Abstract
BACKGROUND AND PURPOSE
The COX isoforms (COX-1, COX-2) regulate human gut motility, although their role under pathological conditions remains unclear. This study examines the effects of COX inhibitors on excitatory motility in colonic tissue from patients with diverticular disease (DD).
EXPERIMENTAL APPROACH
Longitudinal muscle preparations, from patients with DD or uncomplicated cancer (controls), were set up in organ baths and connected to isotonic transducers. Indomethacin (COX-1/COX-2 inhibitor), SC-560 (COX-1 inhibitor) or DFU (COX-2 inhibitor) were assayed on electrically evoked, neurogenic, cholinergic and tachykininergic contractions, or carbachol- and substance P (SP)-induced myogenic contractions. Distribution and expression of COX isoforms in the neuromuscular compartment were assessed by RT-PCR, Western blot and immunohistochemical analysis.
KEY RESULTS
In control preparations, neurogenic cholinergic contractions were enhanced by COX inhibitors, whereas tachykininergic responses were blunted. Carbachol-evoked contractions were increased by indomethacin or SC-560, but not DFU, whereas all inhibitors reduced SP-induced motor responses. In preparations from DD patients, COX inhibitors did not affect electrically evoked cholinergic contractions. Both indomethacin and DFU, but not SC-560, decreased tachykininergic responses. COX inhibitors did not modify carbachol-evoked motor responses, whereas they counteracted SP-induced contractions. COX-1 expression was decreased in myenteric neurons, whereas COX-2 was enhanced in glial cells and smooth muscle.
CONCLUSIONS AND IMPLICATIONS
In control colon, COX-1 and COX-2 down-regulate cholinergic motility, whereas both isoforms enhance tachykininergic motor activity. In the presence of DD, there is a loss of modulation by both COX isoforms on the cholinergic system, whereas COX-2 displays an enhanced facilitatory control on tachykininergic contractile activity.
Keywords: cyclooxygenase-1 and -2, human colon, diverticular disease, excitatory neurotransmission
Introduction
Colonic diverticular disease (DD) is a pathological condition commonly encountered in Western countries, and its complications are a frequent cause of hospitalization (Weizman and Nguyen, 2011). DD is relatively infrequent under the age of 40, after which it increases steadily and reaches over 25% by 60 years (Hemming and Floch, 2010). Although the pathogenesis of DD remains unknown, different factors have been called into play as contributors to its development, such as low fibre diet (Jeyarajah and Papagrigoriadis, 2011), a decrease in tissue compliance, with subsequent colonic wall thickening and lumen narrowing, and an increase in collagen cross-linking (Matrana and Margolin, 2009).
Notably, DD has been associated also with neuromuscular dysfunctions (Bassotti et al., 2001; Bassotti and Villanacci, 2012). Colonic motor abnormalities in DD have been hypothesized to result from changes in enteric nerves, smooth muscle functions (Golder et al., 2003; Bassotti et al., 2005a; Mattii et al., 2013), and low-grade mucosal and myenteric inflammation (Bassotti et al., 2013). In addition, alterations in the excitatory control by enteric cholinergic nerves have been observed in DD, but not by all groups (Tomita et al., 2000; Golder et al., 2003). Functional impairments of enteric non-adrenergic non-cholinergic nerves have been reported (Tomita et al., 2000), and other studies suggest changes in the levels of neuropeptides and 5-HT in the colon of DD patients (Costedio et al., 2008; Simpson et al., 2009). DD appears to be associated also with marked changes in both enteric cholinergic and tachykininergic nerves. In particular, colonic tissues from DD patients displayed an enhanced excitatory control by substance P (SP) and a reduced activity of cholinergic pathways (Guagnini et al., 2006). However, the actual mechanisms underlying such alterations have not been elucidated yet.
It is well known that COX activity from either isoform, COX-1 or COX-2, is deeply involved in the regulation of intestinal neuromotility, and that these enzymes play a significant role in the pathophysiology of altered gut motility under inflammation (Fornai et al., 2005; 2006; 2010). In particular, both COX isoforms are able to exert inhibitory actions on excitatory cholinergic neuromotility, whereas, in the presence of experimental inflammation, these regulatory actions undergo significant rearrangements (Fornai et al., 2006). However, there is a lack of knowledge regarding the putative involvement of enteric COX pathways in the alterations of colonic motility associated with DD. Moreover, the majority of current evidence pertains to colonic circular muscle, whereas alterations occurring in the longitudinal layer during DD have been scarcely investigated.
The present study was designed to achieve the following goals: (i) to characterize the patterns of neuromuscular activity in colonic longitudinal smooth muscle obtained from patients with DD and (ii) to investigate the involvement of COX isoforms in neuromuscular alterations associated with DD, by means of functional, immunohistochemical and molecular analysis.
Methods
Patients and tissue preparation
The experimental protocol was approved by the Ethics Committee of Pisa University Hospital and all patients gave fully informed consent. Specimens of sigmoid colon, taken at least 10 cm away from any lesion in macroscopically normal regions, were obtained from patients undergoing surgery for uncomplicated colonic cancer (four males and six females; aged 33–82 years) and served as controls. Samples of sigmoid colon were taken also from patients undergoing elective left hemicolectomy for DD (six males and nine females; aged 33–87 years). In these patients, the time between last episode onset and surgery ranged from 3 to 12 months. Data regarding the characteristics of patients are summarized in Supporting Information Table S1. These specimens were collected also at least 10 cm away from any macroscopically evident lesion related to inflammation or fibrosis. Care was taken of not dissecting specimens directly from diverticula. All subjects received the same standard anaesthesia consisting of propofol plus fentanyl, both for the induction and for the maintenance phases. Patients had never received radiotherapy or chemotherapy, and had not been treated with steroids or opioids before surgery. Only colonic tissues excised intraoperatively within 60 min from skin incision were used.
Portions of colonic specimens were stripped of mucosa and submucosa, snap-frozen in liquid nitrogen and stored at −80°C for subsequent assays. Other portions were fixed in cold 4% paraformaldehyde, diluted in PBS and employed for routine histology and immunohistochemical studies. The remaining parts were placed into pre-oxygenated Krebs solution (composition, in mM: NaCl 113, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, glucose 11.5, pH 7.4) and transported on ice to the laboratory. Longitudinal muscle strips (3 mm in width, 20 mm in length) were prepared as previously described (Fornai et al., 2005). Morphological and immunohistochemical studies were carried out on paraffin cross sections with circular layer and myenteric ganglia cut longitudinally (Bernardini et al., 2006; 2012). The morphological features of colonic samples were assessed by haematoxylin–eosin (H&E) staining. Immunohistochemical studies were performed by means of the antibodies listed in Supporting Information Table S2.
Recording of longitudinal smooth muscle contractile activity
Contractions of colonic muscle were recorded as previously described (Fornai et al., 2009). Preparations were set up in 10 mL organ baths containing Krebs solution at 37°C, bubbled with 95% O2 + 5% CO2, connected to isotonic transducers (Basile, Comerio, Italy) under constant load (1 g), and allowed to equilibrate for 30 min. Longitudinal muscle contractions were recorded by polygraph (Basile). Transmural electrical stimulation was delivered by a BM-ST6 stimulator (Biomedica Mangoni, Pisa, Italy). Stimuli were applied as 10 s single trains of square wave pulses (0.5 ms, 30 mA). Each preparation was repeatedly challenged with electrical stimulations, and experiments started when reproducible contractions were obtained (usually after 2–3 stimulations).
Preliminary experiments were performed to select the appropriate frequency of electrical stimulation, as well as carbachol and SP concentrations, that elicited submaximal contractions. For this purpose, preparations were challenged with electrical stimuli at increasing frequencies, ranging from 1 to 30 Hz. Frequency–response curves were constructed under the different experimental conditions adopted in the study to test COX inhibitors: (i) standard Krebs solution; (ii) Krebs solution added with guanethidine (adrenergic neurone blocker, 10 μM), Nω-nitro-l-arginine methylester (l-NAME, NOS inhibitor, 100 μM), L-732,138 (NK1 receptor antagonist, 10 μM), GR-159897 (NK2 receptor antagonist, 1 μM) and SB-218795 (NK3 receptor antagonist, 1 μM) to obtain cholinergic motor responses; (iii) Krebs solution containing guanethidine (10 μM), l-NAME (100 μM), GR-159897 (1 μM), SB-218795 (1 μM) and atropine (1 μM), to record contractions driven by endogenous tachykinins (nomenclature follows Alexander et al., 2013). Concentration–response curves to carbachol (0.001–100 μM) or exogenous SP (0.001–10 μM) were constructed in the presence of tetrodotoxin (1 μM). To compare the magnitude of contractions in control preparations with those of tissues from DD patients, the responses obtained from each preparation were normalized to maximal contractions elicited by histamine (100 μM) because the contractile responses induced by histamine in DD patients did not differ significantly from those recorded in control colonic tissues and thus represented suitable reference contractions for the direct comparison of cholinergic and tachykininergic motor responses between control and DD tissues.
The first set of experiments was designed to assay COX inhibitors on contractions elicited by electrical stimulation of cholinergic nerves. Preparations were maintained in Krebs solution containing guanethidine, l-NAME, L-732,138, GR-159897 and SB-218795 to prevent non-cholinergic responses (Fornai et al., 2005). Tissues were then incubated in Krebs solution containing the COX inhibitors indomethacin (1 μM), SC-560 (0.1 μM), DFU (1 μM) or SC-560 plus DFU for 30 min before electrical stimulation. Preparations were incubated with test drugs along two 15 min consecutive periods, with an intervening washing. Drug concentrations were selected on the basis of preliminary experiments, in which increasing concentrations of COX inhibitors (0.01–10 μM) were tested on electrically evoked contractions of control and DD colonic preparations maintained in standard Krebs solutions (see Supporting Information Fig. S1). The selected concentrations were in accordance with those employed in our previous studies on colonic tissues (Fornai et al., 2005; 2006), as well as with selectivity profiles evaluated in vitro (Riendeau et al., 1997; Gierse et al., 2005).
In the second series, COX inhibitors were tested on contractions elicited by electrical stimulation directed at tachykininergic neurons. Tissues were maintained in Krebs solution added with guanethidine, l-NAME, GR-159897, SB-218795 and atropine, to obtain motor responses driven by NK1 receptor activation by endogenous tachykinins. The first stimulation was applied in the absence of other drugs, whereas the second one was applied after 30 min of incubation with COX inhibitors, as reported earlier.
The last set of experiments assessed the effects of COX inhibitors on contractions elicited by extrinsic activation of muscarinic receptors or NK1 receptors on smooth muscle cells. Preparations were maintained in Krebs solution containing tetrodotoxin, and challenged with carbachol (1 μM) or SP (1 μM) at least every 45 min until reproducible responses were obtained. The last two reproducible contractions were taken as control responses, and their mean value was used for calculations of percent variations in the presence of test drugs. In addition, a minimum of 30 min recovery was allowed between the last carbachol or SP application and the incubation with COX inhibitors. After 30 min of incubation with COX inhibitors, as reported earlier, preparations were stimulated with carbachol or SP.
RT-PCR analysis of COX-1 and COX-2
RT-PCR was carried out as previously reported (Fornai et al., 2005). Total RNA was isolated and served as template for single strand cDNA. PCR was performed by primers based upon nucleotide sequence of cloned COX-1 and COX-2 human gene (Hla and Neilson, 1992). PCR was performed by a PCR-Express thermocycler (Hybaid, Ashford, UK) as follows: after 3 min of denaturation at 94°C, 30 cycles of denaturation at 94°C (1 min), annealing at 50°C (1.5 min) and extension at 72°C (2 min) were performed with a final extension at 72°C for 10 min. Untranscribed RNA was included in PCR reactions to verify the absence of genomic DNA. RT-PCR efficiency was evaluated by primers for human β-actin. After PCR, 20 μL of each reaction product was separated on the same 1.5% agarose gel electrophoresis and stained with ethidium bromide. cDNA bands were visualized by UV light and quantitated by densitometric analysis with NIH Image program (Scion Corporation, Frederick, MD, USA), and normalized to β-actin.
Western blot analysis of COX-1 and COX-2
The assay was performed as previously described (Bernardini et al., 2006). Specimens of colonic tissues were homogenized and centrifuged (14,000× g, 4°C). Supernatants were stored at −20°C. Furthermore, 50 μg of lysed tissues was separated by SDS-PAGE and transferred onto PVDF membrane. The blots were then blocked for 1 h and incubated overnight at room temperature with a primary antibody raised against COX-1, COX-2 (Cayman Chemical Co, Ann Arbor, MI, USA) or β-actin (Sigma-Aldrich, Milan, Italy). After repeated washings, a peroxidase-conjugated secondary antibody was added for 1 h at room temperature. Immunoreactive bands were visualized by chemiluminescent reagents (Immobilon, Millipore, MA, USA) and exposed to Kodak Image Station 440 (Eastman Kodak Company, Rochester, NY, USA) for signal and densitometric image analysis. Protein levels were normalized to β-actin.
Immunoperoxidase
Immunostaining for SP and COX isoforms was carried out as previously described (Ippolito et al., 2009; Bernardini et al., 2012). Briefly, after heat-induced epitope retrieval and blockade of non-specific binding, sections were sequentially incubated with primary antibodies, biotinylated immunoglobulins, streptavidin-peroxidase complex and 3-3′-diaminobenzidine tetrahydrochloride (Amresco, Solon, OH, USA), and counterstained with Harris's haematoxylin. The specimens were quantitatively evaluated by two investigators, unaware of the treatment groups. Ten randomly selected microscopic fields for each section were captured by a Leica DMRB light microscope equipped with a digital camera (DFC480; Leica Microsystems, Cambridge, UK) and evaluated by the Image Analysis System ‘L.A.S. software v.4’ (Leica Microsystems). Intensity thresholding was applied depending upon signal-to-noise ratio to highlight the immunoreactive area, which was expressed as percentage of the area examined (percent positive pixels [PPP]) (Bernardini et al., 2012).
Immunofluorescence
COX isoform expression was detected by double immunofluorescence. The immunostaining was preceded by (i) heat-mediated antigen retrieval with microwave for 20 min at 600 W; (ii) tissue autofluorescence blocking step with 1% NaBH4; and (iii) a blocking buffer (BB; composition, bovine serum albumin 1%, fetal bovine serum 2% in PBS) for non-specific binding. Sections were then incubated: first overnight at 4°C in a mixture of the two primary antibodies (HuC/D-COX-1 or HuC/D-COX-2, and GFAP-COX-1 or GFAP-COX-2) diluted in BB; then with the appropriate secondary antibodies for 1 h (Supporting Information Table S2). Sections were then washed and mounted in Vectashield (Vector Lab, Burlingame, CA, USA). Specimens were examined and photographed by a Leica DMRB light fluorescence microscope.
Negative controls
Negative controls were obtained by omitting primary antibodies or substituting them with pre-immune rabbit or mouse serum (1:100). Endogenous peroxidases and avidin-binding activity were assayed by incubating slides with DAB alone or with peroxidase-labelled streptavidin complex/DAB respectively. All reactions were performed at room temperature and in humid chambers, unless otherwise stated.
Statistical analysis
Results are expressed as percent values of control data and given as mean ± SEM. For functional, Western blot and RT-PCR experiments, the significance of differences was evaluated on raw data, prior to percentage normalization, by one-way anova followed by Student–Newman–Keuls test. For immunohistochemical analysis, the results were expressed as mean PPP values and the significance of differences was evaluated by Student's t-test for unpaired data (two-tailed). A P value < 0.05 was considered significant. Calculations were performed by commercial software (GraphPad Prism, version 3.0; GraphPad Software Inc., San Diego, CA, USA).
Materials
Indomethacin, SP, atropine sulphate, l-NAME (Nω-nitro-l-arginine methylester), carbachol hydrochloride, histamine diphosphate and guanethidine were supplied by Sigma Chemical (St Louis, MO, USA); DFU [3-(3-fluorophenyl)-4-(4-methanesulfonyl)-5,5-dimethyl-5H-furan-2-one] was kindly provided by Merck Research Laboratories (Rahway, NJ, USA); SC-560 [5-(4-clorophenyl)-1-(4-metoxyphenyl)-3-trifluoromethyl-pirazole], L-732,138, GR-159897, SB-218795, and tetrodotoxin by Tocris Cookson (Bristol, UK). COX inhibitors were dissolved in dimethyl sulfoxide and further dilutions were made with saline solution. Dimethyl sulfoxide concentration in organ baths never exceeded 0.5%. At this concentration, dimethyl sulfoxide did not affect resting tone, spontaneous contractions or evoked motor responses.
Results
Functional studies
Colonic longitudinal motor activity
During equilibration, most muscle preparations from controls or DD patients displayed spontaneous activity that was low in amplitude and generally stable throughout the experiment. Electrically evoked responses consisted of phasic contractions followed, in most cases, by after contractions of variable amplitude. Atropine (1 μM) abolished phasic contractions or converted them into relaxations, and only after contractions became evident (not shown). Tetrodotoxin (1 μM) abolished electrically induced contractions (−95%). Frequency–response curves, obtained under different conditions, allowed selection of the frequency of 10 Hz, which elicited submaximal contractions (Figure 1A–C). Accordingly, all subsequent experiments, designed to test the effects of COX inhibitors on electrically evoked contractions, were performed at 10 Hz. The exposure of colonic preparations to increasing concentrations of carbachol (0.001–100 μM) or SP (0.001–10 μM), in the presence of tetrodotoxin, resulted also in phasic contractions (Figure 1D and E), which were prevented by atropine or L-732,138 respectively (not shown). In both cases, the construction of concentration–response curves allowed selection of the submaximal concentration of 1 μM for subsequent experiments (Figure 1D and E).
Figure 1.
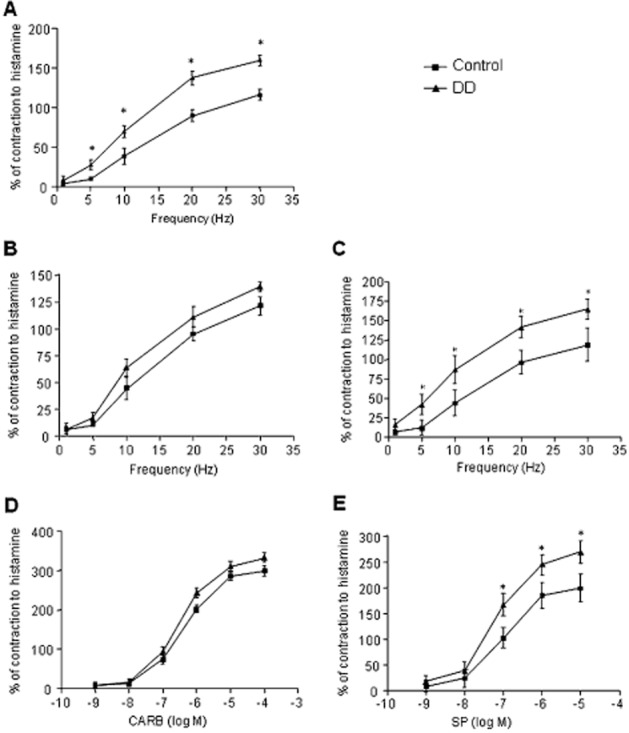
Effects of electrical stimulation (ES, 1–30 Hz) (A–C), carbachol (CARB, 0.001–100 μM) (D) or substance P (SP, 0.001–10 μM) (E) on the motor activity of colonic longitudinal smooth muscle preparations obtained from control subjects or patients with DD. Colonic preparations were maintained in Krebs solution having the following composition: (A) standard control Krebs; (B) addition of guanethidine (10 μM), l-NAME (100 μM), L-732,138 (10 μM), GR-159897 (1 μM) and SB-218795 (1 μM) to record cholinergic contractions; (C) addition of guanethidine (10 μM), l-NAME (100 μM), atropine (1 μM), GR-159897 (1 μM) and SB-218795 (1 μM) to record tachykininergic contractions mediated by NK1 receptors; (D and E) addition of tetrodotoxin (1 μM). Each point represents the mean ± SEM obtained from 6–7 experiments. *P < 0.05, significantly different from control.
Frequency–response curves in the presence of standard Krebs solution displayed an enhanced contractile response of preparations from patients with DD, in comparison with control tissues (Figure 1A). Under cholinergic conditions, the electrically evoked contractions were similar in both control and DD tissues (Figure 1B). Similar results were obtained when colonic preparations were stimulated with increasing concentrations of carbachol (Figure 1D). By contrast, in tissues from DD patients electrically evoked tachykininergic contractions were enhanced, as compared with controls (Figure 1C). Likewise, contractions evoked by exogenous SP were also more pronounced than those recorded in control tissues (Figure 1E).
Effects of COX inhibitors on cholinergic nerve motor pathways
No changes in resting tone were recorded upon incubation of control or DD preparations with indomethacin (1 μM), SC-560 (0.1 μM) or DFU (1 μM). In the presence of guanethidine, l-NAME and NK receptor antagonists, the electrical stimulation evoked phasic contractions, which were abolished, or markedly reduced, by atropine 1 μM. Likewise, these cholinergic responses were suppressed by tetrodotoxin (1 μM). Under these conditions, COX inhibitors enhanced electrically evoked cholinergic contractions of control preparations with effects of different magnitude: indomethacin enhanced the contractions elicited by electrical stimulation (Figure 2A); SC-560 or DFU mimicked this effect, although being less effective (Figure 2A); SC-560 plus DFU enhanced the electrically induced contractions, as effectively as indomethacin (Figure 2A). In colonic preparations from DD patients, indomethacin, SC-560, DFU or SC-560 plus DFU did not affect electrically evoked cholinergic contractions (Figure 2B).
Figure 2.
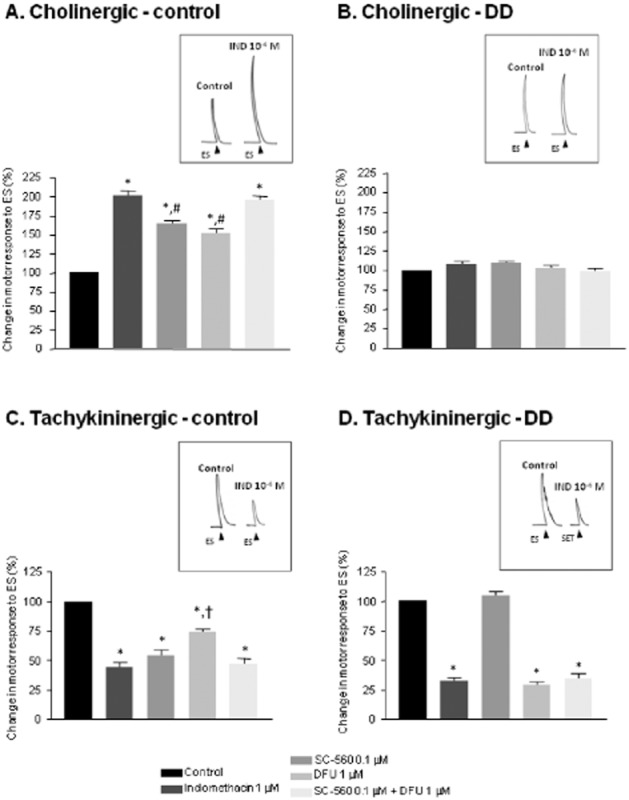
Effects of indomethacin (1 μM), SC-560 (0.1 μM), DFU (1 μM) and SC-560 plus DFU on contractile responses evoked by electrical stimulation (ES) in colonic longitudinal muscle preparations from control subjects (A and C) or patients with diverticular disease (B and D). In A and B, tissues were maintained in Krebs solution containing guanethidine (10 μM), l-NAME (100 μM), L-732,138 (10 μM), GR-159897 (1 μM) and SB-218795 (1 μM) to record cholinergic contractions; in C and D, tissues were in Krebs solution containing guanethidine (10 μM), l-NAME (100 μM), atropine (1 μM), GR-159897 (1 μM) and SB-218795 (1 μM) to record tachykininergic contractions. Tracings in the inset on the right of each panel display the effects of indomethacin (IND, 1 μM) on electrically evoked contractions. Each column represents the mean ± SEM obtained from 7–8 experiments. *P < 0.05, significantly different from control; #P < 0.05, significantly different from indomethacin or SC-560 plus DFU; †P < 0.05, significantly different from indomethacin, SC-560 or SC-560 plus DFU.
Effects of COX inhibitors on tachykininergic nerve motor pathways
Upon incubation of normal or DD preparations with l-NAME, guanethidine, GR-159897, SB-218795 and atropine, electrical stimulation evoked phasic contractions, which were abolished by L-732,138 or tetrodotoxin, indicating the recruitment of neuronal tachykininergic pathways. In this setting, indomethacin (1 μM) or SC-560 (0.1 μM) significantly decreased electrically induced tachykininergic contractions (Figure 2C ) in control preparations. DFU (1 μM) mimicked such an inhibitory effect, although being less effective (Figure 2C). In addition, SC-560 plus DFU decreased the electrically induced contractions, as effectively as indomethacin or SC-560 alone (Figure 2C). In preparations from DD patients, indomethacin, DFU or SC-560 plus DFU exerted similar inhibitory effects on electrically evoked tachykininergic contractions, while SC-560 alone was without effects (Figure 2D).
Effects of COX inhibitors on motor responses elicited by carbachol or SP
Exposure of preparations to carbachol (1 μM) resulted in atropine-sensitive contractions. In control preparations, carbachol-induced contractions were enhanced to a similar extent by indomethacin (1 μM) or DFU (1 μM), whereas SC-560 (0.1 μM) did not elicit significant effects (Figure 3A). Upon incubation with SC-560 plus DFU, carbachol-evoked contractile responses did not differ from those recorded with indomethacin or DFU alone (Figure 3A). In preparations from DD patients, none of the test compounds modified carbachol-induced contractions (Figure 3B).
Figure 3.
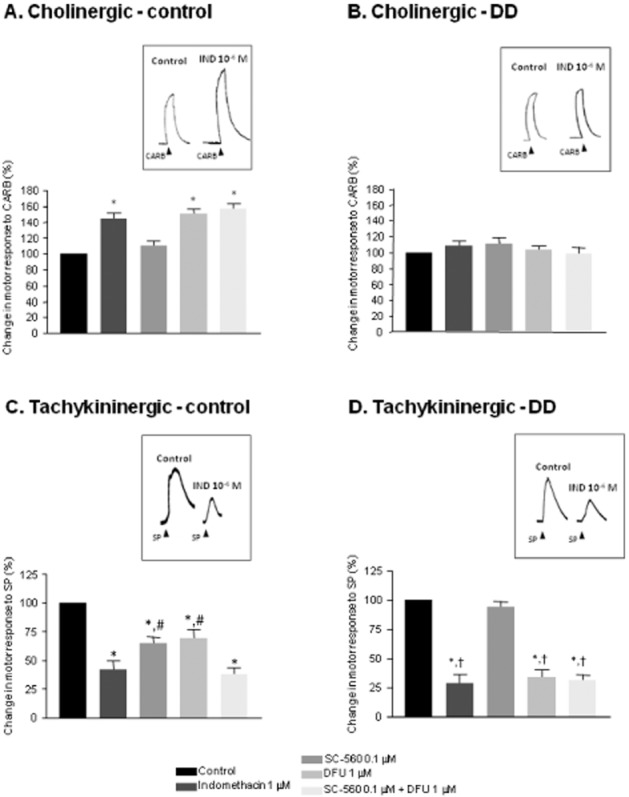
In A and B are shown effects of indomethacin (1 μM), SC-560 (0.1 μM), DFU (1 μM) and SC-560 plus DFU on contractile responses elicited by carbachol (CARB, 1 μM); in C and D, effects of substance P (SP, 1 μM) in colonic longitudinal muscle preparations from control subjects (A and C) or patients with diverticular disease (B and D). Colonic preparations were maintained in Krebs solution containing tetrodotoxin (1 μM). Tracings in the inset on the right of each panel display the effects of indomethacin (IND, 1μM) on contractions evoked by carbachol (CARB, 1 μM) or substance P (SP, 1 μM ). Each column represents the mean ± SEM obtained from 7–8 experiments. *P < 0.05, ssignificantly different from control; #P < 0.05, significantly different from indomethacin or SC-560 plus DFU; †P < 0.05, significantly different from the corresponding values obtained in colonic tissues from control patients.
Treatment of colonic strips with SP (1 μM) resulted in contractile responses that were sensitive to L-732,138, indicating the recruitment of NK1 receptors. Incubation of control strips with indomethacin (1 μM) elicited a significant decrease in SP-evoked contractions (Figure 3C). SC-560 (0.1 μM) or DFU (1 μM) evoked also inhibitory effects, although with lower efficacy than indomethacin (Figure 3C). SC-560 plus DFU decreased SP-induced contractions, as effectively as indomethacin (Figure 3C). In preparations from DD patients, indomethacin, DFU or SC-560 plus DFU caused similar effects in reducing contractions induced by SP. These inhibitory effects were more pronounced than those observed in control tissues (Figure 3D). In this setting, SC-560 alone did not exert significant effects (Figure 3D).
Molecular studies
RT-PCR
RT-PCR showed a basal expression of mRNA encoding COX-1 and COX-2 in control colonic tissues. In the presence of DD, the expression pattern of COX-1 mRNA did not vary, whereas COX-2 was significantly increased (Figure 4A).
Figure 4.
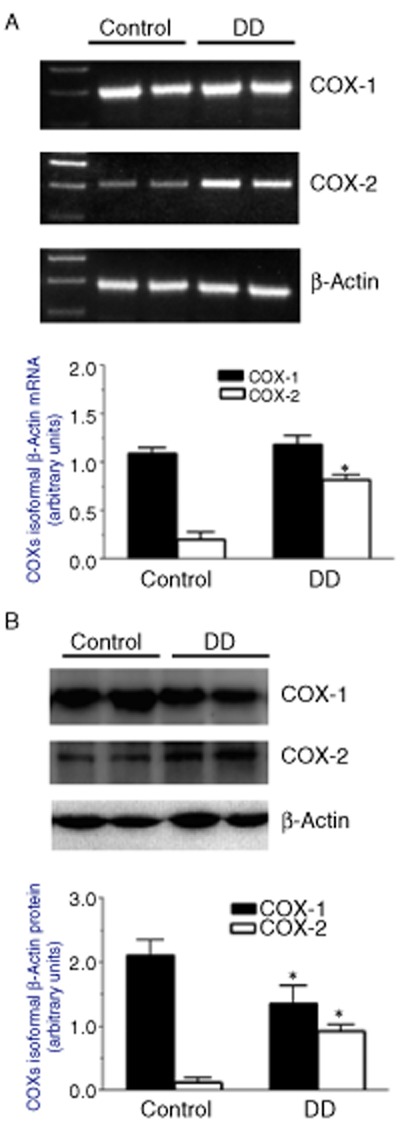
Expression of COX isoforms in colonic neuromuscular layer from control subjects or patients with DD. Representative agarose gel showing RT-PCR products (A, upper panel) for COX-1, COX-2 and β-actin expression level, and column graph displaying the densitometric analysis (A, lower panel) of COX isoform cDNA bands normalized to the expression of β-actin. Representative Western blots (B, upper panel) showing the expression of COX-1, COX-2 and β-actin in colonic neuromuscular tissues and the respective densitometric analysis (B, lower panel). Each column represents the mean ± SEM obtained from 5 experiments. *P < 0.05, significantly different from the corresponding control.
Western blot
COX-1 and COX-2 proteins were both expressed in control colonic tissues. Colonic specimens from patients with DD displayed a reduced COX-1 protein expression, whereas COX-2 protein was increased (Figure 4B).
Immunohistochemistry
SP immunoreactivity was detected in appreciable amounts in nerve endings and neurons of myenteric ganglia of control colon (Figure 5A). This pattern differed in specimens from DD patients, displaying increased levels of SP in myenteric ganglia (Figure 5B and G). The distribution patterns of COX-1 and COX-2 in control colonic specimens were as previously described (Fornai et al., 2005; Bernardini et al., 2006). In particular, COX-1 was detected mainly in HuC/D+ myenteric neurons and, to a lesser extent, in GFAP+ glial cells and some myocytes of longitudinal layer. Colonic samples from DD patients displayed a pronounced reduction of COX-1+ myenteric neurons (Figures 5C, D, G and 6). By contrast, the pattern of COX-2 immunostaining, as observed in GFAP+ glial cells and longitudinal smooth muscle of control samples, was enhanced in myenteric glia and smooth muscle cells in DD colon (Figures 5E–G and 7).
Figure 5.
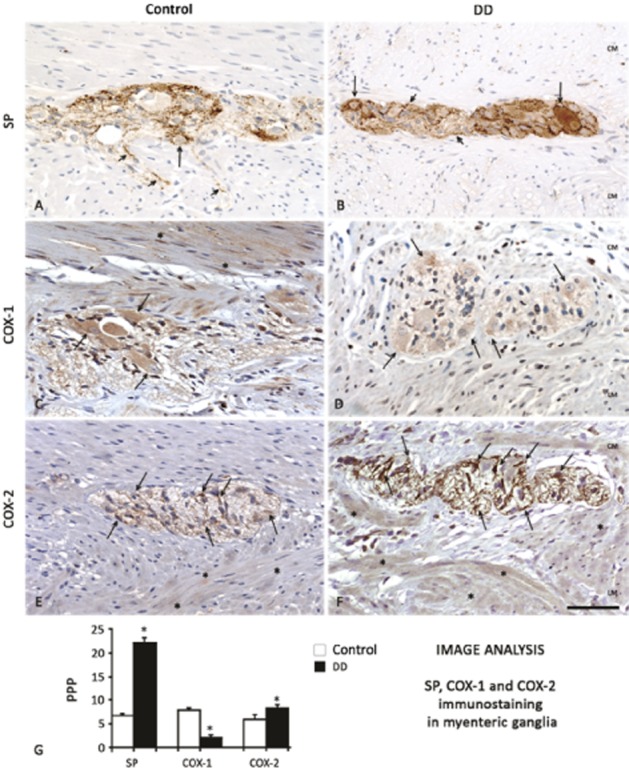
Immunoperoxidase analysis of substance P (SP), COX-1 and COX-2 expression in the neuromuscular layers of colon from control subjects or patients with DD. SP content, constitutively evident in both soma (arrows) and endings (short arrows) in the myenteric ganglia of control colon (A), was markedly increased in DD colon (B). The considerable amount of COX-1, expressed in the cytoplasm of myenteric neurons and smooth muscle cells (C, arrows and asterisks, respectively) in normal colon, was reduced in samples from DD patients (D). By contrast, COX-2 expression, present in small amounts in glia as well as longitudinal muscle of control colon (E, arrows and asterisks, respectively), was increased in glial and smooth muscle cells of DD colon (F, arrows and asterisks respectively). Bar: 50 μm. Panel (G): quantitative analysis of SP, COX-1 and COX-2 expression in myenteric ganglia of control or DD patients. Each column represents the mean of PPP ± SEM obtained from 6–8 experiments. *P < 0.05, significantly different from control.
Figure 6.
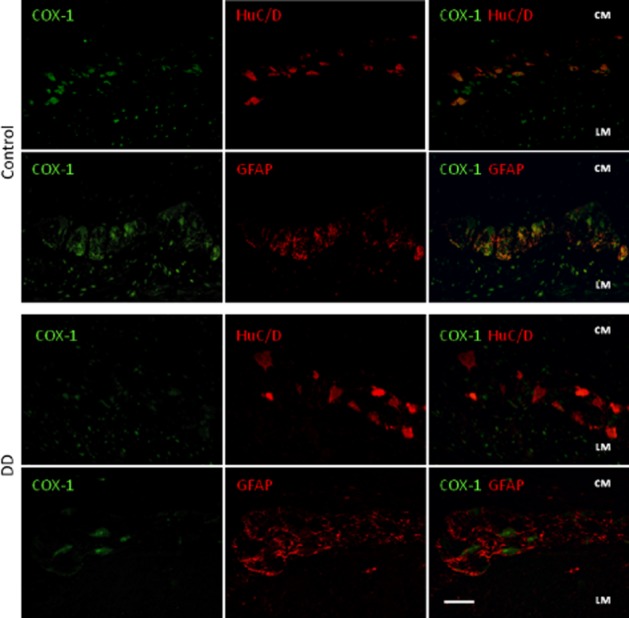
Double immunofluorescence of COX-1 (green)-HuC/D (red neurons) or -GFAP (red glial cells) in myenteric ganglia of colonic specimens from control subjects and DD patients. In control tissue, COX-1 is expressed in most of HuC/D+ neurons, in the nuclei of some myocytes of longitudinal layer (LM), and scarcely in GFAP+ glial cells. In samples of DD colons, COX-1 expression is reduced in HuC/D+ neurons, as demonstrated by merged COX-1-HuC/D images. Bar: 50 μm.
Figure 7.
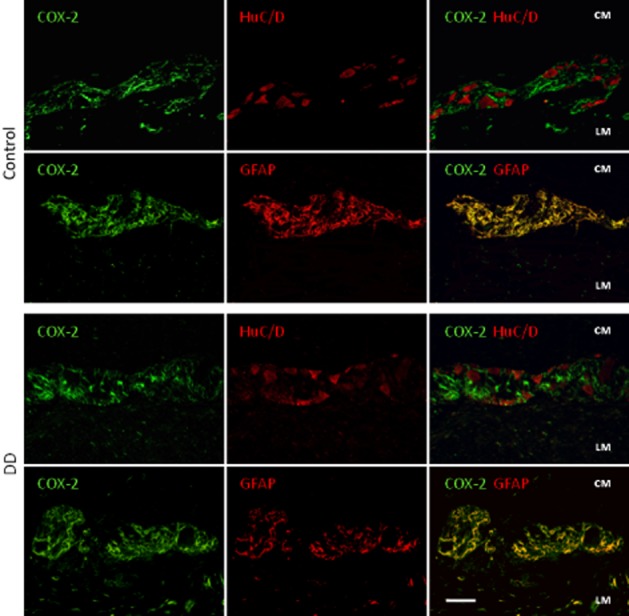
Double immunofluorescence of COX-2 (green)-HuC/D (red neurons) or -GFAP (red glial cells) in myenteric ganglia of colonic specimens from control subjects and DD patients. In control tissue, COX-2 is expressed in GFAP+ glia. In DD colonic samples COX-2-immunoreactivity is enhanced in GFAP+ glia, as demonstrated by merged COX-2-GFAP images. Bar: 50 μm.
Discussion and conclusions
Our study provides evidence that COX-2 plays a differential role in modulating human colonic excitatory cholinergic and tachykininergic motor functions under normal conditions and in the presence of DD. In addition, we have demonstrated, for the first time, that the expression patterns of COX isoforms undergo marked changes in colonic tissues from DD patients, who displayed decreased levels of neuronal COX-1 and an increased expression of muscular COX-2.
Prior to testing the effects of COX inhibitors on colonic motility, care was taken to examine the patterns of motor responses to activation of excitatory pathways in control and DD colon. Thus, frequency–response curves to electrical stimulation were constructed under cholinergic and tachykininergic conditions to evaluate the contractions elicited by myenteric nerves. In addition, concentration–response curves to carbachol or SP were obtained to assess contractions elicited by direct activation of muscarinic or NK1 receptors on smooth muscle cells. The results of these experiments showed that, in the presence of DD, the magnitude of both electrically and carbachol-induced cholinergic responses trended to increase, without reaching statistical significance, as compared with control tissues. These findings agree with previous observations showing that exogenous ACh elicited similar responses in colonic preparations from patients with DD or control subjects (Golder et al., 2003; Burcher et al., 2008). When considering tachykininergic contractions elicited by electrical stimulation or exogenous SP, such motor responses were significantly enhanced in the presence of DD. Consistent with these findings, immunohistochemistry revealed a significant increase in SP expression in the neuromuscular layer of DD colon, as compared with control tissues. In addition, Simpson et al. (2009) previously reported an increased SP expression in colonic muscular layers from DD patients. Taken together with published data, our findings support the view that the colonic longitudinal muscle of DD patients undergoes an enhanced excitatory tachykininergic control, whereas the excitatory cholinergic pathway remains virtually unaffected.
With regard to the changes in tachykininergic responses observed in DD tissues, it was previously observed that, in addition to NK1, NK2 receptors are also expressed in the colon of DD patients (Maselli et al., 2004). In addition, Burcher et al. (2008) showed that neurokinin A or synthetic agonists elicited contractile effects in normal human colon, and that such responses were reduced in colonic preparations from DD patients. Taken together with our findings, these results suggest that, in the presence of DD, the tachykininergic motor responses mediated by NK1 and NK2 receptors undergo differential changes, as NK1-mediated contractions are enhanced, whereas contractions resulting from NK2 receptor activation are reduced. In this respect, the exploration of putative modulatory actions exerted by COX isoforms on NK2-mediated colonic contractions in DD is of great interest, in view of future investigations.
When considering the effects of COX inhibitors on colonic longitudinal muscle, both COX-1 and COX-2 inhibition resulted in an enhancement of cholinergic motor activity under control conditions, whereas these effects were absent in the samples from DD patients. Thus, in normal colon, both COX-1 and COX-2 appear to exert an inhibitory control on cholinergic pathways, which does not occur with DD. As previously reported, COX isoforms work at distinct sites to exert their inhibitory actions on colonic neuromuscular activity under control conditions (Fornai et al., 2005). Indeed, as also shown by the present findings, COX-1 is likely to modulate excitatory cholinergic neurons, whereas COX-2 appears to operate at muscular level. In this context, an important point of novelty of our study is that, in tissues from DD patients, the regulatory actions of COX isoforms no longer occur, as the COX inhibitors did not affect electrically or carbachol-induced motor responses. The reasons underlying the loss of COX control on colonic cholinergic motility in DD patients remain undetermined. However, based upon previous observations, bowel inflammatory conditions are associated with neuromuscular remodelling and marked changes in motility (Neunlist et al., 2003). In line with this concept, our earlier work showed a loss of COX-1 inhibitory control on cholinergic motility during bowel inflammation (Fornai et al., 2006). Barbara et al. (2001) observed that infection by Trichinella spiralis in mice leads to a COX-2-dependent bowel hypercontractility, persisting after infection resolution. Moreover, persistent enteric alterations, following the acute phases of diverticulitis, have been documented, along with changes in colonic neurotransmitter contents (Böttner and Wedel, 2012). Overall, based upon previous knowledge, it is conceivable that colonic tissues from DD patients may undergo marked rearrangements, in terms of modulation by COX isoforms on enteric cholinergic pathways, as a consequence of the past episodes of diverticulitis.
In the present study, another interesting point of novelty came from experiments designed to explore the excitatory tachykininergic control of colonic smooth muscle and its modulation by COX pathways. We first observed that NK1 receptors mediated tachykininergic contractions evoked by either neurogenic tachykinins or exogenously applied SP. Under control conditions, these motor responses were blocked by COX inhibitors, with COX-1 blockade being more effective than COX-2 inhibition. These results, indicating that both COX isoforms exerted an excitatory control on tachykininergic motility in human colon, were consistent with our histomorphological data, showing that COX-1 was mainly expressed at neuronal level and to a lesser extent in smooth muscle cells, and that COX-2 immunopositivity was mainly distributed at glial and muscular level. The neuronal localization of SP suggests that COX-1 was predominantly invovled in the modulation of excitatory tachykininergic nerve pathways, whereas COX-2 was more likely to act at muscular level to modulate post-junctional responses mediated by tachykininergic receptors. However, when considering the effects of COX-1 blockade on myogenic tachykininergic motor responses, we observed that SC-560 blunted SP-induced contractions. Therefore, given the fact that this isoform is also expressed at a muscular level, although to a lower extent, an additional role of COX-1 in the modulation of tachykininergic motor activity at this site cannot be ruled out. In keeping with this view, Amann et al. (2001) previously showed that SP release from guinea pig lung was regulated by COX-1, because it was blunted by indomethacin or SC-560, to a similar extent. When experiments with COX inhibitors were performed on colonic preparations from DD patients, we observed that the excitatory control driven by COX-1 on tachykininergic contractions was no longer active, while that by COX-2 became predominant. In particular, the regulatory actions of COX-2 were evident not only at neural levels but also in longitudinal smooth muscle cells, as shown by the myogenic responses induced by exogenous SP. These conclusions are supported by our immunohistochemical and molecular analysis on colonic tissues from DD patients, which showed a decreased COX-1 expression at neuronal level, with a parallel increment of COX-2 expression in smooth muscle. Of note, Depoortere et al. (2003) have previously observed that, in a rabbit model of colitis, the effects of indomethacin on colonic contractions changed from an excitatory to an inhibitory pattern, indicating a significant rearrangement in the control by COX pathways in the presence of bowel inflammation. In addition, Neunlist et al. (2003) showed that, in patients with ulcerative colitis, there was a shift of colonic myenteric nerves from a predominance of cholinergic to an increased density of SP innervation, suggesting neuroplastic changes were associated with bowel inflammation.
The present observations are relevant clinically. Indeed, it has been repeatedly reported that patients with DD, both symptomatic and asymptomatic, display a pattern of increased colonic motility (Bassotti et al., 2001; 2003), and that in symptomatic subjects there is a significant association between episodes of abdominal pain and colonic motor abnormalities (Bassotti et al., 2005b). Thus, symptoms related to abnormal (hypercontractile) patterns of colonic motor functions in DD patients might depend, at least in part, upon the alterations highlighted in our study. Of course, other abnormalities, such as those related to colonic smooth muscle (Mattii et al., 2013) and mast cells within the colonic layers (Bassotti et al., 2013), are likely also to play pathophysiological roles, and it is possible that the summation of different abnormalities leads to the generation of dysmotility-related (Bassotti and Villanacci, 2012) and sensory (Clemens et al., 2004) abdominal symptoms often reported by patients with DD.
Overall, to the best of our knowledge, our findings provide the first demonstration of a rearranged modulatory control, mediated by COX isoforms, of human colonic excitatory cholinergic and tachykininergic pathways, in the presence of DD. In particular, our data support the concept that, in the presence of DD, both COX isoforms lose their inhibitory control of cholinergic neuromuscular transmission, whereas COX-2 maintains an enhanced excitatory modulation of tachykininergic pathways. Taken together, these findings suggest that, in patients with DD, abdominal symptoms associated with hypercontractility might result, at least in part, from an altered modulation of COX pathways on excitatory motor activity.
In conclusion, the evidence that cholinergic and tachykininergic pathways are both involved in the regulation of excitatory colonic motor activity could be of clinical interest for the development of novel pharmacological compounds acting as anti-spasmodic agents. Moreover, pharmacological blockade of COX-2 is also likely to reduce bowel hypercontractility associated with DD due to the facilitatory effects of this enzyme on excitatory tachykininergic pathways. However, the use of COX inhibitors may increase the risk of diverticular bleeding and complications (Strate et al., 2011), even if there is no current evidence that selective COX-2 inhibitors could induce complications in patients with DD. In addition, our findings on the modulation of excitatory motility by COX isoforms in DD colon might pave the way to future therapeutic approaches based upon the pharmacological modulation of enzymes and/or receptor pathways downstream of COX isoforms, for instance, ligands for prostanoid receptors. Such approaches might be beneficial in resolving dysmotility in DD colon while sparing mucosal integrity and avoiding or lowering the risk of bleeding. Therefore, although it is possible that the use of COX inhibitors for the management of colonic motor disorders associated with DD could have detrimental side-effects, this issue remains controversial and deserves additional investigations.
Glossary
- DD
diverticular disease
- PPP
percent positive pixels
- SP
substance P
Author contributions
M.F., C.B. and N.B. study design; L.A., R.C., G.B., C.I. and C.S. acquisition of data; C.B. and R.C. analysis and interpretation of data; M.F. and R.C. drafting of the manuscript; C.B., N.B. and G.B. critical revision of the manuscript; R.C. statistical analysis; P.B., A.M., M.C. and V.V. technical and material support; C.B., N.B. and G.B. study supervision.
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12733
Figure S1 Results of preliminary dose-ranging experiments. Effects of indomethacin, SC-560 or DFU (0.01–10 μM) on contractile responses induced by 10 s single trains of transmural electrical stimulation (0.5 ms, 30 mA, 10 Hz) in colonic preparations obtained from control subjects or DD patients. Tissues were maintained in standard Krebs solution. In control tissues, indomethacin concentration-dependently enhanced electrically evoked contractions, with a maximal effect at 1 μM. SC-560 and DFU evoked also enhancing actions, with maximal effects at 0.1 and 1 μM respectively. In DD tissues, both indomethacin and DFU blunted the electrically evoked contractions, with maximal effects at 1 μM. By contrast, SC-560 did not affect the contractile responses to electrical stimulation at all tested concentrations. Each column represents the mean ± SEM obtained from 5–6 experiments. *P < 0.05, significantly different from the corresponding contractions observed in the absence of test drugs.
Table S1 Summary of the characteristics of patients.
Table S2 Antibodies employed in the present study.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R, Schuligoi R, Peskar BA. Effects of COX-1 and COX-2 inhibitors on eicosanoid biosynthesis and the release of substance P from the guinea-pig isolated perfused lung. Inflamm Res. 2001;50:50–53. doi: 10.1007/s000110050724. [DOI] [PubMed] [Google Scholar]
- Barbara G, De Giorgio R, Deng Y, Vallance B, Blennerhassett P, Collins SM. Role of immunologic factors and cyclooxygenase 2 in persistent postinfective enteric muscle dysfunction in mice. Gastroenterology. 2001;120:1729–1736. doi: 10.1053/gast.2001.24847. [DOI] [PubMed] [Google Scholar]
- Bassotti G, Villanacci V. Colonic diverticular disease: abnormalities of neuromuscular function. Dig Dis. 2012;30:24–28. doi: 10.1159/000335702. [DOI] [PubMed] [Google Scholar]
- Bassotti G, Battaglia E, Spinozzi F, Pelli MA, Tonini M. Twenty-four hour recordings of colonic motility in patients with diverticular disease: evidence for abnormal motility and propulsive activity. Dis Colon Rectum. 2001;44:1814–1820. doi: 10.1007/BF02234460. [DOI] [PubMed] [Google Scholar]
- Bassotti G, Chistolini F, Morelli A. Pathophysiological aspects of diverticular disease of colon and role of large bowel motility. World J Gastroenterol. 2003;9:2140–2142. doi: 10.3748/wjg.v9.i10.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassotti G, Battaglia E, Bellone G, Dughera L, Fisogni S, Zambelli C, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol. 2005a;58:973–977. doi: 10.1136/jcp.2005.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassotti G, Battaglia E, De Roberto G, Morelli A, Tonini M, Villanacci V. Alterations in colonic motility and relationship to pain in colonic diverticulosis. Clin Gastroenterol Hepatol. 2005b;3:248–253. doi: 10.1016/s1542-3565(04)00614-7. [DOI] [PubMed] [Google Scholar]
- Bassotti G, Villanacci V, Nascimbeni R, Antonelli E, Cadei M, Manenti S, et al. The role of colonic mast cells and myenteric plexitis in patients with diverticular disease. Int J Colorectal Dis. 2013;28:267–272. doi: 10.1007/s00384-012-1554-z. [DOI] [PubMed] [Google Scholar]
- Bernardini N, Colucci R, Mattii L, Segnani C, Fornai M, De Giorgio R, et al. Constitutive expression of cyclooxygenase-2 in the neuromuscular compartment of normal human colon. Neurogastroenterol Motil. 2006;18:654–662. doi: 10.1111/j.1365-2982.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- Bernardini N, Segnani C, Ippolito C, De Giorgio R, Colucci R, Faussone-Pellegrini MS, et al. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J Cell Mol Med. 2012;16:318–327. doi: 10.1111/j.1582-4934.2011.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttner M, Wedel T. Abnormalities of neuromuscular anatomy in diverticular disease. Dig Dis. 2012;30:19–23. doi: 10.1159/000335699. [DOI] [PubMed] [Google Scholar]
- Burcher E, Shang F, Warner FJ, Du Q, Lubowski DZ, King DW, et al. Tachykinin NK2 receptor and functional mechanisms in human colon: changes with indomethacin and in diverticular disease and ulcerative colitis. J Pharmacol Exp Ther. 2008;324:170–178. doi: 10.1124/jpet.107.130385. [DOI] [PubMed] [Google Scholar]
- Clemens CH, Samsom M, Roelofs J, van Berge Henegouwen GP, Smout AJ. Colorectal visceral perception in diverticular disease. Gut. 2004;53:717–722. doi: 10.1136/gut.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costedio MM, Coates MD, Danielson AB, Buttolph TR, 3rd, Blaszyk HJ, Mawe GM, et al. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–1445. doi: 10.1007/s11605-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortere I, Thijs T, Thielemans L, Peeters TL. Mechanisms involved in the loss of excitatory post-stimulus responses by inflammation. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:245–252. doi: 10.1007/s00210-003-0696-5. [DOI] [PubMed] [Google Scholar]
- Fornai M, Blandizzi C, Colucci R, Antonioli L, Bernardini N, Segnani C, et al. Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut. 2005;54:608–616. doi: 10.1136/gut.2004.053322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai M, Blandizzi C, Antonioli L, Colucci R, Bernardini N, Segnani C, et al. Differential role of cyclooxygenase 1 and 2 isoforms in the modulation of colonic neuromuscular function in experimental inflammation. J Pharmacol Exp Ther. 2006;317:938–945. doi: 10.1124/jpet.105.098350. [DOI] [PubMed] [Google Scholar]
- Fornai M, Antonioli L, Colucci R, Ghisu N, Buccianti P, Marioni A, et al. A1 and A2a receptors mediate inhibitory effects of adenosine on the motor activity of human colon. Neurogastroenterol Motil. 2009;21:451–466. doi: 10.1111/j.1365-2982.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- Fornai M, Antonioli L, Colucci R, Bernardini N, Ghisu N, Tuccori M, et al. Emerging role of cyclooxygenase isoforms in the control of gastrointestinal neuromuscular functions. Pharmacol Ther. 2010;125:62–78. doi: 10.1016/j.pharmthera.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Gierse JK, Zhang Y, Hood WF, Walker MC, Trigg JS, Maziasz TJ, et al. Valdecoxib: assessment of cyclooxygenase-2 potency and selectivity. J Pharmacol Exp Ther. 2005;312:1206–1212. doi: 10.1124/jpet.104.076877. [DOI] [PubMed] [Google Scholar]
- Golder M, Burleigh DE, Belai A, Ghali L, Ashby D, Lunniss PJ, et al. Smooth muscle cholinergic denervation hypersensitivity in diverticular disease. Lancet. 2003;361:1945–1951. doi: 10.1016/S0140-6736(03)13583-0. [DOI] [PubMed] [Google Scholar]
- Guagnini F, Valenti M, Mukenge S, Matias I, Bianchetti A, Di Palo S, et al. Neural contractions in colonic strips from patients with diverticular disease: role of endocannabinoids and substance P. Gut. 2006;55:946–953. doi: 10.1136/gut.2005.076372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming J, Floch M. Features and management of colonic diverticular disease. Curr Gastroenterol Rep. 2010;12:399–407. doi: 10.1007/s11894-010-0126-z. [DOI] [PubMed] [Google Scholar]
- Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippolito C, Segnani C, De Giorgio R, Blandizzi C, Mattii L, Castagna M, et al. Quantitative evaluation of myenteric ganglion cells in normal human left colon: implications for histopathological analysis. Cell Tissue Res. 2009;336:191–201. doi: 10.1007/s00441-009-0770-5. [DOI] [PubMed] [Google Scholar]
- Jeyarajah S, Papagrigoriadis S. Review article: the pathogenesis of diverticular disease – current perspectives on motility and neurotransmitters. Aliment Pharmacol Ther. 2011;33:789–800. doi: 10.1111/j.1365-2036.2011.04586.x. [DOI] [PubMed] [Google Scholar]
- Maselli MA, Piepoli AL, Guerra V, Caruso ML, Pezzolla F, Lorusso D, et al. Colonic smooth muscle responses in patients with diverticular disease of the colon: effect of the NK2 receptor antagonist SR48968. Dig Liver Dis. 2004;36:348–354. doi: 10.1016/j.dld.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141–146. doi: 10.1055/s-0029-1236157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattii L, Ippolito C, Segnani C, Battolla B, Colucci R, Dolfi A, et al. Altered expression pattern of molecular factors involved in colonic smooth muscle functions: an immunohistochemical study in patients with diverticular disease. PLoS ONE. 2013;8:e57023. doi: 10.1371/journal.pone.0057023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84–90. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Sundler F, Humes DJ, Jenkins D, Scholefield JH, Spiller RC. Post inflammatory damage to the enteric nervous system in diverticular disease and its relationship to symptoms. Neurogastroenterol Motil. 2009;21:847–858. doi: 10.1111/j.1365-2982.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140:1427–1433. doi: 10.1053/j.gastro.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita R, Fujisaki S, Tanjoh K, Fukuzawa M. Role of nitric oxide in the left-sided colon of patients with diverticular disease. Hepatogastroenterology. 2000;47:692–696. [PubMed] [Google Scholar]
- Weizman AV, Nguyen GC. Diverticular disease: epidemiology and management. Can J Gastroenterol. 2011;25:385–389. doi: 10.1155/2011/795241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Results of preliminary dose-ranging experiments. Effects of indomethacin, SC-560 or DFU (0.01–10 μM) on contractile responses induced by 10 s single trains of transmural electrical stimulation (0.5 ms, 30 mA, 10 Hz) in colonic preparations obtained from control subjects or DD patients. Tissues were maintained in standard Krebs solution. In control tissues, indomethacin concentration-dependently enhanced electrically evoked contractions, with a maximal effect at 1 μM. SC-560 and DFU evoked also enhancing actions, with maximal effects at 0.1 and 1 μM respectively. In DD tissues, both indomethacin and DFU blunted the electrically evoked contractions, with maximal effects at 1 μM. By contrast, SC-560 did not affect the contractile responses to electrical stimulation at all tested concentrations. Each column represents the mean ± SEM obtained from 5–6 experiments. *P < 0.05, significantly different from the corresponding contractions observed in the absence of test drugs.
Table S1 Summary of the characteristics of patients.
Table S2 Antibodies employed in the present study.


