Abstract
Lysophospholipids encompass a diverse range of small, membrane-derived phospholipids that act as extracellular signals. The signalling properties are mediated by 7-transmembrane GPCRs, constituent members of which have continued to be identified after their initial discovery in the mid-1990s. Here we briefly review this class of receptors, with a particular emphasis on their protein and gene nomenclatures that reflect their cognate ligands. There are six lysophospholipid receptors that interact with lysophosphatidic acid (LPA): protein names LPA1 – LPA6 and italicized gene names LPAR1-LPAR6 (human) and Lpar1-Lpar6 (non-human). There are five sphingosine 1-phosphate (S1P) receptors: protein names S1P1-S1P5 and italicized gene names S1PR1-S1PR5 (human) and S1pr1-S1pr5 (non-human). Recent additions to the lysophospholipid receptor family have resulted in the proposed names for a lysophosphatidyl inositol (LPI) receptor – protein name LPI1 and gene name LPIR1 (human) and Lpir1 (non-human) – and three lysophosphatidyl serine receptors – protein names LyPS1, LyPS2, LyPS3 and gene names LYPSR1-LYPSR3 (human) and Lypsr1-Lypsr3 (non-human) along with a variant form that does not appear to exist in humans that is provisionally named LyPS2L. This nomenclature incorporates previous recommendations from the International Union of Basic and Clinical Pharmacology, the Human Genome Organization, the Gene Nomenclature Committee, and the Mouse Genome Informatix.
Keywords: G protein-coupled receptors, lysophospholipids, molecular pharamacology, FTY720 (fingolimod), lipid mediators
Introduction
The biological and pathophysiological functions of the small signalling lipids known as lysophospholipids continues to expand, with roles that involve virtually every vertebrate organ system (Fukushima et al., 2001; Ishii et al., 2004; Choi et al., 2010; Mutoh et al., 2012; Choi and Chun, 2013). The overwhelming majority of effects, and all activities that have led to actual medicines or to compounds that have entered late-stage clinical trials, rely mechanistically on lysophospholipid receptors. All bona fide receptors are of the 7-transmembrane, GPCR class (Table 1 and Figure 1).
Table 1.
Links to online information in the IUPHAR/BPS Guide to PHARMACOLOGY
| Targets | Ligands |
|---|---|
| Akt | [3H]-LPA |
| Cannabinoid receptors | 1-Oleoyl-LPA |
| COX-2 | 2-Oleoyl-LPA |
| EGF receptor | AFD (R) |
| ERK1/2 | Alkyl OMPT |
| GPR34 | AM966 |
| GPR55 | AUY954 |
| GPR174 | Bupivacaine |
| LPA1 receptor | CYM5181 |
| LPA2 receptor | CYM-5442 |
| LPA3 receptor | EGF |
| LPA4 receptor | Oestrogen |
| LPA5 receptor | Farnesyl diphosphate |
| LPA6 receptor | Farnesyl monophosphate |
| Lysophospholipid (LPA) receptors | Fingolimod |
| MAPK | FTY720 |
| Metalloproteinases | FTY720-P |
| MMP9 | IL-13 |
| P2Y10 | IL-17 |
| PLC | IL-6 |
| Protease-activated receptor 1 | IL-2 |
| ROCK | JTE-013 |
| S1P receptors | Ki16425 |
| Sphingosine kinase 1 | LPA |
| S1P1 receptor | LP) |
| S1P2 receptor | LPC |
| S1P3 receptor | LysoPS |
| S1P4 receptor | S1P |
| S1P5 receptor | VEGF |
| Sodium/NHE3 | VPC12249 |
| Urokinase-type plasminogen activator | VPC23019 |
| VPC44116 | |
| W146 |
This table lists protein targets and ligands that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b,d).
Figure 1.
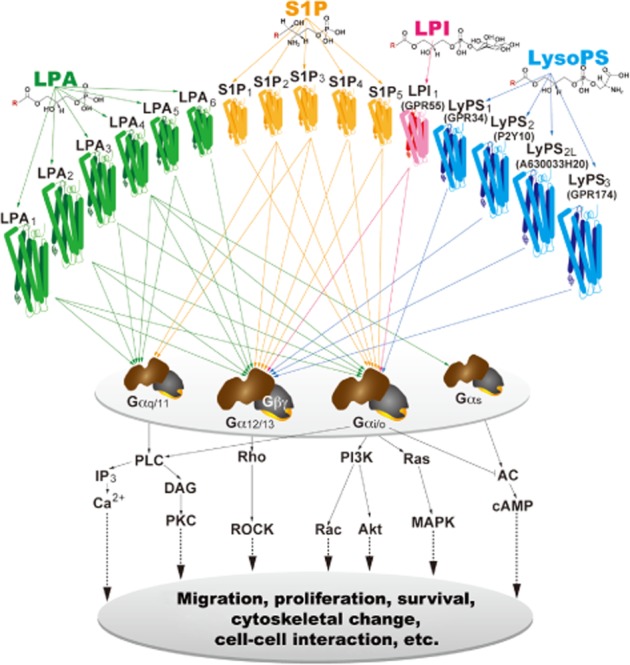
Lysophospholipid receptors and their intracellular signalling pathways. Lysophospholipid ligands (LPA, S1P, LPI and LysoPS) bind to their specific GPCRs, which activate heterotrimeric G-proteins (defined here by their α subunits) to initiate downstream signalling cascades. R in the chemical structures is a variable acyl side chain.
Various orphan receptor names have been used over the years; however, receptor identities have led to two nomenclatures: the first used in pharmacological fields and supported by the International Union of Basic and Clinical Pharmacologists (IUPHAR), and the second used in genetic or genomic fields, as represented by the Human Genome Organization (HUGO), Gene Nomenclature Committee (HGNC), and the Mouse Genome Informatix (MGI) Guidelines for Nomenclature of Genes, based upon the 2011 International Committee on Standardized Genetic Nomenclature for Mice. We briefly review these lysophospholipid receptors and their names, and suggest use of a hybrid nomenclature wherein protein names are referred to by their original IUPHAR names (Chun et al., 2002; 2010; Davenport et al., 2013), while HGNC nomenclatures are used to identify the human genes, and MGI nomenclatures for mice are extended to cover non-human genes (Table 2). In each subheading of this review, the protein name is followed by the human and non-human gene names. Recent additions to the lysophospholipid receptor family include glycerophospholipid species lysophosphatidyl inositol (LPI) and lysophosphatidyl serine (LysoPS); names for these newer receptors and genes have been proposed, which generally follow the receptor protein and gene for other lysophospholipid receptors and have been incorporated in this review. The names of established receptors and their human and non-human gene names start each subsection, while new receptors are treated under a separate heading.
Table 2.
Lysophospholipid receptors
| Ligand | Protein namea | G-proteins | Human | Identityc | Mouse/non-human | Previous orphan namesa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene name | Chr | AA | MMb | Gene name | Chr | AA | MMa | |||||
| LPA | LPA1 | Gs, Gi/o, Gq/11, G12/13 | LPAR1 | 9q31.3 | 364 | 41 109 | 97.3% | Lpar1 | 4 | 364 | 41 119 | vzg-1, edg-2, mrec1.3, lpA1 |
| LPA2 | Gs, Gi/o, Gq/11, G12/13 | LPAR2 | 19p12 | 351 | 39 084 | 83.5% | Lpar2 | 8 | 348 | 38 777 | edg-4, lpA2 | |
| LPA3 | Gs, Gi/o, Gq/11, G12/13 | LPAR3 | 1p22.3-p31.1 | 353 | 40 128 | 91.2% | Lpar3 | 3 | 354 | 40 316 | edg-7, lpA3 | |
| LPA4 | Gs, Gi/o, Gq/11, G12/13 | LPAR4 | Xq21.1 | 370 | 41 895 | 98.4% | Lpar4 | X | 370 | 41 899 | P2Y9/GPR23 | |
| LPA5 | Gs, Gi/o, Gq/11, G12/13 | LPAR5 | 12p13.31 | 372 | 41 347 | 79.0% | Lpar5 | 6 | 372 | 41 394 | GPR92 | |
| LPA6 | Gs, Gi/o, Gq/11, G12/13 | LPAR6 | 13q14 | 344 | 39 392 | 93.0% | Lpar6 | 14 | 344 | 39 439 | P2Y5 | |
| S1P | S1P1 | Gs, Gi/o, Gq/11, G12/13 | S1PR1 | 1p21 | 382 | 42 811 | 94.2% | S1pr1 | 3 | 382 | 42 639 | edg-1, lpB1 |
| S1P2 | Gs, Gi/o, Gq/11, G12/13 | S1PR2 | 19p13.2 | 353 | 38 867 | 90.7% | S1pr2 | 9 | 352 | 38 829 | edg-5, lpB2, AGR16, H218 | |
| S1P3 | Gs, Gi/o, Gq/11, G12/13 | S1PR3 | 9q22.1-q22.2 | 378 | 42 250 | 87.3% | S1pr3 | 13 | 378 | 42 270 | edg-3, lpB3 | |
| S1P4 | Gs, Gi/o, Gq/11, G12/13 | S1PR4 | 19p13.3 | 384 | 41 623 | 81.1% | S1pr4 | 10 | 386 | 42 263 | edg-6, lpC1 | |
| S1P5 | Gs, Gi/o, Gq/11, G12/13 | S1PR5 | 19p13.2 | 398 | 41 775 | 83.8% | S1pr5 | 9 | 400 | 42 331 | edg-8, lpB4, Nrg-1 | |
| LPI | LPI1 | Gs, Gi/o, Gq/11, G12/13 | LPIR1 | 2q37 | 319 | 36 637 | 74.4% | Lpir1 | 1 | 327 | 38 090 | GPR55 |
| LysoPS | LysoPS1 | Gs, Gi/o, Gq/11, G12/13 | LysoPSR1 | Xp11.4 | 381 | 43 860 | 89.0% | Lypsr1 | X | 375 | 43 173 | GPR34 |
| LysoPS2 | Gs, Gi/o, Gq/11, G12/13 | LysoPSR2 | Xq21.1 | 339 | 38 774 | 82.9% | Lypsr2 | X | 328 | 37 244 | P2Y10 | |
| LysoPS3 | Gs, Gi/o, Gq/11, G12/13 | LysoPSR3 | Xq21.1 | 333 | 38 503 | 87.8% | Lypsr3 | X | 335 | 38 761 | GPR174/FKSG79 | |
| LysoPS2L | Gs, Gi/o, Gq/11, G12/13 | N/A | N/A | N/A | N/A | N/A | pending | X | 352 | 40 383 | A630033H20 | |
Chr, chromosome; AA, amino acids; MM, molecular mass. Utilized G-proteins are indicated in black.
Hyperlinks are provided to online information in the IUPHAR/BPS Guide to PHARMACOLOGY.
MMs were obtained from UniProt (UniprotConsortium, 2013).
Identities between human and mouse lysophospholipid receptors were calculated in UniProt (UniprotConsortium, 2013).
Lysophosphatidic acid (LPA) receptors
The many effects of LPA are mediated through the six currently recognized LPA receptors, LPA1–6. These 7-transmembrane GPCRs couple to one or more of the four classes of heterotrimeric G-proteins, commonly defined by their Gα proteins (Gα12/13, Gαq/11, Gαi/o, and Gαs). Less explored is possible signalling through these receptors that do not require heterotrimeric G-proteins (Rajagopal et al., 2005). Activation of these receptors and G-proteins can initiate myriad downstream pathways that in turn, produce a similarly diverse range of biological and pathological effects (Gilman, 1987). The agonists and antagonists for these receptors and their efficacy are summarized in Table 3.
Table 3.
Pharmacological tools for LPA receptors and their efficacy
| Compoundsa | Units (nM) | LPA1 | LPA2 | LPA3 | LPA4 | LPA5 | LPA6 | Assay | References |
|---|---|---|---|---|---|---|---|---|---|
| 1-Oleoyl-LPA | Kd | 69 | 64 | N/A | 100 | 89 | N/A | Binding | Yanagida et al., 2009 |
| EC50 | 64∼200 | 9∼10 | 75∼321 | 26 | 11 | 1495 | Ca2+ | Bandoh et al., 2000; Yanagida et al., 2013 | |
| 2-Oleoyl-LPA | EC50 | ∼200 | ∼10 | ∼10 | N/A | N/A | N/A | Ca2+ | Bandoh et al., 2000 |
| AGP | EC50 | 1500 | 101 | N/A | 303 | 2 | N/A | Ca2+ | Williams et al., 2009 |
| Alkyl OMPT | EC50 | 794 | N/A | 62 | N/A | N/A | N/A | Ca2+ | Qian et al., 2006 |
| VPC31143(R) | EC50 | 59 | 16 | 130 | 341 | 126 | 1484 | Ca2+ | Yanagida et al., 2013 |
| EC50 | 8 | 117 | 322 | N/A | N/A | N/A | GTPγS | Heise et al., 2001 | |
| VPC31144(S) | EC50 | 461 | 2592 | 7123 | 18 | 16 | 4835 | Ca2+ | Yanagida et al., 2013 |
| EC50 | >5000 | 2645 | 4349 | N/A | N/A | N/A | GTPγS | Heise et al., 2001 | |
| Farnesyl diphosphate | IC50,EC50 | N/A | 2100 | 4600 | 1980 | 40b | N/A | Ca2+ | Williams et al., 2009 |
| Farnesyl monophosphate | IC50,EC50 | N/A | 161 | 517 | 1450 | 49b | N/A | Ca2+ | Williams et al., 2009 |
| Ki16425 | (Ki) | (250) | (5600) | (360) | N/A | N/A | N/A | GTPγS | Ohta et al., 2003 |
| VPC12249 | (Ki) | (137) | N/A | (428) | N/A | N/A | N/A | GTPγS | Heise et al., 2001 |
| IC50 (Ki) | 109 (18) | N/A | 175 | N/A | N/A | N/A | GTPγS | Heasley et al., 2004 | |
| AM966 | IC50 | 17 | 1700 | 1600 | 7700 | 8600 | N/A | Ca2+ | Swaney et al., 2010 |
N/A, not applicable.
Hyperlinks are provided to online information in the IUPHAR/BPS Guide to PHARMACOLOGY.
Both farnesyl diphosphate and farnesyl monophosphate are reported to be antagonists for LPA2, 3, 4, but agonist for LPA5.
LPA1/LPAR1/Lpar1
The first receptor identified for any lysophospholipid came from studies on the brain, which identified LPA1 (Hecht et al., 1996), a receptor that mediates the effects of LPA. LPAR1 encodes a receptor of 364 amino acids, with a molecular mass of ∼41 kDa. The human gene is located on chromosome 9 (9q31.3), and consists of at least five exons. A gene variant of Lpar1 (Lpar1-mrec1.3) lacks a predicted 18 amino acids from the amino terminus (Contos and Chun, 1998); however, its function and significance remain unclear. This receptor couples to three Gα proteins – Gαi/o, Gαq/11, and Gα12/13, which can result in the activation of a range of well-known, downstream pathways that include Akt, Rho, MAPK, and PLC. These pathways in turn can account for many of the cellular responses initiated by LPA1 such as changes in cell shape through alterations in the actin cytoskeleton, cell migration, adhesion and cell–cell contact, and Ca2+ mobilization (reviewed in Contos et al., 2000b; Fukushima et al., 2001; Ishii et al., 2004; Choi et al., 2010; Mutoh et al., 2012; Choi and Chun, 2013).
Expression of Lpar1/LPAR1 is widespread, and can be found in most tissues at various stages of development albeit with non-uniform expression (An et al., 1998; Contos et al., 2000b; Ohuchi et al., 2008; Ye, 2008), particularly within the developing nervous system (reviewed in Contos et al., 2000b; Ishii et al., 2004) where it is found in the neuroproliferative ventricular zone (VZ) as well as superficial marginal zone and meninges (Hecht et al., 1996). By birth, the VZ dissipates as does the expression of Lpar1 in this region; however, it reappears in oligodendrocytes that are involved in myelination.
Knockout mice have provided important insights for most of the lysophospholipid receptors, beginning with Lpar1−/− mice that exhibit ∼50% perinatal lethality (Contos et al., 2000a) attributable to olfactory deficits that affect suckling as well as possible central mechanisms that show background strain dependence (Weiner et al., 2001; Estivill-Torrús et al., 2008; Matas-Rico et al., 2008). The developing cerebral cortex in particular is affected by LPA signalling including overall organization (Kingsbury et al., 2003), cell survival, migration, proliferation and process outgrowth (Contos et al., 2000b; Fukushima et al., 2000; 2002; Campbell and Holt, 2001; Kingsbury et al., 2003; Yuan et al., 2003).
Effects on the normal development and organization of the brain have pointed towards LPA influences on central nervous system (CNS) disorders. In particular, neuropsychiatric disorders that could arise prenatally and that could involve bleeding, hypoxia and immunological challenge, as proposed for autism and schizophrenia (Hultman et al., 1999; Cannon et al., 2002; Brimacombe et al., 2007; Byrne et al., 2007), could involve LPA signalling. Proof-of-concept for this idea comes from studies of congenital or fetal hydrocephalus (Yung et al., 2011), one of the most common neurological disorders of newborns and young children, wherein models of FH can be rescued by removal of LPA signalling. Schizophrenia-relevant signals include Lpar1−/− mutant mice that show deficits in pre-pulse inhibition 5-HT levels and glutamatergic synapses (Harrison et al., 2003; Santin et al., 2009; Musazzi et al., 2010; Roberts et al., 2005), while a variant mutant, maLPA1−/−, display a range of other defects (Harrison et al., 2003; Estivill-Torrús et al., 2008; Santin et al., 2009; Castilla-Ortega et al., 2010).
Glia are also influenced by LPA1 signalling. Astrocytes express most LPA receptors (LPA1–5; Shano et al., 2008), and upon treatment with LPA, initiate a wide range of effects in vitro including morphological changes and stabilization of stress fibres (Manning and Sontheimer, 1997; Suidan et al., 1997; de Sampaio et al., 2008) that may contribute to astrogliosis (Sorensen et al., 2003, reviewed in Noguchi et al., 2009). Neuronal differentiation can also be influenced by LPA1 and LPA2 (Spohr et al., 2008). Myelinating cells, oligodendrocytes (Allard et al., 1998; Weiner et al., 1998; Yu et al., 2004) and Schwann cells (SCs) all, express LPA1 and LPA2 (Weiner et al., 2001; Kobashi et al., 2006) and Lpar1(−/−) mutants show increased survival via the Gαi-PI3K-Akt pathway (Weiner and Chun, 1999) and higher levels of Schwann cell apoptosis within the sciatic nerves (Inoue et al., 2004). Myelinating cells, oligodendrocytes, and Schwann cells all express LPA1 and LPA2, and Lpar1(−/−) mutants show increased survival via the Gai-P13K-Akt pathway and higher levels of Schwann cell apoptosis within the sciatic nerves.
LPA receptors have also been linked to neuropathic pain (Inoue et al., 2004) using an animal model of partial sciatic nerve ligation (PSNL) in Lpar1−/− mutants, which may involve demyelination (Inoue et al., 2004; Fujita et al., 2007). Other LPA receptors also appear to participate, including LPA5 (Lin et al., 2012). Moreover, autotaxin (gene name ENPP2/Enpp2) that converts lysophosphatidylcholine (LPC) into LPA (Inoue et al., 2008a,b) also affects neuropathic pain animal models, such that Enpp2+/− mice show protection in a PSNL model (Inoue et al., 2008a). These observations support roles for LPA signalling in neuropathic pain.
LPA signalling is also found to play a role in obesity and fibrosis. LPA signalling can affect both proliferation and differentiation of pre-adipocytes (Valet et al., 1998; Ferry et al., 2003; Simon et al., 2005; Nobusue et al., 2010), and LPA's effects have been observed in adipocytes, including those from db/db mice (type II diabetes obese-diabetic mice; Ferry et al., 2003; Boucher et al., 2005). Fibrosis links to LPA include in the lung, kidney, and liver (Ikeda et al., 1998; Wu and Zern, 2000; Pradere et al., 2007; 2008; Watanabe et al., 2007; Tager et al., 2008). LPA1 is expressed on both cancer cell lines and in tumours, where it can have a variety of effects, both cancer promoting and inhibiting (Yamada et al., 2004; Yu et al., 2008; Li et al., 2009; Shin et al., 2009). LPA1 mutations have been reported in an osteosarcoma cell line (Okabe et al., 2010) and in rat lung and liver tumours (Obo et al., 2009).
LPA2/LPAR2/Lpar2
LPA2 is encoded by LPAR2 on chromosome 19 (19p12) and encodes 348 amino acids for a calculated molecular mass of ∼39 kDa (Contos and Chun, 2000). It is ∼50% identical at the amino acid level to LPA1. Lpar2/LPAR2 is expressed at relatively high levels in leukocytes, kidney, testis, and uterus (An et al., 1998; Contos and Chun, 2000). Relatively low levels are present in most other organs, including the brain (Ohuchi et al., 2008). LPA2 couples to the same heterotrimeric G-proteins as LPA1: Gαi/o, Gαq/11, and Gα12/13 (Contos et al., 2000b), and like LPA1, can promote cell migration and survival (Goetzl et al., 1999; Zheng et al., 2000; 2001; Deng et al., 2002; Panchatcharam et al., 2008). LPA2 may also produce effects via TRIP6, a focal adhesion molecule (Lai et al., 2005; 2007), and both zinc finger or PDZ-domain protein interactions have been reported (Lin and Lai, 2008), along with MAGI3 and Na+/H+ exchanger regulatory factor 2 (NHERF) interactions (Lee et al., 2011). LPA2 signalling may inhibit EGF-induced migration of pancreatic cancer cells through Gα12/13/Rho (Komachi et al., 2009). SCs up-regulate myelin markers like P0 protein via LPA2, including after insult by injury, nerve transection, and in PSNL models of neuropathic pain (Weiner et al., 2001; Inoue et al., 2004). It has also been reported to modulate hippocampal excitatory synaptic transmission (Trimbuch et al., 2009). LPA2, in conjunction with LPA1, can also alter cerebral cortical architecture in ex vivo cultures after exposure to exogenous LPA (Kingsbury et al., 2003), effects of which are lost in Lpar1−/−/Lpar2−/− mutant mouse cultures.
Links to cancer have been reported for LPA2 in promoting neoplasms based upon designed or observed overexpression (Kitayama et al., 2004; Lee and Yun, 2010). LPA2 signalling has also been associated with cancer metastasis and colon endometrial, mesothelia, and ovarian cancer cells (Shida et al., 2003; Jeong et al., 2008; Hope et al., 2009). Instances of cancer inhibition in pancreatic cells have also been reported (Komachi et al., 2009). This influence may involve regulation of a range of factors including Akt/Erk1/2, COX-2, epithelial growth factor receptor, metalloproteinases, VEGF, and urokinase-type plasminogen activator (Huang et al., 2004; Yun et al., 2005; Estrella et al., 2007; Jeong et al., 2008; Shida et al., 2008). Loss-of-function for LPA2 generally appears to be protective against tumourgenesis (Masiello et al., 2006; Estrella et al., 2007; Yu et al., 2008; Zhao et al., 2013).
In the immune system, Lpar2 (similar to Lpar1) is expressed in a variety of immunological organs like the spleen and thymus (Ishii et al., 2004; Kotarsky et al., 2006; Oh et al., 2008), and in lymphocytes (Komachi et al., 2009). LPA2 is expressed in unstimulated T-cells, as compared with LPA1 that is predominantly within stimulated T-cells that can influence cell survival (Goetzl et al., 1999). In unstimulated T-cells, LPA2 is upregulated while LPA1 is downregulated, leading to LPA-induced chemotaxis and inhibition of (Goetzl et al., 2000; Zheng et al., 2000; 2001). In contrast, activated T-cells upregulate LPA1 and downregulate LPA2, leading to inhibited chemotaxis, increased proliferation, and increased IL-2 and IL-13 production upon LPA stimulation (Zheng et al., 2000; Rubenfeld et al., 2006). LPA2 is also expressed on dendritic cells (Panther et al., 2002; Chen et al., 2006).
LPA3/LPAR3/Lpar3
LPAR3/Lpar3 was identified based upon homology to defined LPA receptor genes and cloned using a degenerate, PCR-based cloning strategy (Bandoh et al., 1999; Im et al., 2000b). LPAR3 (human chromosomal locus 1p22.3-p31.1) encodes a 353 amino acid, ∼40 kDa GPCR, which in mice is ∼50% identical in amino acid sequence to LPA1 and LPA2. LPA3 couples to the heterotrimeric Gα proteins Gαi/o and Gαq/11 to mediate downstream signalling pathways including adenyl cyclase activation, PLC activation and Ca2+ mobilization, and MAPK activation (Ishii et al., 2000). LPA3 appears to prefer 2-acyl-LPA containing unsaturated fatty acids (Bandoh et al., 1999; Sonoda et al., 2002).
LPAR3 is expressed in multiple human organs including the brain, heart, lung, ovary, pancreas, prostate, and testis (Bandoh et al., 1999; Im et al., 2000b), as well as mouse lung, testes, kidney, small intestine, spleen, stomach, and heart (Contos et al., 2000b), and during development (Ohuchi et al., 2008). While Lpar3 null mice are viable, they have defects in the immune system reflecting in part LPA3-specific dependent activation of chemotaxis of immature, but not mature, dendritic cells (Chan et al., 2007). They also have effects on zebra fish body asymmetry (Lai et al., 2012) and probably are involved in effects of the nervous system including those involving pain (Ma et al., 2009) and possibly other modalities. However, the most dramatic effect is on embryo implantation and fertility.
Lpar3−/− null female mutants have a prominent reproductive system phenotype whereby normal embryo implantation is disrupted (Ye et al., 2005). Within the uterus, Lpar3 is specifically expressed in luminal endometrial epithelial cells where it is markedly up-regulated during the brief window of embryo implantation, following which its expression is rapidly down-regulated (Ye et al., 2005). The hormones oestrogen and progesterone influence this expression pattern (Hama et al., 2006), and may play a role in allowing embryos to implant within the uterus. Lpar3 null mutant mice were found to have abnormal, delayed implantation of embryos that included crowding along the uterine horn and subsequent reductions in live births that could be attributed to maternal effects of LPA3 loss (Ye et al., 2005). Mechanistic studies demonstrated that LPA3 promotes COX-2 expression; COX-2 is a rate-limiting enzyme for the production of PGs that are known to be important for fertility, although there is evidence that COX–2-independent functions are involved as well (Hama et al., 2007). This may be relevant for the embryo spacing phenotype in Lpar3−/− mice that could interface with cytosolic PLA2α (cPLA2α) or Wnt/β-catenin signalling, in view of the reminiscent phenotypes in null-mutants for these genes (Song et al., 2002; Mohamed et al., 2005). In addition to this maternal phenotype, combined loss of LPA1–3 that are expressed in the testis (Ishii et al., 2004; Ye, 2008) results in loss of germ cells and progeric azoospermia (Ye, 2008), adding to the reproductive spectrum of effects produced by LPA receptor loss from reproductive tissues (reviewed in Ye, 2008).
LPA4/LPAR4/Lpar4
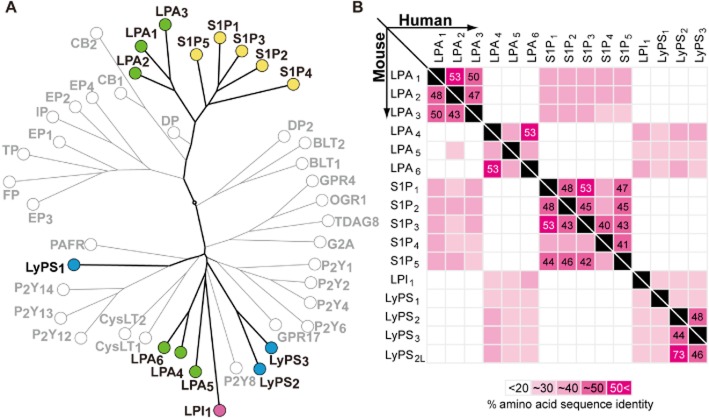
LPA4 is notable because it shares less than 20% amino acid sequence identity with LPA1–3 and S1P1–5, and is phylogenetically far from them and located near the P2Y receptor family (Figure 2). Identification of LPA4 was made by screening orphan receptors, including purine receptor families, using calcium mobilization as a readout for ligand-induced signals (Noguchi et al., 2003). P2Y9 has ∼20% sequence identity to LPA1–3 (Noguchi et al., 2003), yet it responds to LPA and not to assayed nucleosides or nucleotides (Noguchi et al., 2003). LPAR4 is located on chromosome Xq21.1 and encodes a 370 amino acid protein of ∼42 kDa, with mouse Lpar4 being present on the D-region of chromosome X. Lpar4 gene expression is observed in the brain, heart, lung, skin, thymus, and uterus (Ishii et al., 2009b). It is also developmentally expressed within the embryonic brain branchial arches, limb buds, liver, maxillary processes, and somites (Ohuchi et al., 2008).
Figure 2.
Phylogenetic tree of related GPCRs and amino acid sequence identities. (A) A molecular phylogenetic tree of human GPCRs. The selected GPCR protein sequences were analysed for the phylogenetic reconstruction by the ‘All against All’ sequence programme at the Computational Biochemistry Research Group server of the ETH Zürich. (B) Pair-wise matrices comparing amino acid sequences of lysophospholipid receptors. The upper and lower matrices specify identities among lysophospholipid receptors in human and mouse respectively. The amino acid sequence identities are shown in a gray-to-white gradient. The numbers in the boxes were calculated by Clustal Omega (Sievers et al., 2011).
LPA4 couples to Gα-proteins Gαs, Gαi, Gαq, and Gα12/13 (Lee et al., 2007), the latter of which activates Rho/ROCK to induce neurite retraction and stress fibre formation seen with activation of other LPA receptors (Lee et al., 2007; Yanagida et al., 2007). It can also induce cell aggregation and adhesion through N-cadherin (Yanagida et al., 2007) and was the first LPA receptor activating Gαs activity (Lee et al., 2007) to promote intracellular cAMP accumulation. LPA4 can transform cells when co-expressed with oncogenic-promoting genes like c-Myc or Tbx2 (Taghavi et al., 2008). It has also been reported to affect immortalized hippocampal progenitor cells (Rhee et al., 2006).
Null mutant mice for Lpar4 do not show overt abnormalities (Lee et al., 2008) aside from some prenatal loss, probably produced by blood vessel defects that result in abnormal haemorrhage (Sumida et al., 2010). Lymphatic vessels and lymph sacs are also affected during development of the circulatory system (Sumida et al., 2010). Osteoblast differentiation is also inhibited based on cell culture analyses in experiments that knocked down LPAR4 (Liu et al., 2010). Cells from Lpar4−/− mice show reduced cell motility (Lee et al., 2008).
LPA5/LPAR5/Lpar5
LPA5 was the fifth LPA receptor to be reported (Kotarsky et al., 2006; Lee et al., 2006), sharing ∼35% homology with LPAR4, while being more dissimilar to LPAR1–3 (Lee et al., 2006). LPAR5 has a chromosomal location of 12p13.31 and encodes a 372-amino acid protein with a molecular mass of ∼41 kDa, and Lpar5 is located on chromosome 6. LPA5 couples to Gα12/13 and Gαq (Lee et al., 2006) and is expressed broadly, with high expression in dorsal root ganglia (DRG), gastrointestinal lymphocytes, heart, platelets, and spleen (Kotarsky et al., 2006; Lee et al., 2006; Amisten et al., 2008). It is also expressed developmentally in the embryonic mouse brain (Ohuchi et al., 2008).
LPA5-expressing cell lines can induce both neurite retraction and stress fibre formation in response to LPA via the Gα12/13 pathway, including clear receptor internalization (Lee et al., 2006). It also activates Gαq, Gai, leading to intracellular calcium levels (Lee et al., 2006), while also increasing cAMP accumulation via a non-Gαs mechanism, based upon minigene experiments, which implicates other G-protein involvement (Kotarsky et al., 2006; Lee et al., 2006). LPA5 signalling also appears to affect intestinal water absorption (Lin et al., 2010) through effects on intestinal epithelial cells, whereby LPA induces Na+-dependent water absorption via Na+/H+ exchanger 3 (NHE3; see Alexander et al., 2013c) and the NHERF2 that recruits NHE3 to intestinal microvilli (Lin et al., 2010). This receptor has also been implicated in neuropathic pain models through mechanisms that appear to be distinct from effects mediated by LPA1 (Lin et al., 2012).
LPA6/LPAR6/Lpar6
The latest member of the LPA receptor family is LPA6. LPA6 is encoded by LPAR6 on chromosome 13 (13q14) and encodes 344 amino acids for a calculated molecular mass of ∼39 kDa. It is a member of the P2Y receptor family like LPA4, and was known originally by its orphan name P2Y5, which was identified as a human mutation affecting hair growth (Pasternack et al., 2008). Use of a chimeric Gα13 protein enabled detection of LPA6-mediated cAMP accumulation and Rho-dependent morphological alterations, as well as [3H]-LPA binding and LPA-induced [35S]-guanosine 5′-3-O-(thio)triphosphate binding (Yanagida et al., 2009). LPA6 has some preference for 2-acyl-LPA rather than 1-acyl-LPA. The receptor is distinct from the other five in being somewhat refractory to many cell-based tests, as evidenced by the much higher concentrations of LPA required to get a signal (Yanagida et al., 2009). When co-expressed with a promiscuous Gα protein, which activates Gαi, LPA6 stimulated with LPA increased intracellular Ca2+, reduced forskolin-stimulated cAMP and ERK1/2 activation (Lee et al., 2009).
LPA6 was initially identified as being an autosomal dominant genetic factor for hypotrichosis simplex, a complex of rare diseases characterized by familial hair loss in humans. Independent studies identified LPA6 mutations in hypotrichosis patients (Pasternack et al., 2008; Shimomura et al., 2009; Nahum et al., 2011). Conceptually linked reports have implicated lipase member H, associated with decreased LPA production in culture studies that then fail to activate LPA6 (Pasternack et al., 2009; Shinkuma et al., 2010). More recent analyses of this receptor by use of a TGFα shedding assay (Inoue et al., 2012) validate it as an atypical, but legitimate, LPA receptor.
Sphingosine 1-phosphate (S1P) receptors
S1P is a pleiotropic bioactive lipid that is an important regulator of many physiological processes including proliferation, migration, survival, and differentiation and plays important roles in disorders of the immune system and CNS (Maceyka et al., 2012). Most of the actions of S1P are mediated by five specific cognate GPCRs, designated S1P1-S1P5 (Chun et al., 2010; Blaho and Hla, 2011). These receptors bind S1P and dihydro-S1P with high affinity and there is very little evidence for additional endogenous ligands. We have summarized the experimental pharmacological tools for S1P receptors in Table 4.
Table 4.
Pharmacological tools for S1P receptors and their efficacy
| Compoundsa | Units (nM) | S1P1 | S1P2 | S1P3 | S1P4 | S1P5 | Assay | References |
|---|---|---|---|---|---|---|---|---|
| S1P | EC50 | 0.4–79 | 3.8–8.9 | 0.16–2 | 8.6–794 | 0.5–20 | GTPγS | Brinkmann et al., 2002; Sanna et al., 2004; Pan et al., 2006 |
| FTY720-P | EC50 | 0.3–6.3 | N/A | 3.1–4.0 | 0.6–63.1 | 0.3–6.3 | GTPγS | Brinkmann et al., 2002; Pan et al., 2006 |
| AUY954 | EC50 | 1.2 | N/A | 1210 | N/A | 340 | GTPγS | Pan et al., 2006 |
| SEW2781 | EC50 | 13–28.8 | N/A | N/A | N/A | N/A | GTPγS | Sanna et al., 2004; Gonzalez-Cabrera et al., 2008 |
| AFD (R) | EC50 | 2.5 | N/A | 4 | 4 | 1.3 | GTPγS | Brinkmann et al., 2002 |
| CYM5181 | EC50 | 3.4 | N/A | N/A | N/A | N/A | GTPγS | Gonzalez-Cabrera et al., 2008 |
| CYM-5442 | EC50 | 1.2 | N/A | N/A | N/A | N/A | GTPγS | Gonzalez-Cabrera et al., 2008 |
| W146 | EC50 (Ki) | 398 (77) | N/A | N/A | N/A | N/A | GTPγS | Sanna et al., 2006 |
| NIBR-0213 | (Ki) | (2) | N/A | N/A | N/A | N/A | GTPγS | Quancard et al., 2012 |
| VPC03090-P | EC50 (Ki) | (21–24) | N/A | (51–58.7) | 17.7b | 2.4b | GTPγS | Kennedy et al., 2011 |
| VPC23019 | (Ki) | (1) | N/A | (7.6) | N/A | N/A | Bindingc | Davis et al., 2005 |
| VPC44116 | EC50 (Ki) | (30) | N/A | (300) | 6100b | 33b | GTPγS | Foss et al., 2007 |
| JTE-013 | IC50 | N/A | 17 | N/A | N/A | N/A | Bindingc | Osada et al., 2002 |
N/A, not applicable.
Hyperlinks are provided to online information in the IUPHAR/BPS Guide to PHARMACOLOGY.
Ki and IC50 was estimated by determining the competitive binding of radioisotope-labelled S1P.
S1P1/S1PR1/S1pr1
S1P1 was one of the first S1P receptors to be functionally identified (Lee et al., 1998b) and it is the most well studied. Early studies suggested that it might mediate actions of LPA based on its sequence and function (Lee et al., 1998a); however, it is now known to be a selective S1P receptor. S1PR1 is located on chromosome 1 (1p21) and encodes a 382-amino acid of ∼43 kDa that is highly conserved and has 94% sequence identity with the murine receptor. S1PR1 is the only S1PR that couples exclusively to Gαi/o. Although S1PR1 is ubiquitously expressed (Zhang et al., 1999; McGiffert et al., 2002), its most important functions are in the regulation of trafficking of lymphocytes and other haematopoietic cells and vascular development and integrity. Genetic and pharmacological approaches, together with sophisticated intravital staining, have established that S1P1 controls the trafficking and migration of numerous types of haematopoietic cells, including T and B lymphocytes, NK T-cells, dendritic cells, macrophages, neutrophils, haematopoietic progenitors, mast cells, and osteoclasts (Matloubian et al., 2004; Spiegel and Milstien, 2011; Cyster and Schwab, 2012), in both homeostatic and disease settings. Blood and lymph contain high nM levels of S1P, which form a gradient between the much lower levels in tissues (Pappu et al., 2007; Pham et al., 2010). When S1P1 on lymphocytes recognizes high levels of S1P in the blood and lymph, egress of the cells from lymphoid organs into the blood is promoted through activation of the Gαi-phosphatidylinositol-3-kinase pathway and the small GTPase Rac (Spiegel and Milstien, 2011; Cyster and Schwab, 2012). Down-regulation or desensitization of S1P1 enables lymphocytes to subsequently migrate from the blood into tissues (Schwab and Cyster, 2007).
The immunomodulatory drug FTY720/fingolimod, which has been approved by the Food and Drug Administration for the treatment of relapsing forms of multiple sclerosis (MS) (Chun and Hartung, 2010; Chun and Brinkmann, 2011; Cohen and Chun, 2011), is phosphorylated in vivo to FTY720-P, producing the active form of the drug (Brinkmann et al., 2010). FTY720-P is a structural analogue of S1P and an agonist of S1P1, S1P3, S1P4, and S1P5. However, persistent activation of S1P1 by FTY720-P causes its internalization and degradation and thus it acts as a functional antagonist (Graeler and Goetzl, 2002; Matloubian et al., 2004; Oo et al., 2007; Brinkmann et al., 2010; Gonzalez-Cabrera et al., 2012). Down-regulating surface expression of S1P1 on lymphocytes prevents their egress from lymphoid organs and reduces peripheral blood lymphocyte levels (Brinkmann et al., 2010; Gonzalez-Cabrera et al., 2012). Concomitantly, direct CNS actions may be relevant to MS through S1P1 expressed on astrocytes, since conditional removal of this receptor reduces MS-like disease in animals and attenuates FTY720 activity (Choi et al., 2011). Expression of this and other S1P receptors in the CNS supports other activities relevant to MS, and perhaps other CNS disorders (Gardell et al., 2006; Herr and Chun, 2007; Noguchi and Chun, 2011; Soliven et al., 2011; Mutoh et al., 2012; Choi and Chun, 2013; Groves et al., 2013).
S1P1 maintains the integrity of the vascular system (Liu et al., 2000; Camerer et al., 2009; Wang and Dudek, 2009; Abbasi and Garcia, 2013), which is critical for homeostasis and to prevent extravasation of plasma during infections, sepsis and anaphylactic shock, which can be life threatening. Blood S1P enhances vascular barrier function by ligation of S1P1 with subsequent downstream activation of the Rho family of small GTPases, cytoskeletal reorganization, adherens junction and tight junction assembly, and focal adhesion formation (Wang and Dudek, 2009; Abbasi and Garcia, 2013). Depletion of blood S1P in mice induces basal vascular leak and increases lethal responses in anaphylaxis induced by administration of platelet-activating factor or histamine (Camerer et al., 2009). It has been suggested that either S1P continuously activates luminal endothelial S1P1 to maintain tight cell–cell junctions or alternatively, following entry of S1P into the sub-endothelial space via ‘leaky’ endothelium, dynamic S1P1 signalling activates abluminal surface S1P1 to close intercellular gaps. Furthermore, the S1P/S1P1 axis also attenuates LPS-induced acute lung injury in murine and canine models (Wang and Dudek, 2009; Abbasi and Garcia, 2013). Deciphering the mechanisms by which the S1P1 signalling pathway regulates endothelial barrier integrity will help our understanding and treatment of acute inflammatory diseases.
The vital role of S1P1 in vascular maturation and development was demonstrated by knockout of the S1pr1 gene in mice that die in utero because of a defect in the association of mural cells with nascent vessels and incomplete coverage (Liu et al., 2000; Allende et al., 2003). More recently, the role of S1P1 in angiogenesis, the development of new blood vessels, has been slightly revised. It was shown that S1P1 in fact acts independently of mural cells in an endothelial cell-autonomous manner to inhibit sprouting angiogenesis (Shoham et al., 2012). Endothelial S1P1 stabilizes the primary vascular network during development and homeostasis (Gaengel et al., 2012; Jung et al., 2012).
Recently, the crystal structure of S1P1 fused to T4-lysozyme in complex with an antagonist was solved to 2.8 Å resolution (Hanson et al., 2012). Intriguingly, this receptor has a novel N-terminal fold that blocks access of S1P to the binding pocket from the extracellular environment. Therefore, S1P must gain access by entering laterally between helices I and VII within the transmembrane region of S1P1. This work provides the first view of the molecular recognition of S1P (Hanson et al., 2012; Rosen et al., 2013) and may aid in the development of S1P1-specific drugs as well as providing a basis for determining the structure of the other S1P receptors.
S1P2/S1PR2/S1pr2
Now denoted as S1P2, this receptor was previously known as Edg-5, H218, AGR16, and lpB2 and was one of the first to be identified as an S1P receptor (An et al., 1997). The human gene, S1PR2, is located on chromosomal locus 19p13.2 and its sequence is highly conserved across species, with the human receptor containing 353 amino acids and a receptor of ∼39 kDa compared with the murine transcript with 352 (also ∼39 kDa). The S1P2 gene is expressed in a variety of tissues (Zhang et al., 1999; McGiffert et al., 2002) and can couple to multiple G-proteins, although it most efficiently utilizes Gα12/13 to activate the small GTPase Rho. Thus, S1P2 typically inhibits motility through inhibition of Rac. S1P2 has been shown to be involved in S1P-induced cell proliferation, motility and transcriptional activation, usually acting in opposition to S1P1 (Skoura and Hla, 2009; Chun et al., 2010).
S1P2 was initially shown to be required for heart development in zebrafish (Kupperman et al., 2000). It was subsequently reported that S1P2 signals through the Gα13/RhoGEF pathway to promote the migration of myocardial precursor cells (Ye and Lin, 2013), although S1pr2 knockout mice are viable (Ishii et al., 2002), demonstrating species differences. However, these null mutants have multiple severe inner ear defects, leading to deafness and balance problems (Herr et al., 2007; Kono et al., 2007). Using an S1P2 antagonist, JTE013, it was shown that S1P2 promotes vasoconstriction of the spiral modiolar artery, which protects the stria vascularis capillary bed of the inner ear from high perfusion pressure. Several other studies have linked S1P2 to vascular development and remodelling. S1P2 is induced in endothelial cells undergoing hypoxic stress and mice lacking both S1pr1 and S1pr2 exhibit substantially more vascular defects than S1pr1 knockout alone, suggesting that the two receptors may act coordinately during vascular development (Kono et al., 2004). Experiments in developing zebrafish, which have S1PR homologues and S1P levels in the blood that are higher than the KD of the receptors, showed similar results. S1pr1 knockdown interfered with the development of the intersegmental vessels, and this phenotype was enhanced when S1pr2 was suppressed (Mendelson et al., 2013).
S1P2 has also been suggested to play a role in endothelial barrier integrity. In an LPS-induced model of acute lung injury, S1P2 deletion reduced oedema while activation of S1P1 with a specific agonist also reduced oedema (Sammani et al., 2010), suggesting that S1P2 reduces endothelial barrier function in contrast to S1P1, which enhances it. In mice, S1P2 can also promote the recovery from anaphylactic shock, at least in part through counteracting the histamine-induced vasodilatation responsible for hypotension (Olivera et al., 2010; 2013). Accordingly, histamine initiates a negative feedback loop, stimulating production of S1P that acts through S1P2 to increase clearance of histamine by the kidney through excretion.
S1P2 also plays a role in bone maintenance. Bone is remodelled throughout life, with osteoblasts forming bone and osteoclasts resorbing it. Osteoclast precursor cells migrate dynamically between bone and blood, which is controlled by the balance between S1P signalling through S1P1 versus S1P2. While S1P1 promotes osteoclast migration from bone towards high blood levels of S1P (Ishii et al., 2009a), migration away from bone is negatively controlled by S1P2 (Ishii et al., 2010). Insight into how the balance of S1P receptor expression controls bone remodelling was provided by the demonstration that calcetriol, the active form of vitamin D that promotes bone growth, reduces S1P2 expression on osteoclasts (Kikuta et al., 2013). This balance between S1P1 and S1P2 that controls traffic of cells into and out of tissues is becoming paradigmatic. Cyster and colleagues showed that S1P2 promotes the retention of B cells in the germinal centres of lymphoid follicles at the low end of an S1P gradient (Green et al., 2011). Moreover, S1P2 also plays a role in controlling growth and apoptosis of germinal centre B cells through inhibition of Akt (Green et al., 2011).
S1P also has an important role in muscle regeneration through activation of muscle stem cells called satellite cells (Rapizzi et al., 2008). Saba and colleagues demonstrated that S1P biosynthesis is up-regulated following muscle injury (Loh et al., 2012) and activation of S1P2, but not S1P1, promoted muscle regeneration by activating STAT3, which in turn down-regulates the cell cycle inhibitors p21 and p27 allowing for satellite cell growth (Loh et al., 2012). Moreover, Mdx mice, a model for muscular dystrophy, have higher levels of S1P-metabolizing enzymes and lower circulating levels of S1P. However, using a different model of muscle injury induced by bupivacaine, S1P2 was not found to be involved in muscle regeneration (Danieli-Betto et al., 2010). It was suggested that S1P3 promoted, while S1P1 inhibited, muscle regeneration. The conflicting data concerning the specific S1P receptors involved may be due to the different models used or the timing of S1P receptor activation.
S1P2 has also recently been implicated in promoting metastasis. Using genetic and pharmacological approaches, it was shown that bladder cancer xenografts increased systemic S1P levels. This S1P in turn activated S1P2, leading to the down-regulation of Brms1, a known suppressor of metastasis (Ponnusamy et al., 2012). Thus, inhibition of systemic sphingosine kinase 1 and production of S1P and/or S1P2 signalling increased Brms1 expression suppressing lung metastasis (Ponnusamy et al., 2012).
S1P3/S1PR3/S1pr3
S1P3, previously known as Edg-3 and lpB3, was also an early identified S1P receptor (An et al., 1997), with human S1PR3 located at chromosomal locus 9q22.1-q22.2, encoding a 378-amino acid protein of ∼42 kDa, with seven predicted transmembrane domains. It shares 87% identity with the murine S1P3 receptor.
Like S1P2, S1P3 can couple to multiple G-proteins, including Gαi/o, Gαq, and Gα12/13 (Chun et al., 2010), although in cells it most commonly couples to Gαq, leading to the generation of inositol trisphosphate and diacylglycerol with subsequent calcium mobilization and activation of PKC respectively.
Despite fairly broad gene expression (Zhang et al., 1999; McGiffert et al., 2002), global deletion of S1pr3 in mice did not reveal an obvious phenotype or developmental defects (Ishii et al., 2001), although the S1pr2/3 double knockouts have reduced fertility (Ishii et al., 2002). Initially, S1P3 was reported to be highly expressed in breast cancer models where it plays a positive role in cell migration (Chun et al., 2010). Moreover, increased expression of S1P3 in oestrogen receptor (ER)-positive tumour samples correlated with decreased disease-free survival times (Watson et al., 2010). One possible explanation for this is the intriguing finding that in breast cancer cells, oestrogen stimulates S1P release and activation of S1P3 (Sukocheva et al., 2006). This then increases the activity of MMP9, resulting in the release of EGF to signal in an autocrine manner. Additionally, in this system, S1P3 also activates Cdc42 and decreases degradation of, and increases signalling from, the EGF receptor (Sukocheva et al., 2013). Interestingly, an S1P3-blocking monoclonal antibody, 7H9, has been developed that blocks the growth of breast cancer tumours in a xenograft model (Harris et al., 2012).
S1P3 has also been implicated in sepsis. Signalling of the protease-activated receptor 1 on dendritic cells promotes the inflammatory response in sepsis syndrome. Treatment with S1P3-specific antagonists, as well as S1P3 deletion, protects from LPS-induced lethal sepsis (Niessen et al., 2008; Sammani et al., 2010). Although activation of S1P1 increases endothelial barrier enhancement, S1P3 disrupts it (Sammani et al., 2010). Indeed, recent studies associate increased S1P3 expression with sepsis and mortality of intensive care patients (Sun et al., 2012). Finally, several studies indicate that S1P3 is involved in liver fibrosis. S1P, acting through both S1P1 and S1P3, promotes the motility of hepatic stellate cells and their differentiation into hepatic myofibroblasts (Liu et al., 2011), and enhances liver angiogenesis associated with fibrosis (Yang et al., 2013).
S1P4/S1PR4/S1pr4
S1PR4 is located at chromosomal locus 19p13.3, previously known as Edg-6 and lpC1 (Contos et al., 2002) and encodes a 384-amino acid protein of ∼42 kDa in humans that is highly homologous across mammalian species (Van Brocklyn et al., 2000).
S1P4 couples to Gαi and Gα12/13 and promotes cell migration (Graler et al., 2003; Kohno et al., 2003). S1P4 has a restricted tissue distribution and is expressed mainly in haematopoietic tissue, though it was recently reported to be in other tissues, such as the muscle satellite cells, where together with S1P1, it promotes migration in response to S1P (Calise et al., 2012). Expression of S1pr4 has also been reported in rat lungs, but not in renal or mesenteric arteries, and the S1P4 agonist VPC23153 promoted vasoconstriction of both normotensive and hypertensive pulmonary arteries (Ota et al., 2011). Moreover, expression of S1P4 in ER-negative breast cancer cells correlated with poorer prognosis (Ohotski et al., 2012).
S1P4 is also important in megakaryocytes where it is highly induced upon differentiation. Although S1P4 knockout mice have normal platelet levels, their ability to generate platelets after experimentally-induced thrombocytopenia is delayed, suggesting a role for S1P4, in thrombopoiesis (Golfier et al., 2010). Also in these mice, T-cell proliferation and cytokine secretion are not significantly altered (Schulze et al., 2011). Interestingly, S1pr4 knockout mice also have differential responses in various models of inflammation with exacerbated Th2-mediated responses, but reduced Th1-mediated responses. These changes were linked to altered dendritic cell functions, including decreased IL-6 production and IL-17 secretion. S1pr4 deletion also decreased neutrophilia, suggesting a potential role for this receptor in neutrophil migration (Allende et al., 2011).
S1P5/S1PR5/S1pr5
Previously known as Edg-8, lpB4, and Nrg-1, S1PR5 is located at chromosomal locus 19p13.2 and encodes a highly conserved 398-amino acid protein with a calculated molecular mass of ∼39 kD with tissue expression primarily restricted to brain and spleen (Im et al., 2000a; Malek et al., 2001). Like other S1P receptors, it couples to multiple G-proteins, although in its common role of inhibiting migration and promoting cell retraction, it couples to Gα12/13. S1P5 knockout mice are viable and fertile. Intriguingly, they show greatly decreased numbers of circulating NK cells (Walzer et al., 2007). Similar to the role S1P1 plays in T and B cell trafficking, S1P5 promotes the egress of NK cells from bone marrow and lymph nodes into blood and other tissues. Moreover, S1P5 is required for NK recruitment to sites of inflammation (Walzer et al., 2007; Jenne et al., 2009). Furthermore, during NK cell differentiation, S1P5 is expressed, allowing exit from the bone marrow (Mayol et al., 2011). S1P5 knockout mice also lack circulating Ly6C-negative peripheral monocytes, but have normal levels in the bone marrow (Debien et al., 2013). Interestingly, although S1P5 is required for egress of these cells, S1P is not a chemoattractant, suggesting that S1P5 may act during their differentiation.
New lysophospholipid receptors
Efforts to de-orphanize GPCRs led to the identification of putative new members of the lysophospholipid receptor family. These receptors interact with two distinct glycerophospholipids: LPI and LysoPS. Newer technologies to identify receptors, such as the TGFα shedding assay, are being developed and used successfully for both de-orphanization and correction or augmentation of lysophospholipid identities.
LPI receptor: LPI1/LPIR1/Lpir1 (orphan GPR55)
Orphan receptor GPR55 had originally been reported to be a novel cannabinoid receptor (Lauckner et al., 2008); however, it appears that this receptor may in fact act as a LPI receptor based upon recent evaluations (Kotsikorou et al., 2011; Inoue et al., 2012; Aoki, Inoue and colleagues, unpublished). In view of these data, we consider GPR55 as a provisional LPI receptor with receptor name LPI1 and gene names LPIR1/Lpir1 for human and non-human genes respectively. LPIR1 is located on human chromosome 2 (2q37) and encodes a 319-amino acid protein (∼37 kDa). It is currently unclear whether this receptor genuinely acts as a cannabinoid receptor, and efforts are underway to better determine the ligand specificity of this GPCR.
Proposed LysoPS receptors
The following receptors have shown activity using a TGFα shedding assay (Inoue et al., 2012), which strongly support their identity as LysoPS receptors; however, this identity should be considered provisional. In addition, the name of the receptors may require future modification: LysoPSx is utilized here to avoid confusion with lipopolysaccharide that is commonly referred to as LPS. The lysophospholipid LysoPS, has been known as an immune cell stimulus, leading to identification of the first LysoPS receptor from mast cells via de-orphanization of the P2Y family of GPCRs known as GPR34 (Sugo et al., 2006). LyPSR1 is located at chromosomal locus Xp11.4 and encodes a 381-amino acid protein for a calculated molecular mass of ∼44 kD. Receptor identity was confirmed using the TGFα shedding assay (Inoue et al., 2012; Kitamura et al., 2012; Makide and Aoki, 2013), although there is some disagreement in the literature on the veracity of this identity (Ritscher et al., 2012). Genetic deletion of GPR34 does result in immunological dysfunction (Liebscher et al., 2011), consistent with the immunological effects of LysoPS, and combined with positivity in the TGFα assay, its designation as LysoPS1 appears to be warranted. LysoPS1 has been implicated in other cell types such as microglia in the brain (Bedard et al., 2007), and has been linked to diseases or disorders, including a form of night blindness (Jacobi et al., 2000) and cancers of both immune (Ansell et al., 2012) and non-immune origin (Yu et al., 2013). Through the use of the TGFα shedding assay as a screening tool, three other receptors were identified, the first of which was another P2Y orphan receptor, P2Y10. LyPSR2 is located on human chromosome X (Xq21.1) and encodes a 339 amino acid protein (∼39 kDa). Consistent with the biological effects of LysoPS on the immune system and data from analyses of LysoPS1, LysoPS2 also influences the immune system, and appears to show restricted expression in dendritic cells derived from monocytes (Berchtold et al., 1999) and lymphoid lineages (Rao et al., 1999). LysoPS3/LyPSR3/Lypsr3, another orphan receptor (formerly GPR174), was identified as a third LysoPS receptor by TGFα assay (Inoue et al., 2012) and independently supported by classical assays (Sugita et al., 2013). LyPSR3 is located near the LPAR4 and LyPSR2 genes (Xq21.1) and encodes a 333 amino acid protein of ∼39 kDa, which shares about 45% identity with LysoPS2. LyPSR3 has recently been reported as a genetic risk locus for Graves disease (Zhao et al., 2013). During TGFα screening analyses of orphan GPCRs, a mouse cDNA not present in humans, A630033H20, was identified as a LysoPS receptor with predicted homology to LysoPS2 (Inoue et al., 2012). This gene is located between Lypsr2/p2ry10 and Lypsr3/GPR174 on mouse chromosomal locus Xq21.1, which corresponds to the human P2RY10P2 pseudogene. Therefore, nomenclature for a mouse-specific receptor and consequent gene names is neither proposed nor discouraged. A number of lysophospholipid receptor mutants or variants have been reported, such as the mRec1.3 mutant of LPA1 (Contos et al., 2000b; Fukushima et al., 2001) or the original sequence for S1pr3 (Edg-3) that was a variant form present in a cancer cell line (An et al., 1997), and there is currently no uniform recommendation for naming these receptor variants, which could be a topic for future nomenclature efforts.
Concluding remarks
This nomenclature review for lysophospholipid receptors incorporates the recommended, as well as the most common uses of protein and gene names. For receptor proteins, the simple use of the cognate ligand immediately followed by a subscript to designate a receptor subtype is easily extended to receptors for other lysophospholipid ligands, as illustrated by the additions of LPI1 and LysoPS1–3, as was first used for this family based upon IUPHAR recommendations. To easily differentiate proteins from genes and provide an accurate interface with sequence databases such as ENCODE (Maher, 2012; Skipper et al., 2012), the italicized use of the HGNC and MGI nomenclatures are recommended for human and non-human genes respectively. This nomenclature will accommodate the likely addition of new members to the lysophospholipid receptor family via both de-orphanization and revised receptor identities.
Acknowledgments
We thank Danielle Jones and Hope Mirendil for editorial assistance. This work was supported by the National Institutes of Health.
Glossary
- DRG
dorsal root ganglia
- CNS
central nervous system
- HGNC
Gene Nomenclature Committee
- HUGO
Human Genome Organization
- LPA
lysophosphatidic acid
- LPI
lysophosphatidyl inositol
- LysoPS
lysophosphatidyl serine
- MGI
Mouse Genome Informatix
- MS
multiple sclerosis
- PSNL
partial sciatic nerve ligation
- SC
Schwann cell
- S1P
sphingosine 1-phosphate
- VZ
ventricular zone
Conflict of interest
Jerold Chun has received honoraria, consulting fees and/or grant support from: Abbott, Amira Pharmaceuticals, Biogen-Idec, Celgene, GlaxoSmithKline, Johnson and Johnson, Merk, Mitsubishi Tanabe Pharma Corporation, Novaritis, Ono Pharmaceutical Co., Pfizer and Taisho Pharmaceutical Co. Yasuyuki Kihara has no conflict of interest.
References
- Abbasi T, Garcia JG. Sphingolipids in lung endothelial biology and regulation of vascular integrity. Handb Exp Pharmacol. 2013;216:201–226. doi: 10.1007/978-3-7091-1511-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The concise guide to PHARMACOLOGY 2013/14: overview. Br J Pharmacol. 2013a;170:1449–1458. [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013c;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013d;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard J, Barron S, Diaz J, Lubetzki C, Zalc B, Schwartz JC, et al. A rat G protein-coupled receptor selectively expressed in myelin-forming cells. Eur J Neurosci. 1998;10:1045–1053. doi: 10.1046/j.1460-9568.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- Allende ML, Yamashita T, Proia RL. G-protein coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2011;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amisten S, Braun OO, Bengtsson A, Erlinge D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res. 2008;122:47–57. doi: 10.1016/j.thromres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Akasaka T, McPhail E, Manske M, Braggio E, Price-Troska T, et al. t(X;14) (p11;q32) in MALT lymphoma involving GPR34 reveals a role for GPR34 in tumor cell growth. Blood. 2012;120:3949–3957. doi: 10.1182/blood-2011-11-389908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure–activity relationship of cloned LPA receptors. FEBS Lett. 2000;478:159–165. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- Bedard A, Tremblay P, Chernomoretz A, Vallieres L. Identification of genes preferentially expressed by microglia and upregulated during cuprizone-induced inflammation. Glia. 2007;55:777–789. doi: 10.1002/glia.20477. [DOI] [PubMed] [Google Scholar]
- Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, Schuler G, et al. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem Rev. 2011;111:6299–6320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Quilliot D, Praderes JP, Simon MF, Gres S, Guigne C, et al. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diab Tologia. 2005;48:569–577. doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe M, Ming X, Lamendola M. Prenatal and birth complications in autism. Matern Child Health J. 2007;11:73–79. doi: 10.1007/s10995-006-0142-7. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Byrne M, Agerbo E, Bennedsen B, Eaton WW, Mortensen PB. Obstetric conditions and risk of first admission with schizophrenia: a Danish national register based study. Schizophr Res. 2007;97:51–59. doi: 10.1016/j.schres.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta. 2012;1823:439–450. doi: 10.1016/j.bbamcr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Castilla-Ortega E, Sánchez-López J, Hoyo-Becerra C, Matas-Rico E, Zambrana-Infantes E, Chun J, et al. Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol Learn Mem. 2010;94:73–82. doi: 10.1016/j.nlm.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LC, Peters W, Xu Y, Chun J, Farese RV, Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007;82:1193–1200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- Chen R, Roman J, Guo J, West E, McDyer J, Williams MA, et al. Lysophosphatidic acid modulates the activation of human monocyte-derived dendritic cells. Stem Cells Dev. 2006;15:797–804. doi: 10.1089/scd.2006.15.797. [DOI] [PubMed] [Google Scholar]
- Choi JW, Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta. 2013;1831:20–32. doi: 10.1016/j.bbalip.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya) Discov Med. 2011;12:213–228. [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International union of pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International union of basic and clinical pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Chun J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Chun J. Complete cDNA sequence, genomic structure, and chromosomal localization of the LPA receptor gene, lpA1/vzg-1/Gpcr26. Genomics. 1998;51:364–378. doi: 10.1006/geno.1998.5400. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Chun J. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics. 2000;64:155–169. doi: 10.1006/geno.2000.6122. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000a;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000b;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Ye X, Sah VP, Chun J. Tandem genomic arrangement of a G protein (Gna15) and G protein-coupled receptor (s1p(4)/lp(C1)/Edg6) gene. FEBS Lett. 2002;531:99–102. doi: 10.1016/s0014-5793(02)03409-9. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Danieli-Betto D, Peron S, Germinario E, Zanin M, Sorci G, Franzoso S, et al. Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration. Am J Physiol Cell Physiol. 2010;298:C550–C558. doi: 10.1152/ajpcell.00072.2009. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, et al. International union of basic and clinical pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev. 2013;65:967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- Debien E, Mayol K, Biajoux V, Daussy C, De Aguero MG, Taillardet M, et al. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur J Immunol. 2013;43:1667–1675. doi: 10.1002/eji.201343312. [DOI] [PubMed] [Google Scholar]
- Deng W, Balazs L, Wang DA, Van Middlesworth L, Tigyi G, Johnson LR. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology. 2002;123:206–216. doi: 10.1053/gast.2002.34209. [DOI] [PubMed] [Google Scholar]
- Estivill-Torrús G, Llebrez-Zayas P, Matas-Rico E, Santín L, Pedraza C, De Diego I, et al. Absence of LPA1 signaling results in defective cortical development. Cereb Cortex. 2008;18:938–950. doi: 10.1093/cercor/bhm132. [DOI] [PubMed] [Google Scholar]
- Estrella VC, Eder AM, Liu S, Pustilnik TB, Tabassam FH, Claret FX, et al. Lysophosphatidic acid induction of urokinase plasminogen activator secretion requires activation of the p38MAPK pathway. Int J Oncol. 2007;31:441–449. [PubMed] [Google Scholar]
- Ferry G, Tellier E, Try A, Gres S, Naime I, Simon MF, et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162–18169. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss FW, Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita R, Kiguchi N, Ueda H. LPA-mediated demyelination in ex vivo culture of dorsal root. Neurochem Int. 2007;50:351–355. doi: 10.1016/j.neuint.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Weiner JA, Chun J. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev Biol. 2000;228:6–18. doi: 10.1006/dbio.2000.9930. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Ishii I, Contos JJ, Weiner JA, Chun J. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Weiner JA, Kaushal D, Contos JJ, Rehen SK, Kingsbury MA, et al. Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol Cell Neurosci. 2002;20:271–282. doi: 10.1006/mcne.2002.1123. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–599. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Kong Y, Mei B. Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J Immunol. 1999;162:2049–2056. [PubMed] [Google Scholar]
- Goetzl EJ, Kong Y, Voice JK. Cutting edge: differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J Immunol. 2000;164:4996–4999. doi: 10.4049/jimmunol.164.10.4996. [DOI] [PubMed] [Google Scholar]
- Golfier S, Kondo S, Schulze T, Takeuchi T, Vassileva G, Achtman AH, et al. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1-phosphate receptor S1P4. FASEB J. 2010;24:4701–4710. doi: 10.1096/fj.09-141473. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol. 2008;74:1308–1318. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Cahalan SM, Nguyen N, Sarkisyan G, Leaf NB, Cameron MD, et al. S1P(1) receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol Pharmacol. 2012;81:166–174. doi: 10.1124/mol.111.076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- Graler MH, Grosse R, Kusch A, Kremmer E, Gudermann T, Lipp M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J Cell Biochem. 2003;89:507–519. doi: 10.1002/jcb.10537. [DOI] [PubMed] [Google Scholar]
- Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CD, et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol. 2011;12:672–680. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328:9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama K, Aoki J, Bandoh K, Inoue A, Endo T, Amano T, et al. Lysophosphatidic receptor, LPA3, is positively and negatively regulated by progesterone and estrogen in the mouse uterus. Life Sci. 2006;79:1736–1740. doi: 10.1016/j.lfs.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, et al. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol Reprod. 2007;77:954–959. doi: 10.1095/biolreprod.107.060293. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GL, Creason MB, Brulte GB, Herr DR. In vitro and in vivo antagonism of a G protein-coupled receptor (S1P3) with a novel blocking monoclonal antibody. PLoS ONE. 2012;7:e35129. doi: 10.1371/journal.pone.0035129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Reavill C, Brown G, Brown JT, Cluderay JE, Crook B, et al. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol Cell Neurosci. 2003;24:1170–1179. doi: 10.1016/j.mcn.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Heasley BH, Jarosz R, Lynch KR, Macdonald TL. Initial structure-activity relationships of lysophosphatidic acid receptor antagonists: discovery of a high-affinity LPA1/LPA3 receptor antagonist. Bioorg Med Chem Lett. 2004;14:2735–2740. doi: 10.1016/j.bmcl.2004.03.076. [DOI] [PubMed] [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise CE, Santos WL, Schreihofer AM, Heasley BH, Mukhin YV, Macdonald TL, et al. Activity of 2-substituted lysophosphatidic acid (LPA) analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol Pharmacol. 2001;60:1173–1180. doi: 10.1124/mol.60.6.1173. [DOI] [PubMed] [Google Scholar]
- Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8:155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- Herr DR, Grillet N, Schwander M, Rivera R, Muller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci. 2007;27:1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope JM, Wang FQ, Whyte JS, Ariztia EV, Abdalla W, Long K, et al. LPA receptor 2 mediates LPA-induced endometrial cancer invasion. Gynecol Oncol. 2009;112:215–223. doi: 10.1016/j.ygyno.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Huang MC, Lee HY, Yeh CC, Kong Y, Zaloudek CJ, Goetzl EJ. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2) Oncogene. 2004;23:122–129. doi: 10.1038/sj.onc.1206986. [DOI] [PubMed] [Google Scholar]
- Hultman CM, SparAcn P, Takei N, Murray RM, Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case A – control study. BMJ. 1999;318:421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Yatomi Y, Yanase M, Satoh H, Nishihara A, Kawabata M, et al. Effects of lysophosphatidic acid on proliferation of stellate cells and hepatocytes in culture. Biochem Biophys Res Commun. 1998;248:436–440. doi: 10.1006/bbrc.1998.8983. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Ancellin N, O'Dowd BF, Shei GJ, Heavens RP, et al. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem. 2000a;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Harding MA, George SR, O'Dowd BF, Theodorescu D, et al. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol Pharmacol. 2000b;57:753–759. [PubMed] [Google Scholar]
- Inoue A, Ishiguro J, Kitamura H, Arima N, Okutani M, Shuto A, et al. TGFalpha shedding assay: an accurate and versatile method for detecting GPCR activation. Nat Methods. 2012;9:1021–1029. doi: 10.1038/nmeth.2172. [DOI] [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- Inoue M, Ma L, Aoki J, Chun J, Ueda H. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol Pain. 2008a;4:6. doi: 10.1186/1744-8069-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Ma L, Aoki J, Ueda H. Simultaneous stimulation of spinal NK1 and NMDA receptors produces LPC which undergoes ATX-mediated conversion to LPA, an initiator of neuropathic pain. J Neurochem. 2008b;107:1556–1565. doi: 10.1111/j.1471-4159.2008.05725.x. [DOI] [PubMed] [Google Scholar]
- Ishii I, Contos JJ, Fukushima N, Chun J. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol Pharmacol. 2000;58:895–902. doi: 10.1124/mol.58.5.895. [DOI] [PubMed] [Google Scholar]
- Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, et al. Marked perinatal lethality and cellular signaling deficits in mice Null for the two sphingosine 1-phosphate receptors, S1P2/LPB2/EDG-5 and S1P3/LPB3/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009a;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Noguchi K, Yanagida K. Non-Edg family lysophosphatidic acid (LPA) receptors. Prostaglandins Other Lipid Mediat. 2009b;89:57–65. doi: 10.1016/j.prostaglandins.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Jacobi FK, Broghammer M, Pesch K, Zrenner E, Berger W, Meindl A, et al. Physical mapping and exclusion of GPR34 as the causative gene for congenital stationary night blindness type 1. Hum Genet. 2000;107:89–91. doi: 10.1007/s004390000337. [DOI] [PubMed] [Google Scholar]
- Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KJ, Park SY, Seo JH, Lee KB, Choi WS, Han JW, et al. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp Mol Med. 2008;40:607–616. doi: 10.3858/emm.2008.40.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell. 2012;23:600–610. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PC, Zhu R, Huang T, Tomsig JL, Mathews TP, David M, et al. Characterization of a sphingosine 1-phosphate receptor antagonist prodrug. J Pharmacol Exp Ther. 2011;338:879–889. doi: 10.1124/jpet.111.181552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta J, Kawamura S, Okiji F, Shirazaki M, Sakai S, Saito H, et al. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc Natl Acad Sci U S A. 2013;110:7009–7013. doi: 10.1073/pnas.1218799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Makide K, Shuto A, Ikubo M, Inoue A, Suzuki K, et al. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J Biochem. 2012;151:511–518. doi: 10.1093/jb/mvs011. [DOI] [PubMed] [Google Scholar]
- Kitayama J, Shida D, Sako A, Ishikawa M, Hama K, Aoki J, et al. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. 2004;6:R640–R646. doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobashi H, Yaoi T, Oda R, Okajima S, Fujiwara H, Kubo T, et al. Lysophospholipid receptors are differentially expressed in rat terminal Schwann cells, as revealed by a single cell RT-PCR and in situ hybridization. Acta Histochem Cytochem. 2006;39:55–60. doi: 10.1267/ahc.06002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Matsuyuki H, Inagaki Y, Igarashi Y. Sphingosine 1-phosphate promotes cell migration through the activation of Cdc42 in Edg-6/S1P4-expressing cells. Genes Cells. 2003;8:685–697. doi: 10.1046/j.1365-2443.2003.00667.x. [DOI] [PubMed] [Google Scholar]
- Komachi M, Tomura H, Malchinkhuu E, Tobo M, Mogi C, Yamada T, et al. LPA1 receptors mediate stimulation, whereas LPA2 receptors mediate inhibition, of migration of pancreatic cancer cells in response to lysophosphatidic acid and malignant ascites. Carcinogenesis. 2009;30:457–465. doi: 10.1093/carcin/bgp011. [DOI] [PubMed] [Google Scholar]
- Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, et al. Deafness and stria vascularis defects in S1P2 receptor null mice. J Biol Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, et al. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- Kotsikorou E, Lynch DL, Abood ME, Reggio PH. Lipid bilayer molecular dynamics study of lipid-derived agonists of the putative cannabinoid receptor, GPR55. Chem Phys Lipids. 2011;164:131–143. doi: 10.1016/j.chemphyslip.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- Lai SL, Yao WL, Tsao KC, Houben AJ, Albers HM, Ovaa H, et al. Autotaxin/Lpar3 signaling regulates Kupffer's vesicle formation and left-right asymmetry in zebrafish. Development. 2012;139:4439–4448. doi: 10.1242/dev.081745. [DOI] [PubMed] [Google Scholar]
- Lai YJ, Chen CS, Lin WC, Lin FT. c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol Cell Biol. 2005;25:5859–5868. doi: 10.1128/MCB.25.14.5859-5868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YJ, Lin WC, Lin FT. PTPL1/FAP-1 negatively regulates TRIP6 function in lysophosphatidic acid-induced cell migration. J Biol Chem. 2007;282:24381–24387. doi: 10.1074/jbc.M701499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem. 2007;282:4310–4317. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- Lee M, Choi S, Hallden G, Yo SJ, Schichnes D, Aponte GW. P2Y5 is a G(alpha)i, G(alpha)12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am J Physiol Gastrointest Liver Physiol. 2009;297:G641–G654. doi: 10.1152/ajpgi.00191.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Liu CH, Thompson BD, Hla T. Lysophosphatidic acid stimulates the G-protein-coupled receptor EDG-1 as a low affinity agonist. J Biol Chem. 1998a;273:22105–22112. doi: 10.1074/jbc.273.34.22105. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998b;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yun CC. Colorectal cancer cells – proliferation, survival and invasion by lysophosphatidic acid. Int J Biochem Cell Biol. 2010;42:1907–1910. doi: 10.1016/j.biocel.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ritter SL, Zhang H, Shim H, Hall RA, Yun CC. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology. 2011;140:924–934. doi: 10.1053/j.gastro.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, et al. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008;19:5435–5445. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, et al. Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol Cancer Res. 2009;7:1064–1077. doi: 10.1158/1541-7786.MCR-08-0578. [DOI] [PubMed] [Google Scholar]
- Liebscher I, Muller U, Teupser D, Engemaier E, Engel KM, Ritscher L, et al. Altered immune response in mice deficient for the G protein-coupled receptor GPR34. J Biol Chem. 2011;286:2101–2110. doi: 10.1074/jbc.M110.196659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, Lai YJ. Regulation of the LPA2 receptor signaling through the carboxyl-terminal tail-mediated protein–protein interactions. Biochim Biophys Acta. 2008;1781:558–562. doi: 10.1016/j.bbalip.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ME, Rivera RR, Chun J. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J Biol Chem. 2012;287:17608–17617. doi: 10.1074/jbc.M111.330183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, et al. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology. 2010;138:649–658. doi: 10.1053/j.gastro.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yue S, Li C, Yang L, You H, Li L. Essential roles of sphingosine 1-phosphate receptor types 1 and 3 in human hepatic stellate cells motility and activation. J Cell Physiol. 2011;226:2370–2377. doi: 10.1002/jcp.22572. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YB, Kharode Y, Bodine PV, Yaworsky PJ, Robinson JA, Billiard J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem. 2010;109:794–800. doi: 10.1002/jcb.22471. [DOI] [PubMed] [Google Scholar]
- Loh KC, Leong WI, Carlson ME, Oskouian B, Kumar A, Fyrst H, et al. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE. 2012;7:e37218. doi: 10.1371/journal.pone.0037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, et al. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol Pain. 2009;5:64. doi: 10.1186/1744-8069-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher B. ENCODE: the human encyclopaedia. Nature. 2012;489:46–48. doi: 10.1038/489046a. [DOI] [PubMed] [Google Scholar]
- Makide K, Aoki J. GPR34 as a lysophosphatidylserine receptor. J Biochem. 2013;153:327–329. doi: 10.1093/jb/mvt010. [DOI] [PubMed] [Google Scholar]
- Malek RL, Toman RE, Edsall LC, Wong S, Chiu J, Letterle CA, et al. Nrg-1 belongs to the endothelial differentiation gene family of G protein-coupled sphingosine-1-phosphate receptors. J Biol Chem. 2001;276:5692–5699. doi: 10.1074/jbc.M003964200. [DOI] [PubMed] [Google Scholar]
- Manning TJ, Jr, Sontheimer H. Bovine serum albumin and lysophosphatidic acid stimulate calcium mobilization and reversal of cAMP-induced stellation in rat spinal cord astrocytes. Glia. 1997;20:163–172. doi: 10.1002/(sici)1098-1136(199706)20:2<163::aid-glia8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Masiello LM, Fotos JS, Galileo DS, Karin NJ. Lysophosphatidic acid induces chemotaxis in MC3T3-E1 osteoblastic cells. Bone. 2006;39:72–82. doi: 10.1016/j.bone.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Matas-Rico E, García-Diaz B, Llebrez-Zayas P, López-Barroso D, Santín L, Pedraza C, et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci. 2008;39:342–355. doi: 10.1016/j.mcn.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mayol K, Biajoux V, Marvel J, Balabanian K, Walzer T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood. 2011;118:4863–4871. doi: 10.1182/blood-2011-06-362574. [DOI] [PubMed] [Google Scholar]
- McGiffert C, Contos JJ, Friedman B, Chun J. Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p(1) in neurogenesis and s1p(1–3) in angiogenesis. FEBS Lett. 2002;531:103–108. doi: 10.1016/s0014-5793(02)03404-x. [DOI] [PubMed] [Google Scholar]
- Mendelson K, Zygmunt T, Torres-Vazquez J, Evans T, Hla T. Sphingosine 1-phosphate receptor signaling regulates proper embryonic vascular patterning. J Biol Chem. 2013;288:2143–2156. doi: 10.1074/jbc.M112.427344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Di Daniel E, Maycox P, Racagni G, Popoli M. Abnormalities in alpha/beta-CaMKII and related mechanisms suggest synaptic dysfunction in hippocampus of LPA1 receptor knockout mice. Int J Neuropsychopharmacol. 2010;14:941–953. doi: 10.1017/S1461145710001240. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Rivera R, Chun J. Insights into the pharmacological relevance of lysophospholipid receptors. Br J Pharmacol. 2012;165:829–844. doi: 10.1111/j.1476-5381.2011.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum S, Morice-Picard F, Taieb A, Sprecher E. A novel mutation in LPAR6 causes autosomal recessive hypotrichosis of the scalp. Clin Exp Dermatol. 2011;36:188–194. doi: 10.1111/j.1365-2230.2010.03944.x. [DOI] [PubMed] [Google Scholar]
- Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- Nobusue H, Kondo D, Yamamoto M, Kano K. Effects of lysophosphatidic acid on the in vitro proliferation and differentiation of a novel porcine preadipocyte cell line. Comp Biochem Physiol B Biochem Mol Biol. 2010;157:401–407. doi: 10.1016/j.cbpb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Chun J. Roles for lysophospholipid S1P receptors in multiple sclerosis. Crit Rev Biochem Mol Biol. 2011;46:2–10. doi: 10.3109/10409238.2010.522975. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. doi: 10.1016/j.coph.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Obo Y, Yamada T, Furukawa M, Hotta M, Honoki K, Fukushima N, et al. Frequent mutations of lysophosphatidic acid receptor-1 gene in rat liver tumors. Mutat Res/Fundam Mol Mech Mutagen. 2009;660:47–50. doi: 10.1016/j.mrfmmm.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Oh DY, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, et al. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem. 2008;283:21054–21064. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohotski J, Long JS, Orange C, Elsberger B, Mallon E, Doughty J, et al. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2012;106:1453–1459. doi: 10.1038/bjc.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hamada A, Matsuda H, Takagi A, Tanaka M, Aoki J, et al. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev Dyn. 2008;237:3280–3294. doi: 10.1002/dvdy.21736. [DOI] [PubMed] [Google Scholar]
- Okabe K, Hayashi M, Fujii M, Honoki K, Mori T, Fukushima N, et al. Mutations of lysophosphatidic acid receptor genes in human osteosarcoma cells. Pathobiology. 2010;77:278–282. doi: 10.1159/000319875. [DOI] [PubMed] [Google Scholar]
- Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1240. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Dillahunt SE, Rivera J. Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE and IgG-mediated anaphylaxis with minimal effect on its onset. Immunol Lett. 2013;150:89–96. doi: 10.1016/j.imlet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun. 2002;299:483–487. doi: 10.1016/s0006-291x(02)02671-2. [DOI] [PubMed] [Google Scholar]
- Ota H, Beutz MA, Ito M, Abe K, Oka M, McMurtry IF. S1P(4) receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm Circ. 2011;1:399–404. doi: 10.4103/2045-8932.87309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Mi Y, Pally C, Beerli C, Chen A, Guerini D, et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol. 2006;13:1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Panchatcharam M, Miriyala S, Yang F, Rojas M, End C, Vallant C, et al. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ Res. 2008;103:662–670. doi: 10.1161/CIRCRESAHA.108.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panther E, Idzko M, Corinti S, Ferrari D, Herouy Y, Mockenhaupt M, et al. The influence of lysophosphatidic acid on the functions of human dendritic cells. J Immunol. 2002;169:4129–4135. doi: 10.4049/jimmunol.169.8.4129. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Aboud KA, Lee Y-A, Ruschendorf F, Voss K, et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Muller M, Oji V, Traupe H, Sprecher E, et al. In vitro analysis of LIPH mutations causing hypotrichosis simplex: evidence confirming the role of lipase H and lysophosphatidic acid in hair growth. J Invest Dermatol. 2009;129:2772–2776. doi: 10.1038/jid.2009.154. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy S, Selvam SP, Mehrotra S, Kawamori T, Snider AJ, Obeid LM, et al. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med. 2012;4:761–775. doi: 10.1002/emmm.201200244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007;18:3110–3118. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- Pradere JP, Gonzalez J, Klein J, Valet P, Gres S, Salant D, et al. Lysophosphatidic acid and renal fibrosis. Biochim Biophys Acta. 2008;1781:582–587. doi: 10.1016/j.bbalip.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Xu Y, Simper T, Jiang G, Aoki J, Umezu-Goto M, et al. Phosphorothioate analogues of alkyl lysophosphatidic acid as LPA3 receptor-selective agonists. Chem Med Chem. 2006;1:376–383. doi: 10.1002/cmdc.200500042. [DOI] [PubMed] [Google Scholar]
- Quancard J, Bollbuck B, Janser P, Angst D, Berst F, Buehlmayer P, et al. A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem Biol. 2012;19:1142–1151. doi: 10.1016/j.chembiol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Lefkowitz RJ, Rockman HA. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest. 2005;115:2971–2974. doi: 10.1172/JCI26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Garrett-Sinha LA, Yoon J, Simon MC. The Ets factors PU.1 and Spi-B regulate the transcription in vivo of P2Y10, a lymphoid restricted heptahelical receptor. J Biol Chem. 1999;274:34245–34252. doi: 10.1074/jbc.274.48.34245. [DOI] [PubMed] [Google Scholar]
- Rapizzi E, Donati C, Cencetti F, Nincheri P, Bruni P. Sphingosine 1-phosphate differentially regulates proliferation of C2C12 reserve cells and myoblasts. Mol Cell Biochem. 2008;314:193–199. doi: 10.1007/s11010-008-9780-y. [DOI] [PubMed] [Google Scholar]
- Rhee HJ, Nam JS, Sun Y, Kim MJ, Choi HK, Han DH, et al. Lysophosphatidic acid stimulates cAMP accumulation and cAMP response element-binding protein phosphorylation in immortalized hippocampal progenitor cells. Neuroreport. 2006;17:523–526. doi: 10.1097/01.wnr.0000209011.16718.68. [DOI] [PubMed] [Google Scholar]
- Ritscher L, Engemaier E, Staubert C, Liebscher I, Schmidt P, Hermsdorf T, et al. The ligand specificity of the G-protein-coupled receptor GPR34. Biochem J. 2012;443:841–850. doi: 10.1042/BJ20112090. [DOI] [PubMed] [Google Scholar]
- Roberts C, Winter P, Shilliam CS, Hughes ZA, Langmead C, Maycox PR, et al. Neurochemical changes in LPA1 receptor deficient mice – a putative model of schizophrenia. Neurochem Res. 2005;30:371–377. doi: 10.1007/s11064-005-2611-6. [DOI] [PubMed] [Google Scholar]
- Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem. 2013;82:637–662. doi: 10.1146/annurev-biochem-062411-130916. [DOI] [PubMed] [Google Scholar]
- Rubenfeld J, Guo J, Sookrung N, Chen R, Chaicumpa W, Casolaro V, et al. Lysophosphatidic acid enhances interleukin-13 gene expression and promoter activity in T cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L66–L74. doi: 10.1152/ajplung.00473.2004. [DOI] [PubMed] [Google Scholar]
- Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43:394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sampaio ESTC, Choi JW, Gardell SE, Herr DR, Rehen SK, Gomes FC, et al. Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J Biol Chem. 2008;283:7470–7479. doi: 10.1074/jbc.M707758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Bilbao A, Pedraza C, Matas-Rico E, Lopez-Barroso D, Castilla-Ortega E, et al. Behavioral phenotype of maLPA1-null mice: increased anxiety-like behavior and spatial memory deficits. Genes Brain Behav. 2009;8:772–784. doi: 10.1111/j.1601-183X.2009.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze T, Golfier S, Tabeling C, Rabel K, Graler MH, Witzenrath M, et al. Sphingosine-1-phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25:4024–4036. doi: 10.1096/fj.10-179028. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Shano S, Moriyama R, Chun J, Fukushima N. Lysophosphatidic acid stimulates astrocyte proliferation through LPA1. Neurochem Int. 2008;52:216–220. doi: 10.1016/j.neuint.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–1711. [PubMed] [Google Scholar]
- Shida D, Fang X, Kordula T, Takabe K, Lepine S, Alvarez SE, et al. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–6577. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Garzon MC, Kristal L, Shapiro L, Christiano AM. Autosomal recessive woolly hair with hypotrichosis caused by a novel homozygous mutation in the P2RY5 gene. Exp Dermatol. 2009;18:218–221. doi: 10.1111/j.1600-0625.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KJ, Kim YL, Lee S, Kim DK, Ahn C, Chung J, et al. Lysophosphatidic acid signaling through LPA receptor subtype 1 induces colony scattering of gastrointestinal cancer cells. J Cancer Res Clin Oncol. 2009;135:45–52. doi: 10.1007/s00432-008-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkuma S, Akiyama M, Inoue A, Aoki J, Natsuga K, Nomura T, et al. Prevalent LIPH founder mutations lead to loss of P2Y5 activation ability of PA-PLA1alpha in autosomal recessive hypotrichosis. Hum Mutat. 2010;31:602–610. doi: 10.1002/humu.21235. [DOI] [PubMed] [Google Scholar]
- Shoham AB, Malkinson G, Krief S, Shwartz Y, Ely Y, Ferrara N, et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development. 2012;139:3859–3869. doi: 10.1242/dev.078550. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MF, Daviaud D, Pradere JP, Gres S, Guigne C, Wabitsch M, et al. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J Biol Chem. 2005;280:14656–14662. doi: 10.1074/jbc.M412585200. [DOI] [PubMed] [Google Scholar]
- Skipper M, Dhand R, Campbell P. Presenting ENCODE. Nature. 2012;489:45. doi: 10.1038/489045a. [DOI] [PubMed] [Google Scholar]
- Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res. 2009;82:221–228. doi: 10.1093/cvr/cvp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliven B, Miron V, Chun J. The neurobiology of sphingosine 1-phosphate signaling and sphingosine 1-phosphate receptor modulators. Neurology. 2011;76:S9–S14. doi: 10.1212/WNL.0b013e31820d9507. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, et al. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, et al. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J Biol Chem. 2002;277:34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, et al. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol. 2003;64:1199–1209. doi: 10.1124/mol.64.5.1199. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohr TC, Choi JW, Gardell SE, Herr DR, Rehen SK, Gomes FC, et al. Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J Biol Chem. 2008;283:7470–7479. doi: 10.1074/jbc.M707758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita K, Yamamura C, Tabata K, Fujita N. Expression of orphan G-protein coupled receptor GPR174 in CHO cells induced morphological changes and proliferation delay via increasing intracellular cAMP. Biochem Biophys Res Commun. 2013;430:190–195. doi: 10.1016/j.bbrc.2012.11.046. [DOI] [PubMed] [Google Scholar]
- Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, et al. Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun. 2006;341:1078–1087. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- Suidan HS, Nobes CD, Hall A, Monard D. Astrocyte spreading in response to thrombin and lysophosphatidic acid is dependent on the Rho GTPase. Glia. 1997;21:244–252. doi: 10.1002/(sici)1098-1136(199710)21:2<244::aid-glia7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Xia P. Estrogen defines the dynamics and destination of transactivated EGF receptor in breast cancer cells: role of S1P(3) receptor and Cdc42. Exp Cell Res. 2013;319:455–465. doi: 10.1016/j.yexcr.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Sumida H, Noguchi K, Kihara Y, Abe M, Yanagida K, Hamano F, et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116:5060–5070. doi: 10.1182/blood-2010-03-272443. [DOI] [PubMed] [Google Scholar]
- Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, Sammani S, et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol. 2012;47:628–636. doi: 10.1165/rcmb.2012-0048OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010;160:1699–1713. doi: 10.1111/j.1476-5381.2010.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- Taghavi P, Verhoeven E, Jacobs JJ, Lambooij JP, Stortelers C, Tanger E, et al. In vitro genetic screen identifies a cooperative role for LPA signaling and c-Myc in cell transformation. Oncogene. 2008;27:6806–6816. doi: 10.1038/onc.2008.294. [DOI] [PubMed] [Google Scholar]
- Trimbuch T, Beed P, Vogt J, Schuchmann S, Maier N, Kintscher M, et al. Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell. 2009;138:1222–1235. doi: 10.1016/j.cell.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniprotConsortium. Update on activities at the Universal Protein Resource (UniProt) in 2013. Nucleic Acids Res. 2013;41:D43–D47. doi: 10.1093/nar/gks1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet P, Pages C, Jeanneton O, Daviaud D, Barbe P, Record M, et al. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J Clin Invest. 1998;101:1431–1438. doi: 10.1172/JCI806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brocklyn JR, Graler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood. 2000;95:2624–2629. [PubMed] [Google Scholar]
- Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol. 2007;41:616–623. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, et al. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol. 2010;177:2205–2215. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JA, Chun J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc Natl Acad Sci U S A. 1999;96:5233–5238. doi: 10.1073/pnas.96.9.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JA, Hecht JH, Chun J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J Comp Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci. 2001;21:7069–7078. doi: 10.1523/JNEUROSCI.21-18-07069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, et al. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284:17304–17319. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sato K, Komachi M, Malchinkhuu E, Tobo M, Kimura T, et al. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem. 2004;279:6595–6605. doi: 10.1074/jbc.M308133200. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J Biol Chem. 2007;282:5814–5824. doi: 10.1074/jbc.M610767200. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731–17741. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida K, Kurikawa Y, Shimizu T, Ishii S. Current progress in non-Edg family LPA receptor research. Biochim Biophys Acta. 2013;1831:33–41. doi: 10.1016/j.bbalip.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Yang L, Yue S, Liu X, Han Z, Zhang Y, Li L. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol. 2013;59:114–123. doi: 10.1016/j.jhep.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Ye D, Lin F. S1pr2/Galpha13 signaling controls myocardial migration by regulating endoderm convergence. Development. 2013;140:789–799. doi: 10.1242/dev.085340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X. Lysophospholipid signaling in the function and pathology of the reproductive system. Hum Reprod Update. 2008;14:519–536. doi: 10.1093/humupd/dmn023. [DOI] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ma S, Wang L, Zuo B, Li M, Qiao Z, et al. Upregulation of GPR34 expression affects the progression and prognosis of human gastric adenocarcinoma by PI3K/PDK1/AKT pathway. Histol Histopathol. 2013;28:1629–1638. doi: 10.14670/HH-28.1629. [DOI] [PubMed] [Google Scholar]
- Yuan XB, Jin M, Xu X, Song YQ, Wu CP, Poo MM, et al. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat Cell Biol. 2003;5:38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, et al. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol. 2005;289:C2–C11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- Yung Y, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med. 2011;3:99ra87. doi: 10.1126/scitranslmed.3002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Contos JJ, Weiner JA, Fukushima N, Chun J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227:89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]
- Zhao SX, Xue LQ, Liu W, Gu ZH, Pan CM, Yang SY, et al. Robust evidence for five new Graves' disease risk loci from a staged genome-wide association analysis. Hum Mol Genet. 2013;22:3347–3362. doi: 10.1093/hmg/ddt183. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Voice JK, Kong Y, Goetzl EJ. Altered expression and functional profile of lysophosphatidic acid receptors in mitogen-activated human blood T lymphocytes. FASEB J. 2000;14:2387–2389. doi: 10.1096/fj.00-0492fje. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Kong Y, Goetzl EJ. Lysophosphatidic acid receptor-selective effects on Jurkat T cell migration through a Matrigel model basement membrane. J Immunol. 2001;166:2317–2322. doi: 10.4049/jimmunol.166.4.2317. [DOI] [PubMed] [Google Scholar]