Summary
Human Presequence Protease (hPreP) is an M16 metalloprotease localized in mitochondria. There, hPreP facilitates proteostasis by utilizing a ∼13,300Å3 catalytic chamber to degrade a diverse array of potentially toxic peptides, including mitochondrial presequences and amyloid-β (Aβ), the latter of which contributes to Alzheimer's disease pathogenesis. Here we report crystal structures for hPreP alone and in complex with Aβ, which show that hPreP uses size-exclusion and charge complementation for substrate recognition. These structures also reveal hPreP-specific features that permit a diverse array of peptides, with distinct distributions of charged and hydrophobic residues, to be specifically captured, cleaved, and their amyloidogenic features destroyed. SAXS analysis demonstrates that hPreP in solution exists in dynamic equilibrium between closed and open states, with the former being preferred. Furthermore, Aβ binding induces the closed state and hPreP dimerization. Together, these data reveal the molecular basis for flexible yet specific substrate recognition and degradation by hPreP.
Keywords: Alzheimer's disease, M16 metalloproteases, Mitochondria
Introduction
Throughout all domains of life, regulated proteolysis ameliorates the effects of protein damage, misfolding, and aggregation (Powers et al., 2009). Unlike canonical protein-protease networks, M16 metalloproteases, which are Zn2+-dependent and ATP-independent, do not select substrates on the basis of post-translational modifications or embedded degradation tags (Malito et al., 2008; Ravid and Hochstrasser, 2008; Sauer and Baker, 2011). These proteases are vital to an array of biological processes, including clearance of insulin and other peptide hormones by human Insulin Degrading Enzyme (IDE) (Guo et al., 2010), removal of targeting peptides from preproteins by the Mitochondrial Processing Peptidase (MPP, also a component of the cytochrome bc1 complex in plants) (Taylor et al., 2001; Xia et al., 1997), and the catabolism of hemoglobin by Falcilysin (Fln) in the malaria parasite (Murata and Goldberg, 2003). Structural studies reveal M16 proteases share a conserved architecture of two homologous ∼50kDa domains enclosing a large catalytic chamber. Three families, M16A-C, have been characterized on the basis of the connection between these two domains. In M16A, a short loop connects the two domains (Shen et al., 2006), whereas an extended helical linker joins them in M16C (Johnson et al., 2006). M16B proteases lack a linker as the two subunits arise from distinct genes (Taylor et al., 2001) (Figure 1A). As several M16 substrates, e.g. insulin and amyloid-β (Aβ), are involved in the pathogenesis of human disease, dissecting the operating logic of M16 proteases has been a subject of numerous investigations (Falkevall et al., 2006; Manolopoulou et al., 2009).
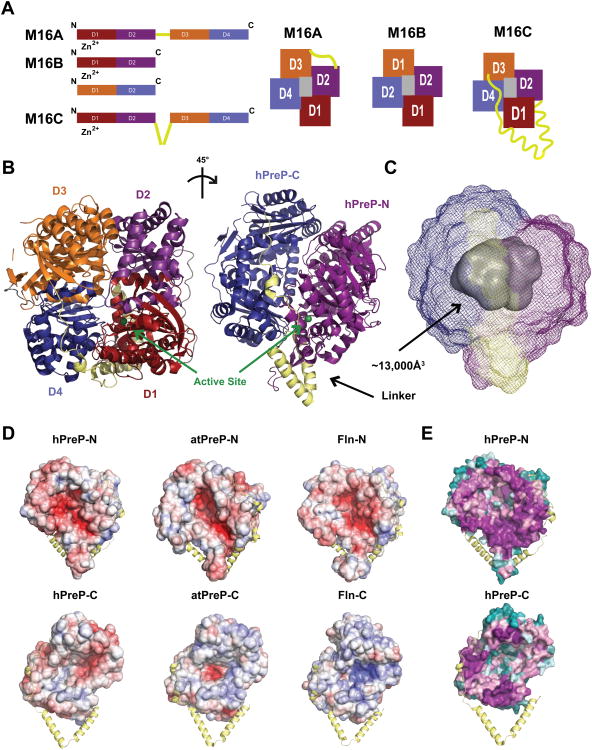
Figure 1. Conserved Mechanisms for Substrate Capture by hPreP.
(A) M16 metalloprotease domain organization. (B) Crystal structure of hPreP (C) Volumetric analysis of the hPreP catalytic chamber, performed with 3V (Voss and Gerstein, 2010). (D) Electrostatic surface representations of hPreP, atPreP (PDB: 2FGE) and Fln (PDB: 3S5H) catalytic chambers at ±6 kT/e, performed with APBS (Baker et al., 2001). Positive surfaces are blue, negative ones red, and neutral ones white. (E) Analysis of M16C catalytic chamber conservation, performed with ConSur (Ashkenazy et al., 2010). Positions are colored on a sliding scale from magenta (most conserved) to teal (degenerate). See also Figure S1 and S2.
Human Presequence Protease (hPreP) is a 117 kDa M16C enzyme that is widely expressed in human tissues (Mzhavia et al., 1999). HPreP primarily localizes to the mitochondrial matrix, where it cuts an array of peptides into recyclable fragments (Alikhani et al., 2011a; Chow et al., 2009; Falkevall et al., 2006). Its substrates include N-terminal mitochondrial targeting peptides or presequences, the clearance of which is vital to proteostasis, as these peptides can insert into mitochondrial membranes, disrupting their electrical potential and uncoupling respiration (Koppen and Langer, 2007; Mossmann et al., 2012). Consistent with this idea, double knockout of A. thaliana PreP (atPreP) 1 and 2 results in a delayed growth phenotype, while yeast PreP deletions display impaired growth on a non-fermentable carbon source under aerobic conditions (Kambacheld et al., 2005; Nilsson Cederholm et al., 2009). Substrate recognition and degradation by PrePs is modeled from the closed-state structure of atPreP, which uses a negatively charged catalytic chamber to engulf substrates peptides of up to ∼65 residues while excluding larger, folded proteins (Falkevall et al., 2006; Johnson et al., 2006; Stahl et al., 2005). Within the chamber, a co-purified peptide was observed, revealing catalysis requires close apposition of conserved residues on both the N and C domains. In the closed state, substrates cannot enter the catalytic chamber and reaction products cannot exit. However, the nature of the conformational switch(es) that PrePs undergo to permit substrate capture is not well understood. HPreP's architecture is expected to be similar to atPreP based on 27% sequence identity. However, hPreP functions in radically different biological contexts from atPreP and correspondingly has distinct substrate cleavage patterns. Thus, hPreP likely employs a unique set of substrate recognition and degradation principles (Chow et al., 2009; Johnson et al., 2006; Malito et al., 2008).
Intriguingly, hPreP degrades several functionally relevant Aβ species (Aβ40, Aβ42, and AβArctic), which are generated by β- and γ-secretase cleavage of the amyloid precursor protein (Falkevall et al., 2006). Aβ aggregates are toxic to the neuron and play a key role in Alzheimer's disease (AD) pathogenesis through intra- and extracellular signaling pathways. Mutations in Aβ production pathways, e.g., at γ-secretase and amyloid precursor protein loci, can lead to Aβ accumulation and familial AD (Huang and Mucke, 2012; Yankner and Lu, 2009). Defects in Aβ clearance pathways may be important to both familial and sporadic or late onset AD (Malito et al., 2008; Saido and Leissring, 2012). Joint action of proteases, including IDE, neprilysin, and cathepsin B, clears Aβ from the extracellular matrix, cytosol, ER, Golgi, endo- and lysosomes (Farris et al., 2003; Iwata et al., 2001; Mueller-Steiner et al., 2006). Recent studies indicate hPreP is the Aβ-degrading protease in mitochondria (Alikhani et al., 2011b; Falkevall et al., 2006; Pinho et al., 2010). Immuno-depletion of hPreP in human brain mitochondria prevents degradation of mitochondrial Aβ; additionally, hPreP activity is diminished in AD brains compared to age-matched controls. Mitochondrial lesions are implicated in AD pathogenesis, and mitochondria may be a site of Aβ accumulation and toxicity through inhibition of Aβ-binding alcohol dehydrogenase and increased production of reactive oxygen species (Lustbader et al., 2004; Manczak et al., 2006). These data suggest a molecular appreciation of hPreP's operating logic can inform our understanding of AD pathogenesis.
While IDE uses its catalytic chamber to specifically recognize and cleave amyloidogenic peptides, the molecular basis for Aβ destruction by hPreP remains elusive. Here we describe the crystal structure of hPreP, which reveals that size-exclusion and charge-complementation are key principles for substrate capture, and identifies those residues within the catalytic chamber used for recognition of diverse peptide motifs. We further show that the dynamics of substrate capture are modulated by the enzyme's unique linker region. Crystallographic and MS analyses reveal how hPreP specifically binds Aβ by relaxing the requirement for a defined orientation of the peptide in the catalytic cleft and using both a hydrophobic exosite and S1 pocket to recognize and degrade Aβ in a selective, non-processive manner. Finally, SAXS analysis reveals the nature of the closed-to-open conformational switch that permits substrate entry into the catalytic chamber, and shows that substrate binding can induce domain closure and dimerization. Together these data reveal the mechanisms hPreP employs for recognition and degradation of amyloidogenic peptides.
Results and Discussion
Crystal Structure of hPreP
To elucidate the molecular basis for hPreP substrate recognition, we determined the crystal structure for isoform 1 of substrate-free hPreP in a closed conformation at 2.0Å (Figure 1B, Table 1). Two key modifications were required to obtain diffracting crystals: surface lysine methylation, which reduced catalytic activity but did not impact protein purity, and introduction of an active site E107Q substitution, which renders hPreP catalytically inactive but substrate-binding competent (Figure S1) (Johnson et al., 2006; Rypniewski et al., 1993). Iodine-based SAD phases were crucial for removing phase bias of the initial molecular replacement solution, and drastically improved the electron density map in key regions, e.g., the hPreP linker, which were otherwise disordered (Figure S2) (Abendroth et al., 2011). Displaying an overall architecture that is highly conserved across the M16C family, hPreP is composed of hPreP-N (aa 33-509) and hPreP-C (aa 576-1037) domains, which are connected by an extended helical hairpin (aa 510-575). HPreP-N and hPreP-C can be further broken down into topologically similar D1-4 (Figure 1B). Although D1-4 superimpose poorly onto one another, pairs D1 (aa 33-288) and D3 (aa 576-844) (RMSD=3.9Å for 862 atom pairs) and D1 and D4 (855-1037) (RMSD=4.2Å for 732 atom pairs) superimpose best onto one another. The other four pairwise comparisons yield RMSDs greater than 5.1Å. This is consistent with ancient gene duplication that used D1 as a template for hPreP-C.
Table 1. Data Collection and Refinement Statistics.
| Crystal | hPreP E107Q | hPreP E107Q + Aβ40 |
|---|---|---|
| PDB ID | 4L3T | 4NGE |
| Data Collection | ||
| Beamline | APS 19ID | APS 19ID |
| Wavelength (Å) | .97895 | 1.045 |
| Space group | C2 | C2 |
| Unit Cell Parameters | ||
| a, b, c (Å) | 245.8, 85.1, 158.5 | 247.3, 86.2, 158.6 |
| α, β, γ (°) | 90, 127.5, 90 | 90, 127.6, 90 |
| Molecules in ASU | 2 | 2 |
| Resolution (Å) | 50 – 2.03 | 50 – 2.70 |
| Rsym (%)a | 8.6 (58.5)b | 18.0 (59.7) |
| I/σ | 17.1 (2.8) | 8.59 (1.8) |
| Redundancyc | 4.1 (3.8) | 2.9 (2.4) |
| Completeness | 98.2 (97.0) | 99.1 (98.2) |
| Unique Reflections | 163827 (8053) | 71876 (3557) |
| Heavy Atom Sites | 54 | 5 |
| Figure of Merit | .83 | .82 |
| Refinement | ||
| Rwork (%)d | 17.1 | 19.1 |
| Rfree (%)e | 21.0 | 23.2 |
| RMS Deviations | ||
| Bond lengths (Å) | .012 | .004 |
| Bond angles (°) | .99 | .85 |
| Number of | ||
| Protein atoms | 15892 | 15986 |
| Solvent molecules | 1154 | 68 |
| Metal cofactors | 2 | 2 |
| Ramachandran plot (%) | ||
| Preferred region | 98.17 | 97.09 |
| Allowed region | 1.83 | 2.91 |
| Disallowed region | 0 | 0 |
| B-factors (Å2) | ||
| Protein | 35.3 | 44.5 |
| Solvent | 46.4 | 49.6 |
Rsym = Σj|<I>-Ij|Σ<I> where Ij is the intensity of the jth reflection and <I> is the average intensity
Values in parentheses indicate the outer resolution shell
Nobs/Nunique
Rwork = Σhkl|Fobs - Fcalc|/Σhkl|Fobs|
Rfree calculated as Rwork, but on 5% data excluded from refinement
The hPreP structure demonstrates that substrate selection by size-exclusion is a conserved mechanism of M16C proteases. HPreP-N and -C enclose a large ∼13,300Å3 chamber for engulfing substrates (Figure 1C). Formation of the active site requires close apposition of hPrePN and –C, whereby the inverted zinc-binding motif on D1 (H104XXEH…E205) and residues R900 and Y906 on D4 form a cleft within the chamber for substrate binding and catalysis (Figure S2) (Johnson et al., 2006). As there is no portal for substrate access in this conformation, substrates too large to fit into the enclosed chamber are excluded on the basis of their size. Available M16C structures, atPreP and Fln, have similarly sized chambers (∼13,000Å3 and ∼12,500Å3, respectively), indicating that the upper limit for substrate size determined for atPreP, ∼65 residues, is conserved across species (Figure S2) (Moberg et al., 2003; Stahl et al., 2005).
Electrostatic interactions play a key role in M16 substrate capture (Johnson et al., 2006; Manolopoulou et al., 2009; Ralat et al., 2010). Interestingly, we observed that while the N-half of M16C chambers are uniformly negatively charged, the C-half is variably charged between species (Figure 1D). The surface of Fln-C is mostly positively charged. HPreP-C has a weakly, variably charged surface while atPreP-C contains a negatively charged pocket surrounded by a weakly positive surface. Furthermore, PreP-N is composed of highly conserved residues while PreP–C is largely degenerate except for those residues that contribute to the catalytic cleft or form contacts with hPreP-N (Figure 1E). These data support a model for substrate selection by unipolar charge complementation between hPreP–N and substrates. The unique charge distribution in hPreP's catalytic chamber may explain why hPreP fails to degrade intact insulin, as hPreP-C lacks a positively charged surface to trap negatively charged insulin in the chamber (Falkevall et al., 2006; Manolopoulou et al., 2009).
Topography of the Catalytic Chamber
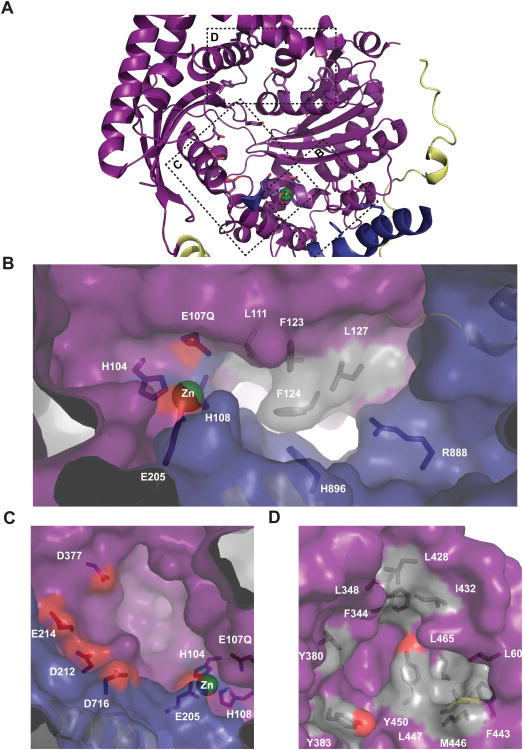
Characteristics of the hPreP catalytic chamber provide a structural basis for the protease's observed substrate degradation patterns (Figure 2A) (Chow et al., 2009). The hPreP-N chamber contains two deep pockets near the active site (Figure 2B). A hydrophobic pocket, consisting of L111, F123, F124, and L127, forms an S1 or S′1 site immediately adjacent to the active site, which explains the preference for bulky hydrophobic residues immediately adjacent to the scissile bond. A second, basic pocket comprised of R888 and H896 12-16Å away from the catalytic zinc, can bind substrate C-termini via salt bridges (Figure 2B). The existence of these pockets explains why cleavage sites with hydrophobic residues in the P1 or P′1 positions can be found 2-5 residues from the C-terminus (Chow et al., 2009; Falkevall et al., 2006). Furthermore, these explain why, despite a high degree of architectural similarity (Figure S2), hPreP has distinct substrate preferences from atPreP, which uses an acidic S′1 pocket to bind basic residues in the P′1 position (Johnson et al., 2006; Stahl et al., 2005). These unique features of hPreP's catalytic cleft may also explain how hPreP effectively degrades peptides as small as leuenkephalin, a pentapeptide, while the minimal substrate length for atPreP is proposed to be 11 residues (Chow et al., 2009; Stahl et al., 2005).
Figure 2. Residues in the hPreP Chamber Facilitate Substrate Recognition.
(A) HPreP-N catalytic chamber. (B) Two pockets (L111, F123, F124, and L127; and H896 and R888) explain observed cleavage site preference for P1 or P′1 hydrophobic residues, and scissile bonds 2-5 residues distal from substrate C-termini. (C) Labeled acidic residues are proximal to the active site and can facilitate interaction with basic substrate residues (D) A network of hydrophobic resides in hPreP-N permits capture of hydrophobic residues.
Presequences are diverse in sequence but have similar physiochemical properties in that they are enriched in positively charged and hydrophobic residues segregated on opposite faces of amphipathic α-helices (Moberg et al., 2004). In the hPreP chamber, residues D716, D212, E214, and D377 form a contiguous, negatively charged surface 15-19Å away from the active site (Figure 2C). Not only would these residues contribute to attracting positively charged residues in presequences, but their arrangement also explains why hPreP prefers arginines 3-5 residues distal to the scissile bond (Chow et al., 2009). Hydrophobic clusters are also present inside the hPreP-N chamber. These include those formed by L60, F443, M446, and L447; by Y380, Y383 and Y450; and by F344, L348, L428 and I432 (Figure 2D). The presence of these residues suggests hydrophobic surfaces facilitate recognition of substrates diverse with respect to the precise location of hydrophobic residues.
Mutational Analysis of the Linker Region
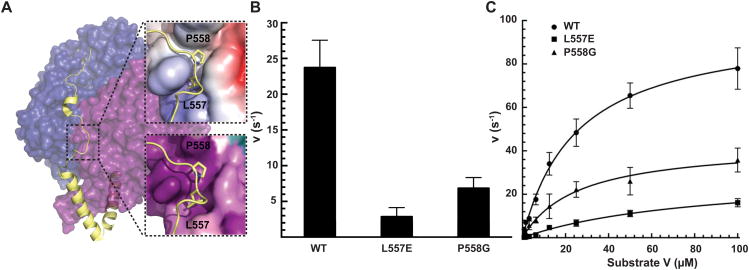
An extended helical hairpin, originating in D2 and stretching across the surface of hPreP to terminate in D3, is the distinguishing structural feature of the M16C family (Figure 1A-B). The importance of the linker for catalytic efficacy and allostery of IDE, an M16A enzyme, is documented (McCord et al., 2013). The PreP linker is likely involved in bringing PreP-N and PreP-C into close apposition for efficient catalysis (Johnson et al., 2006). To test this, we targeted P558 and L557. Both are highly conserved and rest in a conserved hydrophobic pocket on D1 (Figure 3A and S3). We generated mutations to assess the importance of proline's restricted dihedral freedom (P558G) and of the hydrophobic pocket's integrity (L557E) to catalysis. We found that L557E and P558G exhibited ∼20% and 40% the specific activity of WT hPreP, respectively (Figure 3B). Kinetic analysis revealed that both mutants have a reduced Vmax, and L557E has an increased Km (Figure 3C). Fluorescence thermal shift confirmed these effects are specific to hPreP kinetics, as mutants display melting curves and Tm values similar to WT, ruling out the possibility that mutants are simply destabilized (Figure S3). These findings support a role for the linker in catalytic competency, likely by modulating the open-closed conformational switch that is required for efficient catalysis.
Figure 3. Role of the Linker in Catalysis.
(A) L557E and P558G sit in a conserved hydrophobic pocket on the D1 surface. The lower inset is colored by electrostatic surface; the upper one, by conservation. (B) Specific activities of hPreP WT and mutants at an enzyme concentration of 1.25 nM. Activity was determined by monitoring the cleavage of 0.5μM Substrate V at 37 °C. (C) WT and mutant hPreP specific activities at indicated Substrate V concentrations. Datasets were fit to the Michaelis-Menten equation, with Vmax (98, 29, 43 s−1) and Km (26, 82, 26 μM), calculated for WT, L557E, and P558G, respectively. Mean ± SD represents at least 3 experiments. See also Figure S3.
MS Analysis of Aβ Degradation by hPreP
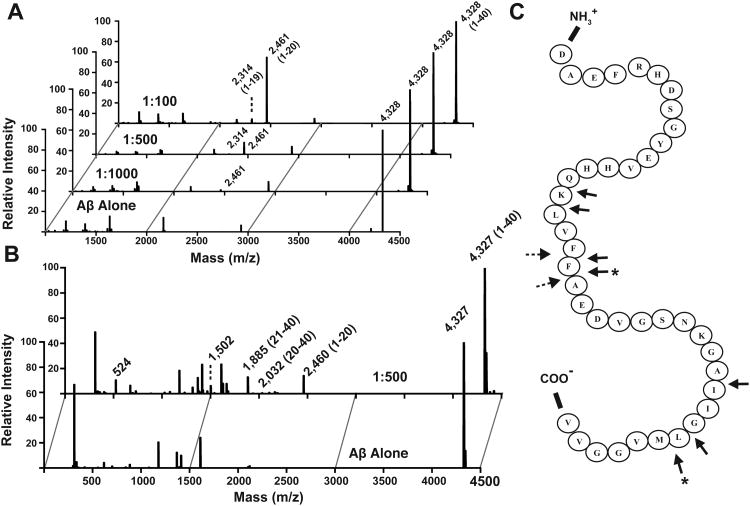
Using Aβ as a model substrate, we examined the sequence of cleavage events that follow substrate capture to resolve whether (1) hPreP degrades substrates stochastically and (2) degradation proceeds along a defined path. Based on previous LC-MS/MS data, the cleavage sites of Aβ40 by hPreP could occur after Q15, K16, F19, F20, A30, G33, and L34 (Falkevall et al., 2006). However, a separate study identified cleavage sites to be restricted to sites following F20 and L34 (Figure 4C) (Chow et al., 2009). Falkevall et al. used prolonged incubation (1 hour) while Chow et al. did not report precise experimental conditions. To discriminate full-length Aβ cleavage sites from those that arise from subsequent cleavages of reaction products, we quenched the Aβ degradation reaction rapidly (∼1 sec). MS and MS2 analysis of the resultant Aβ40 fragments using MALDI-ToF/ToF revealed that Aβ1-20 is the single major cleavage product, increasing in abundance at higher hPreP concentrations (Figure 4A, Table 2). A secondary fragment, Aβ1-19, became noticeable only at high hPreP concentrations. However, we could not detect the corresponding C-terminal Aβ fragments. We then turned to Q-ToF LC-MS/MS to ensure comprehensive identification of degraded Aβ fragments with high mass accuracy. In these spectra, Aβ1-20 and Aβ21-40 are the dominant species observed, and Aβ20-40 the minor one (Figure 4B, Table 2 and S2). Thus, hPreP prefers to cut between F20 and A21 while cleavage between F19 and F20 is a secondary cut site, which reveals Aβ degradation by hPreP is not a stochastic process. The fact that we did not observe most of the previously identified cleavage sites suggests that these are used after Aβ degradation products are available to serve as substrates (Figure 4C). From these data we deduce that Aβ in the hPreP chamber is cleaved once and the resulting products subsequently released. Thus, hPreP degrades Aβ in a selective manner that is not processive.
Figure 4. Mass Analysis of Aβ-degradation by hPreP.
(A) MALDI-ToF/ToF and (B) deconvoluted Q-ToF mass spectra of Aβ40 alone (lower panel) and hPreP-degraded Aβ40 (upper panels). Aβ40 and hPreP were mixed at the indicated molar ratios (1:100-1000). (C) Schematic of hPreP's Aβ40 cleavage sites. Filled arrows denote sites identified by (Falkevall et al., 2006), while dashed arrows indicate cleavage sites identified in this study. Asterisks mark cleavage sites reported by (Chow et al., 2009). See also Figure S4.
Table 2. ToF-Tof and Q-ToF Mass Spectrometry analysis of hPreP degraded Aβa.
| ToF-ToF | ||||
|---|---|---|---|---|
| Aβ | Mobsb | Mcalcb | Errorc | b/y Ions |
| 1-40 | 4328.7 | 4328.2 | -0.5 | N/A |
| 1-20 | 2461.4 | 2461.2 | -0.3 | b5-9,b11-16, b18,b19,y7, y8,y10,y12-20 |
| 1-19 | 2314.4 | 2314.1 | -0.3 | b5-b8,b10-16, b18,y6,y7,y11-19 |
| Q-ToF | ||||
| Aβ | Mobs | Mcalc | Errord | b/y Ions |
| 1-40 | 4327.117 | 4327.148 | -7 | b2,b6,b7,b9,b13-b15,b17,b20-b27,b30-b35,b38, y1-y6,y8-y9,y11,y18-y20, y23-y25,y28-y33 |
| 1-20 | 2460.094 | 2460.161 | -27 | b2,b5-b13,b15-b19, y1-y3,y5,y7-y14,y17-y18 |
| 20-40 | 2032.032 | 2032.066 | -17 | b2-b3,b5,b11-b20, y1-y2,y6-y7,y9-y21 |
| 21-40 | 1884.989 | 1884.998 | -5 | b2-b19,y1-y2,y3-y9, y11-y16,y18-y20 |
Outside Aβ1-40, peaks that exist in Aβ alone are not listed. Those peaks that match predicted Aβ fragments but cannot be confirmed by MS\MS are listed in Table S1
[M+H]+
Error (in Da)
Error (in ppm) = ((Mobs - Mcalc)/Mcalc)(106)).
We also used our MS data to estimate hPreP's catalytic efficiency. Previously, the kcat for Aβ degradation was estimated at ∼0.06 sec−1, with a Km ∼ 2μM using Aβ as an alternate substrate inhibitor for a fluorescent peptide (Chow et al., 2009). Surprisingly, our Q-ToF data show hPreP degrades Aβ40 at a rate of ∼300 sec−1 (Figure 4B, Table S2). Our ToF-ToF data showed a similarly high rate, ∼25-300 sec−1 across different hPreP concentrations (Figure S4, Table S2). This rate is 2-3 orders of magnitude higher than previously reported (Chow et al., 2009), suggesting that hPreP is a highly efficient protease.
Structure of hPreP in Complex with Aβ
To elucidate the structural basis for specific hPreP recognition and degradation of Aβ, we determined the structure of the hPreP E107Q:Aβ40 complex at 2.70Å by MR/SAD, using the arsenic moiety of cacodylated cysteines as the heavy atom (Table 1 and Figure S2) (Dyda et al., 1994). Aβ, bound to the hPreP-N chamber (Figure 5A), was visible by inspection of the initial MR/SAD solution and omit maps (Figure S5). We observed two bulky side chains in the electron density immediately adjacent to the scissile bond, which, as revealed by our mass spectrometry data, correspond to F19 and F20 (Figure 4 and S5). Based on this assignment, we built the remaining visible Aβ residues (Q15-D23), which produced a good fit to the observed density (Figure 5D). The geometry of the active site is such that the peptidyl bond between Aβ F20 and A21 is ideally positioned for hPreP's catalytic base E107—whose ε1 and ε2 oxygens would be 5.0 and 4.0Å away, respectively, based on the position of Q107—to deprotonate a water molecule for nucleophilic attack on the scissile bond (Figure 5D) (Johnson et al., 2006; Shen et al., 2006).
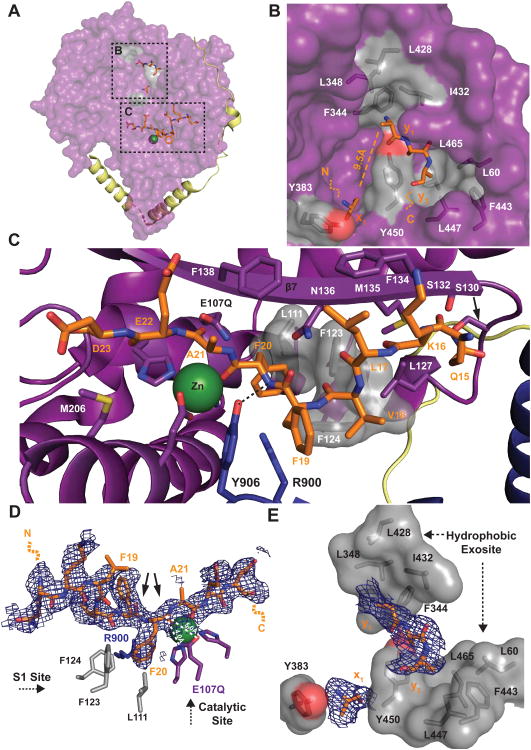
Figure 5. Structural Basis of Aβ Capture and Recognition by HPreP.
(A) Aβ-binding to hPreP-N. (B) Hydrophobic exosite. Four Aβ residues (x1, y1-3) are observed. Hydrophobic residues that comprise the putative side chain binding pockets are displayed in gray. (C) Aβ-binding to the hPreP catalytic cleft. The S1 site consists of L111, F123, F124, and R900. (D-E) 2Fo-Fc electron density maps contoured at 0.5σ for Aβ residues bound to (D) the catalytic cleft and (E) the hydrophobic exosite. Arrows denote preferred Aβ cleavage sites by hPreP. See also Figure S5.
HPreP uses a unique complement of structural features to recognize Aβ in the catalytic cleft. First, hPreP binds Aβ in an orientation opposite to those previously observed in substrate-bound M16 structures (Figure 5C) (Johnson et al., 2006; Shen et al., 2006; Taylor et al., 2001). The relaxed requirement for substrate-orientation in the cleft demonstrates an unexpected degree of flexibility in the manner by which hPreP binds substrates. Second, hydrophobic interactions coordinate substrates in the active site. A hydrophobic pocket formed by L111, F123, and F124 forms a highly conserved S1 site for Aβ F20 in the P1 position (Figure 1E, 5C-D). Interestingly, hPreP repurposes R900 and Y906, which were found to coordinate atPreP substrate backbone carbonyls and are required for efficient catalysis (Johnson et al., 2006). R900 completes the S1 site through a cation-π interaction with Aβ F20, while Y906 forms a hydrogen bond with F20's backbone amine (Figure 5C-D). Finally, hPreP uses a β-strand (β7) to coordinate Aβ in the cleft in a manner that specifically impairs Aβ's ability to aggregate (Figure 5C). While Aβ monomers are largely unstructured, structures of Aβ fibrils seeded from synthetic and human sources show that formation of parallel, intermolecular β-sheets involves the same amyloidogenic segment of Aβ, KVLFF, that we observe binding to the hPreP catalytic cleft (Balbach et al., 2000; Lu et al., 2013; Paravastu et al., 2008; Tjernberg et al., 1996). Thus, the Aβ–bound hPreP structure reveals that hPreP is specialized to recognize and cleave the amyloidogenic segment of Aβ that is prone to form β-sheets, thereby preventing Aβ aggregation in mitochondria.
Interestingly, we observed four unknown Aβ residues binding to a pocket on hPreP-N in both the MR/SAD solution and composite omit maps (Figure 5A and S5). We were unable to resolve these residues' side chains, which indicates that this site anchors multiple Aβ segments. The first of these residues (x1) is separated by 9.5Å (∼3 residues) from the N-terminal of the remaining three residues (y1-3) (Figure 5B). A single bulky side chain is visible in the electron density protruding from y1 (Figure 5E), which is consistent with binding of bulky hydrophobic residues from either the Aβ-N or -C terminus (Figure 4C). Based on this area's enrichment in hydrophobic residues (specifically: L348, L428, I432 and F344; and L465, L60, F443, L447, and Y450) and the presumptive orientation of the Aβ side chains towards them, we term it the “hydrophobic exosite.” This site is likely an evolutionarily recent development, as its conservation is relatively weak (Figure 1E). We propose that the hPreP exosite plays a role in anchoring larger peptides (≥ ∼40 residues) in the chamber, enabling their efficient degradation (Manolopoulou et al., 2009; Ralat et al., 2010). This hydrophobic clamp would confine the interaction of Aβ with hPreP, explaining why the primary cleavage site is between Aβ20 and Aβ21. The existence of this site also offers an explanation for how hPreP is able to efficiently capture Aβ despite Aβ carrying a net negative charge (PI = 5.4) in the mitochondrial matrix (pH ∼ 7.8) that would otherwise be repelled by hPreP-N (Porcelli et al., 2005).
Conformational Profile of hPreP in Solution
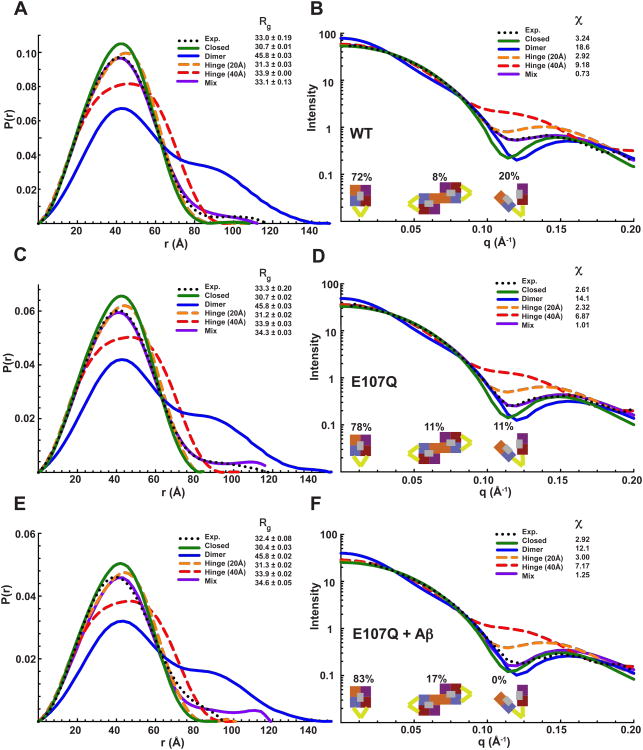
To identify the nature of the conformational switch that permits substrate capture and assess the effect of substrate binding on hPreP dynamics, we analyzed the conformational states of hPreP in solution with SAXS. The observed Rg and Dmax (33 and 117Å, respectively) for WT hPreP were inconsistent with the parameters predicted for a population of closed-state monomers (Rg = 31Å, Dmax = 108Å) or dimers (Rg = 46Å, Dmax = 149Å) (Figure 6A, B). These findings agreed with the poor fit of theoretical scattering curves for closed monomers (χ = 3.2) and dimers (χ = 18.6) to the experimental data, and the lack of intensity dip between 0.09-0.15Å−1. Analysis of the hPreP E107Q yielded similar results (Figure 6C, D), and we conclude that hPreP does not exist exclusively as a closed monomer or dimer in solution.
Figure 6. Conformational Profiles of hPreP in Solution.
Pair distribution functions and scattering curves for WT (A and B), E107Q (C and D), and E107Q + Aβ (E and F). Curve fitting was performed based on atomic models input to CRYSOL (single models) and OLIGOMER (multiple models). The volume fractions shown below the profiles indicate the percent composition by conformational state in solution that yielded the line of best fit for the observed data (mixture). See also Figure S6.
To directly assess the likelihood that hPreP populates multiple states in solution, we modeled open hPreP conformations, systematically increasing the displacement between hPrePN and hPreP-C from 20Å to 50Å, and assessed their ability to account for the experimental data. We tested ‘Parallel Spring’, ‘Hinge’ (a D1/D4 pivot resembling the motion seen in M16A pitrilysin), and ‘Open Book’ (a D2/D3 pivot resembling the motion seen in M16B MPP) motions (Figure S6), but found that no single open state yielded a good fit (χ < 2.0) to the data. We found that the hinge motions reliably produced better fits to the data than other types of motion with equivalent degrees of opening (Figure S6). By testing mixtures of closed monomers, dimers, and 20-50Å hinge models, we were able to generate an excellent fit to the WT data (χ = 0.73) whose fractional occupancy consisted of ∼72% closed monomers, ∼8% closed dimers, ∼20% 40Å hinges (Figure 6A-B, and S6). A similar combination of states yielded the line of best fit for the E107Q mutant, except we observed a higher propensity for closed or partially closed states (Figure 6C-D). Together, these data reveal that hPreP is predominantly a closed monomer, which is consistent with our size exclusion data, with at least one open state in solution (Figure S1). Given the resolution limit for SAXS and the similarity of scattering curves for 20Å parallel spring, hinge, and open book (Figure S6) motions, the precise nature of the movements underlying the open state(s) of hPreP and their role in the hPreP catalytic cycle remain to be determined.
We next assessed whether substrate binding could modify the equilibrium between open and closed hPreP states in solution. Analysis of the scattering curve for the hPreP E107Q:Aβ40 complex revealed that the best fit to the data could be obtained using a combination of monomers (83%) and dimers (17%) exclusively in the closed state (χ = 1.3) (Figure 6E-F, and S6). The fit could not be improved with the addition of ‘open’ states (Figure S6). The fraction of dimers observed in the substrate-bound solution doubled compared to WT, showing that substrate binding can induce hPreP dimerization (Figure 6B and F). These results agreed with the appearance of an intensity dip from 0.09-0.15Å−1 in the experimental data (Figure 6F). Together with the finding that domain closure occurs in the absence of substrate (Figure 1B, 6A-B), these results show substrate binding is sufficient, but not necessary, to induce domain closure, the consequence of which is to lock the enzyme in a catalytically competent state (Aleshin et al., 2009; Johnson et al., 2006).
Comparison of hPreP and IDE
While hPreP and IDE belong to distinct M16 families, they have been considered to be the same functional class in terms of their ability to recognize and degrade amyloidogenic peptides (Aleshin et al., 2009). The structures of hPreP alone and in complex with Aβ confirm that hPreP and IDE both select substrates on the basis of their size, shape, and charge distribution. Furthermore, both hPreP and IDE target Aβ amyloidogenicity for destruction. However, the mechanism for Aβ degradation by hPreP is dramatically different from IDE. This difference may be due to the distinct nature of their exosites. The hPreP exosite recognizes a hydrophobic patch of Aβ or other substrate and acts in concert with the catalytic cleft to constrain cleavage sites. The hydrophobic exosite likely binds unfolded peptide segments, as folded proteins usually do not present flexible, hydrophobic surfaces for capture. This would explain why hPreP prefers to degrade structurally disordered peptides (Falkevall et al., 2006). Consequently, hPreP preferentially cuts Aβ at a specific site non-processively. By comparison, the IDE exosite anchors substrate N-termini via hydrogen bonds. This enables IDE substrates such as insulin, TGF-α, and MIP-1α, to retain defined tertiary structure within the chamber after capture. As the unfolding of these proteins within the IDE chamber is required to stabilize the catalytic cleft, the cleavage of these peptides occurs stochastically at a few pre-defined sites and cleavage proceeds processively (Guo et al., 2010; Manolopoulou et al., 2009; McCord et al., 2013; Ren et al., 2010). Our data also indicate that hPreP is 2-3 fold more efficient than IDE based on the measured specific activities of ∼20 s−1 vs. 10 s−1 for substrate V and ∼25 s−1 vs. 8 s−1 for Aβ (Figure 3B) (Im et al., 2007; McCord et al., 2013). This points towards the utility of both hPreP and IDE as targets for controlling Aβ-load in AD patients. Finally, the oligomerization and conformations of these two enzymes are distinct. HPreP is predominately monomeric and closed while IDE is a dimer that predominantly occupies open states in solution (McCord et al., 2013). Thus, hPreP and IDE likely target distinct sets of substrates in vivo, and hPreP should not be considered to be mitochondrial IDE.
Conclusion
The biology of hPreP is poorly understood. However, high throughput proteomics approaches indicate that hPreP undergoes post-translational modification by phosphorylation and participates in protein-protein interactions with key components of the citric acid cycle (Havugimana et al., 2012; Olsen et al., 2010), either or both of which could enhance the clearance of toxic peptides that impair mitochondrial function. Herein we have developed a molecular picture of hPreP substrate recognition and degradation that provides the structural context for future studies to unravel the roles hPreP in health and disease. These data show that hPreP posses a unique complement of features that enable it to effectively recognize and degrade substrates in a manner that is both flexible, eschewing strict requirements for substrate size, sequence and physiochemical profile, yet specific, utilizing defined structural features to destroy amyloidogenic peptides.
Experimental Procedures
HPreP Cloning, Expression, and Purification
HPreP cDNA was acquired from the Human ORFeome (Open Biosystems) and subcloned into an E. coli expression vector, pProEx, replacing its mitochondrial targeting sequence (aa 1-33) with an N-terminal His6-tag, which we refer to as wild-type (WT). HPreP mutations were generated from this template using a Stratagene QuikChange site-directed mutagenesis kit and verified by sequencing. HPreP WT and E107Q were expressed in Rosetta(DE3) E. coli at 25 °C with 300μM IPTG induction for 20 hours. HPreP mutants were induced with 30μM IPTG for 8 hours at 20 °C (Figure S1 and S3). Proteins were then purified over Ni-NTA affinity, Source Q anion exchange, and Superdex 200 gel filtration columns. Purified samples were flash-frozen in liquid nitrogen and stored at −80 °C. Sample purity was assessed by SDS-PAGE (Figure S1).
Synthetic Aβ40 Production
Aβ40 was synthesized at a 0.25 mmol scale using Fmoc and HBTU/HOBt chemistry on an Applied Biosystems 433A instrument. To increase peptide solubility and facilitate purification, we used a modified version the method described by (Sohma et al., 2004), described in the supplementary experimental procedures. Aβ40 was purified by RP-HPLC, lyophilized, and stored at −20 °C under Argon.
Protein Crystallization
To generate hPreP crystals, hPreP E107Q was subjected to reductive surface lysine methylation (KMe) after anion exchanger or gel filtration (Rypniewski et al., 1993), then further purified by gel filtration. The initial condition was identified from high-throughput crystallization screens using commercially available kits and the Mosquito® platform. Crystals were optimized for diffraction by precipitant, pH, buffer, and additive screening (Figure S1). Crystals were grown at 18° C by hanging-drop vapor diffusion by combining 6 mg/ml KMe hPreP-E107Q in buffer containing 20 mM HEPES pH 7.5, 250 mM NaCl, and 2 mM DTT, with mother liquor containing 15.2% (w/v) PEG 8,000, 15 mM TCEP, 80 mM sodium cacodylate pH 6.5, 160 mM calcium acetate, and 20% (v/v) glycerol in a 1:1 (v/v) ratio. Within a week, crystals reached maximal size, ∼0.1mm in the longest dimension. To obtain the hPreP:Aβ complex, KMe hPreP E107Q was incubated with a five molar excess of Aβ40 for one hour at 18 °C, further purified by gel-filtration, and crystallized as described above.
Data Collection and Structure Determination
Crystals were cryoprotected in mother liquor containing 30% (v/v) glycerol, then flash frozen in liquid nitrogen. Diffraction data were collected at beamline 19ID at Argonne National Laboratory and processed using HKL3000 (Minor et al., 2006). A low quality initial map (Rfree = 48%) was obtained using a homology model of hPreP generated by I-TASSER (Roy et al., 2010) as a molecular replacement search model, followed by executing autobuild in PHENIX (Adams et al., 2010). The electron density map and refinement statistics were considerably improved by combining the phase information from MR with that derived from SAD using Iodide as the heavy atom after briefly soaking the crystals in 200mM KI for <30 seconds (Table 1, Figure S2). By inspection of the anomalous difference map, we discovered dimethylarsenic covalently bound to several cysteine residues (Figure S2) (Dyda et al., 1994). We took advantage this to solve the Aβ-bound structure by MR/SAD using substrate-free hPreP as the search model and Arsenic as the heavy atom (Table 1). Phasing and refinement were performed in PHENIX. Manual rebuilding and editing were done in COOT (Emsley and Cowtan, 2004). MolProbity (Chen et al., 2010) was used to validate stereochemistry.
Kinetic Assays
HPreP enzymatic activity was quantified at 37 °C by monitoring the cleavage of substrate V (7-methoxycoumarin-4-yl-acetyl-RPPGFSAFK-2,4-dinitrophenyl, R&D Systems), a flourogenic bradykinin mimetic, at an excitation wavelength of 327 nm and emission wavelength of 395 nm on a Tecan Safire microplate reader. To initiate the reaction, 1μL 1.25 μM (125 pm) of hPreP was added to the various concentrations of substrate V in 50 mM KPO4, pH 7.3 to a final volume of 100μL. Cleavage was assessed by measuring fluorescence increase at 10-second intervals, with 9 reads/well/time point. To determine the enzymatic activity, background subtraction and linear regression were used (R2 cutoff = 97%). Specific activities (s−1) were calculated by comparing the maximal fluorescence converted from the known quantity of substrate V by hPreP.
Aβ-degradation Assays
Aβ digestion reactions were performed at 37 °C by adding 500 pmol Aβ40 to 0.5-5 pmol of hPreP in a 10μL reaction system. The reaction was immediately quenched (∼1s) by the addition of 10μL stop solution (170 mM EDTA, 0.07% TFA). Samples were then reconstituted with 5μL 0.1% TFA and purified over a C18 column (Millipore). For MALDI-ToF/ToF analysis samples were then mixed with 2.5μL matrix (CHCA) in 70% ACN, 0.1% TFA, and spotted onto the plate for data collection on an AB Sciex ToF/ToF 5800 machine in positive reflector or linear modes over 800 – 6000 (m/z). Data were analyzed by MMass (Strohalm et al., 2010), fragments matched with FindPept using a 0.5 Da tolerance, and b/y ions identified with Molecular Weight Calculator. Q-ToF LC-MS/MS analysis used 1 pmol hPreP and 500 pmol Aβ40. The reaction system was reconstituted with 10μL 0.1% TFA, analyzed on an Agilent ChipCube II (300SB C18 Zorbax, 43mm × 75μm ID, 5μm particle size, 40 nl trapping column) by elution with 5-65% ACN over 8.0 min at 2.5μl/min. Data were collected over 300-2400 (m/z) on an Agilent 6540 QToF LC-MS/MS machine in ESI+ mode, then analyzed, deconvoluted, b/y ions identified, and fragments matched using a 30 ppm tolerance in MassHunter (Agilent).
SAXS Data Collection and Analysis
Data was collected at Argonne National Laboratory's Advanced Photon Source, beamline 18ID, at 23 °C using a Mar 165 CCD detector, an incident X-ray wavelength of 1.033Å, and protein concentration of 0.5 mg/ml. For Aβ-binding experiments, hPreP was preincubated with two molar excess of Aβ40 for 20 minutes. Data were reduced using custom macros for IgorPro (WaveMetrics, Inc.) written by the BioCAT staff, then analyzed using ATSAS (Petoukhov et al., 2012). We used PRIMUS and GNOM to determine the Rg in reciprocal and real space, respectively. Theoretical scattering curves were calculated for models and fit to the experimental data using CRYSOL, and OLIGOMER was used to determine sample's percent composition by conformational state
Supplementary Material
Highlights.
Crystal structures for human Presequence Protease alone and in complex with Aβ
HPreP substrates are selected by size, shape, and charge distribution
The hPreP catalytic chamber recognizes and destroys Aβ amyloidogenicty
SAXS reveals hPreP open states and substrate binding dynamics
Acknowledgments
We are grateful to Lauren McCord, Raymond Hulse, and Adam Eisenberg for technical assistance, and to Phoebe Rice for critical evaluation of experimental data. We thank the staffs of the SBC and BioCAT of the Advanced Photon Source at Argonne National Laboratory, operated by UChicago Argonne, LLC, for the U.S. Dept. of Energy, Office of Biological and Environmental Research, under contract DE-AC02-06CH1135, for their help in data collection. This research was supported by NIH R01 GM81549 to WJT and NIH P41 GM103622 to BioCAT. JVK, WGL, and WJT designed and performed research, and analyzed the data. KPS, ABS, and SCM contributed reagents, and data collection and analysis expertise. JVK and WJT wrote the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abendroth J, Gardberg AS, Robinson JI, Christensen JS, Staker BL, Myler PJ, Stewart LJ, Edwards TE. SAD phasing using iodide ions in a high-throughput structural genomics environment. J Struct Funct Genomics. 2011;12:83–95. doi: 10.1007/s10969-011-9101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleshin AE, Gramatikova S, Hura GL, Bobkov A, Strongin AY, Stec B, Tainer JA, Liddington RC, Smith JW. Crystal and solution structures of a prokaryotic M16B peptidase: an open and shut case. Structure. 2009;17:1465–1475. doi: 10.1016/j.str.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhani N, Berglund AK, Engmann T, Spanning E, Vogtle FN, Pavlov P, Meisinger C, Langer T, Glaser E. Targeting capacity and conservation of PreP homologues localization in mitochondria of different species. J Mol Biol. 2011a;410:400–410. doi: 10.1016/j.jmb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Alikhani N, Guo L, Yan S, Du H, Pinho CM, Chen JX, Glaser E, Yan SS. Decreased proteolytic activity of the mitochondrial amyloid-beta degrading enzyme, PreP peptidasome, in Alzheimer's disease brain mitochondria. J Alzheimers Dis. 2011b;27:75–87. doi: 10.3233/JAD-2011-101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529–533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbach JJ, Ishii Y, Antzutkin ON, Leapman RD, Rizzo NW, Dyda F, Reed J, Tycko R. Amyloid fibril formation by A beta 16-22, a seven-residue fragment of the Alzheimer's beta-amyloid peptide, and structural characterization by solid state NMR. Biochemistry. 2000;39:13748–13759. doi: 10.1021/bi0011330. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KM, Gakh O, Payne IC, Juliano MA, Juliano L, Isaya G, Hersh LB. Mammalian pitrilysin: substrate specificity and mitochondrial targeting. Biochemistry. 2009;48:2868–2877. doi: 10.1021/bi8016125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyda F, Hickman AB, Jenkins TM, Engelman A, Craigie R, Davies DR. Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases. Science. 1994;266:1981–1986. doi: 10.1126/science.7801124. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, Eneqvist T, Tjernberg L, Ankarcrona M, Glaser E. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem. 2006;281:29096–29104. doi: 10.1074/jbc.M602532200. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Manolopoulou M, Bian Y, Schilling AB, Tang WJ. Molecular basis for the recognition and cleavages of IGF-II, TGF-alpha, and amylin by human insulin-degrading enzyme. J Mol Biol. 2010;395:430–443. doi: 10.1016/j.jmb.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, et al. A census of human soluble protein complexes. Cell. 2012;150:1068–1081. doi: 10.1016/j.cell.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H, Manolopoulou M, Malito E, Shen Y, Zhao J, Neant-Fery M, Sun CY, Meredith SC, Sisodia SS, Leissring MA, et al. Structure of substrate-free human insulin-degrading enzyme (IDE) and biophysical analysis of ATP-induced conformational switch of IDE. J Biol Chem. 2007;282:25453–25463. doi: 10.1074/jbc.M701590200. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Bhushan S, Stahl A, Hallberg BM, Frohn A, Glaser E, Eneqvist T. The closed structure of presequence protease PreP forms a unique 10,000 Angstromŝ3 chamber for proteolysis. EMBO J. 2006;25:1977–1986. doi: 10.1038/sj.emboj.7601080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambacheld M, Augustin S, Tatsuta T, Muller S, Langer T. Role of the novel metallopeptidase Mop112 and saccharolysin for the complete degradation of proteins residing in different subcompartments of mitochondria. J Biol Chem. 2005;280:20132–20139. doi: 10.1074/jbc.M500398200. [DOI] [PubMed] [Google Scholar]
- Koppen M, Langer T. Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol. 2007;42:221–242. doi: 10.1080/10409230701380452. [DOI] [PubMed] [Google Scholar]
- Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular structure of beta-amyloid fibrils in Alzheimer's disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Malito E, Hulse RE, Tang WJ. Amyloid beta-degrading cryptidases: insulin degrading enzyme, presequence peptidase, and neprilysin. Cell Mol Life Sci. 2008;65:2574–2585. doi: 10.1007/s00018-008-8112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of Abeta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manolopoulou M, Guo Q, Malito E, Schilling AB, Tang WJ. Molecular basis of catalytic chamber-assisted unfolding and cleavage of human insulin by human insulin-degrading enzyme. J Biol Chem. 2009;284:14177–14188. doi: 10.1074/jbc.M900068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord LA, Liang WG, Dowdell E, Kalas V, Hoey RJ, Koide A, Koide S, Tang WJ. Conformational states and recognition of amyloidogenic peptides of human insulin-degrading enzyme. Proc Natl Acad Sci USA. 2013;110:13827–13832. doi: 10.1073/pnas.1304575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- Moberg P, Nilsson S, Stahl A, Eriksson AC, Glaser E, Maler L. NMR solution structure of the mitochondrial F1beta presequence from Nicotiana plumbaginifolia. J Mol Biol. 2004;336:1129–1140. doi: 10.1016/j.jmb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Moberg P, Stahl A, Bhushan S, Wright SJ, Eriksson A, Bruce BD, Glaser E. Characterization of a novel zinc metalloprotease involved in degrading targeting peptides in mitochondria and chloroplasts. Plant J. 2003;36:616–628. doi: 10.1046/j.1365-313x.2003.01904.x. [DOI] [PubMed] [Google Scholar]
- Mossmann D, Meisinger C, Vogtle FN. Processing of mitochondrial presequences. Biochim Biophys Acta. 2012;1819:1098–1106. doi: 10.1016/j.bbagrm.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Murata CE, Goldberg DE. Plasmodium falciparum falcilysin: a metalloprotease with dual specificity. J Biol Chem. 2003;278:38022–38028. doi: 10.1074/jbc.M306842200. [DOI] [PubMed] [Google Scholar]
- Mzhavia N, Berman YL, Qian Y, Yan L, Devi LA. Cloning, expression, and characterization of human metalloprotease 1: a novel member of the pitrilysin family of metalloendoproteases. DNA Cell Biol. 1999;18:369–380. doi: 10.1089/104454999315268. [DOI] [PubMed] [Google Scholar]
- Nilsson Cederholm S, Backman HG, Pesaresi P, Leister D, Glaser E. Deletion of an organellar peptidasome PreP affects early development in Arabidopsis thaliana. Plant Mol Biol. 2009;71:497–508. doi: 10.1007/s11103-009-9534-6. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer's beta-amyloid fibrils. Proc Natl Acad Sci USA. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoukhov MV, Franke D, Shkumatov AV, Tria G, Kikhney AG, Gajda M, Gorba C, Mertens HDT, Konarev PV, Svergun DI. New developments in the ATSAS program package for small-angle scattering data analysis. J Appl Crystallogr. 2012;45:342–350. doi: 10.1107/S0021889812007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho CM, Bjork BF, Alikhani N, Backman HG, Eneqvist T, Fratiglioni L, Glaser E, Graff C. Genetic and biochemical studies of SNPs of the mitochondrial Abeta-degrading protease, hPreP. Neurosci Lett. 2010;469:204–208. doi: 10.1016/j.neulet.2009.11.075. [DOI] [PubMed] [Google Scholar]
- Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Bioph Res Co. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- Ralat LA, Kalas V, Zheng Z, Goldman RD, Sosnick TR, Tang WJ. Ubiquitin is a novel substrate for human insulin-degrading enzyme. J Mol Biol. 2010;406:454–466. doi: 10.1016/j.jmb.2010.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Nat Rev Mol Cell Biol. 2008;9:679–690. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Guo Q, Guo L, Lenz M, Qian F, Koenen RR, Xu H, Schilling AB, Weber C, Ye RD, et al. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme. EMBO J. 2010;29:3952–3966. doi: 10.1038/emboj.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypniewski WR, Holden HM, Rayment I. Structural consequences of reductive methylation of lysine residues in hen egg white lysozyme: an X-ray analysis at 1.8-A resolution. Biochemistry. 1993;32:9851–9858. doi: 10.1021/bi00088a041. [DOI] [PubMed] [Google Scholar]
- Saido T, Leissring MA. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med. 2012;2:a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- Shen Y, Joachimiak A, Rosner MR, Tang WJ. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006;443:870–874. doi: 10.1038/nature05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohma Y, Sasaki M, Hayashi Y, Kimura T, Kiso Y. Design and synthesis of a novel water-soluble Aβ1-42 isopeptide: an efficient strategy for the preparation of Alzheimer's disease-related peptide, Aβ1-42, via O-N intramolecular acyl migration reaction. Tetrahedron Lett. 2004;45:5965–5968. [Google Scholar]
- Stahl A, Nilsson S, Lundberg P, Bhushan S, Biverstahl H, Moberg P, Morisset M, Vener A, Maler L, Langel U, et al. Two novel targeting peptide degrading proteases, PrePs, in mitochondria and chloroplasts, so similar and still different. J Mol Biol. 2005;349:847–860. doi: 10.1016/j.jmb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Strohalm M, Kavan D, Novak P, Volny M, Havlicek V. mMass 3: a cross-platform software environment for precise analysis of mass spectrometric data. Anal Chem. 2010;82:4648–4651. doi: 10.1021/ac100818g. [DOI] [PubMed] [Google Scholar]
- Taylor AB, Smith BS, Kitada S, Kojima K, Miyaura H, Otwinowski Z, Ito A, Deisenhofer J. Crystal structures of mitochondrial processing peptidase reveal the mode for specific cleavage of import signal sequences. Structure. 2001;9:615–625. doi: 10.1016/s0969-2126(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Tjernberg LO, Naslund J, Lindqvist F, Johansson J, Karlstrom AR, Thyberg J, Terenius L, Nordstedt C. Arrest of beta-amyloid fibril formation by a pentapeptide ligand. J Biol Chem. 1996;271:8545–8548. doi: 10.1074/jbc.271.15.8545. [DOI] [PubMed] [Google Scholar]
- Voss NR, Gerstein M. 3V: cavity, channel and cleft volume calculator and extractor. Nucleic Acids Res. 2010;38:W555–562. doi: 10.1093/nar/gkq395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Lu T. Amyloid beta-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:4755–4759. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.