Abstract
IL-6 is a pleiotropic cytokine that participates in normal functions of the immune system, haematopoiesis, metabolism, as well as in the pathogenesis of metabolic and cardiovascular diseases. Both pro- and anti-inflammatory roles of IL-6 have been described, which are distinguished by different cascades of signalling transduction, namely classic and trans-signalling. The present review summarizes the basic principles of IL-6 signalling and discusses its roles in diabetes and associated cardiovascular complications, with emphasis on the different outcomes mediated by the two modes of IL-6 signalling and the value of developing therapeutic strategies to specifically target the deleterious trans-signalling of IL-6.
Keywords: interleukin-6, inflammation, diabetes, cardiovascular diseases
Introduction
Investigations into the versatile biological activities of IL-6 expanded two and half decades ago. The function of IL-6 was first considered to be exerted in the acute phase of the inflammatory response but this cytokine was later found to be active in haematopoiesis, production of monoclonal antibodies by B-cells, development of cytotoxic T-cell and the transition from innate to acquired immunity (Van Snick, 1990). The role of IL-6 is not restricted to the immune system, as it is also involved in neuronal differentiation and regeneration, liver regeneration and regulation of metabolic process. Although IL-6 is mostly regarded as a pro-inflammatory cytokine that promotes inflammation under various pathological conditions, its anti-inflammatory and regenerative properties have been increasingly recognized (Scheller et al., 2011). On the contrary, a significant elevation of plasma IL-6 level has been characterized as a marker for metabolic disorder and cardiovascular disease. However, the exact in vivo pathophysiological significance of IL-6 remains unsolved. This article reviews the complexity of IL-6 action in the pathogenesis and development of type 2 diabetes mellitus (T2DM) and associated cardiovascular complications, hoping to shed light on the potential therapeutic strategies targeting IL-6 signalling in combating metabolic vascular diseases.
Classic signalling of IL-6
IL-6 is normally not expressed constitutively, but its expression is extensively induced by a spectrum of stimuli such as viral and bacterial infection, pro-inflammatory cytokines (TNF-α and IL-1), angiotensin II, oxidative stress and physical exercise. The circulating concentration of IL-6 in humans ranges from approximately 1 pg·mL−1 in healthy individuals, with a several-fold surge in chronic inflammation, a hundred-fold after physical exercise, to fatal levels of greater than 1 μg·mL−1 in sepsis (Hack et al., 1989; Waage et al., 1989; Ostrowski et al., 1998; Ruotsalainen et al., 2010).
The classic IL-6 signalling is initiated by the binding of IL-6 to the membrane-bound IL-6 α-receptor (IL-6R), which exhibits a low-binding affinity and does not possess an intrinsic kinase activity (receptor nomenclature follows Alexander et al., 2013). This interaction of IL-6 with IL-6R recruits another membrane component, the glycoprotein 130 (gp130) to form a high-affinity and signalling-competent assembly of the three molecules (illustrated in Figure 1, left) (Boulanger et al., 2003). IL-6R is expressed only by a few types of cells, mainly hepatocytes, pancreatic islet cells, macrophages, neutrophils and certain subtypes of T-cells (Doganci et al., 2005; Ellingsgaard et al., 2008; Waetzig and Rose-John, 2012). The glycoprotein gp130 is a ubiquitously expressed signal transducer shared by all members of the IL-6 family, including IL-11 leukaemia inhibitory factor, ciliary neurotrophic factor, oncostatin-M and cardiotrophin-1 (Taga and Kishimoto, 1997). Formation of the IL-6/IL-6R/gp130 complex induces phosphorylation of JAKs in the cytoplasmic domains of gp130, and subsequent phosphorylation of docking sites for the transcription factor STAT. STAT3 and, to a lesser extent, STAT1, are recruited to gp130, phosphorylated and, in turn, translocated to the nucleus and then bind to DNA of target genes including acute phase proteins in liver, ‘suppressor of cytokine signalling’ proteins that negatively regulate JAK/STAT signalling, and a variety of other genes (illustrated in Figure 1, middle) (Heinrich et al., 1998). In addition to JAK/STAT activation, the SRC homology domain 2-containing tyrosine phosphatase 2 (SHP-2) is recruited to its binding site on gp130 and phosphorylated to initiate the MAPK cascade (Heinrich et al., 2003; McFarland-Mancini et al., 2010). Moreover, IL-6 can activate the PI3K/Akt pathway in hepatocytes, cardiomyocyte and certain cancer cell lines, where it confers protective effects against cell apoptosis (Hideshima et al., 2001; Zhang et al., 2003; Smart et al., 2006; Chou et al., 2013).
Figure 1.
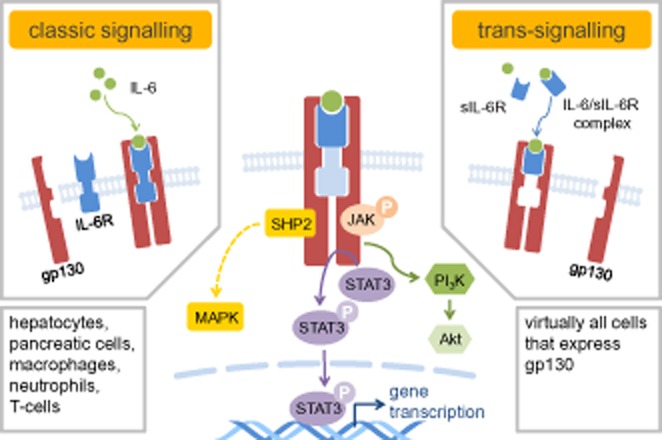
The intracellular IL-6 signal transduction is initiated by dimerization of gp130 through binding of IL-6/IL-6R (classic signalling, left panel) or IL-6/sIL-6R (trans-signalling, right panel) to gp130. Phosphorylation of tyrosine residues in gp130 upon dimerization leads to activation of three gp130 signalling pathways: JAK/STAT pathway (middle), SHP2/MAPK pathway (left) and PI3K/Akt pathway (right). SHP2, SRC homology domain 2-containing tyrosine phosphatase 2; PI3K, phosphatidylinositol 3-kinase.
Trans-signalling of IL-6
IL-6R also exists in a soluble form (sIL-6R) lacking the transmembrane and cytoplasmic domains, produced by either shedding of membrane-bound IL-6R or alternative splicing of IL-6 mRNA, with hepatocytes and immune cells as major sources (Peters et al., 1998). IL-6 binds to sIL-6R with affinity similar to that of the membrane-bound IL-6R and initiates subsequent signalling via engagement with gp130 (illustrated in Figure 1, right) (Rose-John and Heinrich, 1994). Therefore, the IL-6/sIL-6R complex acts as an agonist of gp130-mediated IL-6 signalling. This process, called trans-signalling, expands the spectrum of potential targets of IL-6 to virtually any cell type, because of the ubiquitous expression of gp130.
A further complication is that gp130 also naturally occurs in a soluble form. This can associate with the IL-6/sIL-6R complex and leads to inhibition of IL-6 trans-signalling, without affecting the classic signalling mode (Narazaki et al., 1993; Garbers et al., 2011b). Soluble gp130 (sgp130) is generated by alternative splicing and is detectable in the circulation of healthy individuals at 100–400 ng·mL−1, buffering the systemic response to circulating IL-6 (Narazaki et al., 1993; Jostock et al., 2001).
The anti-inflammatory and regenerative activities of IL-6 are generally mediated by the classic signalling mode, whereas pro-inflammatory responses in many pathological conditions involve trans-signalling (Rabe et al., 2008; Rose-John, 2012). Tocilizumab, a humanized IL-6R-specific mouse antibody that blocks both membrane-bound and soluble IL-6R, is the only drug approved, so far, that targets IL-6 signalling and has been shown to be effective in treating rheumatoid arthritis, a condition where increased IL-6 levels are correlated with disease progression (Ash and Emery, 2012). The major side effects include the increased risk of infections, mild increase in lipid, liver malfunction and body weight gain (Tanaka et al., 2012). Given the dys-regulation of IL-6 production in the pathogenesis of autoimmune, chronic inflammatory and even malignant diseases, blocking IL-6R or antagonizing IL-6 is therefore considered to be a rational therapeutic strategy. In addition to the proven efficacy and safety in treating rheumatoid arthritis, tocilizumab and several anti-IL-6R antibodies or IL-6 antagonists still waiting for approval are in clinical trials for the treatment of Castleman's disease, ankylosing spondylitis, diabetes mellitus and are being assessed for their benefits in reducing cardiovascular risks in patients with rheumatoid arthritis (Tanaka et al., 2012; Waetzig and Rose-John, 2012).
During the past decade, there has been increasing interest in understanding the IL-6 trans-signalling pathway because this pathway could be a target for therapeutic intervention to inhibit inflammation. Recent animal studies showed that selective inhibition of IL-6 trans-signalling by sgp130Fc, a recombinant version of sgp130, did ameliorate IL-6-driven inflammation in various diseases, with the same or even higher therapeutic efficacy and better side effect profile than global blockade of IL-6 signalling (Waetzig and Rose-John, 2012). In addition, high levels of the IL-6/sIL-6R complex in synovial fluid has been reported in patients with rheumatoid arthritis, which is responsible for joint destruction (Kotake et al., 1996), indicating the potentially important contribution of the inhibition of IL-6 trans-signalling to the clinical efficacy of tocilizumab. Due to the success of tocilizumab and the promise of selective inhibition of trans-signalling, studies are now increasingly being redirected to develop drugs that specifically inhibit IL-6 trans-signalling, without affecting the classic signalling pathway.
IL-6 in obesity and diabetes
Low-grade chronic inflammation in obesity, reflected by a two- to threefold increase in the systemic level of cytokines including IL-6, appears to precede and is a risk factor of the subsequent development of insulin resistance and T2DM (Spranger et al., 2003; Wang et al., 2013; Lowe et al., 2014). T2DM is a metabolic disorder characterized by hyperglycaemia due to the failure of pancreatic beta-cells to compensate for peripheral insulin resistance (Pradhan et al., 2001). Consequently, hyperglycaemia, dyslipidaemia and chronic inflammation jointly precipitate cardiovascular complications including atherosclerosis, coronary artery disease, stroke and peripheral arterial disease, which are the major causes of morbidity and mortality in T2DM (Haffner et al., 1998; Grundy et al., 1999). IL-6 has been identified as an independent predictor of T2DM and associated cardiovascular events (Spranger et al., 2003; Lowe et al., 2014). Adipocytes and macrophages residing in adipose tissue are the major sources for the elevated plasma IL-6 concentration up to 2–3 pg·mL−1 in patients with obesity and T2DM (Pradhan et al., 2001; Spranger et al., 2003). Nevertheless, the existing evidence is not enough to establish a causal association between IL-6 levels and the progression to metabolic and cardiovascular disorders. Due to its pleiotropic actions in various tissues and organs, the exact role of IL-6 in the pathogenesis of diabetes must be examined carefully in a cell- and tissue-specific manner, but allowing for the possibility of crosstalk between affected tissues and organs.
IL-6 classic signalling regulates metabolism
IL-6, considered as a pro-inflammatory cytokine and a predictor of T2DM, was originally considered to mediate adverse metabolic effects, contributing to insulin resistance and deteriorating glucose homeostasis (Bastard et al., 2000). However, when a large body of earlier studies is reviewed, the role of IL-6 in insulin resistance still remains controversial. Hepatocytes, skeletal muscle cells and adipocytes, the main cell types involved in the regulation of peripheral insulin sensitivity and glucose homeostasis, respond differently to IL-6. The ability of IL-6 to reduce insulin sensitivity in hepatocytes by interfering with insulin signalling is supported by strong experimental evidence (illustrated in Figure 2, left), whereas the results on adipocytes and skeletal muscle cells are not always consistent (Kristiansen and Mandrup-Poulsen, 2005). The higher responsiveness of hepatocytes, compared with other cell types, may be related to the presence of membrane-bound IL-6R. It should be noted that these studies are mainly performed in vitro on cell lines and for a short term, and with use of the supraphysiological concentrations of IL-6 that are much higher than those involved in low-grade chronic inflammation. Also, the experimental conditions may not mimic closely the chronic pathophysiological situations, where IL-6 acts in synergy with other cytokines and mediates crosstalk between different cell types and tissues, during inflammatory reactions. Moreover, in those early studies the level of sIL-6R and sgp130 is not assessed in targeted pathological models, nor is IL-6 trans-signalling taken into consideration, which is especially critical to investigate cells lacking membrane-bound IL-6R.
Figure 2.
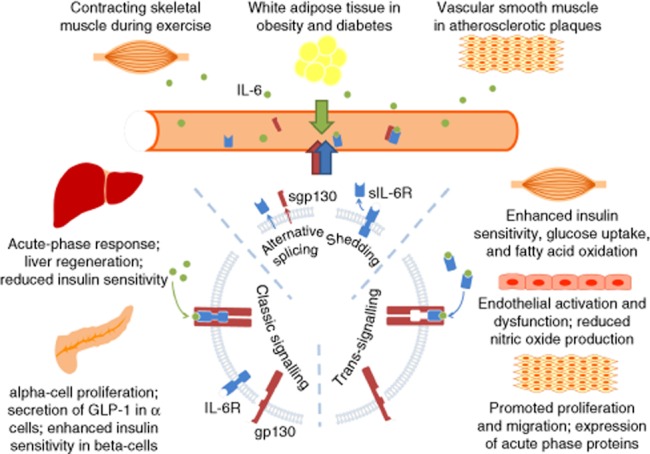
The pleiotropic role of IL-6 is demonstrated by its differential actions on various tissues and organs, which partly depends upon whether the classic signalling or trans-signalling is involved. The diagram illustrates features relevant to metabolic regulation and disorders, and cardiovascular complications. There are three sources of elevated plasma IL-6 – adipose tissue in diabetes and obesity, contracting skeletal muscle during exercise and vascular smooth muscle cells in atherosclerotic plaques (upper panel). Given the presence of membrane-bound IL-6R in hepatocytes and pancreatic islet cells, IL-6 is most likely to elicit its effects in liver and pancreas through classic signalling (left); whereas in skeletal muscle and the vascular wall, IL-6 acts through trans-signalling, because these cells lack expression of membrane-bound IL-6R (right).
In the present context, it is important to note that IL-6-deficient mice develop mature-onset obesity, hepatic inflammation and systemic insulin resistance (Wallenius et al., 2002; Matthews et al., 2010). In line with these observations, increased cholesterol and triglyceride levels, accompanied by weight gain, were observed in patients with rheumatoid arthritis treated by tocilizumab, in addition to immunosuppression, as the main unwanted effect of global IL-6 inhibition (Richez et al., 2012; Md Yusof and Emery, 2013; Strang et al., 2013). Moreover, IL-6 released from contracting skeletal muscle during exercise can increase circulating levels to 100 pg·mL−1 and this response is much greater than those of other cytokines involved (Pedersen, 2000; Steensberg et al., 2000). The undoubted benefit of exercise on prevention and amelioration of obesity, diabetes and cardiovascular diseases points to a beneficial role of circulating IL-6 as an endocrine factor in regulating metabolism (Febbraio et al., 2004). Now, the elevation of IL-6 has been increasingly recognized to activate pathways that facilitate energy turnover and enhance insulin sensitivity.
Several recent studies emphasizing the physiological relevance of IL-6 in vivo and chronic effects of moderate elevations of plasma IL-6 have contributed to our awareness of the paradox regarding both benefits and harms of IL-6. In humans, IL-6 increased fatty acid oxidation and glucose transporter 4 (GLUT4) translocation to plasma membrane, hence promoting glucose uptake in skeletal muscle via AMP-activated protein kinase (Carey et al. (2006); illustrated in Figure 2, right). Holmes et al. (2008) carried out a study to mimic chronic elevation of IL-6 in rats via either continuous IL-6 infusion or twice-daily intermittent injection and found that treatment for 14 days with IL-6, by either method, increased basal insulin sensitivity, improved glucose tolerance and enhanced fat oxidation through up-regulation of PPARα and uncoupled protein-2 in skeletal muscle, which accounts for over 90% of the insulin-stimulated glucose uptake into cells. Ellingsgaard et al. (2008) reported that membrane-bound IL-6R was expressed in alpha-cells of pancreatic islets (secreting glucagon), and also in beta-cells (secreting insulin), albeit to a lesser extent. The same research group has recently demonstrated that elevation of plasma IL-6, released from either contracting skeletal muscle or white adipose tissues, stimulates the secretion of glucagon-like peptide-1 (GLP-1) from intestinal L cells and pancreatic alpha-cells via the classic signalling pathway to induce insulin secretion in the beta-cells, resulting in improved glycaemic control (illustrated in Figure 2, left) (Ellingsgaard et al., 2011). Furthermore, IL-6 knockout reduced the level of pancreatic GLP-1 in mice; neutralization of IL-6 in wild-type mice fed with high-fat diet and, in db/db mice, impaired this obesity-induced alpha-cell adaptation and further disrupted glucose homeostasis. In addition, raised circulating IL-6 promoted pancreatic alpha-cell proliferation in response to a high-fat diet (Ellingsgaard et al., 2008). Although increased alpha-cell mass is normally expected to cause an increase in glucagon production, elevated IL-6 increased PC1/3 expression in alpha-cells, leading to a shift in peptide production from glucagon towards GLP-1, thus promoting functional beta-cell compensation to maintain proper insulin secretion and glucose homeostasis (Ellingsgaard et al., 2008; 2011). It is of interest to note that even in obese and diabetic mice with chronic and sustained elevation of plasma IL-6, acute administration of IL-6 still exerted similar beneficial effects on GLP-1 production and improved insulin sensitivity and glucose homeostasis (Ellingsgaard et al., 2011). An adipose tissue and skeletal muscle endocrine-islet axis may exist, allowing for metabolic adaptation to the increased insulin demand in obesity and the improved beta-cell function in response to physical exercise.
In addition, the anti-inflammatory and immunosuppressive role of IL-6 in both local and systemic inflammatory responses has been widely accepted (Kristiansen and Mandrup-Poulsen, 2005). During exercise, muscle-derived IL-6 is likely to inhibit the expression of TNF-α and IL-1β, hence offering protection against the risk of insulin resistance induced by these two pro-inflammatory cytokines (Pedersen, 2007). Moreover, IL-6 is elevated in concert with a number of anti-inflammatory cytokines such as IL-10, and may regulate the level of pro-inflammatory cytokines (Xing et al., 1998). The association between IL-6 and progression to T2DM may merely reflect an attempt to counteract the chronic inflammation triggered by other inflammatory mediators.
IL-6 trans-signalling mediates inflammation leading to cardiovascular complications
Plasma concentration of the sIL-6R in healthy individuals ranges between 50 and 80 ng·mL−1, and increases during viral infection (Honda et al., 1992), although another study reported a decrease of plasma sIL-6R in bacterial infection (Frieling et al., 1995). Elevation of sIL-6R and sgp130 is also associated with metabolic syndrome and endothelial cell inflammation (Weiss et al., 2013). Although little is known about how sIL-6R is being altered under pathological conditions, the potential importance of sIL-6R-mediated trans-signalling should not be neglected. It is believed that the availability of sIL-6R, rather than that of IL-6, determines the switch-on of the deleterious impact of IL-6 and the extent to which the inflammatory response progresses. However, the plasma concentration of sIL-6R does not necessarily represent the whole extent of IL-6 trans-signalling. The local ratio of IL-6 and sIL-6R and the ratio of IL-6R to gp130 are important in determining the outcome of IL-6 signalling. Further investigation into the dynamics of sIL-6R and IL-6 trans-signalling will lead to better understanding of the balance between anti-inflammatory and pro-inflammatory roles of IL-6 under pathophysiological conditions.
Administration of tocilizumab to patients with rheumatoid arthritis benefits endothelial function and attenuates aortic stiffness (Protogerou et al., 2011). As endothelial cells and smooth muscle cells of the vascular wall lack membrane-bound IL-6Rs, the consistently reported, deleterious, action of IL-6 in vasculature is most likely to be mediated through binding to sIL-6R and subsequent activation of gp130 signalling, namely trans-signalling.
Regardless of whether IL-6 is elevated in response to metabolic adaptation or infectious stress, its presence in the circulation will affect the cells first encountered, i.e., the vascular endothelium. IL-6 suppresses insulin-stimulated NO production in endothelial cells through enhancing production of TNF-α (illustrated in Figure 2, right). This effect appears to be relatively specific as IL-6 does not increase TNF-α levels in skeletal muscle and adipose tissue (Yuen et al., 2009). Moreover, IL-6 inhibits endothelium-dependent NO-mediated relaxations in systemic blood vessels of pregnant rats (Orshal and Khalil, 2004) and impairs the vasodilator effect of insulin through activation of JNK and ERK1/2 (Andreozzi et al., 2007). Given the lack of membrane-bound IL-6R on both endothelial cells and smooth muscle cells, IL-6 trans-signalling is most likely to mediate these detrimental effects. Therefore, therapeutic strategies targeting IL-6 trans-signalling might be able to specifically block the pro-inflammatory effect of IL-6 while retaining its beneficial action on regulating metabolism, with the promise of ameliorating inflammation and improving metabolic adaptation in diabetes and related cardiovascular complications.
On the contrary, the soluble form of gp130, sgp130, acts as an endogenous inhibitor, specifically against IL-6 trans-signalling, but not classic signalling or other IL-6 family members (Schuett et al., 2012). Patients with coronary artery disease have significantly lower plasma levels of endogenous sgp130, suggesting that a compromised counterbalancing of IL-6 trans-signalling may contribute to atherogenesis in humans (Schuett et al., 2012). However, the change of sgp130 level under different pathological conditions has not been thoroughly investigated. Notably, the inhibition of IL-6 trans-signalling by sgp130Fc produces beneficial effects in several mouse models of human diseases with elevated inflammation (Rose-John, 2012), especially in atherosclerosis, a major cardiovascular complication associated with obesity and diabetes.
As the consequence of an imbalanced lipid metabolism and chronic inflammation in the arterial wall, atherosclerosis is manifested as atherosclerotic plaques and luminal narrowing of arteries, increasing risks for stroke and coronary artery disease. The atherogenesis is initiated by endothelial dysfunction and structural alterations at those sites in the vasculature susceptible to disturbed laminar flow (Chiu and Chien, 2011), and subsequent infiltration of intimal immune cells (Weber and Noels, 2011). Vascular smooth muscle cell (VSMC) proliferation and migration from media to intima plays a critical role in neointima formation after vascular injury and subsequent development of atherosclerotic plaques (Hansson et al., 2006). The IL-6 level is elevated locally in atherosclerotic plaques (Schieffer et al., 2000), with VSMCs as the major source of IL-6 production in plaques (Chiu et al., 2007; Loppnow et al., 2011). IL-6 acts in an autocrine manner via trans-signalling to further accelerate inflammatory responses in VSMCs, including expression of acute phase proteins, proliferation and migration, which are critical in early atherosclerosis (illustrated in Figure 2, right) (Ikeda et al., 1993; Wang et al., 2007; Chava et al., 2009). Also, IL-6 promotes endothelial activation by inducing expression of chemoattractant proteins and adhesion molecules that recruits immune cells into sub-intimal space (illustrated in Figure 2, right) (Marin et al., 2001; Hansson, 2005). Moreover, IL-6 activates macrophages to migrate and differentiate, which potentially accelerates atherogenesis (Bacon et al., 1990).
The deleterious effect of IL-6, probably through trans-signalling, makes sgp130Fc a promising candidate for the treatment of atherosclerosis. In hypercholesterolaemic Ldlr−/− mice, sgp130Fc treatment reduces atherosclerosis, without affecting body weight or the serum lipid profile (Schuett et al., 2012). Also, endothelial activation and intimal smooth muscle cell infiltration were decreased in sgp130Fc-treated mice, resulting in a marked reduction of monocyte recruitment and subsequent progression of atherosclerotic plaques (Schuett et al., 2012). These findings illustrate the critical involvement of IL-6 trans-signalling in atherosclerosis and it warrants further investigation of sgp130Fc as a novel therapeutic agent for the treatment of atherosclerosis and related diseases.
Notably, IL-6 is positively associated with coronary artery disease (Saremi et al., 2009; Tehrani et al., 2013). Two recently published studies in The Lancet further suggest a causal pro-inflammatory role of IL-6 in coronary artery disease based upon genetic investigations. A single nucleotide polymorphism, Asp358Ala, of IL-6R affects its proteolytic cleavage, leading to a reduced level of its membrane-bound form in hepatocytes, monocytes or macrophages, and concomitant elevation of its soluble form in circulation (Collaboration IRGCERF et al., 2012; Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al., 2012). Reduction of membrane-bound IL-6R attenuates the classic pathway that induces production of acute phase proteins such as C-reactive protein and fibrinogen, correlating to a reduction of the risk of coronary artery disease (Collaboration IRGCERF et al., 2012; Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al., 2012). However, these studies are limited by their lack of examination of the trans-signalling, because elevated sIL-6R, as a side-effect, is likely to enhance the extent of trans-signalling and may also alter other components of IL-6 signalling, such as sgp130. In this context, the beneficial effect provided by the attenuation of classic signalling to reduce production of acute phase proteins may override the deleterious effects of trans-signalling in the development of atherosclerotic plaques. However, the possibility that sgp130 levels increase in response to the elevated sIL-6R and counterbalance sIL-6R-mediated trans-signalling cannot be ruled out. Moreover, the Asp358Ala mutant results in a similar reduction in circulating concentrations of inflammatory biomarkers, such as C-reactive protein and fibrinogen, as the effect of tocilizumab treatment, whereas the alteration of blood lipids to a pro-atherogenic profile in tocilizumab-treated patients was not seen in carriers of the Asp358Ala mutation (Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al., 2012). This again indicates a critical role of IL-6 in metabolic regulation and the necessity to distinguish the two downstream signalling pathways and the consequent effects on metabolic regulation and cardiovascular risks in response to IL-6. These new results add additional weight to the consideration of selectively inhibiting the pro-inflammatory pathway of IL-6 as an attractive strategy to reduce cardiovascular risks.
Regulation of balance between classic and trans-signalling
Instead of simply inhibiting trans-signalling, the tuning of balance between classic and trans-signalling might be more beneficial under conditions of obesity and diabetes. For instance, two membrane-bound proteases, disintegrin and metalloprotease (ADAM)-10 and ADAM-17, are responsible for sIL-6R shedding in humans (Garbers et al., 2011a; Waetzig and Rose-John, 2012), and the expression of ADAM17 was raised in arterial tissue from patients with atherosclerosis (Canault et al., 2006). TNF-α converting enzyme (TACE), the counterpart of human ADAM17 in mice, is elevated in high-fat diet-induced obese mice (Voros et al., 2003), whereas heterozygous Tace+/− mice are protected from diet-induced obesity (Serino et al., 2007). Treatment with the TACE inhibitor improves insulin sensitivity, corrects hyperglycaemia and inhibits vascular inflammation (Federici et al., 2005). Therefore, ADAM over-activity probably shifts the IL-6 signalling balance towards trans-signalling, thus contributing to vascular inflammation and diabetes.
Perspectives
With more results on the pro-inflammatory action of IL-6 trans-signalling available and the state-of-the-art evaluation of classic signalling in metabolic regulation, it is possible to better understand the complexity of both the physiological and pathological properties of IL-6. However, how some key components such as endogenous sIL-6R and sgp130 respond to inflammation is still unclear. In addition, insights into the upstream regulation of the balance between classic and trans-signalling pathways will be extremely valuable. Also, several important issues with regard to the delicate risk-benefit balance between these two divergent pathways downstream of the common point of gp130 activation, as well as the regulatory mechanism, are yet to be resolved. Additional complexity arises from the extensive crosstalk between cells and tissues secreting IL-6 upon stimulation and effectors that respond differently, due to the presence or absence of classic signalling. Moreover, the biological outcome of IL-6 activation also depends upon its interaction with the many other cytokines concomitantly stimulated in response to inflammation, oxidative stress or physical exercise.
From a pharmacological point of view, the success of tocilizumab in alleviating inflammation in rheumatoid arthritis has supported the promise of targeting IL-6 in obesity and T2DM where chronic inflammation contributes to the disease progression. As IL-6 signalling transduction through either membrane-bound or soluble IL-6R ends in the activation of gp130, the pharmacological strategy to discriminate between classic and trans-signalling must have a target beyond the common point. As growing evidence supports the notion that inhibiting trans-signalling is as effective as or even better than global inhibition in many IL-6-driven diseases, the design of selective inhibitors of IL-6 trans-signalling through sIL-6R which do not interfere with the function of membrane-bound IL-6R is a logical and promising approach to new treatments. Fortunately, nature has provided us a solution with the existence of the endogenous sgp130 that sterically prevents the association of the IL-6/sIL-6R complex with membrane-bound gp130 and hence specifically inhibits trans-signalling. Recombinant proteins that mimic the property of sgp130 have been developed and are in clinical trials for the treatment of inflammatory diseases. In summary, blocking the unwanted IL-6 trans-signalling, while maintaining the physiological classic signalling, holds out the promise of developing selective trans-signalling inhibitors in order to alleviate diabetes-related cardiovascular complications.
Acknowledgments
This work was supported by grants from Hong Kong Research Grant Council (CUHK464712), National Basic Research Program of China (2012CB517805) and CUHK Focused Investment Scheme B.
Glossary
- ADAM10
disintegrin and metalloprotease-10
- ADAM17
disintegrin and metalloprotease-17
- GLP-1
glucagon-like peptide-1
- gp130
glycoprotein 130
- IL-6R
IL-6 α-receptor
- sgp130
soluble glycoprotein 130
- sIL-6R
soluble IL-6 α-receptor
- TACE
TNF-α converting enzyme
- T2DM
type 2 diabetes mellitus
- VSMC
vascular smooth muscle cell
Conflict of interest
The authors declare that no conflict of interest exists.
References
- Andreozzi F, Laratta E, Procopio C, Hribal ML, Sciacqua A, Perticone M, et al. Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol Cell Biol. 2007;27:2372–2383. doi: 10.1128/MCB.01340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. Br J Pharmacol. 2013;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash Z, Emery P. The role of tocilizumab in the management of rheumatoid arthritis. Expert Opin Biol Ther. 2012;12:1277–1289. doi: 10.1517/14712598.2012.707178. [DOI] [PubMed] [Google Scholar]
- Bacon K, Gearing A, Camp R. Induction of in vitro human lymphocyte migration by interleukin 3, interleukin 4, and interleukin 6. Cytokine. 1990;2:100–105. doi: 10.1016/1043-4666(90)90003-c. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, et al. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187:82–91. doi: 10.1016/j.atherosclerosis.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- Chava KR, Karpurapu M, Wang D, Bhanoori M, Kundumani-Sridharan V, Zhang Q, et al. CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2009;29:809–815. doi: 10.1161/ATVBAHA.109.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY, Tsai MC, et al. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood. 2007;110:519–528. doi: 10.1182/blood-2006-08-040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CH, Lai SL, Chen CN, Lee PH, Peng FC, Kuo ML, et al. IL-6 regulates Mcl-1L expression through the JAK/PI3K/Akt/CREB signaling pathway in hepatocytes: implication of an anti-apoptotic role during liver regeneration. PLoS ONE. 2013;8:e66268. doi: 10.1371/journal.pone.0066268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration IRGCERF. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, et al. The IL-6R alpha chain controls lung CD4 + CD25 + Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A. 2008;105:13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53:1643–1648. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, et al. Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest. 2005;115:3494–3505. doi: 10.1172/JCI26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieling JT, van Deuren M, Wijdenes J, van der Meer JW, Clement C, van der Linden CJ, et al. Circulating interleukin-6 receptor in patients with sepsis syndrome. J Infect Dis. 1995;171:469–472. doi: 10.1093/infdis/171.2.469. [DOI] [PubMed] [Google Scholar]
- Garbers C, Janner N, Chalaris A, Moss ML, Floss DM, Meyer D, et al. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem. 2011a;286:14804–14811. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, Thaiss W, Jones GW, Waetzig GH, Lorenzen I, Guilhot F, et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem. 2011b;286:42959–42970. doi: 10.1074/jbc.M111.295758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, et al. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. IL-6-type cytokine signalling through the gp130/JAK/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- Holmes AG, Mesa JL, Neill BA, Chung J, Carey AL, Steinberg GR, et al. Prolonged interleukin-6 administration enhances glucose tolerance and increases skeletal muscle PPARalpha and UCP2 expression in rats. J Endocrinol. 2008;198:367–374. doi: 10.1677/JOE-08-0113. [DOI] [PubMed] [Google Scholar]
- Honda M, Yamamoto S, Cheng M, Yasukawa K, Suzuki H, Saito T, et al. Human soluble IL-6 receptor: its detection and enhanced release by HIV infection. J Immunol. 1992;148:2175–2180. [PubMed] [Google Scholar]
- Ikeda U, Ikeda M, Seino Y, Takahashi M, Kasahara T, Kano S, et al. Expression of intercellular adhesion molecule-1 on rat vascular smooth muscle cells by pro-inflammatory cytokines. Atherosclerosis. 1993;104:61–68. doi: 10.1016/0021-9150(93)90176-u. [DOI] [PubMed] [Google Scholar]
- Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- Kotake S, Sato K, Kim KJ, Takahashi N, Udagawa N, Nakamura I, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl. 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- Loppnow H, Buerke M, Werdan K, Rose-John S. Contribution of vascular cell-derived cytokines to innate and inflammatory pathways in atherogenesis. J Cell Mol Med. 2011;15:484–500. doi: 10.1111/j.1582-4934.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Woodward M, Hillis G, Rumley A, Li Q, Harrap S, et al. Circulating Inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes. 2014;63:1115–1123. doi: 10.2337/db12-1625. [DOI] [PubMed] [Google Scholar]
- Marin V, Montero-Julian FA, Gres S, Boulay V, Bongrand P, Farnarier C, et al. The IL-6-soluble IL-6Ralpha autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: an experimental model involving thrombin. J Immunol. 2001;167:3435–3442. doi: 10.4049/jimmunol.167.6.3435. [DOI] [PubMed] [Google Scholar]
- Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, Watson N, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- Md Yusof MY, Emery P. Targeting interleukin-6 in rheumatoid arthritis. Drugs. 2013;73:341–356. doi: 10.1007/s40265-013-0018-2. [DOI] [PubMed] [Google Scholar]
- Narazaki M, Yasukawa K, Saito T, Ohsugi Y, Fukui H, Koishihara Y, et al. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82:1120–1126. [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension. 2004;43:434–444. doi: 10.1161/01.HYP.0000113044.46326.98. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK. Exercise and cytokines. Immunol Cell Biol. 2000;78:532–535. doi: 10.1111/j.1440-1711.2000.t01-11-.x. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. IL-6 signalling in exercise and disease. Biochem Soc Trans. 2007;35(Pt 5):1295–1297. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- Peters M, Muller AM, Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood. 1998;92:3495–3504. [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. 2011;219:734–736. doi: 10.1016/j.atherosclerosis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Rabe B, Chalaris A, May U, Waetzig GH, Seegert D, Williams AS, et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111:1021–1028. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- Richez C, Barnetche T, Khoryati L, Duffau P, Kostine M, Contin-Bordes C, et al. Tocilizumab treatment decreases circulating myeloid dendritic cells and monocytes, 2 components of the myeloid lineage. J Rheumatol. 2012;39:1192–1197. doi: 10.3899/jrheum.111439. [DOI] [PubMed] [Google Scholar]
- Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300(Pt 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen E, Stancakova A, Vauhkonen I, Salmenniemi U, Pihlajamaki J, Punnonen K, et al. Changes in cytokine levels during acute hyperinsulinemia in offspring of type 2 diabetic subjects. Atherosclerosis. 2010;210:536–541. doi: 10.1016/j.atherosclerosis.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Saremi A, Anderson RJ, Luo P, Moritz TE, Schwenke DC, Allison M, et al. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT) Atherosclerosis. 2009;203:610–614. doi: 10.1016/j.atherosclerosis.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:281–290. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- Serino M, Menghini R, Fiorentino L, Amoruso R, Mauriello A, Lauro D, et al. Mice heterozygous for tumor necrosis factor-alpha converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes. 2007;56:2541–2546. doi: 10.2337/db07-0360. [DOI] [PubMed] [Google Scholar]
- Smart N, Mojet MH, Latchman DS, Marber MS, Duchen MR, Heads RJ. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc Res. 2006;69:164–177. doi: 10.1016/j.cardiores.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang AC, Bisoendial RJ, Kootte RS, Schulte DM, Dallinga-Thie GM, Levels JH, et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis. 2013;229:174–181. doi: 10.1016/j.atherosclerosis.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- Tehrani DM, Gardin JM, Yanez D, Hirsch CH, Lloyd-Jones DM, Stein PK, et al. Impact of inflammatory biomarkers on relation of high density lipoprotein-cholesterol with incident coronary heart disease: Cardiovascular Health Study. Atherosclerosis. 2013;231:246–251. doi: 10.1016/j.atherosclerosis.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Voros G, Maquoi E, Collen D, Lijnen HR. Differential expression of plasminogen activator inhibitor-1, tumor necrosis factor-alpha, TNF-alpha converting enzyme and ADAMTS family members in murine fat territories. Biochim Biophys Acta. 2003;1625:36–42. doi: 10.1016/s0167-4781(02)00589-4. [DOI] [PubMed] [Google Scholar]
- Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waetzig GH, Rose-John S. Hitting a complex target: an update on interleukin-6 trans-signalling. Expert Opin Ther Targets. 2012;16:225–236. doi: 10.1517/14728222.2012.660307. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu Z, Li Q, Karpurapu M, Kundumani-Sridharan V, Cao H, et al. An essential role for gp130 in neointima formation following arterial injury. Circ Res. 2007;100:807–816. doi: 10.1161/01.RES.0000261350.61711.9e. [DOI] [PubMed] [Google Scholar]
- Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- Weiss TW, Arnesen H, Seljeflot I. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism. 2013;62:1008–1013. doi: 10.1016/j.metabol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen DY, Dwyer RM, Matthews VB, Zhang L, Drew BG, Neill B, et al. Interleukin-6 attenuates insulin-mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor-alpha expression. Diabetes. 2009;58:1086–1095. doi: 10.2337/db08-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Y, Shen B. PI3-K/Akt pathway contributes to IL-6-dependent growth of 7TD1 cells. Cancer Cell Int. 2003;3:1. doi: 10.1186/1475-2867-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


