Abstract
Myogenic precursors in adult skeletal muscle (satellite cells) are mitotically quiescent but can proliferate in response to a variety of stresses including muscle injury. To gain further understanding of adult myoblasts, we analyzed myogenesis of satellite cells on intact fibers isolated from adult rat muscle. In this culture model, satellite cells are maintained in their in situ position underneath the fiber basement membrane. In the present study patterns of satellite cell proliferation, expression of myogenic regulatory factor proteins, and expression of differentiation-specific, cytoskeletal proteins were determined, via immunohistochemistry of cultured fibers. The temporal appearance and the numbers of cells positive for proliferating cell nuclear antigen (PCNA) or for MyoD were similar, suggesting that MyoD is present in detectable amounts in proliferating but not quiescent satellite cells. Satellite cells positive for myogenin, α-smooth muscle actin (αSMactin), or developmental sarcomeric myosin (DEVmyosin) appeared following the decline in PCNA and MyoD expression. However, expression of myogenin and αSMactin was transient, while DEVmyosin expression was continuously maintained. Moreover, the number of DEVmyosin+ cells was only half of the number of myogenin+ or αSMactin+ cells—indicating, perhaps, that only 50% of the satellite cell descendants entered the phase of terminal differentiation. We further determined that the number of proliferating satellite cells can be modulated by basic FGF but the overall schedule of cell cycle entry, proliferation, differentiation, and temporal expression of regulatory and structural proteins was unaffected. We thus conclude that satellite cells conform to a highly coordinated program when undergoing myogenesis at their native position along the muscle fiber.
INTRODUCTION
Satellite cells are situated between the basement membrane and the plasma membrane of the myofiber and are thought to be the primary source of myogenic cells in postnatal and adult skeletal muscle (Mauro, 1961, 1979; Bischoff, 1974; reviewed in Campion, 1984; Schultz, 1989; Grounds and Yablonka-Reuveni, 1993). Individual myofibers become encased by a basement membrane during late embryogenesis and it is at that stage that the distinction of satellite cells by their morphology and location is first possible (reviewed in Grounds and Yablonka-Reuveni, 1993). In vivo studies analyzing the incorporation of [3H]thymidine into satellite cell nuclei and, subsequently, into fiber nuclei, indicated that at least some of the satellite cells are proliferative in young animals, contributing additional nuclei to the fibers (Moss and Leblond, 1971). In the adult, satellite cells are mitotically quiescent but can reinitiate proliferative activity in response to injury, and their progeny eventually fuse into preexisting or new myofibers (Snow, 1977; Schultz et al., 1978, 1985; Carlson and Faulkner, 1983). Muscle trauma involving fiber death and wound-related processes is not the only condition that leads to satellite cell proliferation; activation of these precursors also occurs in response to stresses such as stretch and compensatory hypertrophy, exercise, and denervation (see summary in Bischoff, 1989; Antonio and Gonyea, 1993; Snow, 1981, 1990).
Myogenic cultures of cells isolated from normal juvenile or adult postnatal muscle have been commonly presumed to be cultures of satellite cells (Bischoff, 1974; Yablonka-Reuveni et al., 1987; Hartley et al., 1992; Johnson and Allen, 1993). In such in vitro models the myogenic precursors become mitotically active upon culturing and their progeny eventually differentiate and fuse into multinucleated fibers. A number of growth factors including fibroblast growth factor, insulin-like growth factor, transforming growth factor-β, and platelet-derived growth factor have been shown to be involved in the proliferation and differentiation of satellite cell progeny in such cultures (Clegg et al., 1987; Allen and Boxhorn, 1989; Yablonka-Reuveni et al., 1990; Yablonka-Reuveni and Seifert, 1993; reviewed in Grounds and Yablonka-Reuveni, 1993). Studies of cultured satellite cells from mammalian and avian species revealed many unique features of these cells compared to fetal myoblasts and have led to the proposal that satellite cells represent a subset of myoblasts which become dominant during late embryogenesis (reviewed in Cossu and Molinaro, 1987; Hartley and Yablonka-Reuveni, 1992; Stockdale, 1992; Yablonka-Reuveni, 1994). The cell culture studies contributed immensely to our current understanding of the regulation of satellite cells, but the association between the satellite cells and their neighboring fibers is not maintained in such cultures. Interactions between the satellite cells and the myofibers could be important for maintaining the satellite cell in a quiescent or proliferative state (Bischoff, 1990a,b).
We have adopted the rat single fiber system pioneered by Bekoff and Betz (1977) and further developed by Bischoff (1986, 1989) in order to more critically evaluate myogenesis of adult satellite cells in their native position without the complexity of the intact tissue. In this model the fibers are isolated with their basement membrane, retaining their few satellite cells in the original site underneath the basement membrane (Bischoff, 1989). These satellite cells can be induced to proliferate as demonstrated by incorporation of [3H]thymidine and autoradiography (Bischoff, 1986, 1990b). Progeny of the activated satellite cells may fuse with each other to form new myotubes underneath the existing basement membrane but they do not fuse with their associated fibers as long as the fibers are intact (Bischoff, 1986, 1990a). The studies of Bischoff focused primarily on the proliferative state of satellite cells, distinguishing between the proliferating cells along the fiber and the nonproliferating nuclei of the myofiber itself via authoradiography following the administration of [3H]thymidine. We were interested in identifying simpler means for quantitative visualization of both proliferating and differentiating satellite cells on the fiber. This alternative approach would then be used to determine whether myogenesis of satellite cells in their native position conforms to the regulatory program predicted from cell culture studies.
This paper reports on the utilization of indirect immunofluorescence to trace myogenesis of satellite cells in the isolated fiber model and the use of this approach to analyze the role of growth factors in adult myogenesis. To distinguish proliferating cells from the rest of the myofiber nuclei we used an antibody against proliferating cell nuclear antigen (PCNA). PCNA is an auxiliary protein to DNA polymerase δ whose levels correlate with DNA synthesis during the cell cycle, becoming maximal during the S phase (Bravo et al., 1987, reviewed in Baserga, 1991). We further reasoned that upon activation, satellite cells may undergo a primordial program of myogenesis and thus may express specific muscle characteristics which are not expressed by the neighboring fiber; perhaps this expression would allow a distinction between the satellite cell and the fiber. With this in mind we analyzed the expression of the myogenic regulatory factor proteins MyoD (Davis et al., 1987; Tapscott et al., 1988) and myogenin (Wright et al., 1989; Edmondson and Olson, 1989)—members of the basic helix-loop-helix family of myogenic transcription factors. The four known members of this family are thought to be important in myogenic determination and in the progression from proliferation to differentiation during myogenesis (Rudnicki et al., 1993; reviewed in Olson, 1992, 1993; Sassoon, 1993; Weintraub, 1993; Emerson, 1993). MyoD and myogenin are not expressed by satellite cells in normal uninjured adult muscle (Grounds et al., 1992; Füchtbauer and Westphal, 1992). In the present fiber study, we also analyzed satellite cells for the expression of cytoskeletal or sarcomeric proteins which are associated with myogenic differentiation during development but are not expressed in the adult. These proteins included α-smooth muscle actin (αSMactin), which was shown to be transiently expressed in developing mouse and rat muscle (Woodcock-Mitchell et al., 1988; Babai et al., 1990), and developmental sarcomeric myosin heavy chain(s) (DEVmyosin), which has been detected in developing but not in adult muscle (Marini et al., 1991).
Results presented here suggest that myogenesis of satellite cells maintained in their native position follows a program of highly synchronized, sequential expression of myogenic regulatory factor proteins and cytoskeletal/sarcomeric proteins. This program persists even when cell proliferation is enhanced in the presence of basic fibroblast growth factor (bFGF).
METHODS
Animals
Adult rats (males, 2.5 to 3 months old, 250–280 g, Sprague-Dawley) were used throughout the study.
Isolation and Culture of Rat Muscle Fibers
Single muscle fibers with associated satellite cells were prepared from the flexor digitorum brevis (FDB) muscle of the hind foot of adult rats according to Bischoff (1986, 1989) with slight modifications. Muscles from both hind feet of one rat were used for each preparation. The outer connective tissue was removed and the muscles were immersed in a 5-ml solution of 0.2% collagenase (type 1, lot 10H-0466 from Sigma, St. Louis, MO; resuspended in Eagle’s minimal essential medium (MEM)). Digestion was for 2 hr at 37°C without agitation. The collagenase-treated muscle was transferred into 3 ml of MEM containing 10% horse serum, teased, and further triturated with a wide-mouth Pasteur pipet in a way similar to that described by Bischoff (1986, 1989). Fibers were then allowed to settle at 1g for 15 min at room temperature through 10 ml of MEM containing 10% horse serum in 15-ml Sorvall polycarbonate tubes. Fiber precipitation was repeated a total of three times to free the fibers from debris and connective tissue cells liberated by the digestion and teasing. Final fiber sediment, in about 0.6 to 0.7 ml of residual medium, was aliquoted into twelve to fourteen 35-mm tissue culture plates coated with 0.1 ml of isotonic Vitrogen solution which was made isotonic (pH 7.0) by the addition of 1 vol of 7× DMEM to 6 vol of stock Vitrogen 100 (Celtrix Laboratories, Palo Alto, CA). About 50 μl of fiber suspension, dispensed using a trimmed, wide mouth micropipet tip to prevent fiber breakage, was added to the center of the plates immediately following Vitrogen coating. Plates were gently swirled to allow even spreading of fiber aliquots throughout the plates and incubated for 20 min at 37°C to allow formation of Vitrogen gel and adherence of fibers to the matrix. Cultures then received 1.0 ml of basal medium (MEM containing 20% controlled process serum replacement (CPSR2, Sigma) and 1% horse serum (Sigma; preselected for supporting clonal growth of chicken myoblasts; for details see Yablonka-Reuveni and Seifert, 1993). For routine cultures, medium was changed every 2–3 days, but when the effect of bFGF and/or of bFGF blocking antibody was studied, the medium and additives were replenished daily to allow adequate supply of the reagents. Human recombinant, yeast-produced bFGF was kindly provided by Dr. S. Hauschka (Department of Biochemistry, University of Washington, Seattle, WA) and was added to the medium of the fiber cultures at 2 ng/ml. Higher bFGF concentrations in the range of 5 to 10 ng/ml had mitogenic effects on satellite cells in the intact fiber system identical to those obtained with 2 ng/ml FGF (data not shown). Activity blocking antibody against bFGF (IgG fraction, made in rabbit immunized with human recombinant bFGF and cross-reacted with rat bFGF, developed by Lindner and Reidy, 1991) as well as control rabbit IgG fraction were kindly provided by Drs. V. Lindner and M. Reidy (Department of Pathology, University of Washington). These anti-bFGF and control rabbit IgG were added to the fiber cultures at 100 μg/ml.
Antibody Staining and Counting of Positive Cells on Isolated Fibers
Single and double immunolabeling of fiber cultures was performed using indirect immunofluorescence. Cultures were rinsed with MEM at room temperature, fixed for 10 min at 4°C with ice-cold, 100% methanol, and air-dried at room temperature for 10 min. Cultures were then kept at 4°C, in sterile Tris-buffered saline containing normal goat serum (TBS-NGS: 0.05 M Tris, 0.15 M NaCl, 1% normal goat serum, pH 7.4) to block nonspecific antibody binding. Following a minimum of 24 hr in TBS-NGS, fiber cultures were rinsed (3×) with Tris-buffered saline containing Tween 20 (TBS-T20; 0.05 M Tris, 0.15 M NaCl, 0.05% Tween 20, pH 7.4). For reaction with single antibodies, cultures were then incubated with the primary antibody (listed below) for 1 hr at room temperature and the incubation was continued overnight at 4°C. Cultures were then rinsed (3×) with TBS-T20 and incubated at room temperature for 1–2 hr with fluorescein-conjugated secondary antibodies diluted with the blocking buffer TBS-NGS. FITC-conjugated rabbit anti-mouse IgG diluted 1:100 (obtained from Organon-Technika Cappel, Downington, PA) was used for all monoclonal primary antibodies; Fluorescein-conjugated donkey anti-rabbit IgG diluted 1:75 (obtained from Jackson ImmunoResearch Laboratories, West Grove, PA) was used for the one polyclonal primary antibody utilized in the study (i.e., anti-MyoD). Secondary antibodies were selected based on minimum background with the fiber cytoplasm. Cultures were rinsed again with TBS-T20 and mounted in VECTAS-HIELD mounting medium from Vector Laboratories (Burlingame, CA).
Double immunofluorescence was conducted to verify interpretations of single labeling experiments (see Results). In such instances the cultures were reacted first with the appropriate monoclonal antibody followed by a reaction with the secondary antibody as discussed above, except that the secondary antibody was a rhodamine-conjugated goat anti-mouse IgG (Organon-Technika, Cappel). Cultures were then reacted overnight with both the monoclonal antibody and a rabbit polyclonal antibody (anti-MyoD, see description below) followed by reaction with both the rhodamine-conjugated goat anti-mouse IgG and the fluorescein-conjugated donkey anti-rabbit IgG. Routine use of rhodamine-conjugated secondary antibodies for single or double staining of fiber cultures was avoided; fibers demonstrated high autofluorescence using the rhodamine filter system, leading to tedious data collection and eye strain. The two cycles of reaction with the monoclonal antibody and the rhodamine-conjugated secondary antibody enhanced the specific staining while the background fluorescence demonstrated by the fiber itself remained at the same level as that following one cycle only.
Nuclei of fiber cultures were counterstained with 4,6-diamidino-2-phenylindole (DAPI, 1 μg/ml) and visualized with Hoescht filters as previously described (Hartley et al., 1991). Observations were made with a Zeiss photomicroscope equipped for epifluorescence, and Kodak EL 135 film (400 ASA) was used for photography. For each time point of an individual experiment, a minimum of 30 fibers in at least two or three parallel 35-mm plates were used to count fiber-associated nuclei (or cells) positive for the different antibodies. Experiments were repeated several times. Counting of fiber cultures was done using a 25X objective. Positive cells were scored as the number of positives on each individual fiber. These numbers were then averaged per 10 fibers and used in a format shown in Fig. 4. Alternatively, results with individual fibers were analyzed as shown in Fig. 5.
Primary Antibodies
The following primary antibodies diluted in TBS-NGS were used to study fiber cultures:
Two different mouse monoclonal antibodies against PCNA were utilized. In the first step of the investigation we used an anti-PCNA (mAb 19A2) from Coulter Corporation (Hialea, FL). In later stages we used an anti-PCNA (mAb 19F4) from Boehringer Mannheim (Indianapolis, IN). This antibody was shown to immunostain nuclei of proliferating cells in primary cultures of rat satellite cells (Johnson and Allen, 1993; Yablonka-Reuveni, unpublished) and to detect a 34-kDa protein in extracts of such rat cultures (Johnson and Allen, 1993).
A mouse monoclonal antibody against rodent myogenin (mAb F5D, hybridoma supernatant form) which was developed and provided by Dr. W. Wright, University of Texas. This antibody is described in Cusella-De Angelis et al. (1992). In mass culture of adult rat satellite cells, this antibody recognizes the nuclei of single cells and myotubes (see, for example, Fig. 3).
A mouse monoclonal antibody against αSMactin which was originally developed by Skalli et al. (1986) and was purchased from Sigma (mAb 1A4). The antibody is specific for this actin isoform in many species including rodents. Fibers in cross sections of FDB muscle from either 3-week-old or 2- to 3-month-old rat are negative for αSMactin; blood vessels in these sections, as expected, are positive for the antigen (Yablonka-Reuveni, unpublished). In mass cultures of adult rat satellite cells, the antibody recognizes cytoplasm of some mononucleated cells and myotubes (see, for example, Fig. 3).
A mouse monoclonal antibody against developmental but not adult isoforms of sarcomeric myosin heavy chain (DEVmyosin) in various species including rodents (mAb F1584C10). The antibody was originally prepared against fetal bovine myosin and was shown to recognize myosin heavy chain in embryonic and fetal but not adult human muscle (Marini et al., 1991). Antibody was kindly provided by Dr. J. J. Leger and F. Pons (Faculty of Pharmacy, INSERM, Montpellier, France). Using this antibody we demonstrated that about 50% of the fibers in FDB muscle of 3-week-old rats are positive, but fibers positive for this antibody are rare in FDB muscle from 2- to 3-month-old rats. In mass cultures of adult rat satellite cells, this antibody recognizes the cytoplasm of some mononucleated cells and myotubes.
A rabbit polyclonal antibody against rodent MyoD was prepared and provided by Dr. S. Alemà (Institute of Cell Biology, CNR, Rome, Italy). To prepare this antibody, a complete mouse MyoD protein was expressed in Escherichia coli as a fusion product with 210 amino acids of the phage protein T10. The protein used to immunize rabbits was further isolated by gel electroelution. The antibody immunoprecipitates a protein of 45 kDa, immunostains nuclei of mouse cells expressing MyoD mRNA (for example C2 cells), and supershifts the MyoD band in gel retardation assays of C2 nuclear extracts. Patterns obtained with this antibody in the different assays are similar to those obtained with a polyclonal antibody against MyoD of Tapscott et al. (1988) and with a monoclonal antibody against MyoD of Dias et al. (1992) (S. Alemà, personal communication; also our laboratory regarding immunostaining). In mass cultures of adult rat satellite cells the antibody recognizes nuclei of single cells; only infrequent myotubes are positive for the antibody.
RESULTS
Morphology of Fiber Cultures and Stainability with Antibodies
When fibers were cultured in the basal medium (MEM containing 20% CPSR2 and 1% horse serum) they remained intact for at least 8 days, exhibiting cross-striations throughout culture history. Individual cells were rarely observed outside of the fibers and their growth was drastically suppressed in the basal medium (see Figs. 1 and 2 for fibers cultured for 2 to 5 days; fibers cultured for 8 days look similar). In contrast, fibers maintained in MEM supplemented with 10% horse serum or 10% horse serum/5% chicken embryo extract lost their normal morphology after several days in culture, exhibited vesicles to various degrees, and ultimately degenerated. Furthermore, the latter two horse serum-rich media supported extensive growth of single cells outside of the fibers, and with additional time these fiber cultures came to resemble a standard myogenic primary culture with mononucleated cells and myotubes, with few remnant original fibers (see Fig. 3). New myotubes joining the ends of the isolated fibers could be detected in the two kinds of media containing 10% horse serum media, but no formation of such myotubes (or sprouting) at the ends of original fibers, discussed by Bischoff (1986, 1990), was observed in the basal medium.
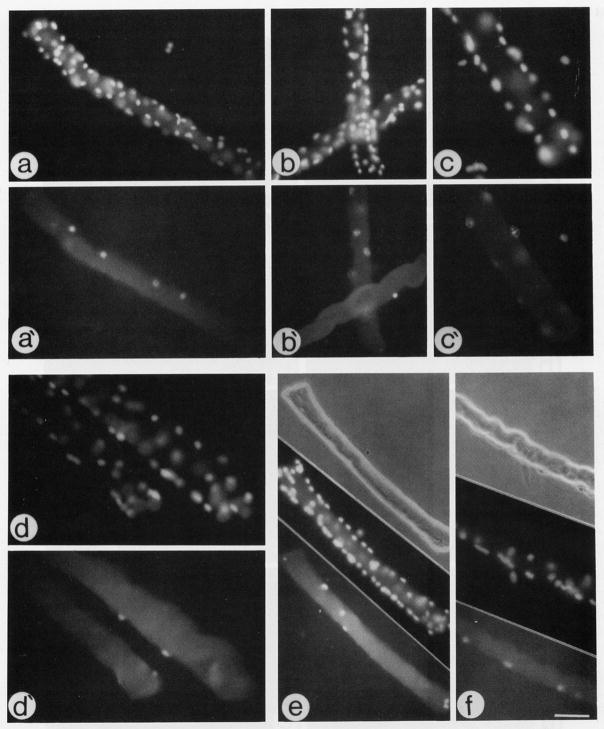
Fig. 1.
Micrographs of fiber cultures maintained in basal medium and reacted via indirect immunofluorescence with an antibody against PCNA (a′–c′), an antibody against MyoD (d′), and antibody against myogenin (e, f). (a–d) Fluorescent micrographs of the same fibers in a′–d′, respectively, stained with DAPI to highlight all nuclei in the micrographic field. (e and f) Three parallel micrographs demonstrating a phase micrograph of an individual fibers (top), a micrograph of the fiber nuclei stained with DAPI (middle), and a micrograph of the fiber reacted with an antibody against myogenin (bottom). Fibers reacted with anti-PCNA or anti-MyoD were maintained in culture for 2 days; fibers reacted with anti-myogenin were maintained in culture for 3 days. Bar, 68 μm for a, a′, b, b′, and e; 43 μm for c, c′, d, d′, and f. Please note that not all positive nuclei or cells on the fibers are in the same focal plane; therefore, not all positive nuclei or cells are in focus in the different micrographs.
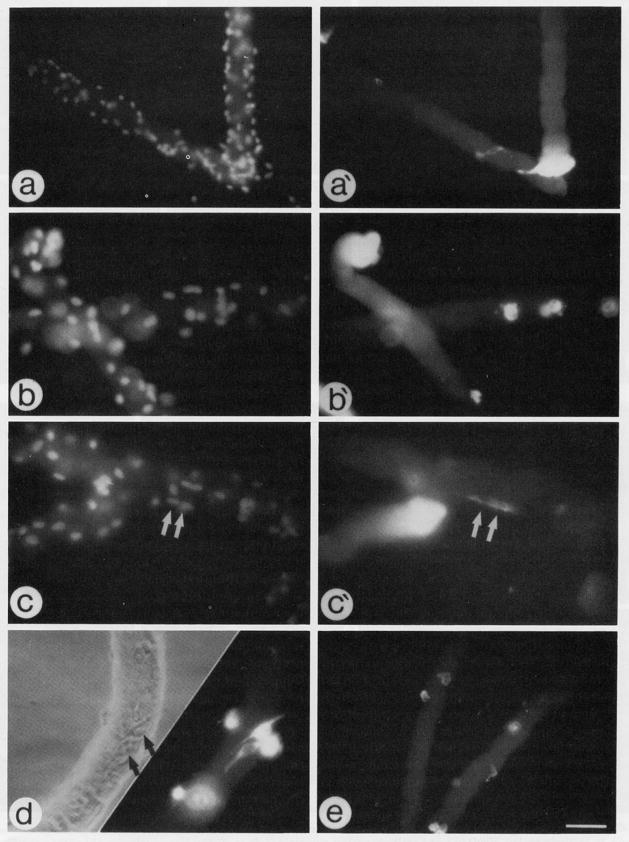
Fig. 2.
Micrographs of fiber cultures maintained in basal medium and reacted via indirect immunofluorescence with an antibody against DEVmyosin (a′–c′, d) and an antibody against αSMactin (e). (a–c) Fluorescent micrographs of the same fibers in a′–c′, respectively, stained with DAPI to highlight all nuclei in the micrographic field; arrows in c and c′ indicate two fused myosin-positive cells. (d) Parallel phase and fluorescent (anti-myosin stain) micrographs; arrows in d point out the position of the two myosin-positive cells. Fibers reacted with anti-myosin or anti-actin antibodies were maintained in culture for 5 or 3 days, respectively. Bar, 68 μm for a, a′, and e; 43 μm for panels b, b′, c, c′, and d. Please note that not all positive nuclei or cells on the fibers are in the same focal plane; therefore, not all positive nuclei or cells are in focus in the different micrographs.
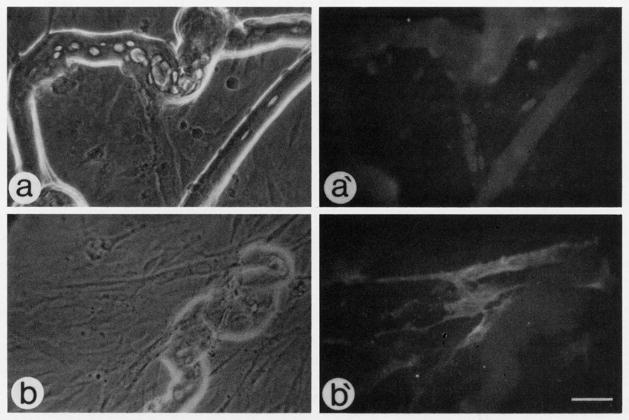
Fig. 3.
Micrographs of fiber cultures isolated from adult rat and maintained in medium containing 10% horse serum and 5% chicken embryo extract for 8 days. (a and a′) Phase and fluorescent micrographs of cultures reacted with the antibody against myogenin via indirect immunofluorescence. (b and b′) Phase and fluorescent micrographs of cultures reacted with the antibody against αSMactin via indirect immunofluorescence. In both cases cultures resemble myogenic mass cultures with mononucleated cells and myotubes; remnants of the original fibers are present as well. Bar, 43 μm.
We then searched for antibodies which would distinguish between satellite cells (either proliferating or differentiating) and the rest of the myofiber. Since the adult rat myofiber contains only few quiescent satellite cells in vivo (two or three cells prior to proliferation (Bischoff, 1989)), we reasoned that any specific antibody marker should react with only small number of cells or nuclei, reflecting the original satellite cell or their progeny. An antibody marker, even if specific for skeletal muscle, which reacts with the entire fiber cytoplasm or all myonuclei, would be unusable for these experiments. Figs. 1 and 2 demonstrate patterns of fiber stainability with several antibodies which seem to detect only small number of nuclei or cells, thus presumably visualizing satellite cells or their direct descendants. The fibers were maintained in basal medium for 2 to 5 days prior to fixation and are counterstained with DAPI to visualize the multinuclei within the myofiber. Antibodies against PCNA (Figs. 1a′, 1b′, and 1c′), MyoD (Fig. 1d′) and myogenin (Fig. 1, lower portion of e and f) stained discrete nuclei; antibodies against DEVmyosin (Figs. 2a′, 2b′, 2c′, and 2d) and αSMactin (Fig. 2e) stained cytoplasm of discrete cells. Age of cultures used for reaction with the different antibodies was based on the temporal appearance of cells positive for the different proteins. These temporal appearance patterns are further discussed in the next section and are shown in a more quantitative manner in Fig. 4.
Upon staining of progressively older fiber cultures with the antibody against DEVmyosin we could also identify infrequent small myotubes along the original fiber (presumably underneath the fiber basement membrane) which were separated from the original fiber (see for example Figs. 2c and 2c′ for 5-day cultures). This is in agreement with the observations made by Bischoff (1986, 1990a) which indicated that some progeny of satellite cells can fuse with each other underneath the basement membrane of the isolated intact fiber but fusion of the cells with the associated fibers does not take place—despite the fact that the progeny of satellite cells on intact fibers eventually become fusion competent, they remain primarily as mononucleated cells as long as the fibers are intact. It is also important to note that the antibody against DEVmyosin, but not any of the other antibodies tested, often reacted with the terminal regions of the cultured fibers (see, for example, Figs. 2a′, 2b′, and 2c′).
Parallel fiber cultures reacted only with secondary antibodies did not exhibit nuclear or cytoplasmic stain of individual cells. Staining of the fibers with antibodies against a variety of other antigens, such as laminin, α2-macroglobulin, desmin, and all isoforms of sarcomeric myosin, for which the fibers are positive, did not reveal any staining patterns resembling individual satellite cells, or proved problematic due to their reactivity with the entire fiber as well.
Quantification of Satellite Cells on Isolated Fibers Maintained in Basal Medium
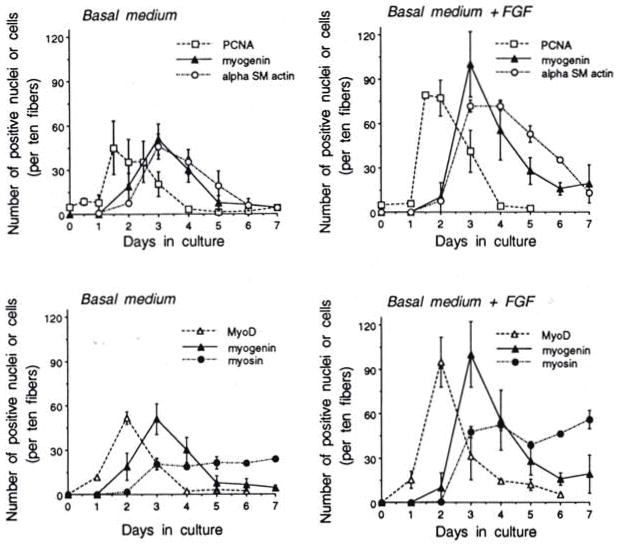
We employed the stainability of the single cells on the isolated fibers to quantify the temporal expression of PCNA, MyoD, myogenin, αSMactin, and DEVmyosin. Figure 4 (left two panels) demonstrates these results with fibers maintained in basal medium. Results are separated into two panels because of the density of the data points; myogenin is shown in both panels for comparison. The results indicate that the temporal increase and decline of PCNA+ cells coincided with that of MyoD+ cells; the temporal appearance and decline of myogenin+ cells coincided with that of αSMactin a day later than cells positive for PCNA or MyoD. Cells positive for DEVmyosin become apparent within 24 hr following the appearance of myogenin+ or αSMactin+ cells. Quantification of cells positive for PCNA, MyoD, myogenin, or αSMactin results in graphs with similar dimensions. This suggests that these four different characteristics reflect the same cells as they traverse from exhibiting detectable levels of PCNA and MyoD to exhibiting detectable levels of myogenin and αSMactin. We further analyzed fiber cultures by double immunostaining as shown in Table 1. Day 2 fiber cultures were reacted with anti-MyoD/anti-PCNA and Day 3 fiber cultures were reacted with anti-MyoD/anti-myogenin. Results indicate that the majority of the cells coexpress both MyoD and PCNA at Day 2 in culture when expression of both antigens peaks. On Day 3 in culture the majority of the cells are positive for myogenin only. However there is a substantial number of MyoD+/myogenin+ cells at Day 3 in culture. The presence of the MyoD+/myogenin+ cells suggests that some, if not all, of the satellite cells express both factors subsequent to expressing MyoD only and prior to becoming myogenin+ only.
Fig. 4.
Temporal appearance of cells or nuclei positive for PCNA, MyoD, myogenin, αSMactin, and DEVmyosin on cultured rat fibers. Left two panels describe results with fibers maintained in basal medium only. Right two panels describe results with fibers maintained in basal medium to which bFGF was added at 2 ng/ml. Medium (±bFGF) was changed daily. Cells or nuclei positive for the different proteins were revealed with appropriate antibodies employing indirect immunofluorescence. Fiber cultures were routinely monitored using a 25X objective. Numbers reflect averages for 10 fibers, but routinely for each time point, within an individual experiment, a minimum of 30 fibers were analyzed in two or three culture plates. Results are based on several experiments and standard deviation is based on the different experiments. Standard deviation within parallel plates of the same experiments was no more than 5%. See Fig. 5 for the total number of fibers analyzed to construct the time points in the various panels of Fig. 4.
TABLE 1.
Number of Positive Nuclei Detected by Double Immunofluorescence of Cultured Fibers
| Antibodies tested | Reactivity of nuclei | Day 2 |
Day 3 |
||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Anti-MyoD/Anti-PCNA | MyoD+ only | 36 | 9.39 | — | — |
| PCNA+ only | 18 | 4.70 | — | — | |
| MyoD+/PCNA+ | 329 | 85.90 | — | — | |
| Anti-MyoD/Anti-myogenin | MyoD+ only | — | — | 11 | 2.69 |
| Myogenin+ only | — | — | 344 | 84.31 | |
| MyoD+/myogenin+ | — | — | 53 | 12.99 | |
| Total positive nuclei | 383 | 408 | |||
| Total fibers | 60 | 60 | |||
To further investigate whether myogenin is indeed expressed by progeny of the proliferating cells and not by some of the cultured myofiber nuclei, we examined the effect of eliminating cell proliferation on subsequent expression of myogenin. Fiber cultures were continuously exposed upon establishment to 10 μm cytosine arabinoside—a drug which kills cycling cells. Nuclei reacting with the anti-PCNA, or subsequently with the anti-myogenin, were almost completely eliminated (data not shown). We also demonstrated that exposure of fiber cultures to 5-bromo-2-deoxyuridine (50 μM), a drug which was shown to prevent differentiation of myoblasts in mass culture upon continuous incorporation into the DNA (Stockdale et al., 1964; Tapscott et al., 1988), drastically reduced the numbers of MyoD+ nuclei and extinguished myogenin+ nuclei; no effect was observed on the appearance and disappearance of PCNA+ cells on the fibers. Collectively, the above described experiments which have indicated that there is a link between DNA replication and expression of MyoD and myogenin provide further support that the two myogenic regulatory factor proteins are expressed in cells during or subsequent to their proliferation and not in myonuclei.
The graph summarizing the number of DEVmyosin+ cells (Fig. 4, lower left panel) is different in shape than the other four graphs. The cells expressing detectable levels of DEVmyosin peak together with cells expressing myogenin or αSMactin, but this myosin expression is not transient; once the number of myosin+ cells peaks, this number seems to remain relatively constant. Furthermore, maximum number of DEVmyosin+ cells is about half of that expressing myogenin or αSMactin, suggesting that perhaps only about 50% of the satellite cells which had entered myogenesis eventually express detectable levels of DEVmyosin. This unique pattern of DEVmyosin expression (graph reaching plateau at about 50% of the peak number of myogenin+ cells) is consistent for various conditions. We observed it for bFGF-treated adult fibers (see details in next section) and for fibers from growing rats (3-week-old; Yablonka-Reuveni and Rivera, unpublished data).
As shown in Fig. 4, few PCNA+ cells were present on fibers that were fixed immediately following their culturing (0 days in culture). These infrequent PCNA+ cells can be cells which already expressed PCNA prior to the isolation and/or began expressing PCNA during the isolation procedure or following it. The number of these cells does not change during the first 24 hr in culture.
The Number of Proliferating and Subsequently Differentiating Satellite Cells on Cultured Fibers Is Enhanced by the Addition of bFGF
The pattern of myogenic marker expression by satellite cells on intact fibers was also tested in the presence of bFGF. This FGF-related investigation was performed not only to examine the effect of the growth factor but also to determine the consistency of the protein markers used above. For the FGF experiments, fiber cultures were maintained in basal medium fortified with bFGF at 2 ng/ml as detailed under Methods. Results are shown in Fig. 4 (two right panels). Experiments were always conducted in parallel with control cultures maintained in basal medium as shown in the left two panels of Fig. 4. The number of cells positive for PCNA, MyoD, myogenin, αSMactin, and DEVmyosin was enhanced by about twofold in the presence of bFGF. However, the temporal appearance of positive cells was similar to that of the control cultures regardless of the protein marker examined. The appearance and decline of PCNA+ cells coincided with that of MyoD+ cells; likewise, the appearance and decline of myogenin+ cells coincided with the αSMactin+ cells. As in control cultures, cells positive for DEVmyosin reached a plateau level at the same time that cells positive for myogenin or αSMactin peaked, and the number of myosin+ cells was about half of the number of myogenin+ or αSMactin+ cells. Time spent in culture prior to the increase in PCNA+ or MyoD+ cells was identical in the absence or presence of FGF, and the timing of the transition from the proliferative to the differentiative compartment was similar. Kinetics of the αSMactin+ cells suggest that the expression of this actin may lag slightly behind myogenin. Such a lag is also some-what suggested by the control graph.
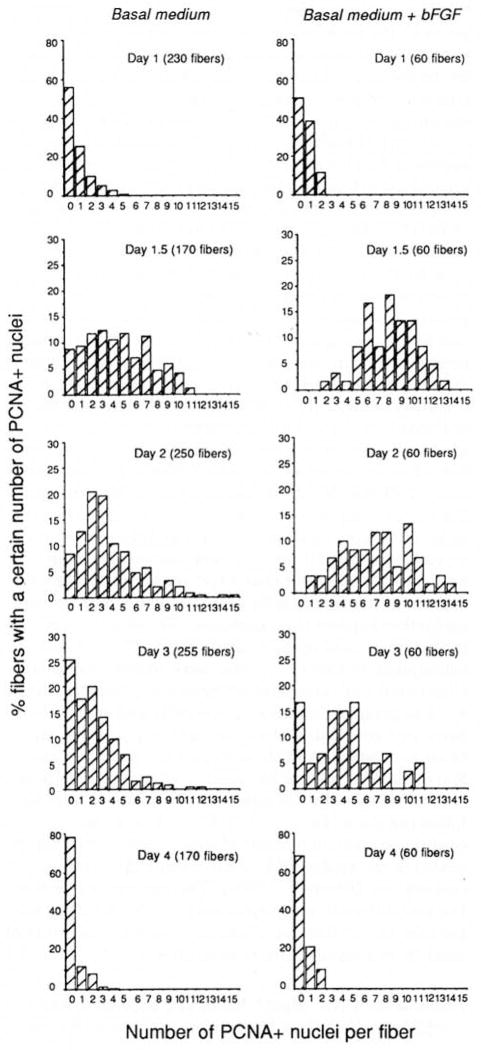
As shown in Fig. 4, the increase from basal level to peak level of PCNA+ cells occurs between 24 and 36 hr in culture in both control and FGF-treated fibers but, in the presence of FGF, the number of positive cells per fiber is higher. This difference can be due to accelerated cell pro-liferation and/or to the fact that FGF promotes more quiescent satellite cells to enter the cell cycle. To gain more insight regarding the effect of FGF we analyzed the data contributing to Fig. 4 by the frequency of fibers containing a given number of positive satellite cells. Figure 5 demonstrates this analysis for the PCNA results. The figure indicates that on Days 1.5 and 2, all fibers in cultures fortified with FGF have PCNA+ nuclei. In cultures lacking exogenous FGF, 10% of the fibers never contained PCNA+ cells on Days 1.5 and 2. Furthermore, in cultures fortified with bFGF, there are more fibers with higher number of PCNA+ cells than in cultures maintained in basal medium only. Even on Day 3, there is still a distinct difference in the distribution of PCNA+ nuclei between the fibers that were cultured in the presence or absence of bFGF. This pattern of more positive fibers with higher numbers of positive nuclei is demonstrated for the myogenin data as well (analyses not shown). The results suggest that more satellite cells are recruited to the cell cycle in the presence of bFGF; however, the possibility of an FGF-accelerated cell cycle cannot be ruled out.
Fig. 5.
Analysis of the frequency of fibers containing a specific number of PCNA+ nuclei when fibers are cultured in basal medium in the absence or presence of 2 ng/ml bFGF. Left and right columns describe results in the absence or presence of bFGF, respectively. Total number of fibers for each analysis is indicated in the figures. Data in this figure are the same ones contributing to Fig. 4.
“Baseline” Proliferation of Satellite Cells on Fibers in Basal Medium Is Regulated by bFGF-Immunorelated Material
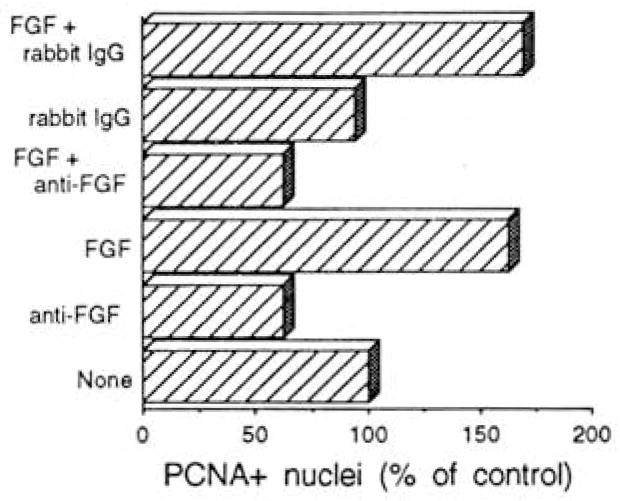
We were interested to determine if the baseline proliferation of satellite cells (that is, in the absence of added bFGF, demonstrated in Fig. 4 in the upper left panel) is also mediated by bFGF. To pursue this investigation we cultured adult rat fibers in basal medium in the presence of an antibody which specifically blocks the mitogenic effect of bFGF. As was shown by Lindner and Reidy (1991) this antibody does not block the mitogenic activity of other growth factors such as platelet-derived growth factor and epidermal growth factor or of calf serum. Culture medium and additives were replenished after 24 hr to allow ample supply of additives, and cultures were fixed 48 hr after initial plating and examined for PCNA+ nuclei as shown in Fig. 6. The antibody against bFGF suppressed the number of positive nuclei by about 40–50%. As expected, bFGF alone promoted the number of PCNA+ cells, but the addition of bFGF in the presence of the anti-bFGF did not overcome the reduction in PCNA+ cells caused by the antibody. Control rabbit IgG had no significant effect on the number of PCNA+ nuclei in either the absence or presence of bFGF. Collectively, the results of the different treatments suggest that nearly 50% of the baseline proliferation of satellite cells on intact fibers is promoted by a mitogen which is immunorelated to bFGF. Because bFGF is not routinely detectable in the serum used, we propose that this bFGF-related mitogen is contributed by the isolated fiber.
Fig. 6.
Analysis of the number of satellite cells on isolated rat fibers following the addition of an antibody that can block the activity of bFGF. Antibody and control IgG were added at 100 μg/ml and bFGF was added at 2 ng/ml. Fiber cultures were exposed to additives continuously (with a change of basal medium ± additives at 24 hr) and fixed 48 hr following initial culturing. Results are based on two independent experiments and each treatment within each experiment was performed on two or three parallel culture plates. Standard deviation between experiments is not shown but was less than 5–10%.
DISCUSSION
The present study was undertaken in order to characterize how myogenesis of adult satellite cells progresses when the cells are maintained in their native position between the basement membrane and the plasma membrane of the muscle fiber. We first characterized the reactivity of cultured adult rat muscle fibers with antibodies to specific muscle proteins. Staining patterns and frequency of positive cells or nuclei have suggested that the antibodies specifically recognize satellite cells associated with the individual fibers. The antibody-staining approach has been further utilized to analyze the progression of activated satellite cells through the different compartments of the myogenic program and the effect of bFGF during myogenesis of satellite cells on intact fibers. Our results suggest that myogenesis of satellite cells in their native position follows a highly coordinated, multistep program of regulatory and structural protein expression. Proliferation of satellite cells on intact fibers is supported by bFGF but the time course of progression through the different program compartments is not modified by this mitogen. Clearly, our quantification of satellite cells with the different antibodies is limited to the cells that express detectable levels of protein. Nevertheless, we demonstrated that the number of cells detected on intact fibers by antibodies against PCNA, MyoD, myogenin, and αSMactin is similar, suggesting that all four markers visualize the same cells (although the cells might be at different points of myogenesis). Results with double immunostaining (Table 1) and the finding that FGF enhanced to the same degree the number of cells positive for the four antibodies further support this conclusion. The observation that in the presence of cytosine arabinoside PCNA+ cells, or subsequent myogenin+ cells, were almost completely eliminated indicates that myogenin expression is confined to progeny of the PCNA+ cells and further supports our conclusion that the antibody-positive structures are indeed satellite cells and not myofiber nuclei. Moreover, the maximum number of satellite cells per fiber as revealed by the immunocytochemistry approach following the addition of bFGF (see Fig. 5) does not exceed the maximum number of satellite cells per fiber revealed in the studies of Bischoff using [3H]thymidine incorporation (Bischoff, 1990b). The agreement between the two different assay systems provides a strong support for the utilization of our approach to the study of satellite cell myogenesis on intact fibers.
Patterns of PCNA, MyoD, Myogenin, αSMactin, and DEVmyosin Protein Expression on Cultured Fibers
When we began the present investigation of intact fibers, we expected that an antibody against PCNA would react with nuclei of proliferating satellite cells, but it was unclear whether antibodies against MyoD and myogenin would localize to nuclei of satellite cells and/or fiber nuclei. Likewise, it was unclear whether the satellite cells or the whole fiber would contain cytoplasmic proteins, expressed during embryonic but not adult myogenesis. The similarity in the temporal appearance and disappearance of PCNA+ and MyoD+ cells on intact fibers (with or without bFGF) suggests that it is the proliferating satellite cells which express the myogenic regulatory factor protein MyoD. Analyzing the myogenic cell line C2, Tapscott et al. (1988) concluded that MyoD protein was expressed by proliferating myoblasts, but not all proliferating myoblasts expressed MyoD. We also monitored the reactivity of mass cultures of rat satellite cells dissociated from isolated fibers and of C2 cells with the antibody against MyoD used in the present fiber study. We concluded that MyoD protein was expressed by proliferating cells and that not all proliferating cells expressed MyoD. Furthermore, MyoD expression continued in mononucleated cells after PCNA expression ceased (unpublished data). It is only in the fiber model that we could document such a close correlation between PCNA and MyoD expression. Despite this close correlation, the present fiber study is not sufficient to rule out the possibility that it is because of this MyoD expression that satellite cell proliferation would cease (see Olson, 1992, for a review regarding the inhibitory effect of MyoD on cell proliferation).
As the number of PCNA+ cells or MyoD+ cells on intact fibers declines, myogenin+ cells appear. The striking similarity of the shape of the graphs representing MyoD+ cells and myogenin+ cells with a 24-hr lag in reactivity (with or without the addition of bFGF) suggests that the cells spend only 24 hr in the proliferating, MyoD+ compartment, followed by a switch to the myogenin+ compartment. The double staining experiments shown in Table 1 suggest that coexpression of both MyoD and myogenin proteins is likely to occur during the transition from the MyoD+ phase to the myogenin+ phase. The fact that the onset of MyoD protein expression precedes the onset of myogenin protein expression has been previously noted for primary mouse myoblasts isolated from embryonic, fetal, and newborn stages (Cusella-De Angelis, 1992; Smith et al., 1993) and for primary rat myoblasts isolated from adult muscle (our unpublished studies). However, it is only in the present study of isolated fibers that a very synchronous transition from MyoD+ cells to myogenin+ cells has been observed.
The similarity in the kinetics of myogenin+ cells and αSMactin+ cells on single fibers suggests that myogenin expression by satellite cells following exit from the cell cycle coincides with αSMactin expression. We therefore hypothesize that during myogenesis on single fibers, progeny of satellite cells enter a compartment where they are positive for both myogenin and αSMactin. It is important to note that although myogenin and αSMactin are transiently expressed by cells on intact fibers, the expression of these two proteins is maintained by myotubes in mass cultures of rat satellite cells (see for example Fig. 3).
Which compartment along the myogenic program might the transient expression of myogenin reflect? Using the rat myoblasts L6, Wright et al. (1989) demonstrated that the level of myogenin transcripts peaks just prior to the onset of the increase in transcripts for myosin heavy chains or fusion of myoblasts into myotubes. This myogenin expression prior to myosin expression was named “mid-decision” and has been considered as the first step of differentiation during myogenesis (Wright et al., 1989). In the latter study, as myosin transcripts further accumulated and fusion continued, the level of myogenin transcript declined to a baseline level. Similarly, in the present rat fiber study, the number of satellite cells positive for myogenin (or αSMactin+) peaked during the third day of culture and declined on the fourth day to a baseline level; DEVmyosin expression by satellite cells was initiated just shortly after myogenin and αSMactin but was maintained while the expression of the other two proteins was transient. We therefore propose that the expression of myogenin and αSMactin by satellite cells on intact fibers marks a compartment of cells nearing terminal differentiation or cells at mid-decision. In progressively older fiber cultures the number of DEVmyosin+ cells was higher than the number of myogenin+ cells (or MyoD+ cells). This suggests that the final DEVmyosin+ cells do not express myogenin (or MyoD). However, this does not exclude the possibility that the DEVmyosin+ cells may express one or both of the other two known myogenic regulatory factor proteins (MRF4 and myf-5).
When DEVmyosin+ cells reached a plateau they numbered only about half of the peak number of cells which were myogenin+ or αSMactin+. What might than be the fate of the other 50% of the myogenin+ cells which did not transit into DEVmyosin expression? It is unlikely that these cells fused with the existing fibers as in this fiber model, satellite cell progeny do not fuse with the associated mature fibers despite the fact that they can fuse with each other and form new myotubes (Bischoff, 1986, 1990a). Furthermore, examining fibers following the decline in myogenin+ nuclei, we could identify on the fibers mononucleated cells which do not express DEVmyosin. Quantification of the DEVmyosin– cells requires an efficient visualization means that can capture all of these cells; we are now attempting to develop such an approach. It is still arguable that the additional 50% of the cells which do not express DEVmyosin underwent terminal differentiation but expressed undetectable levels of DEVmyosin, or that they expressed other isoforms of myosin not detected by the antibody. Our mass culture experiments, however, do not support the latter possibility, as they indicate that the same number of mononucleated differentiated cells can be detected by the antibody against DEVmyosin and by the MF20 antibody (Bader et al., 1982) which recognizes all forms of sarcomeric myosin heavy chains (data not shown). It is thus possible that not all myogenin-expressing cells in the fiber cultures are bound for terminal differentiation. Indeed, our assays of a reactivity with an antibody against myogenin cannot indicate whether myogenin in the cells is in its active form, whether other important molecules are present to participate in the myogenic activity of myogenin, and whether the myogenin-expressing cells developed the final block that will make the cells terminally differentiated (see reviews by Olson (1992, 1993) and Weintraub (1993) regarding the role of phosphorylation and other regulatory proteins in modulating the function of the myogenic regulatory factors). The notion that only 50% of the satellite cell progeny eventually terminally differentiated is in agreement with the studies of Moss and Leblond (1971) who, analyzing [3H]thymidine incorporation in growing rat muscle, suggested that following each division of satellite cells only 50% of the progeny can fuse with the fiber while the second cell can continue to proliferate. In the present study the satellite cell progeny which are not bound for terminal differentiation (expression of DEVmyosin) do not continue to proliferate either. We are presently examining the possibility that further proliferation of these cells requires additional growth factors which are not present in the fiber culture or, even if are present, they are not presented to the cells in a suitable form.
How Do the Results with the Temporal Expression of Myogenic Regulatory Factor Proteins on Single Fibers Compare with Data Gleaned from in Vivo Models?
Our studies described in this paper have indicated that it is the satellite cells which express MyoD and myogenin following culturing of isolated fiber and that these two proteins are not detected in the fibers prior to activation of satellite cells. Various studies at the mRNA level have identified few, if any, transcripts of MyoD and myogenin in uninjured, adult muscle (Hinterberger et al., 1991; Eftimie et al., 1991; Buonanno et al., 1992; Beilharz et al., 1992). However, conclusions from in vivo studies regarding the nature of entities expressing MyoD and myogenin at the mRNA or protein levels following overt injury or other types of stress seem to vary depending on the model used. Similar to the results of present fiber study, in situ hybridization studies on mouse skeletal muscle concluded that quiescent myoblasts in mature muscle do not express MyoD and myogenin transcripts, but that MyoD and myogenin transcripts are present in mononuclear cells following injury to the muscle by crushing. In the latter study transcripts peaked within 1–2 days postinjury and declined to pre-injury levels by 8 days; myofibers did not express detectable levels of such transcripts prior to or following injury (Grounds et al., 1992). Immunocytochemical localization of MyoD and myogenin in mouse following muscle grafting identified the antigens first in mono-nucleated cells (presumably satellite cells) and subsequently in nuclei of regenerating fibers (Füchtbauer and Westphal, 1992). In contrast, mRNA transcript localization in chicken muscle following a brief period of muscle stretch suggested that the MyoD homologue is expressed by nuclei within the fibers (Eppley et al., 1993). Likewise, it was suggested that MyoD and myogenin protein antigens are expressed in myofibers of rat diaphragm following denervation, being first detected about 2.5 days following denervation (Weis, 1994). The differences in the conclusions of the various studies might be related to differences in gene activation due to variability in the injury/stress imposed. However, it is yet unclear whether the expression of MyoD by myo-nuclei following stretch resulted in MyoD protein synthesis as well. Also, taking in consideration both the long period of time between denervation and expression of MyoD/myogenin proteins in the study by Weis (1994) and the fact that denervation causes increase in satellite cell numbers (Snow, 1981), it is still possible that satellite cells have been involved in the MyoD/myogenin expression following denervation.
bFGF Promotes a Greater Number of Satellite Cells to Enter the Cell Cycle but Does Not Modify the Overall Schedule of Myogenesis
In the present study we also analyzed the effect of bFGF on myogenesis of satellite cells. It has been previously demonstrated that bFGF is a potent mitogen of satellite cells in culture (Allen and Boxhorn, 1989; DiMario and Strohman, 1988), of myogenic cell lines derived from satellite cells (Clegg et al., 1987; Yablonka-Reuveni, 1994), and of satellite cells on isolated intact fibers (Bischoff, 1986). The present study enhances the original study of Bischoff by extending the investigation to both proliferation and differentiation of satellite cells on intact fibers in response to bFGF. The present study has indicated that bFGF promotes proliferation of more satellite cells on more fibers and subsequently contributes to the appearance of more differentiated satellite cells on more fibers, but bFGF does not modify the time spent in the different compartments of proliferation and differentiation—it does not affect the highly regulated program that the satellite cells undergo on the isolated fibers. As discussed above, in routine mass cultures of myoblasts derived from satellite cells, bFGF acts as a potent mitogen. Furthermore, bFGF can inhibit myogenic differentiation (Clegg et al., 1987; Olwin and Rapraeger, 1991). Future experiments will be required to determine the basis for the difference between the fiber model versus the mass culture model in regard to the mitogenic response of satellite cells to bFGF.
The bFGF-blocking studies additionally demonstrated that proliferation of satellite cells in the basal medium is at least partially controlled by bFGF or an antigenically related substance; in the presence of the antibody, proliferation was reduced by close to 50% compared to control fibers. Since FGF is not present in the serum used, we propose that the fiber unit itself (fiber cytoplasm and/or fiber basement membrane and/or satellite cells) contains the bFGF-immunorelated material. We have not conducted an exhaustive investigation to isolate such an endogenous mitogen from the fibers, but there are several studies that detected bFGF in adult mouse muscle by immunolocalization. bFGF was detected in the myofiber periphery (potentially extracellular matrix), in nuclei within intact myofibers, and in dystrophin-positive cells in close association with the myofibers (potentially satellite cells) (DiMario et al., 1989; Anderson et al., 1991). Furthermore, transcripts for bFGF (and acidic FGF) were detected in myoblasts of mouse- and rat-derived cell lines, suggesting that endogenously produced FGFs promote proliferation of the FGF-producing myoblasts (Moore et al., 1991). A recent study has further demonstrated that bFGF can be released from the cytoplasm of muscle fibers following injury and that the extent of the release is related to the level of injury; all bFGF was released following lethal damage to the fiber, but only some bFGF was released following a gentler disturbance to the plasma membrane (Clarke et al., 1993). Hence, in the present study, the proliferation of the satellite cells in basal medium (which can be reduced by an antibody against bFGF) might be regulated by bFGF released from “wounded” regions in some of the fibers. Proliferation of more satellite cells upon addition of bFGF, as seen in the present study, could perhaps occur in vivo upon release of more bFGF from the fibers following further muscle trauma.
Collectively, the present study adds support to the proposal that bFGF is an important regulator of satellite cell proliferation and that at least some of this FGF is endogenously produced in the muscle fiber unit.
In summary, the study presented here has established means to analyze myogenesis of satellite cells in their in situ position by the muscle fiber. This approach provides an assay system to investigate the role of various agents which might be involved in the control of myogenesis in growing and adult muscle. Furthermore, the present model might be useful in identifying agents that can prolong the proliferative phase of myoblasts in their in situ position. This could prove useful for both growth and repair of muscle.
Acknowledgments
We thank Dr. R. Bischoff for helpful suggestions on the isolation of rat fibers. We are also grateful to Drs. J. N. Buskin and R. A. Seifert for thoughtful comments on the manuscript, to Dr. A. Reuveni for valuable advice on graph presentation, to Dr. S. Alemá for making the anti-MyoD antibody available to us prior to its publication, to Drs. L. L. Leger and F. Pons for the anti-DEV myosin antibody, to Dr. W. Wright for the anti-myogenin antibody, to Drs. V. Lindner and M. Reidy for the anti-bFGF antibody, and to Dr. S. Hauschka for bFGF. This work was supported by grants to Z.Y.-R. from the Muscular Dystrophy Association, the National Institutes of Health (AR39677), and the Co-operative State Research Service–U.S. Department of Agriculture (Agreement No. 93-37206-9301). During the course of the study Z.Y.-R. was also supported by a grant-in-aid from the American Heart Association.
References
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Liu L, Kardami E. Distinctive patterns of basic fibroblast growth factor (bFGF) distribution in degenerative and regenerative areas of dystrophic (mdx) striated muscles. Dev Biol. 1991;147:96–109. doi: 10.1016/s0012-1606(05)80010-7. [DOI] [PubMed] [Google Scholar]
- Antonio J, Gonyea WJ. Skeletal muscle fiber hyperplasia. Med Sci Sports Exercise. 1993;25:1333–1345. [PubMed] [Google Scholar]
- Babai F, Musevi-Aghdam J, Schurch W, Royal A, Gabbiani G. Coexpression of α-sarcomeric actin, α-smooth muscle actin and desmin during myogenesis in rat and mouse embryos. I Skeletal muscle. Differentiation. 1990;44:132–142. doi: 10.1111/j.1432-0436.1990.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunohistochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R. Growth regulation of the PCNA gene. J Cell Sci. 1991;98:433–436. doi: 10.1242/jcs.98.4.433. [DOI] [PubMed] [Google Scholar]
- Beilhartz MW, Lareu R, Garrett KL, Grounds MD, Fletcher S. Quantitation of muscle precursor cell activity in skeletal muscle by Northern analysis of MyoD and myogenin expression: Application to dystrophic (MDX) mouse muscle. Mol Cell Neurosci. 1992;3:326–333. doi: 10.1016/1044-7431(92)90029-2. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Betz W. Properties of isolated adult rat muscle fibers maintained in tissue culture. J Physiol. 1977;271:537–547. doi: 10.1113/jphysiol.1977.sp012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat Rec. 1974;180:645–662. doi: 10.1002/ar.1091800410. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells in intact myofibers in culture. Dev Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Analysis of muscle regeneration using single myofibers in culture. Med Sci Sports Exercise. 1989;21:S164–S172. [PubMed] [Google Scholar]
- Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990a;109:943–952. doi: 10.1242/dev.109.4.943. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Cell cycle commitment of rat muscle satellite cells. J Cell Biol. 1990b;111:201–207. doi: 10.1083/jcb.111.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Apone L, Morasso MI, Beers R, Brenner HR, Eftimie R. The MyoD family of myogenic factors is regulated by electrical activity: Isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Res. 1992;20:539–544. doi: 10.1093/nar/20.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion DR. The muscle satellite cell: A review. Int Rev Cytol. 1984;87:225–251. doi: 10.1016/s0074-7696(08)62444-4. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. The regeneration of skeletal muscle fibers following injury: A review. Med Sci Sports Exercise. 1983;15:187–198. [PubMed] [Google Scholar]
- Clarke MSF, Khakec R, McNeil PL. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci. 1993;106:121–133. doi: 10.1242/jcs.106.1.121. [DOI] [PubMed] [Google Scholar]
- Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. Growth factor control of skeletal muscle differentiation: Commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987;105:949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Molinaro M. Cell heterogeneity in the myogenic lineage. Curr Top Dev Biol. 1987;23:185–208. doi: 10.1016/s0070-2153(08)60625-0. [DOI] [PubMed] [Google Scholar]
- Cusella-De Angelis MG, Lyons G, Sonnino C, De Angelis L, Vivarelli E, Farmer K, Wright WE, Molinaro M, Bouche M, Buckingham M, Cossu G. MyoD:Myogenin independent differentiation of primordial myoblasts in mouse somites. J Cell Biol. 1992;116:1243–1255. doi: 10.1083/jcb.116.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfectcd cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dias P, Parham DM, Shapiro DN, Tapscott SJ, Houghton PJ. Monoclonal antibodies to the myogenic regulatory protein MyoD1: Epitope mapping and diagnostic utility. Cancer Res. 1992;52:6431–6439. [PubMed] [Google Scholar]
- DiMario J, Buffinger N, Yamada S, Strohman RC. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science. 1989;244:688–690. doi: 10.1126/science.2717945. [DOI] [PubMed] [Google Scholar]
- DiMario J, Strohman RC. Satellite cells from dystrophic (mdx) mouse muscle are stimulated by fibroblast growth factor in vitro. Differentiation. 1988;39:42–49. doi: 10.1111/j.1432-0436.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci USA. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson CP. Embryonic signals for skeletal myogenesis: Arriving at the beginning. Curr Opinion Cell Biol. 1993;5:1057–1064. doi: 10.1016/0955-0674(93)90092-5. [DOI] [PubMed] [Google Scholar]
- Eppley ZA, Kim J, Russell B. A myogenic regulatory gene, qmf1, is expressed by adult myonuclei after injury. Am J Physiol. 1993;265:C397–C405. doi: 10.1152/ajpcell.1993.265.2.C397. [DOI] [PubMed] [Google Scholar]
- Füchtbauer EM, Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn. 1992;193:34–39. doi: 10.1002/aja.1001930106. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Molecular and cellular biology of muscle regeneration. In: Partridge T, editor. Molecular and Cell Biology of Muscular Dystrophy. Chapman & Hall; London: 1993. pp. 210–256. [Google Scholar]
- Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yablonka-Reuveni Z. Myoblasts from embryonic and adult skeletal muscle regulate myosin expression differently. Dev Biol. 1991;148:249–260. doi: 10.1016/0012-1606(91)90334-y. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yablonka-Reuveni Z. Skeletal muscle satellite cells appear during late chicken embryogenesis. Dev Biol. 1992;153:206–216. doi: 10.1016/0012-1606(92)90106-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Yablonka-Reuveni Z. Evidence for a distinct adult myogenic lineage in skeletal muscle. Comments Dev Neurobiol. 1992;1:391–404. [Google Scholar]
- Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF. Expression of the muscle regulatory MRF4 during somite and skeletal myofiber development. Dev Biol. 1991;147:144–156. doi: 10.1016/s0012-1606(05)80014-4. [DOI] [PubMed] [Google Scholar]
- Johnson SE, Allen RE. Proliferation cell nuclear antigen (PCNA) is expressed in activated rat skeletal muscle satellite cells. J Cell Physiol. 1993;154:39–43. doi: 10.1002/jcp.1041540106. [DOI] [PubMed] [Google Scholar]
- Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci USA. 1991;88:3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini JF, Pons F, Leger J, Loffreda N, Anoal M, Chevallay M, Fardeau M, Leger JJ. Expression of myosin heavy chain isoforms in Duchenne muscular dystrophy patients and carriers. Neuromusc Disord. 1991;1:397–409. doi: 10.1016/0960-8966(91)90003-b. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Muscle Regeneration. Raven Press; New York: 1979. [Google Scholar]
- Moore JW, Dionne C, Jaye M, Swain JL. The mRNAs encoding acidic FGF, basic FGF and FGF receptor are coordinately downregulated during myogenic differentiation. Development. 1991;111:741–748. doi: 10.1242/dev.111.3.741. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as a source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–436. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154:216–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol. 1993;7:1369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- Olwin BB, Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol. 1991;118:631–639. doi: 10.1083/jcb.118.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PNJ, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sassoon DA. Myogenic regulatory factors: Dissecting their role and regulation during vertebrate embryogenesis. Dev Biol. 1993;156:11–23. doi: 10.1006/dbio.1993.1055. [DOI] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radioautographic study. J Exp Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8:217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- Schultz E. Satellite cell behavior during skeletal muscle growth and regeneration. Med Sci Sports Exercise. 1989;21:S181–S186. [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Treciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against α-smooth muscle actin: A new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TH, Block NE, Rhodes SJ, Konieczny SF, Miller JB. A unique pattern of expression of the four muscle regulatory factor proteins distinguishes somitic from embryonic, fetal and newborn mouse myogenic cells. Development. 1993;117:1125–1133. doi: 10.1242/dev.117.3.1125. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II An autoradiographic study. Anat Rec. 1977;188:201–218. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- Snow MH. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anat Rec. 1981;207:593–604. doi: 10.1002/ar.1092070407. [DOI] [PubMed] [Google Scholar]
- Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat Rec. 1990;227:437–446. doi: 10.1002/ar.1092270407. [DOI] [PubMed] [Google Scholar]
- Stockdale FE. Myogenic cell lineages. Dev Biol. 1992;154:284–298. doi: 10.1016/0012-1606(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Stockdale F, Okazaki K, Nameroff M, Holtzer H. 5-Bromodeoxyuridine: Effect on myogenesis in vitro. Science. 1964;146:533–535. doi: 10.1126/science.146.3643.533. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: A nuclear phosphoprotein requiring a myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: Redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Weis J. Jun, Fos, MyoD1, and myogenin proteins are increased in skeletal muscle fiber nuclei after denervation. Acta Neuropathol. 1994;87:63–70. doi: 10.1007/BF00386255. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J, Mitchell JJ, Low RB, Kieny M, Sengel P, Rubbia L, Skalli O, Jackson B, Gabbiani G. α-Smooth muscle actin is transiently expressed in embryonic rat cardiac and skeletal muscles. Differentiation. 1988;39:161–166. doi: 10.1111/j.1432-0436.1988.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to myoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Development and postnatal regulation of adult myoblasts. Microsc Res Tech. 1994 doi: 10.1002/jemt.1070300504. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 1990;111:1623–1629. doi: 10.1083/jcb.111.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Quinn LS, Nameroff M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev Biol. 1987;119:252–259. doi: 10.1016/0012-1606(87)90226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seifert RA. Proliferation of chicken myoblasts is regulated by specific isoforms of platelet-derived growth factor: Evidence for differences between myoblasts from mid and late stages of embryogenesis. Dev Biol. 1993;156:307–318. doi: 10.1006/dbio.1993.1079. [DOI] [PubMed] [Google Scholar]