Abstract
BACKGROUND AND PURPOSE
CR4056 is a novel imidazoline-2 (I2) ligand exhibiting potent analgesic activity in animal models of pain. In this study, we investigated the effects of CR4056 in a well-established model of postoperative pain where rats develop hyperalgesia in the injured hind paw.
EXPERIMENTAL APPROACH
By measuring paw withdrawal threshold to mechanical pressure, we studied the pharmacology of CR4056, potential sex differences in pain perception and response to treatment, and the pharmacodynamic interaction of CR4056 with morphine.
KEY RESULTS
Oral CR4056 and subcutaneous morphine dose-dependently reversed the hyperalgesic response. Analgesic effects of CR4056 were completely suppressed by the non-selective imidazoline I2/α2-adrenoceptor antagonist idazoxan, were partially reduced (∼30%; P < 0.05) by the selective α2-adrenoceptor antagonist yohimbine, but were not influenced by the non-selective I1/α2-adrenoceptor antagonist efaroxan or by the μ opioid receptor antagonist naloxone. We found no differences in responses to CR4056 or morphine between male and female rats. However, females had a lower pain threshold than males, and needed lower doses of drugs to reach a significant analgesia. When CR4056 and morphine were combined, their median effective doses were lower than expected for additive effects, both in males and in females. Isobolographic analysis confirmed a synergism between CR4056 and morphine.
CONCLUSIONS AND IMPLICATIONS
CR4056 is a novel pharmacological agent under development for postoperative pain both as stand-alone treatment and in association with morphine. CR4056 has successfully completed Phase I studies for tolerability and pharmacokinetics in healthy volunteers, and is currently entering the first proof-of-concept study in patients.
Keywords: CR4056, imidazoline-2 receptors, postoperative pain, drug synergism, sex differences
Introduction
Imidazoline-2 (I2) binding sites, also referred to as I2 receptors, are widely distributed in mammalian cells of the central and peripheral nervous system (Molderings, 1997; receptor nomenclature follows Alexander et al., 2013). At a cellular level, I2 receptor ligands have been mainly associated with an inhibitory modulation of MAO activity (Tesson et al., 1995; Ozaita et al., 1997). Remarkably, metabolism of catecholamines by sensory neurons contributes to peripheral neuropathies, suggesting that an MAO inhibitor, devoid of adverse effects in the CNS, could be useful for the treatment of chronic pain (Dina et al., 2008). Indeed, pharmacological manipulations that increase the synaptic levels of noradrenaline and 5-HT have gained prominence in the management of chronic pain, including neuropathic pain and fibromyalgia (Kuner, 2010). Another relevant activity of I2 receptors is the modulation of the opioid system, which may occur at different levels. First, recent evidence has shown how I2 ligands mimic the effect of opioid receptor agonists by increasing beta-endorphin secretion in the rat adrenal medulla (Hwang et al., 2005; Chang et al., 2010). Second, a role for I2 receptors in pain control stems from the interaction between imidazoline ligands and the opioid system in the locus ceruleus neurons (Ruiz-Durántez et al., 2003). This interaction is probably involved in the prevention of tolerance and addiction to opioids (Wu and Raja, 2011).
CR4056 is a novel I2 receptor ligand characterized by potent analgesic activity in different animal models of inflammatory and neuropathic pain (Ferrari et al., 2011; Thorn et al., 2012; Li et al., 2014). The efficacy of CR4056 was thoroughly investigated in a rat model of neuropathic pain that parallels the clinical condition of patients treated with the chemotherapeutic agent bortezomib (Meregalli et al., 2012).
In the present study, we investigated the effects of acute oral administration of CR4056 in a rat model of postoperative pain (Brennan et al., 1996). The pharmacology of the incisional pain in rats has been well characterized in a study by Whiteside et al. (2004). Their work shows how, from a pharmacological point of view, postoperative pain is clearly distinct from pure inflammatory or neuropathic pain. Indeed, when hyperalgesic or allodynic responses are measured 24 h after surgery, both inflammatory and neuropathic mechanisms contribute to the pain balance (Whiteside et al., 2004). This condition, from a translational point of view, is quite common in patients undergoing a major surgical event where, as expected, pain evoked by pressure is greater than pain experienced at rest.
In these patients, opioid analgesics still remain the treatment of choice during the intraoperative and postoperative period. Morphine is by far the most commonly used opioid, although its long-term use in chronic pain conditions is limited by a relevant spectrum of adverse effects, including constipation, dependence and tolerance (Anderson and Palmer, 2006; Oderda et al., 2007; Sadhasivam and Chidambaran, 2012). Because multimodal analgesia has been shown to be an effective strategy to improve postoperative pain management (Buvanendran and Kroin, 2009), we included in this study a protocol of co-administration of morphine and CR4056. If different drugs contribute to pain control through different mechanisms, the optimal analgesia could be reached at lower doses of each drug, thus decreasing the chance for adverse effects to occur. Further work in our study was devoted to analysing potential sex differences in the perception of pain and response to treatment in this animal model. This is actually a matter of debate because relevant discrepancies do exist among animal as well as human studies (Craft, 2003; Kroin et al., 2003; Aubrun et al., 2005; Dahan et al., 2008; Fillingim et al., 2009).
Methods
Animal subjects
All animal care and experimental procedures described were in compliance with international laws and policies (Directive 2010/63/EU revising Directive 86/609/EEC on the protection of animals used for scientific purposes; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996), and were approved by the Rottapharm Review Board. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of N = 336 animals were used in the experiments described here. Male and female Sprague-Dawley rats (Charles River, Calco, Italy) weighing 250–300 g were housed with ad libitum access to food and water, in a temperature-controlled room with a 12 h light/dark cycle, at least 1 week before the surgical procedure.
Brennan's model of postoperative pain
Rats were anaesthetized with 2% isoflurane in pure oxygen inside an induction chamber. Once unconscious, rats were removed and placed on a non-rebreathing anaesthetic circuit with mask delivery of isoflurane in pure oxygen throughout the procedure. Paw incision was performed as described by Brennan et al. (1996), with minor modifications. A 1 cm longitudinal incision was made through skin and fascia of the plantar face of the right hind paw, starting at 0.5 cm from the proximal edge of the heel. The plantar muscle was then elevated with forceps and incised longitudinally with the blade, keeping attention to leave muscle origins intact. A group of un-operated sham rats was always present in each experiment. Paw withdrawal threshold (i.e. the pain threshold) to mechanical pressure was determined by the Randall-Selitto method (Analgesy-Meter, Ugo Basile, Comerio, VA, Italy) 24 h after surgery. According to the weight of the rats enrolled in the study, we set the Randall-Selitto device on level 2 (range: 0–750 g). CR4056 was suspended in 0.5% methyl cellulose (MC) and administered orally. Mechanical hyperalgesia was measured 30, 90, 180 and 360 min after CR4056 administration. Idazoxan, efaroxan, yohimbine, atipamezole and naloxone were administered i.p. or s.c. 15 min before CR4056 administration. Morphine was administered s.c.
The interaction of CR4056 with morphine was evaluated by administering fixed proportions of CR4056 and morphine (1:1 for male rats, 3:1 for female rats) selected on the basis of their respective ED50 when administered alone. When CR4056 and morphine were co-administered, since these drugs induce their analgesic effect with a different temporal response, we synchronized the peak effect of each drug by administering CR4056 1 h before morphine. Thus, in these types of experiments, mechanical hyperalgesia was measured 90, 180 and 360 min after CR4056 administration corresponding to 30, 120 and 300 min after morphine administration.
Data analysis
Throughout the manuscript, data are given either as mean and SEM or as ED50 and 95% confidence interval (95% CI). To evaluate the statistical significance of the anti-hyperalgesic effects of drugs (alone or in combination), data analysis was performed on the crude mechanical threshold values expressed in grams. Dose-response curves and experiments with receptor antagonists were analysed by two-way analysis of variance: repeated measures (RM) two-way anova with treatment as the inter-subject variable and time as the intra-subject variable. F and P values for the main effect of treatment are given in the text. Post hoc comparisons were made using a multiple comparison within each experimental time point (Tukey's multiple comparisons test), with P < 0.05 considered statistically significant (GraphPad Prism software, version 6.0; GraphPad Software Inc., San Diego, CA, USA). A Student's t-test was run when mean withdrawal threshold was to be compared between two experimental groups (i.e. sham un-operated rats vs. control operated rats; male rats vs. female rats), with P < 0.05 considered statistically significant.
The dose that produced 50% of the anti-hyperalgesic effect (ED50) was calculated at the time corresponding to the peak effect (90 min for CR4056, 30 min for morphine, either alone or in combination) using a standard linear regression analysis of the log dose-response curve, constrained between 100% (i.e the mean withdrawal threshold in sham un-operated rats) and 0% (i.e. the mean withdrawal threshold in control operated rats). The regression analyses were performed on the single data points (six animals at each of at least three doses) and not on the group means.
The interaction of CR4056 with morphine was evaluated by isobolographic analysis, which was carried out as described by Tallarida et al. (1989). The isobologram was constructed by connecting the ED50 of CR4056 plotted on the abscissa with the ED50 of morphine plotted on the ordinate to obtain the additivity line. On this line, a theoretical additive ED50 (ED50 add) was calculated (Pinardi et al., 2001; Miranda et al., 2002) using the following formula:
where R is the potency ratio of CR4056 alone to morphine alone, P1 is the proportion of CR4056 and P2 is the proportion of morphine in the total mixture.
The variance of ED50 add was calculated from the fraction (FR) of the ED50 in the combination as:
From this variances, 95% confidence limits of the theoretical additive ED50 were calculated. To evaluate the statistical significance of the synergistic effect, the theoretical values calculated as described above were compared by a Student's t-test with the ED50 values experimentally obtained for the drug mixture. The interaction index (I.I.) was calculated as the ratio experimental ED50/theoretical ED50 (Miranda et al., 2008). Values lower than 1 indicate synergistic interactions.
Isobolograms and dose-response curves were plotted using SigmaPlot version 12.0 (Systat Software Inc., San Jose, CA, USA).
Materials
The following chemicals were used: morphine (S.A.L.A.R.S., Como, Italy), naloxone, efaroxan, yohimbine, idazoxan, naproxen (SigmaAldrich, Milan, Italy) and atipamezole (Abcam PLC, Cambridge, UK). CR4056 was synthesized by the Medicinal Chemistry Department of Rottapharm. CR4056 was suspended in 0.5% methyl cellulose (MC) and administered orally (5 mL·kg−1); naproxen was dissolved in distilled water and administered orally (5 mL·kg−1); all the other drugs were dissolved in saline for i.p. or s.c. administration.
Results
CR4056 dose-response efficacy in male rats: comparison with morphine
Twenty-four hours after surgery, male rats showed hyperalgesia to mechanical stimuli. The mean withdrawal threshold in the injured paw was halved compared with that measured in the hind paw of sham rats (302.5 ± 29.7 g vs. 610.0 ± 18.5 g; Student's t-test: P < 0.01). Under these experimental conditions, oral CR4056 (range 1–10 mg·kg−1) significantly [RM two-way anova: F(3, 20) = 13.99; P < 0.0001] and dose-dependently reversed the established hyperalgesia (ED50 = 1.63 mg·kg−1; 95% CI = 1.07–2.47) (Figure 1A). Oral naproxen (30 mg·kg−1), previously reported to be poorly active in reducing postoperative pain (Whiteside et al., 2004), did not show significant effects at any time point analysed. Conversely, subcutaneous morphine (range 0.5–6 mg·kg−1) significantly [RM two-way anova: F(4, 25) = 14.40; P < 0.0001) and dose-dependently reversed the established hyperalgesia (ED50 = 1.27 mg·kg−1; 95% CI = 0.93–1.73) (Figure 1B).
Figure 1.
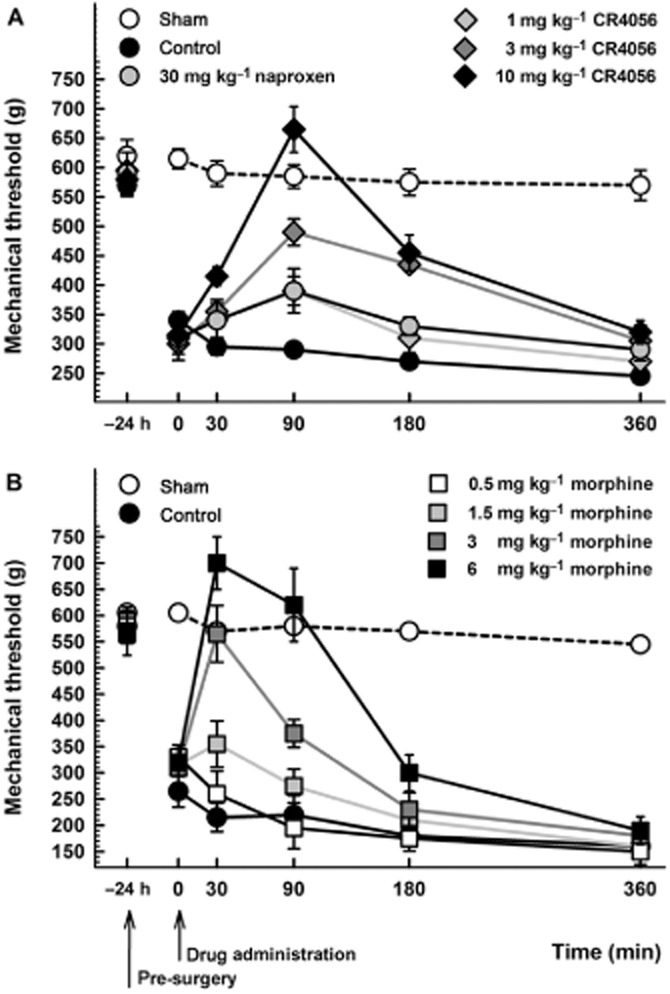
(A) Anti-hyperalgesic effect of CR4056 on postoperative pain-induced mechanical hyperalgesia in male rats (Randall-Selitto test). CR4056 was orally administered 24 h after surgery. Naproxen (30 mg·kg−1; oral) was used as comparison. Data represent the mean withdrawal threshold expressed in grams ± SEM (n = 6 per group). (B) Anti-hyperalgesic effects of morphine on postoperative pain-induced mechanical hyperalgesia in male rats (Randall-Selitto test). Morphine was subcutaneously administered 24 h after surgery. Data represent the mean withdrawal threshold expressed in grams ± SEM (n = 6 per group).
Pharmacology of CR4056-induced analgesia
The analgesic effect induced by CR4056 was completely suppressed by the non-selective imidazoline I2/α2-adrenoceptor antagonist idazoxan (3 mg·kg−1, i.p.; Figure 2A). Yohimbine (2 mg·kg−1, i.p.; Figure 2C), a selective α2-adrenoceptor antagonist, partly but significantly reduced (by about 30%; Tukey's multiple comparisons test: P < 0.05) the effect of CR4056. Similar results were obtained with atipamezole (1 mg·kg−1, s.c.; data not shown), an α2–adrenoceptor antagonist with negligible affinity for I2 receptors (Diaz et al., 1997; Pertovaara et al., 2005). Conversely, the non-selective I1/α2-adrenoceptor antagonist efaroxan (1 mg·kg−1, i.p.; Figure 2B) and the μ opioid receptor antagonist naloxone (3 mg·kg−1, i.p.; Figure 2D) were unable to alter the analgesic response induced by CR4056.
Figure 2.
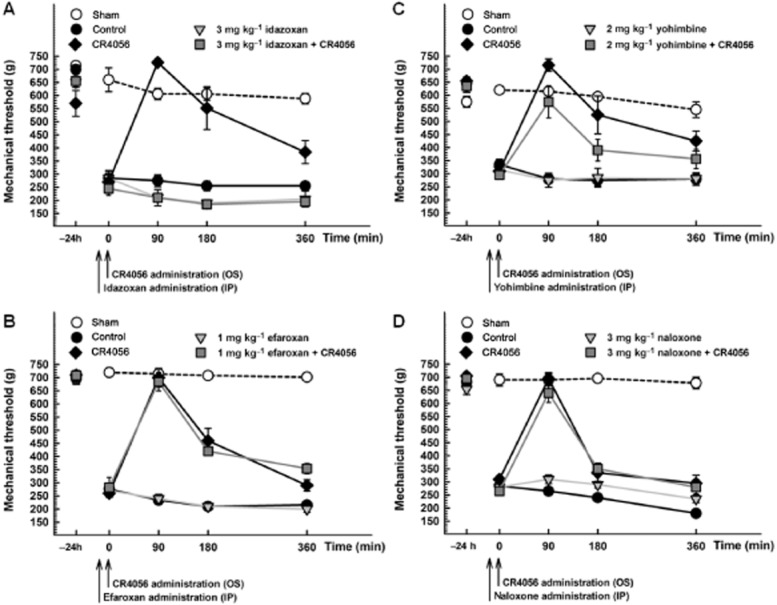
Effects of idazoxan [A: RM two-way anova: F(3, 20) = 41.77; P < 0.0001], efaroxan [B: RM two-way anova: F(3, 20) = 53.71; P < 0.0001], yohimbine [C: RM two-way anova: F(3, 20) = 25.17; P < 0.0001] and naloxone [D: RM two-way anova: F(3, 20) = 26.25; P < 0.0001] on the analgesic activity induced by 10 mg·kg−1 oral CR4056. All the antagonists were administered i.p. 15 min before CR4056. Data represent the mean withdrawal threshold expressed in grams ± SEM (n = 6 per group).
CR4056 dose-response efficacy in female rats: comparison with morphine
At baseline, female Sprague-Dawley rats showed a significantly lower paw withdrawal threshold to mechanical stimuli versus male rats (P < 0.01; Figure 3). Twenty-four hours after surgery, female rats still showed lower threshold levels than those recorded in male rats, so that the effect of surgery on mechanical threshold was similar in female and male rats (Figure 3). And even in females, oral CR4056 significantly [RM two-way anova: F(3, 20) = 41.91; P < 0.0001] and dose-dependently reversed the established hyperalgesia (ED50 = 0.93 mg·kg−1; 95% CI = 0.75–1.14) (Figure 4A). According to the protocol adopted for male rats, we administered to female rats a subcutaneous treatment with morphine (range 0.125–1 mg·kg−1). Morphine significantly [RM two-way anova: F(4, 25 = 19.09, P < 0.0001] and dose-dependently reversed the established hyperalgesia (ED50 = 0.25 mg·kg−1; 95% CI = 0.18–0.35) (Figure 4B).
Figure 3.
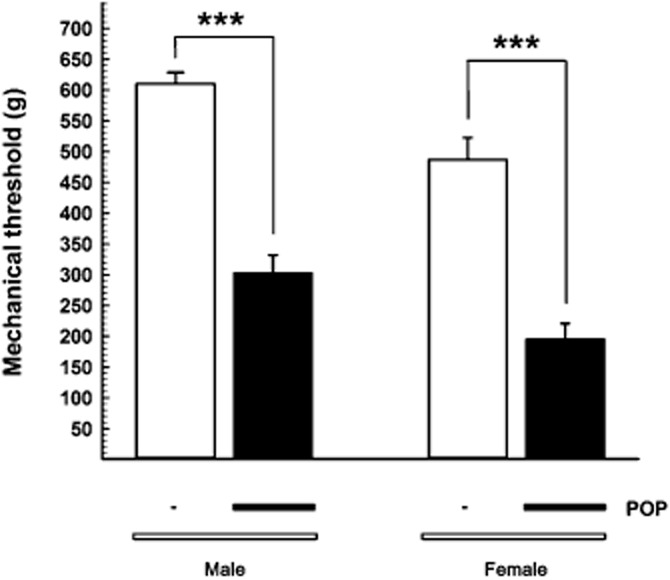
Sex differences in postoperative pain-induced mechanical hyperalgesia in Sprague-Dawley rats. The mean withdrawal threshold (expressed in grams ± SEM) 24 h after surgery, shown by the black bar below the columns (POP, postoperative pain), was compared with that in sham un-operated rats (no black bar). Thresholds were lower in female rats both in sham and POP groups. ***P < 0.001. Student's t-test; n = 12 per group.
Figure 4.
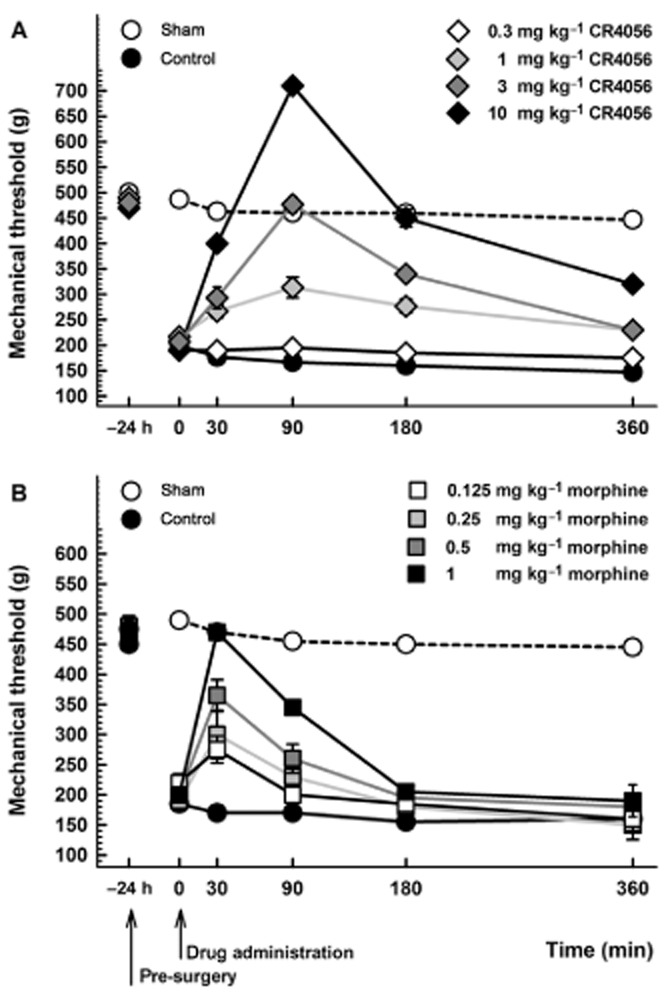
(A) Anti-hyperalgesic effect of CR4056 on postoperative pain-induced mechanical hyperalgesia in female rats (Randall-Selitto test). CR4056 was orally administered 24 h after surgery. Data represent the mean withdrawal threshold expressed in grams ± SEM (n = 6 per group). (B) Anti-hyperalgesic effect of morphine on postoperative pain-induced mechanical hyperalgesia in female rats (Randall-Selitto test). Morphine was subcutaneously administered 24 h after surgery. Data represent the mean withdrawal threshold expressed in grams ± SEM (n = 6 per group).
Morphine/CR4056 co-treatment: dose-response efficacy and isobolographic analysis
We examined the effects of co-administered morphine and CR4056, and determined the type of interaction between the two treatments. Similarly to morphine or CR4056 alone, their combination produced a significant, dose-dependent analgesic effect 24 h after surgery both in male rats [Figure 5A; RM two-way anova: F(4, 25) = 27.61; P < 0.0001] and in female rats [Figure 5B; RM two-way anova: F(4, 25) = 9.89; P < 0.0001]. Isobolographic analysis revealed a significant synergistic interaction that is independent of sex. In fact, when CR4056 and morphine were combined, their ED50 values were about fivefold lower than those measured after administration of each drug alone, both in male rats (0.28 mg·kg−1; 95% CI = 0.22–0.36 vs. 1.63 mg·kg−1; 95% CI = 1.07–2.47 for CR4056; 0.28 mg·kg−1; 95% CI = 0.22–0.36 vs. 1.27 mg·kg−1; 95% CI = 0.93–1.73 for morphine) and in female rats (0.15 mg·kg−1; 95% CI = 0.10–0.24 vs. 0.93 mg·kg−1; 95% CI = 0.75–1.14 for CR4056; 0.05 mg·kg−1; 95% CI = 0.03–0.08 vs. 0.25 mg·kg−1; 95% CI = 0.18–0.35 for morphine). And they were significantly lower than the ED50 values predicted assuming an additive effect, both in male rats (Figure 6A; Student's t-test: P < 0.01) and in female rats (Figure 6B; Student's t-test: P < 0.01). The I.I. was <0.4 for both drugs.
Figure 5.
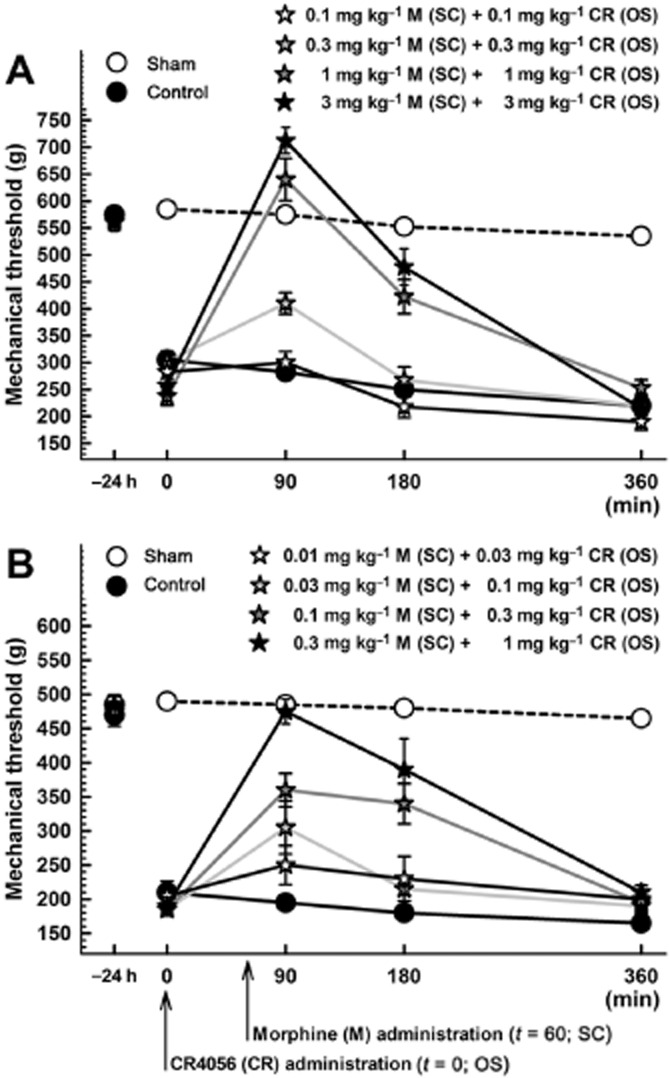
Anti-hyperalgesic effect of the combination oral CR4056/subcutaneous morphine on postoperative pain-induced mechanical hyperalgesia (Randall-Selitto test) in male (A) and female (B) rats. Since CR4056 and morphine showed a different temporal response when administered alone, we synchronized the peak effect of each drug by administering CR4056 1 h before morphine. Thus, mechanical hyperalgesia was measured 90, 180 and 360 min after CR4056 administration corresponding to 30, 120 and 300 min after morphine administration. Data represent the mean withdrawal threshold expressed in grams ± SEM (n = 6 per group). OS, oral administration; SC, subcutaneous administration.
Figure 6.
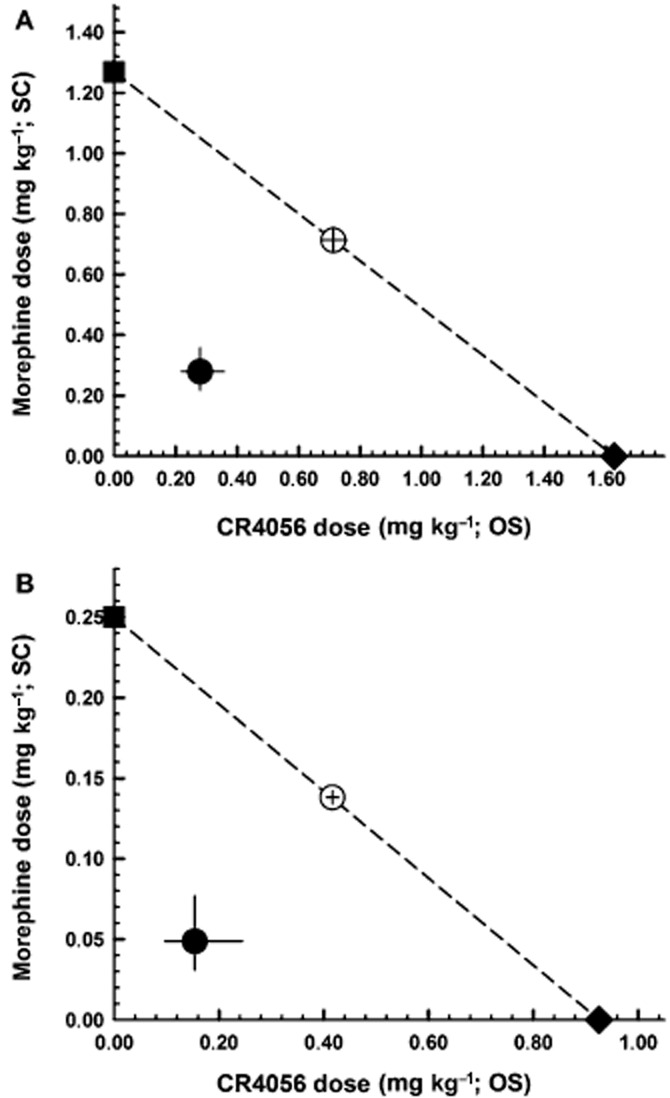
Isobolograms for the combination of oral CR4056 with subcutaneous morphine in male (A) and female (B) rats. Open circles correspond to the theoretical ED50 with 95% CI; filled circles corresponds to the experimental co-treatment ED50 with 95% CI; filled square corresponds to the experimental ED50 for morphine alone; and filled diamond corresponds to the experimental ED50 for CR4056 alone. I.I. was 0.39 for both CR4056 and morphine in male rats. In female rats, the calculated I.I. was 0.37 and 0.35 for CR4056 and morphine respectively. OS, oral administration; SC, subcutaneous administration.
Discussion
Imidazoline I2 receptors have been studied for more than two decades (Mallard et al., 1992; Miralles et al., 1993; Carpéné et al., 1995; Tesson et al., 1995), but only in recent years they have truly emerged as a promising target for the treatment of different conditions, including depression and pain (Ferrari et al., 2011; Li and Zhang, 2011; Li et al., 2011; 2014; Han et al., 2012; Meregalli et al., 2012; Tonello et al., 2012; Garau et al., 2013; Min et al., 2013). The main finding of this study is that a single oral treatment with the I2 receptor ligand CR4056 significantly reduced mechanical hyperalgesia caused by surgical lesion of the hind paw in rats, using Brennan's model of postoperative pain. Since CR4056 was previously reported to be effective in both inflammatory and neuropathic pain models (Ferrari et al., 2011; Meregalli et al., 2012; Li et al., 2014), this study identified CR4056 as a broad-spectrum analgesic drug.
In agreement with what was reported for the capsaicin-induced hyperalgesia model in the rat (Ferrari et al., 2011), the effect of CR4056 in Brennan's model of postoperative pain seems to be mechanism-related. In fact, idazoxan, a mixed I2/α2-adrenoceptor antagonist, completely suppressed the analgesic activity of CR4056, suggesting that binding to the I2 receptor is a primary requirement for its efficacy. Conversely, when we tried to block the analgesic effect of CR4056 with substances that do not interact with imidazoline I2 receptors, we failed to show a significant antagonism. A relevant exception was the moderate (about 30%) but significant effect of yohimbine, a classical α2-adrenoceptor antagonist, administered at the maximum possible dose not inducing obvious behavioural changes (Arrant et al., 2013; Zheng and Rinaman, 2013). As yohimbine did not antagonize the analgesic effect of CR4056 in the capsaicin model (Ferrari et al., 2011), we decided to test in the postoperative model an additional α2-adrenoceptor antagonist, atipamezole, which has negligible affinity for I2 receptors (Pertovaara et al., 2005), still obtaining a partial but significant block of the effects of CR4056. A similar pattern of I2 receptor signal modulation was previously shown by Diaz et al. (1997) in a different pain model. They reported that BU224, a potent I2 receptor ligand, inhibited the response of dorsal horn neurones in a dose-dependent manner, and that this effect was completely reversed by idazoxan but only partly reversed by yohimbine and atipamezole. In their discussion, these authors suggested that the actions of BU224 were mostly mediated through spinal imidazoline receptors selectively blocked by a putative antagonist to these receptors, such as idazoxan. Conversely, α2-adrenoceptor antagonists, such as yohimbine and atipamezole, antagonized the in vivo effects of drugs modulating noradrenergic pathways either directly by interacting with α2-adrenoceptors or indirectly by inhibiting the noradrenergic catabolism (Schreiber et al., 1998; Onttonen and Pertovaara, 2000; Li et al., 2007). Like other imidazoline receptor modulators, CR4056 is a functional and reversible inhibitor of MAO-A, because it modulates enzyme activity through allosteric I2 sites present in a discrete population of MAO proteins (Carpéné et al., 1995; Tesson et al., 1995; Ferrari et al., 2011). Accordingly, it is possible that the increased levels of endogenous noradrenaline found in different areas of the central nervous system after oral administration of CR4056 in rats (Ferrari et al., 2011) activate presynaptic α2 autoreceptors, which in turn are blocked by exogenous yohimbine and/or atipamezole. But the different levels of inhibition observed in our experiments with yohimbine and idazoxan (i.e. partial vs. complete) supports the hypothesis that CR4056 exerts its analgesic effects mainly through a mechanism strictly related to I2 receptors.
Remarkably, the recent interest in the I2 receptor as a promising target in drug discovery prompted Min et al. (2013) to assess for the first time the safety profile of two widely used I2 receptor ligands, namely 2-(2-benzofuranyl)-2-imidazoline (2-BFI) and 2-(4, 5-dihydroimidazol-2-yl)quinolone (BU224). They found that these compounds produce epileptic seizures in two strains of mice. Because these effects were not antagonized by the prototypical I2 receptor antagonist idazoxan, the authors suggested that the epileptogenic potential of 2-BFI and BU224 is not related to I2 receptors but rather to a different shared mechanism. No neurobehavioral changes were instead associated with CR4056 during preclinical safety pharmacology studies, and specifically in the behavioural Irwin test in rodents, where the compound was administered orally up to a dose 100 times greater than the dose producing analgesic activity (Meregalli et al., 2012). Despite the different safety profile of CR4056 compared with 2-BFI and BU224, which makes the first one a candidate drug, all of them markedly attenuated the place escape/avoidance behaviour at a dose that significantly attenuated the hyperalgesic response in rat models of inflammatory and neuropathic pain (Li et al., 2014). This result is particularly relevant since it represents the first evidence that the efficacy of this novel class of putative analgesics may not be limited to the evoked component of pain but may also include the spontaneous/affective component, that is the one commonly measured in clinical trials of postoperative pain.
According to a recent review, postoperative pain in humans is clinically treated with a mixture of drugs, including non-steroidal anti-inflammatory drugs, opioids and peripheral anaesthetics (Wu and Raja, 2011). Interestingly, epidemiological and clinical studies show that women are at greater risk for many pain conditions, and there is some suggestion that postoperative pain may be more severe in women than in men (Fillingim and Maixner, 1995; Riley et al., 1998; Fillingim et al., 2009). In addition, sex differences in the response to stimuli or in the response to analgesics have been claimed in a number of human and animal studies (Fillingim and Maixner, 1995; Aubrun et al., 2005; Dahan et al., 2008; Campesi et al., 2012; Patil et al., 2013). So we did an additional series of experiments in female rats and compared the results with those obtained in male rats.
In 2003, Kroin and colleagues had already analysed potential differences between male and female Sprague-Dawley rats with regard to postoperative pain and analgesic response. That study had shown no differences in postoperative pain perception or in the response to various analgesic drugs, such as morphine, gabapentin and clonidine (Kroin et al., 2003). In the present study, female Sprague-Dawley rats had a significantly lower paw withdrawal threshold to mechanical stimuli than male rats, before and after surgery. As a consequence, there were no sex differences in the net hyperalgesic response. Conversely, female rats were significantly more sensitive to treatments than males, as the ED50 of subcutaneous morphine and oral CR4056 was five- and twofold lower, respectively, in females versus males. In this regard, the differences between our results and those obtained by Kroin et al. (2003) may be justified on the basis of the following considerations. First, we examined mechanical hyperalgesic responses registered by a Randall-Selitto apparatus while they reported on mechanical allodynic responses registered manually by von Frey filaments. Compared with monofilaments, the Randall-Selitto apparatus has a bigger contact surface that can recruit sensory nerve endings in a different manner (Khalsa, 2004). Second, we performed dose-response experiments (four increasing doses of morphine) with a time-dependent (30, 90, 180 and 360 min) evaluation of mechanical threshold, while Kroin et al. (2003) evaluated the effect of two doses of morphine at a single time point (30 min), and the route of morphine administration was also different (s.c. vs. i.p.). However, it is relevant to emphasize that the sex differences we found were limited to drug potency, as there were no differences in the efficacy of treatments.
Another relevant finding of our study was the synergistic interaction between morphine and CR4056. In a previous study (Ferrari et al., 2011), we already analysed the nature of the morphine–CR4056 interaction in the capsaicin model of pain in male Wistar rats. In that study, the combination of morphine with CR4056 was clearly synergistic, being more effective than predicted on the basis of a simple additive effect. The present results confirmed the synergistic nature of morphine–CR4056 interaction also in the postoperative model of pain in both male and female Sprague-Dawley rats. When these agents were co-administered, the doses required to achieve the ED50 were about five times lower than those required when each drug was administered alone. This finding is important from a translational point of view because combination therapy may decrease the required dose of individual drugs, thus limiting the occurrence of adverse effects.
In conclusion, this study has demonstrated a significant analgesic effect of CR4056 in a surgical model of pain in rats, and a synergistic interaction between CR4056 and morphine that was independent of sex.
Acknowledgments
The authors wish to thank Dr. Albino Bonazzi for his institutional reporting and useful discussion on this project, and Mrs. Laura Radaelli for her skilful secretarial assistance. Preliminary results of this study were presented at the 32nd annual scientific meeting of the American Pain Society (Menghetti et al., J Pain 14(4S):S44, 2013), held in New Orleans, LA, USA, and at the 8th congress of the European Federation of IASP Chapters (Lanza et al., EFIC Proc., p. No. 812, 2013) held in Florence, Italy.
Glossary
- 2-BFI
2-(2-benzofuranyl)-2-imidazoline
- 95% CI
95% confidence interval
- BU224
2-(4, 5-dihydroimidazol-2-yl)quinolone
- I2
imidazoline-2
- I.I
interaction index
- RM
repeated measures
Contributions
M. L., F. F., D. T. and I. M. contributed in the process of data acquisition and in drafting the article. M. L., F. F. and G. C. contributed in the conception and design of the study, in the analysis and interpretation of data, and in the final approval of the version to be submitted. G. C. contributed also in obtaining the funding. M. L. (marco.lanza@rottapharm.com) and G. C. (gianfranco.caselli@rottapharm.com) declare to take responsibility for the integrity of the work as a whole, from inception to finished article.
Conflict of interest
IM is a graduate visiting student with no competing interests. All the other authors are scientists from the research unit of the Rottapharm group.
This study was funded by the Rottapharm group. However, the Rottapharm group as a corporate entity had no role in the conduct of the study; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ, Palmer GM. Recent pharmacological advances in paediatric analgesics. Biomed Pharmacother. 2006;60:303–309. doi: 10.1016/j.biopha.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Arrant AE, Schramm-Sapyta NL, Kuhn CM. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res. 2013;256:119–127. doi: 10.1016/j.bbr.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–593. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- Campesi I, Fois M, Franconi F. Sex and gender aspects in anesthetics and pain medication. Handb Exp Pharmacol. 2012;214:265–278. doi: 10.1007/978-3-642-30726-3_13. [DOI] [PubMed] [Google Scholar]
- Carpéné C, Collon P, Remaury A, Cordi A, Hudson A, Nutt D, et al. Inhibition of amine oxidase activity by derivatives that recognize imidazoline I2 sites. J Pharmacol Exp Ther. 1995;272:681–688. [PubMed] [Google Scholar]
- Chang CH, Wu HT, Cheng KC, Lin HJ, Cheng JT. Increase of beta-endorphin secretion by agmatine is induced by activation of imidazoline I(2A) receptors in adrenal gland of rats. Neurosci Lett. 2010;468:297–299. doi: 10.1016/j.neulet.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: ‘from mouse to man’. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- Diaz A, Mayet S, Dickenson AH. BU-224 produces spinal antinociception as an agonist at imidazoline I2 receptors. Eur J Pharmacol. 1997;333:9–15. doi: 10.1016/s0014-2999(97)01118-7. [DOI] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Alessandri-Haber N, Bogen O, Chen X, Green PG, et al. Neurotoxic catecholamine metabolite in nociceptors contributes to painful peripheral neuropathy. Eur J Neurosci. 2008;28:1180–1190. doi: 10.1111/j.1460-9568.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, et al. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum. 1995;4:209–221. [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garau C, Miralles A, García-Sevilla JA. Chronic treatment with selective I2-imidazoline receptor ligands decreases the content of pro-apoptotic markers in rat brain. J Psychopharmacol. 2013;27:123–134. doi: 10.1177/0269881112450785. [DOI] [PubMed] [Google Scholar]
- Han Z, Cheng ZH, Liu S, Yang JL, Xiao MJ, Zheng RY, et al. Neurovascular protection conferred by 2-BFI treatment during rat cerebral ischemia. Biochem Biophys Res Commun. 2012;424:544–548. doi: 10.1016/j.bbrc.2012.06.152. [DOI] [PubMed] [Google Scholar]
- Hwang SL, Liu IM, Tzeng TF, Cheng JT. Activation of imidazoline receptors in adrenal gland to lower plasma glucose in streptozotocin-induced diabetic rats. Diabetologia. 2005;48:767–775. doi: 10.1007/s00125-005-1698-2. [DOI] [PubMed] [Google Scholar]
- Khalsa PS. Biomechanics of musculoskeletal pain: dynamics of the neuromatrix. J Electromyogr Kinesiol. 2004;14:109–120. doi: 10.1016/j.jelekin.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroin JS, Buvanendran A, Nagalla SK, Tuman KJ. Postoperative pain and analgesic responses are similar in male and female Sprague-Dawley rats. Can J Anaesth. 2003;50:904–908. doi: 10.1007/BF03018737. [DOI] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- Li C, Sekiyama H, Hayashida M, Takeda K, Sumida T, Sawamura S, et al. Effects of topical application of clonidine cream on pain behaviours and spinal Fos protein expression in rat models of neuropathic pain, postoperative pain, and inflammatory pain. Anesthesiology. 2007;107:486–494. doi: 10.1097/01.anes.0000278874.78715.1d. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol. 2011;658:49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y, Winter JC. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I2 receptor ligands. Eur J Pharmacol. 2011;669:59–65. doi: 10.1016/j.ejphar.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Qiu Y, Peng BW, Zhang Y. Anti-hyperalgesic effects of imidazoline I2 receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol. 2014;171:1580–1590. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard NJ, Hudson AL, Nutt DJ. Characterization and autoradiographical localization of non-adrenoceptor idazoxan binding sites in the rat brain. Br J Pharmacol. 1992;106:1019–1027. doi: 10.1111/j.1476-5381.1992.tb14450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B, et al. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–167. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Peng BW, He X, Zhang Y, Li JX. Gender difference in epileptogenic effects of 2-BFI and BU224 in mice. Eur J Pharmacol. 2013;718:81–86. doi: 10.1016/j.ejphar.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles A, Olmos G, Sastre M, Barturen F, Martin I, Garcia-Sevilla JA. Discrimination and pharmacological characterization of I2-imidazoline sites with [3H]idazoxan and alpha-2 adrenoceptors with [3H]RX821002 (2-methoxy idazoxan) in the human and rat brains. J Pharmacol Exp Ther. 1993;264:1187–1197. [PubMed] [Google Scholar]
- Miranda HF, Sierralta F, Pinardi G. Neostigmine interactions with non steroidal anti-inflammatory drugs. Br J Pharmacol. 2002;135:1591–1597. doi: 10.1038/sj.bjp.0704599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda HF, Prieto JC, Puig MM, Pinardi G. Isobolographic analysis of multimodal analgesia in an animal model of visceral acute pain. Pharmacol Biochem Behav. 2008;88:481–486. doi: 10.1016/j.pbb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Molderings GJ. Imidazoline receptors: basic knowledge, recent advances, and future prospects for therapy and diagnosis. Drug Future. 1997;22:757–772. [Google Scholar]
- Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–406. doi: 10.1345/aph.1H386. [DOI] [PubMed] [Google Scholar]
- Onttonen T, Pertovaara A. The mechanical antihyperalgesic effect of intrathecally administered MPV-2426, a novel alpha2 -adrenoceptor agonist, in a rat model of postoperative pain. Anesthesiology. 2000;92:1740–1745. doi: 10.1097/00000542-200006000-00034. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Olmos G, Boronat MA, Lizcano JM, Unzeta M, García-Sevilla JA. Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2-imidazoline receptors in rat liver. Br J Pharmacol. 1997;121:901–912. doi: 10.1038/sj.bjp.0701214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil MJ, Green DP, Henry MA, Akopian AN. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience. 2013;253:132–141. doi: 10.1016/j.neuroscience.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A, Haapalinna A, Sirviö J, Virtanen R. Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist. CNS Drug Rev. 2005;11:273–288. doi: 10.1111/j.1527-3458.2005.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinardi G, Sierralta F, Miranda HF. Interaction between the antinociceptive effect of ketoprofen and adrenergic modulatory systems. Inflammation. 2001;25:233–239. doi: 10.1023/a:1010923820109. [DOI] [PubMed] [Google Scholar]
- Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Ruiz-Durántez E, Torrecilla M, Pineda J, Ugedo L. Attenuation of acute and chronic effects of morphine by the imidazoline receptor ligand 2-(2-benzofuranyl)-2-imidazoline in rat locus coeruleus neurons. Br J Pharmacol. 2003;138:494–500. doi: 10.1038/sj.bjp.0705052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics. 2012;13:1719–1740. doi: 10.2217/pgs.12.152. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Getslev V, Weizman A, Pick CG. The antinociceptive effect of moclobemide in mice is mediated by noradrenergic pathways. Neurosci Lett. 1998;253:183–186. doi: 10.1016/s0304-3940(98)00638-7. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45:947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Tesson F, Limon-Boulez I, Urban P, Puype M, Vandekerckhove J, Coupry I, et al. Localization of I2-imidazoline binding sites on monoamine oxidases. J Biol Chem. 1995;270:9856–9861. doi: 10.1074/jbc.270.17.9856. [DOI] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I2 receptor agonists in rats. Br J Pharmacol. 2012;166:1936–1945. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonello R, Villarinho JG, da Silva Sant'Anna G, Tamiozzo L, Machado P, Trevisan G, et al. The potential antidepressant-like effect of imidazoline I2 ligand 2-BFI in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:15–21. doi: 10.1016/j.pnpbp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, et al. Pharmacological characterisation of a rat model of incisional pain. Br J Pharmacol. 2004;141:85–91. doi: 10.1038/sj.bjp.0705568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–2225. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- Zheng H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze requires hindbrain noradrenergic neurons that target the anterior ventrolateral bed nucleus of the stria terminalis. Eur J Neurosci. 2013;37:1340–1349. doi: 10.1111/ejn.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]


