.5 (PGP9.5), NPY and phospho-PKD in human, mouse and rat lung tissue was assessed on paraffin-embedded sections. Sections were deparaffinized, rehydrated and treated with either 0.05% trypsin (PGP9.5), heat-induced antigen retrieval in buffer pH 6 (NPY) or pH 9 (phospho-PKD). Non-specific binding was blocked with 10% BSA prior to overnight incubation with primary antibodies at 4°C: PGP9.5 (Biotrend – Destin, FL, USA, 1:400), NPY (Sigma – Vienna, Austria, 1:300) and phospho-PKD (Cell Signaling – Danvers, MA, USA, 1:100). After washing steps, sections were incubated with anti-rabbit immunoglobulin labelled with Alexa Fluor 594 (Invitrogen – Vienna, Austria, 1:500), Alexa Fluor 488 (Invitrogen) or HRP-conjugated anti-rabbit immunoglobulin (ImPRESS, Vector Laboratories, Burlingame, CA, USA) for 1 h. Sections were washed and either mounted with DAPI-containing medium (Vector Laboratories) or counterstained with methylgreen (Sigma).
elisa
Circulating levels of NPY in human serum from donors and IPAH patients were determined using a commercially available elisa kit (Usnc Life Science, Wuhan, China) according to the manufacturer's instructions.
RNA isolation and real-time PCR
RNA was isolated from samples of lung or PA using TriFast RNA isolation buffer (PeqLab, Erlangen, Germany) followed by RNA purification using the peqGOLD Total RNA isolation kit (PeqLab). Total RNA was reverse transcribed using iScript kit (BioRad, Hercules, CA, USA) according to manufacturer's instructions. Real-time PCR was performed using a LightCycler 480 (Roche, Wien, Austria) with QuantiFast SYBR PCR kit (Qiagen, Hilden, Germany). Primers sequences are given in Supporting Information Table S3. PBGD (hydroxymethylbilane synthase) served as a housekeeping gene (Fink et al., 1999). Cycling conditions were as follows: 6 min at 95°C, [5 s at 96°C, 5 s at 59°C and 10 s at 72°C] ×45. Melting curve analysis and gel electrophoresis were performed to confirm the exclusive amplification of the expected PCR product. The ΔCt values for each target gene were calculated as ΔCt = Ct housekeeping gene – Ct target gene. The ΔΔCt values were calculated as ΔΔCt = ΔCt sample – mean ΔCt of control group.
Isolated, perfused and ventilated lungs from mice
Experiments on isolated perfused lungs from mice were performed as described by Nagaraj et al., (2009). Adult C57BL/6 mice (Charles River, Sulzfield, Germany) were anaesthetised by isoflurane and killed by overdose of intra-peritoneal injection of ketamine (200 mg kg−1) and xylazine (20 mg kg−1), given i.p.. Lungs were removed from the thorax under artificial ventilation, and the pulmonary circulation perfused with Krebs-Henseleit buffer (KHB, 120 mM NaCl, 4.3 mM KCl, 1.1 mM KH2PO4, 2.4 mM CaCl2, 1.3 mM MgCl2, 25 mM NaHCO3 and 13.3 mM glucose, as well as 5% (weight/volume) hydroxyethylamylopectin (molecular weight 200,000 Da) and pH adjusted to 7.4 by bubbling with normoxic gas mixture containing 5% CO2). The lungs were mounted in a water-heated chamber (IPL-1, Hugo-Sachs Electronics, March-Hugstetten, Germany) with negative pressure ventilation. An initial steady state period of 15 min (perfusion with KHB at flow rate 1 mL min−1) was taken as baseline. For concentration response experiments with adrenaline, lungs were perfused with 0.05, 0.5, 5 and 10 μg mL−1 of each concentration followed by washout. Initial adrenaline concentration range was based on previously published reports (Shen et al., 2005, Goldman et al., 1989). For all further experiments, 0.5 μg mL−1 adrenaline was used to pre-constrict the pulmonary vessels. Afterwards, increasing concentrations of NPY (1, 10 and 100 nM, Bachem, Bubendorf, Switzerland) were applied and each concentration was followed by a washout period with 0.5 μg mL−1 adrenaline. Initial NPY concentration range was based on previously published reports (Zukowska-Grojec et al., 2009) and 100 nM NPY was selected for all further experiments. For the antagonist studies, lungs were first perfused with adrenaline and 100 nM NPY. Following NPY washout, lungs were perfused with inhibitor for 3 min. Then perfusion with inhibitor in the presence of NPY was started, followed by washout and application of higher inhibitor concentrations (Y1 receptor antagonist BIBO 3304: 0.01, 0.1 and 1 μM: Y5 receptor antagonist CGP 71683: 0.1 and 1 μM; both from Tocris, Bristol, UK). Amplitude of NPY induced PAP elevation was calculated as a difference between maximal pressure elevation upon NPY application and baseline 1 min before NPY application (see Supporting Information Fig. S2). For the control and inhibitory experiments, the difference was calculated at matching time points. Amplitude of the first NPY induced vasoconstriction was set to 100% and the amplitudes of the following vasoconstrictions were re-calculated in percent relative to the first one.
Vascular reactivity measurements
Adult male C57BL/6J mice aged 8–12 weeks (Charles River Laboratories) were anaesthetized by isoflurane and killed as described above. Tertiary intra-pulmonary arteries from the left and right mouse lungs were isolated. The arteries were cleaned of surrounding tissue and cut into segments 3–4 mm in length. The PA were positioned between the jaws in wire myograph chambers (Danish MyoTechnology, Aarhus, Denmark) containing Krebs-Henseleit buffer (KHB) and connected to force transducers for the measurement of isometric tension (PowerLab, ADInstruments, Oxford, UK). A basal tension of 2 mN was applied to the arteries for an initial 45 min stabilization period. KHB containing 120 mM KCl was used to determine viability of tissues. Arteries were stimulated three times using 120 mM KCl to obtain reproducible contractions. Following stable contraction with 0.5 μg·mL−1 adrenaline (minimum 35 min), 100 nM NPY was added. For antagonist experiments, arteries were pre-constricted with adrenaline, then antagonists were applied to individual arteries BIBO 3304 (0.01, 0.1, 1 and 10 μM) or CGP 71683 (0.1 and 1 μM) or vehicle for 10 min subsequently followed by NPY stimulation. Concentration range for Y1 receptor and Y5 receptor antagonists was based on a previously published report (Dumont et al., 2006). The NPY-induced contraction (either alone or in presence of antagonists) was calculated relative to maximum adrenaline-induced contraction set to 100%.
Cell culture and cell stimulation
Primary human pulmonary artery smooth muscle cells (hPASMC) were isolated from small calibre PA (below the fourth branch of the main PA) from IPAH patients and donor lungs. The purity of hPASMC cultures was confirmed using immunofluorescent staining for α-smooth muscle actin (minimum 95% of cells stained positive). All experiments were performed with cells in passage one to five and growth arrested by serum deprivation for 24 h. In experiments with BIBO 3304 and CGP 71683, cells were incubated with indicated concentration of antagonists for 1 h prior to NPY stimulation.
Live cell calcium (Ca2+) imaging
Live cell Ca2+ imaging was performed according to (Balint et al., 2013). Human PASMC were cultured on 25-mm-diameter, gelatin-coated glass cover slides until sub-confluence. The slides were then transferred to the experimental solution (Ringer, containing 5.5 mM KCl, 140.5 mM NaCl, 0.5 mM KH2PO4, 0.5 mM Na2HPO4, 10 mM glucose, 10 mM HEPES, 1.5 mM CaCl2, 1 mM MgCl2, pH 7.4). After 25 min of Fura-2/AM incubation, the single glass cover slide was mounted on the stage of a Zeiss 200 M inverted epifluorescence microscope coupled to a PolyChrome V monochromator (Till Photonics, Grafelfing, Germany) light source in a sealed, temperature-controlled RC-21B chamber (Warner Instruments, Hamden, CT, USA). Fluorescence images were obtained with alternate excitation at 340 and 380 nm. Emitted light was collected at 510 nm by an Andor Ixon camera. The acquired images were stored and subsequently processed offline with TillVision software (Till Photonics). Cells were perfused continuously with Ringer solution (0.5 mL/min). After obtaining baseline stable fluorescence signal measurements, PASMC were pre-treated with adrenaline (0.5 μg/mL) in Ringer solution. Afterwards, cells were perfused with 100 nM NPY in the presence of adrenaline or only with adrenaline (see Supporting Information Fig. S2). In another set of experiments, NPY 100 nM was infused without pre-treating the cells (NPY). To study the effect of Y1 receptor antagonism, cells were pretreated with adrenaline and 100 nM NPY. After NPY washout, cells were perfused with 1 μM BIBO 3304 for 3 min followed by NPY stimulation.
Measurements were made every 3s. Background fluorescence was recorded from each cover slip and subtracted before calculation. Maximal and minimal values of fluorescence were determined at the end of experiment by treating the cells with 10 μM ionomycin and 10 mM EGTA. Cells that did not respond to ionomycin were discarded. For quantification of NPY effect on intracellular Ca2+, calcium signal for the entire duration of NPY administration was averaged. For other groups (control, adrenaline, NPY and inhibitor effects), corresponding values of fluorescence were calculated.
Western blotting
Cells were lysed in RIPA lysis buffer (Sigma Aldrich, Vienna, Austria) containing protease and phosphatase inhibitors (Roche). Equal amounts of proteins were loaded per lane and separated by SDS–PAGE and transferred to a PVDF membrane. After blocking with 5% BSA in TBS-T buffer, the membrane was incubated overnight at 4°C with one of the following antibodies: anti-phospho-p38, anti-p-38, anti-phosphoPKD/PKCμ, anti-PKD/PKCμ and anti-α-tubulin (all from Cell Signaling). After 1 h incubation with peroxidase-labelled secondary antibody (Cell Signaling), proteins were detected using ECL Prime Kit (GE Healthcare, Vienna, Austria).
Proliferation
Human PASMC (10 000 cells per well) were seeded in 96-well plates. Afterwards, cells were starved (VascuLife Basal Medium, Lifeline Cell Technology, Frederick, MD, USA, 0% FCS, 1% antibiotic) for 24 h. NPY was added and after 24 h, the proliferation of hPASMC was determined by [3H]-thymidine (BIOTREND, Cologne, Germany, Chemikalien GmbH) incorporation as an index of DNA synthesis and measured as increase in radioactivity by a scintillation counter (Wallac 1450 MicroBeta TriLux Liquid Scintillation Counter & Luminometer, PerkinELmer, Waltham, MA, USA). Each independent experiment was performed in 10 replicates on cells isolated from six different donors and six IPAH patients and repeated twice.
Data analysis
Data are represented as box and whisker plots or mean ± SEM. Statistical analysis was performed in GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) using the paired t-test, for experiments comparing two groups, and anova Kruskal–Wallis with the Dunn multiple comparison post test, or two-way anova with Bonferroni post tests, for experiments comparing three or more groups. P < 0.05 was considered statistically significant. Calculation of IC50 values was performed in GraphPad Prism 5 using non-linear, four parameter, regression analysis with variable slope.
Results
Arteries from IPAH patients display increased innervation
Vasoconstriction and remodelling of PA are the main characteristics of human IPAH. We speculated that vessel innervation could play a direct role in the control of pulmonary vascular tone and remodelling process in adult IPAH patients. Staining with an antibody against PGP9.5, a general neuronal marker, revealed increased number of PGP9.5 positive profiles within the PA from IPAH patients (Figure 1A–D). Similar results were obtained with an additional staining against NPY (Figure 1E–H). These results imply increased sympathetic innervation in remodelled IPAH arteries compared to donors.
Figure 1.
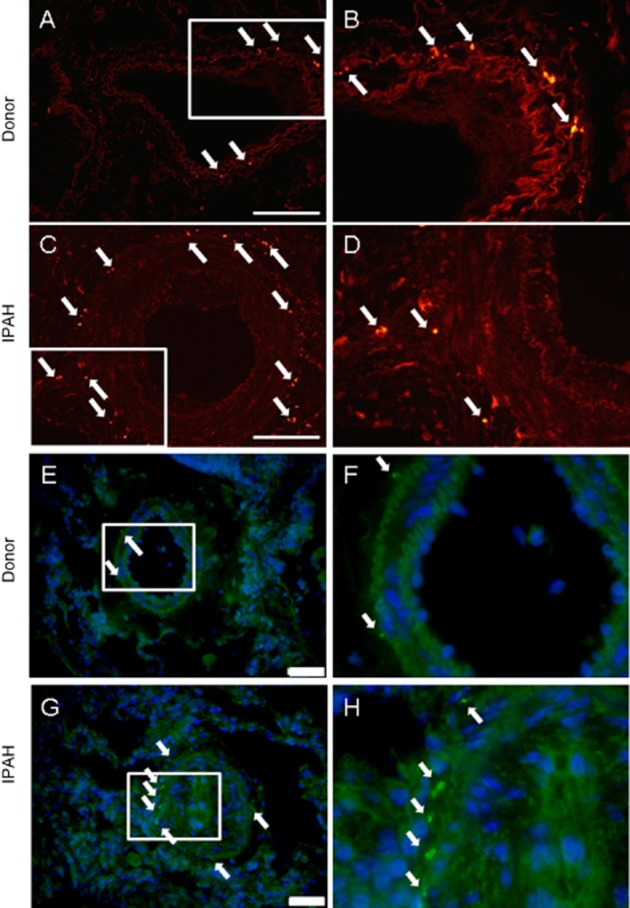
IPAH remodelled arteries displayed higher number of sympathetic nerve endings. Immunofluorescent staining of donor and IPAH lung sections against (A–D) PGP9.5 neuronal marker and (E–H) NPY. Right panels represent high power magnification of boxed area in left panels. Arrows indicate positive staining. Representative images from five different patients per group. Scale bar represents 200 μm for A, C or 50 μm for E, G.
Expression levels of NPY1R are increased in IPAH patients
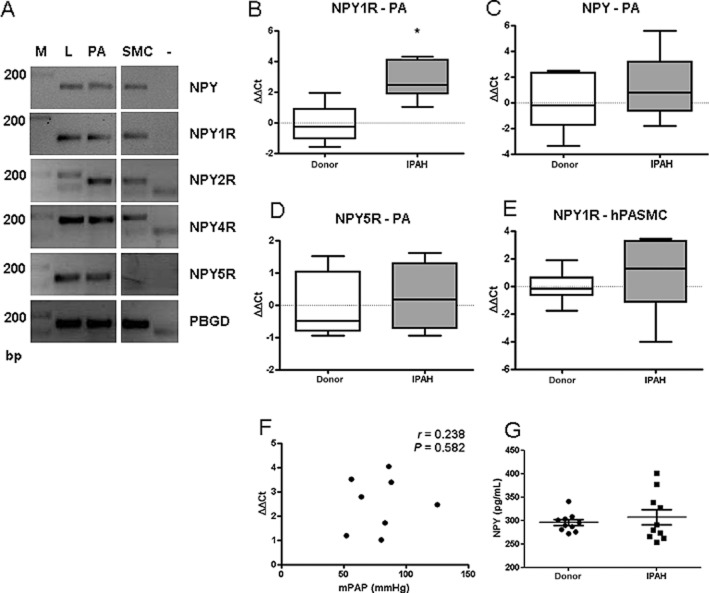
Being the predominant neuropeptide system innervating the pulmonary vasculature (Allen et al., 1989), we focused further on the NPY/Y1 receptor axis. Control human lungs expressed NPY and all four subtypes of NPY receptors (Figure 2A). In contrast, hPASMC expressed Y1, Y2 and Y4, but not Y5 receptors. NPY was at the limit of detection (Figure 2A). The low or absence of NPY expression in hPASMC corresponds with the immunohistochemical data, which showed that NPY was predominantly localized to neuronal endings and inflammatory cells. Expression levels of Y1 receptors were significantly up-regulated in human PA isolated from IPAH patients (Figure 2B); however, the mRNA levels of NPY and Y5 receptors were unchanged between donor and IPAH samples (Figure 2C, D). Furthermore, there was no difference in Y1 receptor expression levels between donor and IPAH hPASMC (Figure 2E) and no correlation of mean PAP with Y1 receptor expression levels in PA isolated from IPAH patients in our patient cohort (Figure 2F). Furthermore, circulating levels of NPY were not significantly different between donors and IPAH patients (Figure 2G).
Figure 2.
Expression of NPY Y1 receptors is increased in IPAH patients. (A) Representative gel image of PCR products for NPY ligand and receptors, in human lung tissue. PBGD serves as a positive control. Marker (M), lung homogenate (L), PA, PASMC and negative control (–). mRNA expression levels in PA from donor and IPAH lungs of (B) Y1 receptors (C) NPY and (D) Y5 receptors (n = 4–10). (E) Y1 receptor mRNA expression level in donor and IPAH hPASMC (n = 9–10). (F) Correlation of Y1 receptor gene expression in PA samples versus mean PAP (mPAP) in IPAH patient cohort. (G) Determination of circulating NPY levels in serum from donors and IPAH patients (n = 10). *P < 0.05.
Expression levels of Y1 receptors are elevated in hypoxic mouse PH model
In order to experimentally assess the human data, we performed NPY immunostaining and measured expression levels of Y1 receptors and NPY in a hypoxia-induced PH mouse model. NPY staining was mostly observed in endothelium layer and strong immunoreactivity in inflammatory cells (Figure 3A, B). Y1 receptor expression was increased after 21 days of hypoxia exposure (Figure 3C). As hypoxia-induced changes in mice are reversible (Hislop and Reid, 1977) and in order to mimic a potentially therapeutic reverse remodelling process, we re-exposed hypoxic animals to normoxia. Re-exposure revealed down-regulation of Y1 receptors expression (Figure 3E). Although mRNA levels of NPY were not increased in chronic hypoxia (Figure 3C), expression of NPY was significantly down-regulated in acute phase of re-exposure of hypoxic animals to normoxia (Figure 3F).
Figure 3.
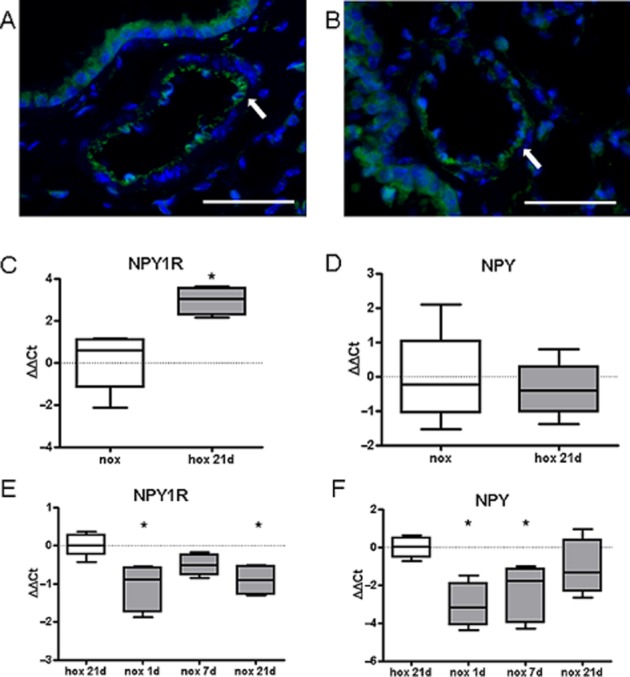
Expression of Y1 receptors is increased in hypoxia-induced pulmonary hypertension in mice. Representative immunofluorescence images of NPY localization in pulmonary vessels of mice exposed to (A) normoxia or (B) 21 days hypoxia (scale bar represents 50 μm). mRNA expression levels of (C) Y1 receptors and (D) NPY ligand in lung homogenates of mice exposed to normoxia (nox) or 21 days hypoxia (hox 21d), (n = 4–8). Gene expression of (E) Y1 receptors and (F) NPY in lung homogenates from mice first exposed to 21 days hypoxia and re-exposed for 1, 7 and 21 days to normoxia (n = 4–6). *P < 0.05.
Expression levels of Y1 receptors and NPY ligand is increased in rat PH models
We next investigated whether the previously observed changes could be reproduced in more severe animal models of PH, which more closely resemble end-stage human disease. Corresponding to the mouse data, NPY immunostaining was observed mainly in non-neuronal cells in rats given SU5416 and exposed to hypoxia (Figure 4A, B). Furthermore, we could observe significant up-regulation of both Y1 receptors and NPY in lung homogenates (Figure 4C, D). Similar results were obtained in monocrotaline-treated rats. Here, accumulation of NPY-positive inflammatory cells (Figure 4E, F) and higher expression levels of NPY ligand and Y1 receptors were observed (Figure 4G, H). Taken together, increased expression levels of Y1 receptors, in both human disease and different animal PH models, suggest a possible role of the NPY-Y1 receptor axis in the pathogenesis of PH.
Figure 4.
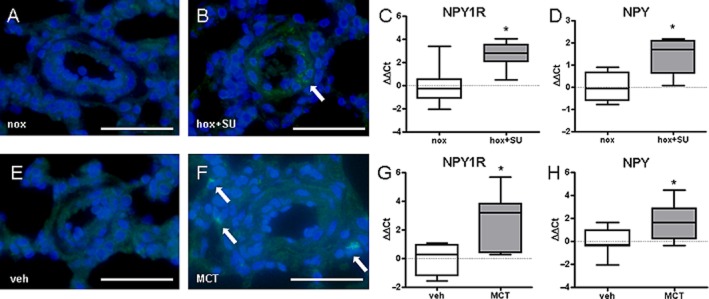
Expression of NPY and Y1 receptors is increased in rat models of pulmonary hypertension. Representative immunofluorescence images of NPY localization in pulmonary vessels from rats exposed to (A) normoxia (nox) or (B) hypoxia+SU5416 (hox+SU). Gene expression of (C) Y1 receptors and (D) NPY in lung homogenates from rats exposed to nox or hox+SU (n = 4–7). Representative immunofluorescence images of NPY localization in pulmonary vessels from (E) vehicle (veh) and (F) monocrotaline (MCT)-treated rats. Gene expression of (G) Y1 receptors and (H) NPY in lung homogenates from veh and MCT-treated rats (n = 4–10). *P < 0.05. Scale bare represents 50 μm. Arrows indicate positive staining.
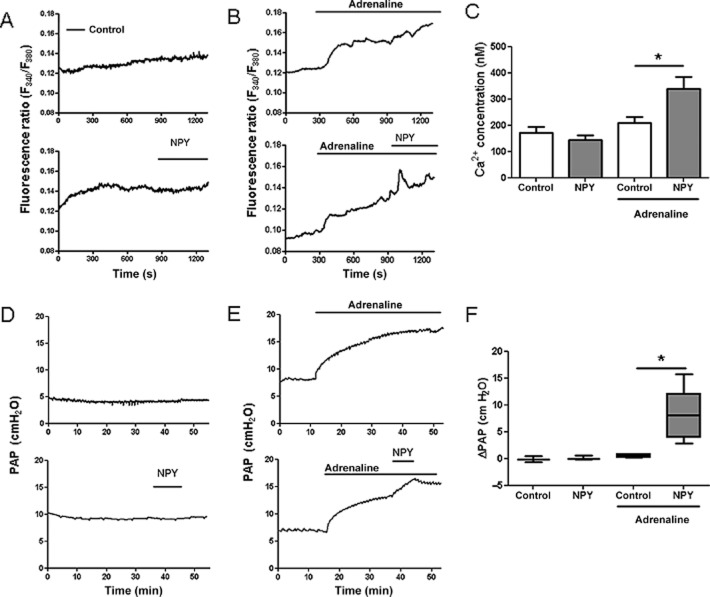
NPY stimulation synergistically increases adrenaline-induced rise in intracellular Ca2+ and pulmonary vasoconstriction
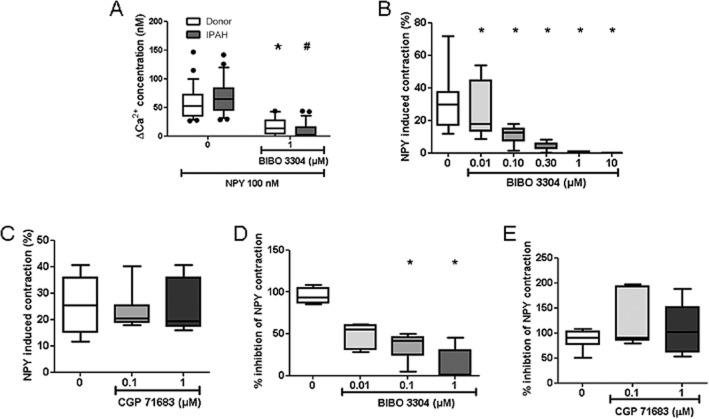
Next, we investigated whether these changes have either adaptive or pathogenic role in PH. The functional role was first examined in isolated hPASMC. As shown in Figure 2A, hPASMC expressed Y1 receptors but not Y5 receptors. Stimulation of hPASMC with NPY caused a significant and transient increase in intracellular Ca2+ only in the presence of adrenaline (Figure 5A–C). The effect of NPY on intracellular Ca2+ prompted us to investigate acute effects of NPY stimulation on pulmonary circulation in an ex vivo setting. In isolated, ventilated and perfused mouse lungs, NPY ligand led to enhanced vasoconstriction of adrenaline pre-constricted lung vessels (Figure 5D–F). NPY-induced calcium influx was mediated by Y1 receptors with no significant difference in response between control and IPAH hPASMC (Figure 6A). Furthermore, NPY-induced additional vasoconstriction in adrenaline pre-constricted isolated mouse PA (Supporting Information Fig. S2C). This effect could be concentration-dependently abolished by pretreatment with the specific Y1 receptor antagonist, BIBO 3304 (Figure 6B) with an apparent IC50 value of 0.08 μM. In contrast, the specific Y5 receptor antagonist, CGP 71683, did not alter NPY-induced mouse PA vasoconstriction (Figure 6C). Similarly, in isolated, ventilated and perfused mouse lungs, NPY-enhanced vasoconstriction of adrenaline pre-constricted lung vessels was blocked with BIBO 3304 with an IC50 value of 0.09 μM (Figure 6D), while CGP 71683 had no effect (Figure 6E).
Figure 5.
NPY stimulation increased intracellular calcium and caused pulmonary vasoconstriction in the presence of adrenaline. Human PASMC were loaded with Fura-2 calcium-sensitive dye, perfused with solutions containing NPY (100 nM), adrenaline (0.5 μg·mL−1) or both and monitored with inverted epifluorescence microscope. (A, B) Representative fluorescence ratio tracings over time in human PASMC. (C) Intracellular calcium concentrations upon NPY stimulation in the presence or absence of adrenaline (n = 14–15 cells in each group, from four different donors). (D, E) Representative tracings of PAP over time in isolated, perfused and ventilated mouse lungs. (F) Changes in PAP upon NPY stimulation in the presence or absence of adrenaline (n = 3–5 per group). *P < 0.05.
Figure 6.
The Y1 receptor antagonist blocked NPY-induced intracellular calcium increase and pulmonary vasoconstriction. Human PASMC (from three different donors and IPAH patients) were loaded with Fura-2/AM calcium-sensitive dye, perfused with solutions containing NPY (100 nM), adrenaline (adrenaline; 0.5 μg·mL−1) and monitored with inverted epifluorescence microscope. (A) Change in intracellular calcium concentration upon NPY stimulation in the presence or absence of the Y1 receptor antagonist BIBO 3304 (n ≥ 20 cells in each group). Changes in isolated intrapulmonary mouse pulmonary artery contraction upon NPY stimulation in the presence or absence of (B) BIBO 3304 or (C) the Y5 receptor antagonist CGP 71683 (n ≥ 7 per group). Changes in PAP over time in isolated, perfused and ventilated mouse lungs under adrenaline and NPY stimulation and in the presence or absence of (D) BIBO 3304 or (E) CGP 71683 (n ≥ 5 per group). * or # (in the case of IPAH PASMC) denotes P < 0.05.
NPY stimulation of hPASMC leads to increased PKD activation and proliferation
In addition to vasoconstriction, another hallmark of PH is pulmonary vascular remodelling, which is characterized by aberrant hPASMC proliferation. Therefore, we investigated direct effect of NPY on human PASMC. NPY stimulation increased hPASMC proliferation as analysed by 3H-thymidine incorporation, with no significant difference in response between control and IPAH PASMC (Figure 7A). This effect was specifically blocked with Y1 receptor (Figure 7B), but not the Y5 receptor (Figure 7C) antagonist, cumulatively confirming the specificity of this pharmacological approach and the notion that NPY acts on hPASMC via Y1 receptors. Next, we performed Western blot studies to assess the phosphorylation status of downstream signalling molecules following Y1 receptor activation. Human PASMC stimulated with NPY displayed increased phosphorylation of p38 and PKD kinases (Figure 7D, E), indicating activation of these signalling cascades. This effect was partially blocked by the Y1 receptor antagonist BIBO 3304 (Figure 7F, G). In accordance with in vitro data, we observed increased staining against phospho-PKD in IPAH pulmonary arteries compared with donor (Figure 7H, I). These results imply that NPY could have a direct pathological role in pulmonary vascular remodelling process, independent of co-adrenergic activation.
Figure 7.
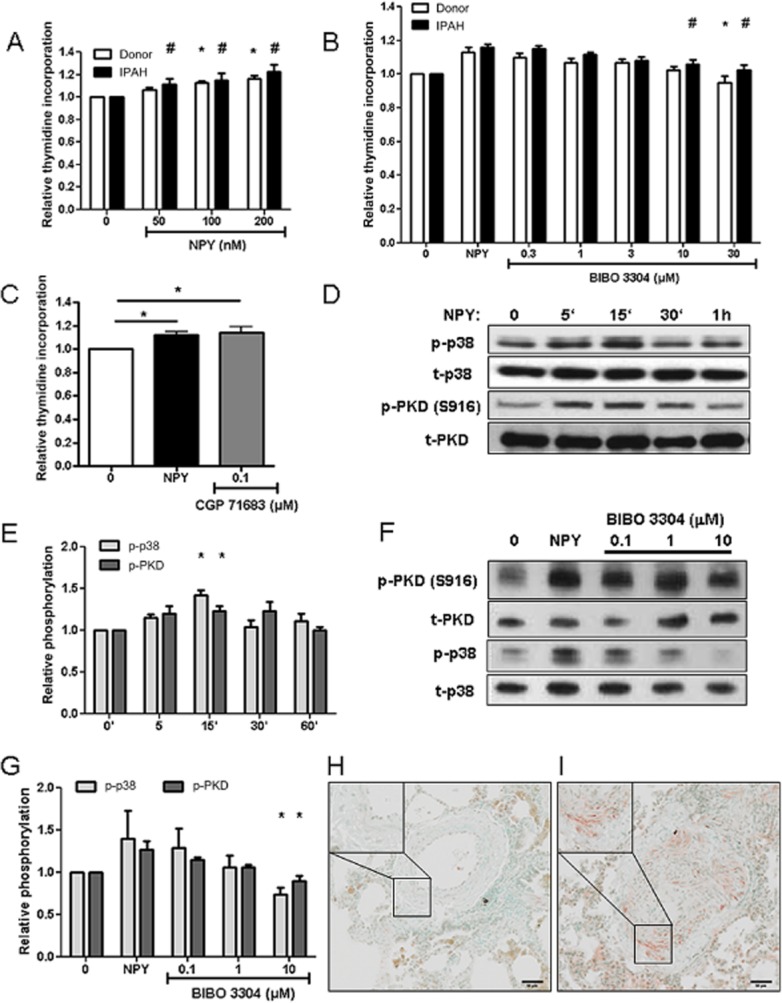
NPY stimulation activated PKD and p38 signalling pathways and increased hPASMC proliferation. (A) Donor and IPAH hPASMC (n ≥ 11 independent experiments) were stimulated with the indicated concentrations of NPY. Graph represents the amount of 3H-labelled thymidine incorporation as an index of cell proliferation. Serum-starved hPASMC were pretreated for 1 h with indicated concentrations of BIBO 3304 (B, n ≥ 9 independent experiments) or CGP 71683 (C, n ≥ 7 independent experiments) inhibitors, followed by overnight incubation with 200 nM NPY. (D) Representative western blot image of phosphorylation of PKD, at serine S916 position and p38 kinase. Human PASMC were serum-starved and stimulated with 200 nM NPY for indicated time. Determination of total PKD and p38 protein levels served as corresponding loading control. (E) Quantification of (D) (n ≥ 5 independent experiments). (F) Representative western blot image of PKD and p38 phosphorylation after 10 min 200 nM NPY stimulation and pretreatment with indicated BIBO 3304 concentrations. (G) Quantification of (F) (n ≥ 7 independent experiments). Representative images of immunohistochemical staining of human pulmonary artery from donor (H) and IPAH patients (I) against phosphoPKD (n = 5 for each group). * or # (in the case of IPAH PASMC) denotes P < 0.05.
Discussion and conclusions
PH is a devastating condition with incompletely understood pathomechanism and few treatment options. Here, we show the possible involvement of the NPY-Y1 receptor axis and its downstream signalling pathway in the processes underlying PH.
Even though early reports have shown increased sympathetic innervation in remodelled neonatal PH vessels (Allen et al., 1989), the role of neuronal factors in PH has been mostly neglected. Our study is the first to show increased sympathetic/NPY-ergic innervation of remodelled PA in adult PH patients. This is accompanied by increased expression of Y1 receptors, both in human PH samples and animal models. Furthermore, the observed change in Y1 receptor expression during recovery from hypoxia-induced PH in mice, suggests that down-regulation of Y1 receptor expression could also be involved in a resolution process of the disease. Unfortunately, the lack of specific commercially available antibodies prevented confirmation of the expression data at the protein level.
Despite unchanged expression and circulating levels of NPY in IPAH patients, it is tempting to speculate that the increased expression of Y1 receptors in PH could lead to hypersensitivity to NPY stimulation and subsequent pulmonary vasoconstriction. Accordingly, increased sympathetic activation has been observed in PAH patients (Velez-Roa et al., 2012; Ciarka et al., 2013). This could be a contributing pathological factor in the early stages of IPAH or other forms of PH. Particularly interesting in this respect are borderline PAH patients who have PAP at rest within normal range, but show a steeper than expected increase in PAP during exercise, suggestive of early pulmonary vasculopathy (Kovacs et al., 2009). We could therefore speculate that sympathetic activation under stress conditions coupled with increased Y1 receptor expression could constitute a vicious pathological cycle leading to exacerbated pulmonary vasoconstriction in these patients. In line with this speculation, others have reported that synergistic effects of different vasoconstrictors may only operate when the pulmonary arteries are under the influence of increased vascular tone as in PH (MacLean and Morecroft, 2001). Furthermore, we observed that NPY expression was not confined exclusively to neuronal endings in pulmonary vessels, as reported earlier (Zukowska-Grojec et al., 1998b; Holler et al., 2008). This raises the possibility that, in addition or independent of innervation, non-neuronal sources, such as inflammatory cells, could play a role in PH by activating Y1 receptors. In fact, non-neuronal cells are likely to be the major NPY source in rodent PH models, which contain no or very limited sympathetic innervation of intrapulmonary arteries (Kummer, 2009).
The Y1 receptor is a GPCR coupling to Gi proteins, leading to decreased cAMP levels in vascular smooth muscle cells (Reynolds and Yokota, 1988) and vasoconstriction. Signalling through Y1 receptors and inhibition of adenylate cyclase could further exacerbate pulmonary vasoconstriction. An additional mechanism by which NPY mediates vasoconstriction is by increasing intracellular calcium, although this seems to be species and cell-type specific. In accordance with Wier et al. (2009), we observed an increase in intracellular calcium and pulmonary vasoconstriction with NPY in the presence of adrenaline. In our experimental setting, NPY stimulation alone had no vasoactive effect. In contrast, in porcine aorta smooth muscle cells, NPY alone was sufficient to induce rise in intracellular calcium, which was mediated by Y1 receptors and PLC and inositol 1,4,5-trisphosphate (Shigeri et al., 1995). Similarly, in human heart and aortic smooth muscle cells, NPY stimulation caused a rise in basal resting intracellular Ca2+ mainly via the activation of Y1 receptors (Jacques et al., 2000). On the other hand, in rabbit PASMC, NPY did not stimulate the elevation of intracellular calcium, nor potentiate noradrenaline-induced elevation of intracellular calcium (Reynolds and Yokota, 1988). Taken together, it seems that a rise in cytosolic calcium, caused or potentiated by NPY, can result with hPASMC contraction in vitro and pulmonary vasoconstriction in vivo. In our experimental set-up, this vasoactive effect was mediated by Y1, rather than Y5 receptors, as demonstrated in experiments using the specific antagonists.
In addition to acute haemodynamic aspects, another hallmark of PH is pulmonary vascular remodelling, characterized by aberrant vessel wall cell proliferation and apoptosis. These processes eventually lead to vessel lumen narrowing and obstruction. NPY stimulation is involved in pathologic remodelling of systemic arteries, an effect mediated by Y1 receptors and independent of co-adrenergic stimulation (Shigeri and Fujimoto, 2005). In accordance, we show that NPY alone, concentration-dependently stimulated hPASMC proliferation, implying a pathogenic role for the NPY- Y1 receptor axis in pulmonary vascular remodelling. Even though Y1 receptor expression levels were higher in PA isolated from IPAH patients compared with donors, this difference was no longer present in cultured cells. This observation most likely explains the absence of higher NPY-induced responses in IPAH hPASMC compared with donors. In rat aortic smooth muscle cells, the mitogenic effect of NPY is bimodal. On one hand, Y1 receptors activate calcium-dependent PKC and calcium/calmodulin-dependent kinase II, and on the other hand, Y5 receptors activate a calcium-independent inhibition of the adenylyl cyclase-PKA pathway (Pons et al., 2008). Our observations that hPASMC express Y1 but not Y5 receptors, and that antagonism of Y1 receptors attenuated NPY-induced vasoconstriction and hPASMC proliferation, indicates the importance of Y1 receptors in mediating NPY effects in hPASMC.
On a molecular level, we identified PKD (PKD/PKCμ) as a potential key mediator of Y1 receptors downstream signalling. This is further supported by the observation of increased staining against phospho-PKD in remodelled PA from IPAH patients compared with donors. In addition to NPY, PKD can be activated by PDGF-BB, a known and potent mediator of pulmonary vascular remodelling (Abedi et al., 1998). Taken together, this implies that the activation of PKD might be a convergent downstream point for several different pathomechanisms in PH. PKD has already been implicated in pathological vascular hypertrophy by inactivating the histone deacetylase HDAC5 (Xu et al., 2009) and promoting vascular smooth muscle cell proliferation by ERK5 and c-Fos activation (Geng et al., 2009). Furthermore, increased phosphorylation of p38 MAPK could be mediated by PKD (Hao et al., 2012). Finally, activation of p38 could additionally lead to attenuated cAMP formation in hPASMC (El-Haroun et al., 2008), a contributing mechanism in NPY vasoconstrictive action.
In conclusion, the observed increased NPYergic innervation and expression of NPY Y1 receptors could represent potential hypersensitivity to NPY, secreted either by neuronal endings or non-neuronal cells in PH. NPY vasoconstrictive and trophic effects on PA and hPASMC are dependent on Y1 receptor activation. Furthermore, NPY effects on hPASMC, mediated in part by the activation of PKD and p38 signalling pathways, could form a molecular basis for a pathological role for the NPY/Y1 receptor axis in pulmonary vascular remodelling.
Acknowledgments
The work was supported by Ludwig Boltzmann Gesellschaft and ECCPS (to G. K.). N. W. was supported by DFG and ECCPS. We thank Ida Niklasson, Maria Schloffer and Julia Schittl for excellent technical assistance, and Vasile Foris and Maria Tscherner for their help with patient characterization and serum collection.
Glossary
- hPASMC
human pulmonary arterial smooth muscle cells
- IPAH
idiopathic pulmonary arterial hypertension
- KHB
Krebs-Henseleit buffer
- PA
pulmonary artery/arteries
- PAP
pulmonary artery pressure
- PH
pulmonary hypertension
Author contributions
G. Kwapiszewska initiated the project and designed the study. S. Crnkovic, L. M. Marsh and G. Kwapiszewska wrote the manuscript. A. Olschewski was involved in data analysis, commented and approved the manuscript. S. Crnkovic, B. Egemnazarov, P. Jain, U. Seay, N. Gattinger and G. Kwapiszewska performed the experiments, acquired and analysed data. Z. Bálint was involved in data analysis and commented on the manuscript. G. Kovacs, B. Ghanim, W. Klepetko, R. T. Schermuly and N. Weissmann were involved in tissue sample procurement and data analysis.
Conflict of interest
All authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12751
Figure S1 Pulmonary vascular remodelling in IPAH and rodent PH models. Representative double immunohistochemical staining against endothelial marker von Willebrand factor (brown) and smooth muscle cell marker α−smooth muscle actin (purple) in human donor (A) and IPAH (B) lung tissue; normoxia (C) and hypoxia (D) exposed mice; normoxia (E) and Su-hypoxia exposed rats (F); vehicle (G) and monocrotaline-treated rats (H). Black scale bars indicate 100 μm (A, B); 20 μm (C–F) and 50 μm (G, H).
Figure S2 (A) Schematic representation of the experimental setup for calcium measurements. (B) Schematic representation of the experimental set-up of isolated perfused lung experiments. (C) Representative tracings of contraction force in isolated mouse intrapulmonary arteries under adrenaline and NPY stimulation.
Table S1 IPAH patient characteristics. Values are presented as (mean ± SD).
Table S2 Patient characteristics used for elisa determination of circulating NPY levels. Values are presented as (mean ± SD).
Table S3 Primer sequences used in this study.
Appendix S1 Supplementary Material and Methods.
References
- Abe K, Tilan JU, Zukowska Z. NPY and NPY receptors in vascular remodeling. Curr Top Med Chem. 2007;7:1704–1709. doi: 10.2174/156802607782340948. [DOI] [PubMed] [Google Scholar]
- Abedi H, Rozengurt E, Zachary I. Rapid activation of the novel serine/threonine protein kinase, protein kinase D by phorbol esters, angiotensin II and PDGF-BB in vascular smooth muscle cells. FEBS Lett. 1998;427:209–212. doi: 10.1016/s0014-5793(98)00427-x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KM, Wharton J, Polak JM, Haworth SG. A study of nerves containing peptides in the pulmonary vasculature of healthy infants and children and of those with pulmonary hypertension. Br Heart J. 1989;62:353–360. doi: 10.1136/hrt.62.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint Z, Zabini D, Konya V, Nagaraj C, Vegh AG, Varo G, et al. Double-stranded RNA attenuates the barrier function of human pulmonary artery endothelial cells. PLoS ONE. 2013;8:e63776. doi: 10.1371/journal.pone.0063776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Zhang FF, Xu J, Xie DJ, Zhou L, Nguyen T, et al. Pulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension) J Am Coll Cardiol. 2013;62:1092–1100. doi: 10.1016/j.jacc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- Ciarka A, Doan V, Velez-Roa S, Naeije R, van de Borne P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181:1269–1275. doi: 10.1164/rccm.200912-1856OC. [DOI] [PubMed] [Google Scholar]
- Dumitrascu R, Weissmann N, Ghofrani HA, Dony E, Beuerlein K, Schmidt H, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113:286–295. doi: 10.1161/CIRCULATIONAHA.105.581405. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Cadieux A, Doods H, Fournier A, Quirion R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral nervous systems: BIB03304 (Y1) and CGP71683A (Y5) Can J Physiol Pharmacol. 2000;78:116–125. [PubMed] [Google Scholar]
- Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- El-Haroun H, Clarke DL, Deacon K, Bradbury D, Clayton A, Sutcliffe A, et al. IL-1beta, BK, and TGF-beta1 attenuate PGI2-mediated cAMP formation in human pulmonary artery smooth muscle cells by multiple mechanisms involving p38 MAP kinase and PKA. Am J Physiol Lung Cell Mol Physiol. 2008;294:L553–L562. doi: 10.1152/ajplung.00044.2006. [DOI] [PubMed] [Google Scholar]
- Fink L, Stahl U, Ermert L, Kummer W, Seeger W, Bohle RM. Rat porphobilinogen deaminase gene: a pseudogene-free internal standard for laser-assisted cell picking. Biotechniques. 1999;26:510–516. doi: 10.2144/99263rr02. [DOI] [PubMed] [Google Scholar]
- Geng J, Zhao Z, Kang W, Wang W, Liu G, Sun Y, et al. Hypertrophic response to angiotensin II is mediated by protein kinase D-extracellular signal-regulated kinase 5 pathway in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2009;388:517–522. doi: 10.1016/j.bbrc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Goldman WF, Wier WG, Blaustein MP. Effects of activation on distribution of Ca2+ in single arterial smooth muscle cells. Determination with fura-2 digital imaging microscopy. Circ Res. 1989;64:1019–1029. doi: 10.1161/01.res.64.5.1019. [DOI] [PubMed] [Google Scholar]
- Hao F, Wu DD, Xu X, Cui MZ. Histamine induces activation of protein kinase D that mediates tissue factor expression and activity in human aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2012;303:H1344–H1352. doi: 10.1152/ajpheart.00500.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A, Reid L. Changes in the pulmonary arteries of the rat during recovery from hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1977;58:653–662. [PMC free article] [PubMed] [Google Scholar]
- Holler J, Zakrzewicz A, Kaufmann A, Wilhelm J, Fuchs-Moll G, Dietrich H, et al. Neuropeptide Y is expressed by rat mononuclear blood leukocytes and strongly down-regulated during inflammation. J Immunol. 2008;181:6906–6912. doi: 10.4049/jimmunol.181.10.6906. [DOI] [PubMed] [Google Scholar]
- Jacques D, Sader S, El-Bizri N, Chouffani S, Hassan G, Shbaklo H. Neuropeptide Y induced increase of cytosolic and nuclear Ca2+ in heart and vascular smooth muscle cells. Can J Physiol Pharmacol. 2000;78:162–172. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, Troster N, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180:881–886. doi: 10.1164/rccm.200904-0563OC. [DOI] [PubMed] [Google Scholar]
- Kummer W. Pulmonary vascular innervation and its role in responses to hypoxia: size matters! Proc Am Thorac Soc. 2011;8:471–476. doi: 10.1513/pats.201101-013MW. [DOI] [PubMed] [Google Scholar]
- Kwapiszewska G, Wygrecka M, Marsh LM, Schmitt S, Trosser R, Wilhelm J, et al. Fhl-1, a new key protein in pulmonary hypertension. Circulation. 2008;118:1183–1194. doi: 10.1161/CIRCULATIONAHA.107.761916. [DOI] [PubMed] [Google Scholar]
- Lang M, Kojonazarov B, Tian X, Kalymbetov A, Weissmann N, Grimminger F, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS ONE. 2012;7:e43433. doi: 10.1371/journal.pone.0043433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh LA, Blackshear PJ. Neuropeptide Y stimulation of myosin light chain phosphorylation in cultured aortic smooth muscle cells. J Biol Chem. 1990;265:18393–18399. [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean MR, Morecroft I. Increased contractile response to 5-hydroxytryptamine1-receptor stimulation in pulmonary arteries from chronic hypoxic rats: role of pharmacological synergy. Br J Pharmacol. 2001;134:614–620. doi: 10.1038/sj.bjp.0704273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man FS, Handoko ML, Guignabert C, Bogaard HJ, Vonk-Noordegraaf A. Neurohormonal axis in patients with pulmonary arterial hypertension: friend or foe? Am J Respir Crit Care Med. 2013;187:14–19. doi: 10.1164/rccm.201209-1663PP. [DOI] [PubMed] [Google Scholar]
- Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell NW, Archer SL, Defelice A, Evans S, Fiszman M, Martin T, et al. Anticipated classes of new medications and molecular targets for pulmonary arterial hypertension. Pulm Circ. 2013;3:226–244. doi: 10.4103/2045-8932.109940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj C, Tang B, Balint Z, Wygrecka M, Hrzenjak A, Kwapiszewska G, et al. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J. 2013;41:85–95. doi: 10.1183/09031936.00211811. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Sperk G, Herzog H, Holzer P. Delayed stress-induced differences in locomotor and depression-related behaviour in female neuropeptide-Y Y1 receptor knockout mice. J Psychopharmacol. 2010;24:1541–1549. doi: 10.1177/0269881109104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons J, Lee EW, Li L, Kitlinska J. Neuropeptide Y: multiple receptors and multiple roles in cardiovascular diseases. Curr Opin Investig Drugs. 2004;5:957–962. [PubMed] [Google Scholar]
- Pons J, Kitlinska J, Jacques D, Perreault C, Nader M, Everhart L, et al. Interactions of multiple signaling pathways in neuropeptide Y-mediated bimodal vascular smooth muscle cell growth. Can J Physiol Pharmacol. 2008;86:438–448. doi: 10.1139/y08-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds EE, Yokota S. Neuropeptide Y receptor-effector coupling mechanisms in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988;151:919–925. doi: 10.1016/s0006-291x(88)80369-3. [DOI] [PubMed] [Google Scholar]
- Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Cheng KT, Leung YK, Kwok YC, Kwan HY, Wong CO, et al. Epinephrine-induced Ca2+ influx in vascular endothelial cells is mediated by CNGA2 channels. J Mol Cell Cardiol. 2008;45:437–445. doi: 10.1016/j.yjmcc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Fujimoto M. Neuropeptide Y stimulates DNA synthesis in vascular smooth muscle cells. Neurosci Lett. 1993;149:19–22. doi: 10.1016/0304-3940(93)90337-k. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Nakajima S, Fujimoto M. Neuropeptide YY1 receptors-mediated increase in intracellular Ca2+ concentration via phospholipase C-dependent pathway in porcine aortic smooth muscle cells. J Biochem. 1995;118:515–520. doi: 10.1093/oxfordjournals.jbchem.a124938. [DOI] [PubMed] [Google Scholar]
- Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez-Roa S, Ciarka A, Najem B, Vachiery JL, Naeije R, van de Borne P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation. 2004;110:1308–1312. doi: 10.1161/01.CIR.0000140724.90898.D3. [DOI] [PubMed] [Google Scholar]
- Weisel FC, Kloepping C, Pichl A, Sydykov A, Kojonazarov B, Wilhelm J, et al. Impact of s-adenosylmethionine decarboxylase 1 on pulmonary vascular remodeling. Circulation. 2014;129:1510–1523. doi: 10.1161/CIRCULATIONAHA.113.006402. [DOI] [PubMed] [Google Scholar]
- Wier WG, Zang WJ, Lamont C, Raina H. Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp Physiol. 2009;94:31–37. doi: 10.1113/expphysiol.2008.043638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, et al. Angiotensin II stimulates protein kinase D-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2355–2362. doi: 10.1161/ATVBAHA.107.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Fisher TA, Ji H. Mechanisms of vascular growth-promoting effects of neuropeptide Y: role of its inducible receptors. Regul Pept. 1998a;75–76:231–238. doi: 10.1016/s0167-0115(98)00073-1. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998b;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Pulmonary vascular remodelling in IPAH and rodent PH models. Representative double immunohistochemical staining against endothelial marker von Willebrand factor (brown) and smooth muscle cell marker α−smooth muscle actin (purple) in human donor (A) and IPAH (B) lung tissue; normoxia (C) and hypoxia (D) exposed mice; normoxia (E) and Su-hypoxia exposed rats (F); vehicle (G) and monocrotaline-treated rats (H). Black scale bars indicate 100 μm (A, B); 20 μm (C–F) and 50 μm (G, H).
Figure S2 (A) Schematic representation of the experimental setup for calcium measurements. (B) Schematic representation of the experimental set-up of isolated perfused lung experiments. (C) Representative tracings of contraction force in isolated mouse intrapulmonary arteries under adrenaline and NPY stimulation.
Table S1 IPAH patient characteristics. Values are presented as (mean ± SD).
Table S2 Patient characteristics used for elisa determination of circulating NPY levels. Values are presented as (mean ± SD).
Table S3 Primer sequences used in this study.
Appendix S1 Supplementary Material and Methods.