Significance
CALAA-01 is a targeted nanoparticle containing siRNA that is a first-in-class experimental therapeutic for cancer. To our knowledge, it is the first targeted, polymer-based nanoparticle-carrying siRNA to be systemically administered to humans. Results from a human phase Ia/Ib clinical trial are presented and correlated to preclinical animal data to provide an initial assessment of how this class of experimental therapeutics is translated from animals to humans.
Keywords: translational medicine, DNA proliferation, DNA replication, maximum tolerance dose, dose limiting toxicity
Abstract
Nanoparticle-based experimental therapeutics are currently being investigated in numerous human clinical trials. CALAA-01 is a targeted, polymer-based nanoparticle containing small interfering RNA (siRNA) and, to our knowledge, was the first RNA interference (RNAi)–based, experimental therapeutic to be administered to cancer patients. Here, we report the results from the initial phase I clinical trial where 24 patients with different cancers were treated with CALAA-01 and compare those results to data obtained from multispecies animal studies to provide a detailed example of translating this class of nanoparticles from animals to humans. The pharmacokinetics of CALAA-01 in mice, rats, monkeys, and humans show fast elimination and reveal that the maximum concentration obtained in the blood after i.v. administration correlates with body weight across all species. The safety profile of CALAA-01 in animals is similarly obtained in humans except that animal kidney toxicities are not observed in humans; this could be due to the use of a predosing hydration protocol used in the clinic. Taken in total, the animal models do appear to predict the behavior of CALAA-01 in humans.
Therapeutics that use RNA interference (RNAi) as their mechanism of action offer opportunities for inhibiting virtually any gene target. Several RNAi-based therapeutics have been and are currently being investigated in human clinical trials (1). To date, results from two clinical investigations with cancer patients have been reported (2, 3). In 2008, a cyclodextrin polymer-based nanoparticle formulation containing siRNA (CALAA-01) entered the clinic and, to our knowledge, was the first example of systemic administration of a cationic polymer–siRNA nanoparticle therapeutic to humans (4). Additionally, CALAA-01 is the first, to our knowledge, systemically administered nanoparticle of any kind to show the presence of nanoparticles localized in patient tumors in amounts that correlated with dose levels given to the patients and demonstrate gene inhibition by RNAi (2). Given that this class of nanoparticle shows potential to deliver siRNAs to extrahepatic tumors, future clinical translations of this type of therapeutic agent will rely upon answering questions pertaining to the toxicity and efficacy of this type of multicomponent formulation. To this end, we present data from the phase Ia/Ib clinical trial of CALAA-01 and correlate the data from humans with preclinical studies across several nonhuman species to answer key questions about the clinical translation of CALAA-01.
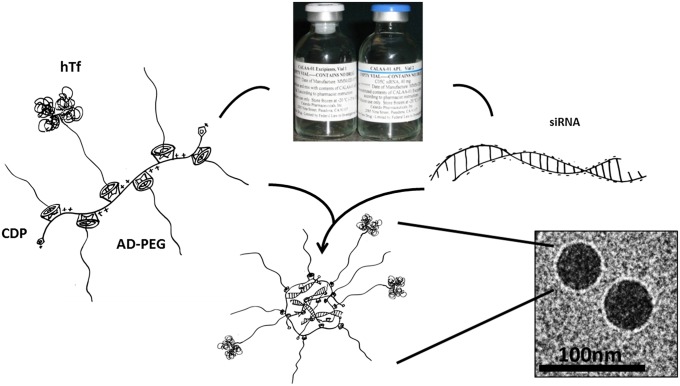
CALAA-01 is a four-component system that is manufactured as a two-vial formulation for clinical dosing (Fig. 1). Vial 1 contains a mixture of three delivery components: (i) a linear, cationic cyclodextrin-based polymer (CDP), (ii) a hydrophilic polymer [adamantane polyethylene glycol (AD-PEG)] used to promote nanoparticle stability in biological fluids, and (iii) a human transferrin protein (hTf)-targeting ligand (AD-PEG-hTf) displayed on the exterior of the nanoparticle to engage Tf receptors (hTfR) on the surface of the cancer cells. The hTfR is known to be up-regulated in human tumor cells, including melanoma (5, 6). Vial 2 contains (iv) siRNA designed to reduce the expression of the M2 subunit of ribonucleotide reductase (RRM2). RRM2 is an established anticancer target (7, 8), and i.v. administration of CALAA-01 inhibits the growth of human tumor xenografts in mice by inhibiting the expression of RRM2 (9) (SI Appendix, Fig. S1). Vials 1 and 2 are mixed at the bedside, and the components self-assemble into ∼75-nm nanoparticles (10). The formulated nanoparticles are administered intravenously and are designed to target tumor tissue via the enhanced permeability and retention effect (11, 12).
Fig. 1.
Schematic of nanoparticle assembly. CALAA-01 is manufactured as two vials. Vial 1 contains the polymer delivery components: a cationic CDP polyethylene glycol modified with a terminal adamantane group (AD-PEG) and some AD-PEG conjugated to human transferrin. Vial 2 contains siRNA designed to reduce the expression of the M2 subunit of ribonucleotide reductase (RRM2). When the vials are mixed together in the pharmacy, the components self-assemble into a nanoparticle. Cyro-electron micrografts of CALAA-01 are shown.
The nature and complexity of CALAA-01 raised a number of questions during its clinical translation: Would delivery of unmodified siRNA to patients result in severe immunostimulation? Would the positively charged polymer component of CALAA-01 result in coagulopathic effects and kidney toxicity in patients? Would the off-target deposition of CALAA-01 in the liver result in hepatotoxicity? Would the pharmacokinetic/pharmacodynamic properties scale across species (from animals to humans) based on body mass or body-surface area? Would the toxicities observed in the preclinical toxicity studies translate into toxicity in patients? Here, we present data from both humans and animals to provide insights into answering these and other questions of importance for translating nanotherapeutics of this class.
Results
Overview of Human Phase Ia/Ib Clinical Trial.
Nineteen patients were enrolled in the phase Ia clinical trial between May 2008 and September 2010 (SI Appendix, Table S1). Dose escalation from 3 to 30 mg/m2 (dose is listed as the amount of siRNA) in 15 patients was carried out between May 2008 and May 2009 with no observed dose-limiting toxic events (DLTs). Then there was an approximately 1-y enrollment gap until September 2010. Upon trial resumption, the subsequent two patients in the 30 mg/m2 cohort experienced DLTs. The final two patients in the phase Ia trial were treated at the previously well-tolerated 24 mg/m2 dose level; however, these doses were not well tolerated by either patient.
Consequently, the clinical protocol was amended into a phase Ib to include an additional cohort with a modified dose schedule that was predicated on the idea that initial exposure to CALAA-01 at lower dose levels may desensitize patients to the innate immune response that might be responsible for the adverse events (SI Appendix, Table S1). Patients were to receive a lower dose of CALAA-01 for cycle 1 (18 mg/m2—well tolerated by patients during phase Ia) which, if there were no safety concerns, would be followed by higher doses (27 mg/m2) in subsequent cycles. This strategy was designed to improve the tolerability of CALAA-01 to patients while maintaining its potential efficacy. Five patients were enrolled in phase Ib between September 2011 and July 2012. Two patients in this cohort experienced DLTs, and a decision was made to end the study.
CALAA-01 Clinical Trial Patient Demographics and Tumor Response.
Table 1 and SI Appendix, Tables S2–S4, summarize the patient demographics. All clinical characteristics and demographics were similar across cohorts and dose levels. Twenty-four patients received at least one dose of CALAA-01. Mean duration on treatment was 36.6 d (median 28.4 d). Mean number of cycles completed was 1.8 (median of 2 cycles) (SI Appendix, Table S5). The primary reason for discontinuation from the study was progressive disease in seven (29%) patients as evidenced by increase in tumor size (SI Appendix, Table S6). During the study, 19 (79%) patients had at least one posttreatment scan and were considered evaluable for tumor response by RECIST v1.0. No objective tumor responses were observed; the best response per RECIST v1.0 criteria reported by the investigators was stable disease (SD) for 4 mo, a change of prior course, in one melanoma patient treated at the highest dose level. The patient received a total of six cycles of CALAA-01 at 30 mg/m2 before discontinuing due to progressive disease.
Table 1.
Patient demographics
| Variable | No. of patients |
| Total study population | 24 |
| Phase 1a (3–30 mg/m2) | 19 |
| Phase 1b (18–27 mg/m2) | 5 |
| Median age (y) | 63.5 (range 47–83) |
| Male/female | 19/5 |
| ECOG performance status 0/1 | 11/12 |
| Prior therapies | 21 |
| Investigational drugs | 13 |
| Chemotherapy | 24 |
| Radiotherapy | 12 |
| Tumor types | |
| Melanoma | 5 |
| Gastrointestinal | 8 |
| Prostate | 2 |
| Others* | 9 |
| Median no. of cycles/patient | 2 (range 1–5) |
ECOG, Eastern Cooperative Oncology Group.
Dose-Limiting Toxicities in the Clinic.
In cycle 1, two patients in the 30-mg/m2 dose cohort experienced DLTs. One patient had metastatic prostate adenocarcinoma to the lungs and paraaortic lymph nodes and had previously received radiation therapy and progressed on bicalutamide. This patient developed grade 3 ischemic colitis, grade 2 diarrhea, and grade 1 fever and was immediately discontinued from the study. The second DLT occurred in a patient with metastatic melanoma who had previously received radiation therapy and high-dose IFN. The patient developed grade 4 fatigue, grade 2 flu-like symptoms, grade 2 muscular spasm, and grade 1 nausea and was immediately discontinued from the study. Both patients were hospitalized and fully recovered after receiving concomitant medication.
In phase Ib, the protocol was amended to include an additional cohort with a modified dose schedule (18 mg/m2 in cycle 1 followed by 27 mg/m2 in subsequent cycles) to possibly reduce the innate immune response, improve the tolerability, and maintain the potential efficacy of the study drug. Two patients in phase Ib experienced DLTs. One patient with undifferentiated small-cell carcinoma of the cervix with metastasis to the lungs experienced grade 3 hypersensitivity reactions after the third and fourth doses of 18 mg/m2 in cycle 1, necessitating infusion termination during dose 4. Another patient with rectal adenocarcinoma with metastasis to the lungs, who previously received radiation therapy and five systemic therapies, showed grade 3 fatigue during cycle 1, and dose was reduced to 15 mg/m2 on cycle 2 to minimize adverse events (AEs). Only two of the five patients enrolled in phase Ib completed cycle 1 and progressed to the 27-mg/m2 dose level.
Overall Safety Profile.
All AEs are summarized in SI Appendix, Table S7. The most common treatment-related AEs of any grade with incidence in greater than 15% of patients were fatigue (n = 12), chills (n = 12), and fever (n = 10). Grade 3/4-related AEs occurring in multiple patients included lymphopenia (n = 3) and fatigue (n = 2). Grade 3/4-related AEs during CALAA-01 infusions included hypersensitivity, ischemic colitis, diarrhea, and fever (n = 1 each). Another treatment-related grade 3 AE included hyponatremia (n = 1). Possibly related serious AEs included grade 2 sinus bradycardia in one patient, grade 2 hematuria, and grade 1 pericardial effusion in one patient. Overall five (21%) patients discontinued study participation due to an adverse event. The ability to conclusively associate siRNA plasma concentration data with adverse events in this study was limited. Four patients experienced DLTs in cycle 1; the plasma concentrations observed in these patients were in the same range as others who did not experience DLTs. Furthermore, there was no correlation between elevated plasma cytokine levels and severity of patient AEs.
Pharmacokinetics of CALAA-01 in Humans and Animals.
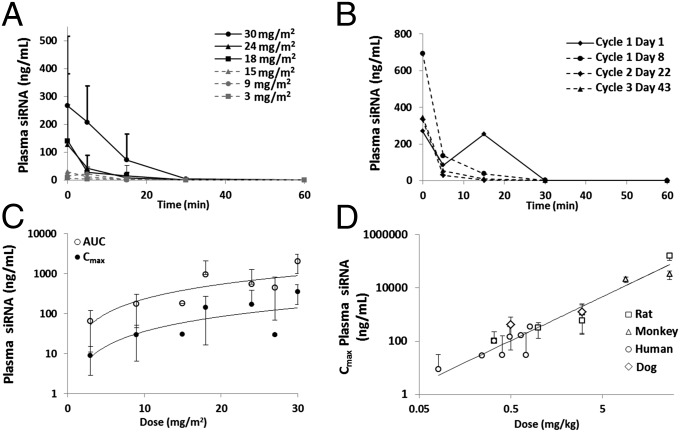
Human pharmacokinetic (PK) data are illustrated in Fig. 2. We observed a rapid decline of the plasma concentrations of the siRNA component of CALAA-01 to below the limit of detection by 30 min after the end of infusion in almost all patients (Fig. 2A). These findings are consistent with the preclinical data in mice (13), rats, and monkeys (SI Appendix, Fig. S2A). Plasma siRNA concentration data after CALAA-01 infusion was obtained in multiple treatment cycles from seven subjects and appeared to be consistent within subjects across cycles. Fig. 2B shows the plasma siRNA concentration after four individual doses (across three cycles) of CALAA-01 in a single patient. The rate of plasma siRNA concentration decline after each dose was similar. There was no apparent accumulation of CALAA-01 siRNA upon multiple dosing in any patient.
Fig. 2.
PK assessment of CALAA-01 in patients and across species. (A) Time course of average plasma concentration of the siRNA component of CALAA-01 following the end of infusion for all dosing cohorts. (B) Time course of plasma CALAA-01 siRNA following the end of infusion from cycles 1–3 of CALAA-01 from one patient who received 30 mg/m2 CALAA-01. Plasma concentration curves are similar at each cycle. Lines connecting data points are guides for the eye only. (C) AUC and Cmax relationship to dose in humans. (D) Scaling of CALAA-01 Cmax across four different species.
Data shown in Fig. 2C demonstrate the relationship between the dose of CALAA-01 to the plasma siRNA area under the curve (AUC) and the maximal concentration (Cmax) after i.v. infusion. AUC and Cmax increased with dose, consistent with the preclinical data in rats and monkeys. Cmax and AUC appeared to be linearly related in all species (SI Appendix, Fig. S2B). Fig. 2D illustrates the Cmax dose scaling of CALAA-01 across four species (rat, dog, monkey, and human). These data were best fit when Cmax was plotted against dose in weight (mg/kg) rather than dose in body-surface area (mg/m2). Conversion on mg/m2 bases was carried out as proposed by Reagan-Shaw et al. (14).
CALAA-01 Causes Minimal Liver and Kidney Toxicity in Humans.
The primary toxicities observed in preclinical studies with CALAA-01 were liver and kidney toxicities as evidenced by dose-dependent elevations in liver enzymes, creatinine, and blood urea nitrogen (BUN) in rats and monkeys (SI Appendix, Figs. S3 and S4). The polymer delivery components of CALAA-01 (vial 1) when administered without siRNA (vial 2) induced similar elevations in liver enzymes, creatinine, and BUN; however, in monkeys, the full CALAA-01 formulation (vial 1 + vial 2) trended toward increased liver and kidney toxicity compared with the polymer delivery components alone. Postmortem tissue examination of monkeys and rats treated with CALAA-01 or polymer delivery components alone revealed similar pathologic changes in liver (increased monocytes/Kupffer cell hypertrophy) and kidney (tubular necrosis). These data suggest that the polymer delivery components of CALAA-01 were primarily responsible for the liver and kidney toxicity observed. To mitigate the possibility of kidney toxicity in patients, the treatment protocol included a pretreatment i.v. hydration bolus of 500 mL of 5% (wt/vol) dextrose in water before CALAA-01 infusions, and the patients were encouraged to drink 2–3 L of fluids per day while participating in the study.
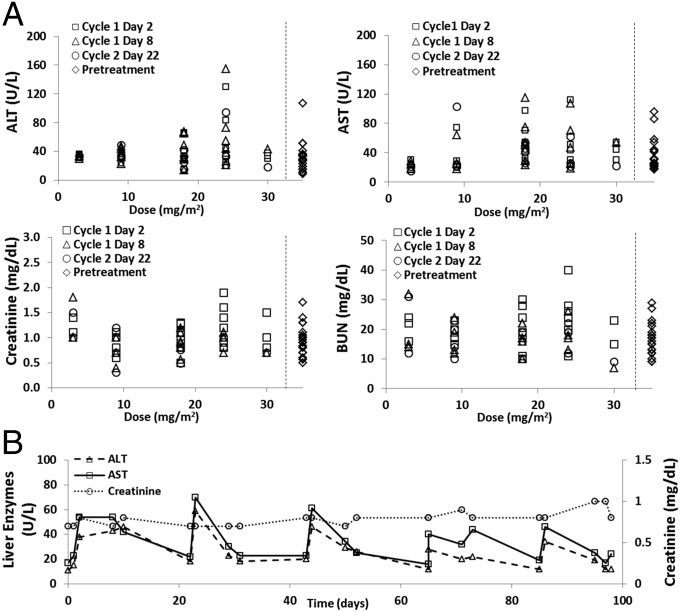
The human serum chemistry data are presented in Fig. 3A. CALAA-01 treatment resulted in minimal and clinically insignificant dose elevations in serum aspartate transaminase (AST) and alanine amino transferase (ALT) (several patients had elevated liver enzymes at pretreatment), and no elevation in BUN or serum creatinine over pretreatment values was observed. When followed longitudinally over five cycles of therapy, serum AST and ALT were noted to increase at the start of each treatment cycle and return to baseline in the interim whereas serum creatinine remained stable (Fig. 3B).
Fig. 3.
Minimal evidence for liver or kidney toxicity of CALAA-01 in humans. (A) Human serum liver enzymes and kidney markers plotted against dose level. Data are from various cycle dates 24 h post CALAA-01 infusion. Pretreatment values are presented to the right of the dotted lines. (B) Liver enzymes and kidney markers over 98 d in a patient that received six cycles of CALAA-01 at 30 mg/m2. Lines connecting data points are guides for the eye only. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen.
CALAA-01 Treatment Resulted in Dose-Dependent Platelet Count Decreases in Both Animals and Humans.
SI Appendix, Figs. S5 and S6, provide data that summarize the effects of CALAA-01 treatment on monkey and rat coagulation parameters and platelet count. CALAA-01 treatment in both rats and monkeys resulted in transient prolongation of activated partial thromboplastin time (APPT) and a marked drop in platelet count. Fibrinogen levels were depressed following CALAA-01 treatment in monkeys, but were elevated following treatment in rats. Prothrombin time (PT) was unaffected in either species. Treatment with the CALAA-01 polymer delivery components alone induced identical changes in these coagulation parameters, except for PT in monkeys, which was elevated following polymer delivery component treatment but not CALAA-01 treatment. These data suggest that the polymer delivery components of CALAA-01 were likely responsible for alterations in coagulation parameters and platelet counts following CALAA-01 infusion.
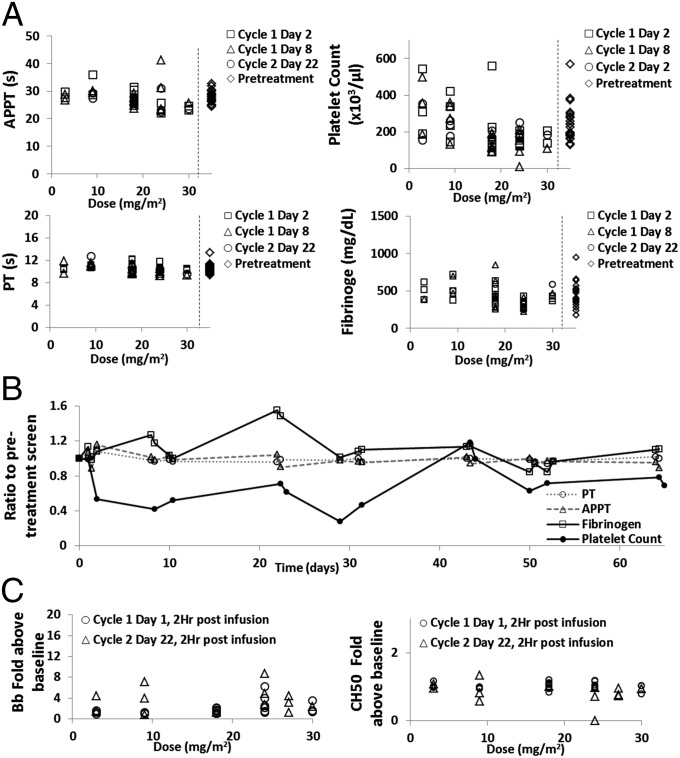
Fig. 4 A and B summarize the effects of CALAA-01 treatment on human coagulation parameters and platelet count. As in the preclinical animal models, CALAA-01 treatment resulted in marked dose-dependent, but clinically insignificant, decline in platelet counts; however, other coagulation parameters (APPT, PT, fibrinogen) where unaffected. When followed longitudinally over four cycles in a single patient (30 mg/m2 doses), platelet count initially declined over cycles 1 and 2; however, it then recovered over the subsequent two treatment cycles. No long-term effects on APPT, PT, or fibrinogen levels were observed in this patient.
Fig. 4.
Effects of CALAA-01 on human coagulation and complement. (A) Coagulation values plotted against dose level of CALAA-01. Data are from various cycle dates 6 h post CALAA-01 infusion for PT and APPT and 24 h post infusion for platelets and fibrinogen. Pretreatment values are presented to the right of the dotted lines. (B) Coagulation values over 65 d in a patient that received four cycles of CALAA-01 at 30 mg/m2. Lines connecting data points are guides for the eye. (C) Complement levels plotted against dose level of CALAA-01. APPT, activated partial thromboplastin time; CH50, total hemolytic complement capacity in serum; PT, prothrombin time.
CALAA-01 Treatment Did Not Alter Serum-Complement Function in Animals and Humans.
SI Appendix, Table S8, provides data that summarizes the effects of CALAA-01 treatment on monkey serum complement activity. Plasma Bb (activated complement factor B) is a specific and sensitive marker for activation of the alternative complement pathway, which has been shown to be most appropriate for use in detecting complement activation induced by oligonucleotides, as this class of molecules has been shown to specifically induce alternative pathway activation (15). No appreciable elevation in serum Bb was observed over baseline increases (baseline increases result from restraining the animals for treatment). CH50 reflects total hemolytic complement capacity and is measured ex vivo; it reflects the residual capacity of the entire system to form membrane attack complexes when triggered ex vivo, and the occurrence of a complement activation event in vivo will therefore result in a decrease in CH50 (i.e., due to consumption of complement factors). A marked reduction in CH50 for most of the polymer delivery component-treated monkeys was observed at the end of infusion with complete or nearly complete recovery by 6 h postinfusion. There were also reductions in CH50 for several CALAA-01–treated animals, but there was no clear dose dependence in terms of the incidence of animals with distinct decreases in CH50 or the group mean values. These data suggest that the polymer delivery components’ ability to activate complement is mitigated when formulated as the CALAA-01 nanoparticle, consistent with previously published in vitro complement activation studies (10).
Fig. 4C shows data that summarize the effect of CALAA-01 treatment on human complement activity. No appreciable change in level of Bb split product or CH50 activity was observed following CALAA-01 dosing in humans. Of note, a single patient in the 24-mg/m2 dose cohort had CH50 pretreatment values at near 0 and had a large relative drop in CH50 after the day 22 dose; however, the absolute change was minimal.
Effects of CALAA-01 Treatment on Other Hematologic Parameters in Animals and Humans.
SI Appendix, Figs. S7 and S8, provides data that summarize the effects of CALAA-01 treatment on monkey and rat hematologic parameters. In both species, dose-dependent decreases in serum hemoglobin and red blood cell count were observed following CALAA-01 treatment. These effects were similar in groups dosed with the polymer delivery components alone. In monkeys, treatment with both CALAA-01 and polymer delivery components alone resulted in reduced reticulocyte counts (a measure of new red blood cell production). These data suggest that, in monkeys, the polymer delivery components of CALAA-01 interfere with new red blood cell production, thereby preventing replenishment of the red cells lost following the repeated phlebotomy necessary to obtain the serum toxicology specimens. Dose-dependent white blood cell count elevations were also observed in monkeys and rats treated with either CALAA-01 or polymer delivery components alone.
SI Appendix, Fig. S9, shows data that summarizes the effects of CALAA-01 treatment on human hematologic parameters. No dose-dependent effects on serum hemoglobin, red blood cell count, or lymphocyte counts were observed. A single patient receiving 24 mg/m2 of CALAA-01 was found to have severe anemia that was possibly related to CALAA-01 treatment. Two subjects in the phase Ib cohort were found to have lymphopenia that was deemed probably treatment related.
Mild, Dose-Dependent Elevations in Serum Cytokines Following CALAA-01 Treatment Were Observed in Animals and Humans.
The siRNA component of CALAA-01 is not chemically modified. Much preclinical work has demonstrated that nonchemically modified siRNAs can be immunostimulatory under certain conditions (16). Specifically, systemic administration of siRNA with lipid carriers can cause significant immunostimulation. However, when administered intravenously alone, nonchemically modified siRNAs do not necessarily result in immunostimulation in mice (17). Additionally, other cationic polymer-based siRNA/nanoparticle systems have been shown to not elicit a pronounced immune response in vivo (18). Preclinical data characterizing the immune response to CALAA-01 dosing in monkeys has previously been reported (19). Elevated levels of IL-6 were observed at the highest dosing group (27 mg/kg). Increasing amounts of IL-12 and IFN-γ were observed in one monkey at 9 and 27 mg/kg, respectively. However, the increases in these cytokines were not quantitatively impressive or suggestive of intensive immunostimulation.
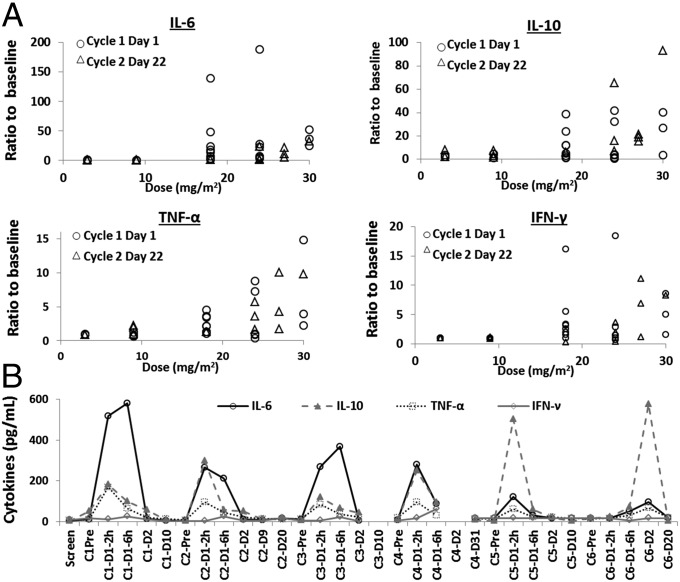
Fig. 5A shows data that summarize the assessment of CALAA-01 treatment-induced immune responses in humans. Plasma and serum were obtained from study patients at prescribed time points and analyzed for cytokines (IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-12p70, TNF-α, and IFN-γ). These results indicate apparent treatment-related elevations in some cytokines—namely IL-6, IL-10, TNF-α, and IFN-γ. No other cytokines showed any apparent trends or changes in response to CALAA-01 administration. These elevations were all transient in nature, with cytokine levels returning to baseline by 24 h after the end of infusion in all cases (SI Appendix, Fig. S10). Fig. 5B shows the serum IL-6, IL-10, TNF-α, and IFN-γ levels post dose over six cycles of treatment in a single patient (30 mg/m2 doses). IL-6 response attenuated with each dose whereas IL-10 response increased upon continued dosing. IL-6 and TNF-α are each indicative of an inflammatory response; the fact that both were transiently elevated in some patients suggests that CALAA-01 induces an inflammatory response in some patients at some of the higher dose levels. IL-10 is indicative of a T helper 2 (Th2)-mediated immune response; however, IL-4 is indicative of the same type of response, and CALAA-01–dependent changes in IL-4 levels were not observed. Overall, the results suggest that CALAA-01 may induce a Th2-mediated immune response in some subjects at some of the higher dose levels examined in the clinical study.
Fig. 5.
CALAA-01 treatment resulted in mild dose-dependent immunostimulation. (A) Various cytokine levels, expressed as ratio over baseline, plotted against CALAA-01 dosing level. (B) Cytokine levels over six cycles of CALAA-01 treatment at 30 mg/m2 in a single patient. Data from each cycle were collected immediately before CALAA-01 infusion and 2, 6, and 24 h post infusion. The x axis is labeled as cycle number (C#), overall time since treatment initiation (D#), and hours post infusion as appropriate.
Discussion
PK Correlations Between Animal Models and Patients.
A question in the emerging field of nanoparticle-based therapeutics is how doses should be scaled from preclinical animal models to patients. Should scaling be based on body weight or on body-surface area? Studies with liposome nanoparticles suggest that allometric scaling based on body weight works reasonably well (20). However, the AUC of a polymer-based nanoparticle of camptothecin (CRLX101) scaled best across species based on body-surface area (21). Here, we find that allometric scaling across species best correlates with body weight rather than with body-surface area. Scaling of CALAA-01 is based on Cmax rather than AUC. We chose Cmax for correlation because the rapid elimination of CALAA-01 resulted in too few data points for accurate AUC calculation (however, Cmax and AUC were found to be linearly related).
The clearance mechanism of CALAA-01 is different from other nanoparticle-based therapeutics. The monocyte phagocytic system is thought to be primarily responsible for nanoparticle clearance (20). However, CALAA-01 is cleared mainly through the kidney due to its interaction with the renal filtration barrier (13). Therefore, PK observations of CALAA-01 may be generalizable only to other nanoparticles that are held together by electrostatic interactions between the carrier molecules (positively charged) and the siRNA (negatively charged).
Toxicity Correlations Between Preclinical Models and Clinical Results.
Based on the preclinical animal data, the major concerns for CALAA-01 in the clinic were kidney and liver toxicity. Surprisingly, no changes in serum creatinine or BUN were observed in patients treated with CALAA-01. It is possible that the preinfusion IV hydration protocol was sufficient to mitigate this toxicity. Alternatively, it is possible that humans are less susceptible to the polymer delivery component-induced kidney toxicity or that CALAA-01 dose levels were below those required to induce kidney injury in humans. There was no evidence of overt liver toxicity in the clinic. However, in some patients there did appear to be a slight and clinically insignificant rise in serum liver enzyme at the start of each cycle. Regardless, neither of these two major toxicity concerns resulted in AEs in the clinic.
Other toxicity concerns from the preclinical animal data included decreases in platelet counts, reticulocyte count, and increased APPT (primarily in monkeys). In patients, dose-dependent, but clinically insignificant, decreases in platelet counts were observed. Anemia was observed in two patients; however, there was no dose dependence. Additionally, no effects on coagulation values (APPT, PT, or fibrinogen level) were observed in patients. These effects correlated well with those observed in animals, except for the effects on APPT (marked in monkeys, but not in rats).
Understanding Preclinical and Clinical Toxicity Profiles.
We can conclude from the preclinical toxicity studies that one or more of the delivery components within CALAA-01, rather than the siRNA component (active pharmaceutical ingredient), were primarily responsible for the adverse effects observed. In almost every parameter measured, administration of the polymer delivery components alone to animals resulted in similar results as with CALAA-01 administration (e.g., liver/kidney tox, platelet drop, APPT rise). To demonstrate the biologic feasibility of liver and kidney toxicity of polymer components, we examined the biodistribution of a fluorescently labeled formulation of the CALAA-01 excipients in mice (SI Appendix, Fig. S11). Bright fluorescence signal was observed throughout the kidney (localized primarily to glomeruli and tubule lumens) and liver sinusoids. Weak fluorescence signal was detected in several other organs as well. These data lead us to speculate that purification of any free polymer delivery component material remaining after CALAA-01 assembly in the formulation may provide a reduced toxicity profile and should be investigated.
It should be noted that in monkeys, but not in rats, fully formulated CALAA-01 trended toward increased liver and kidney toxicity compared with equivalent amounts of free polymer delivery components alone. We speculate that this increased toxicity resulted from the conglomeration of the polymer delivery components in a nanoparticle formulation rather than from any effect of the siRNA.
The majority of AEs resulting from CALAA-01 in humans were primarily hypersensitivity and sequelae of acute immune responses (flushing, fever, fatigue, etc.). Here, too, we suspect that the delivery components of CALAA-01 were the culprit. In both rats and dogs, CALAA-01 administration resulted in hypersensitivity reactions (flushing, skin edema, increased respiratory rates). SI Appendix, Table S9, shows histamine release following CALAA-01 treatment in male beagle dogs. The delivery components alone resulted in a 10-fold higher histamine release than CALAA-01. Uchida et al. have shown how cationic polymers can induce histamine release in rat mast cells (22). The histamine release following CALAA-01 was mitigated by standard pretreatment with antihistamines and steroids. All patients in the study received a similar pretreatment before CALAA-01 administration.
The potential mechanisms for the observed DLTs are less clear. Although the dose-limiting fatigue experience by one patient was consistent with the milder fatigue observed during dose escalation in other patients, the other DLTs (ischemic colitis, lymphopenia, severe hypersensitivity reaction, and hyponatremia) were not expected based on dose level or preclinical toxicity data. Furthermore, two of the patients who experienced DLTs were in the phase Ib cohort and received only doses of 18 mg/m2 CALAA-01. During dose escalation in phase Ia, this dose was very well tolerated by all patients. However, three of the four patients to receive only this dosage of CALAA-01 during phase Ib experienced grade 3 toxicities, including two DLTs.
It is possible that these toxicities were simply not observed during dose escalation in phase Ia due to the potentially stochastic nature of these AEs. In SI Appendix, Table S1, we illustrate the date of first dose of cycle 1 for every patient in the study compared with the AEs that they experienced. The ∼1-y enrollment gap is represented by the thick black line in SI Appendix, Table S1. Despite the treatment dose trend after this gap essentially being a dose de-escalation phase (30 to 24 to 18 mg/m2) starting in July 2010, there was a notable trend in increases in the number of higher severity and acute toxic events despite the general dose reduction over this time. Thus, an alternative explanation of this AE trend is the possibility that an aspect of CALAA-01 changed because it was manufactured at the beginning of the trial (only a single batch was manufactured for this trial). Delivery components from a different (nonclinical) batch of CDP, AD-PEG, and siRNA components stored and analyzed at the M.E.D. laboratory (California Institute of Technology) have shown excellent stability for times longer than the phase Ia/b clinical trial. However, Tf-PEG-Ad conjugates have shown structural alterations. Thus, if there were any aspect of the clinical CALAA-01 formulation that did change over this time period, we speculate that the transferrin-targeting agent is the most likely component of CALAA-01 to have done so (possibly mis-folded, aggregated, or degraded) in a manner not captured via analytics that were used (more sophisticated analytic methods were used by the M.E.D. laboratory), thereby resulting in the more severe AEs being observed during the late phase Ia and Ib trials. Admittedly, these post hoc analyses are speculative because we were not able to analyze the clinical materials by the analytical methods (used at the California Institute of Technology) at their time of use; however, we believe that quality control assays designed to test for these possibilities would be appropriate for any future protein-targeted nanotherapeutics.
Summary.
Taken in total, the evidence provided from animal studies for CALAA-01 do appear to accurately assess the behavior observed in the clinic. Because the DLTs in animals were in the kidney, a prehydration protocol was used in the clinic to protect the kidneys, and the DLTs observed in animals were not observed in humans. CALAA-01 was well tolerated during the initial dose escalation portion of the phase Ia study. The delivery system used in CALAA-01 does provide for targeted delivery of functional siRNA (2). However, the full potential of CALAA-01 was not evaluated in this phase Ia/Ib clinical study.
Materials and Methods
Complete details of materials and methods are provided in SI Appendix.
Patient Enrollment.
Twenty-four patients were enrolled in the study. Eligible patients were at least 18 y of age with histologically or cytologically confirmed solid malignancy that is metastatic or unresectable, refractory to standard therapy, or for which no standard curative or palliative therapy is available. The additional eligibility criteria included were Eastern Cooperative Oncology Group (ECOG) performance status ≤1; life expectancy of at least 6 mo; adequate marrow, hepatic. and renal function as well as acceptable hematological and biochemical values. All eligible patients had recurring tumors or tumors that radiation therapy has failed. Patients who received prior adjuvant, neoadjuvant, or any other therapy for metastatic disease needed to be fully recovered from prior diagnostic or therapeutic surgery for at least 30 d before initial dosing. No restriction was placed on the number of cycles or regimens of prior therapy. Patients who were allergic to Benadryl (Diphenhydramine; McNEIL-PPC Inc.), Zantac (Ranitidine; Boehringer Ingelheim), Zofran (Ondansetron; GlaxoSmithKline), dexamethasone, other drugs in these classes or contrast media required for protocol testing were not eligible.
The study was approved by the institutional review board or ethics committee at each participating center (South Texas Accelerated Research Therapeutics, San Antonio, TX; University of California, Los Angeles Jonsson Comprehensive Cancer Center, Los Angeles, CA; and City of Hope Comprehensive Cancer Center, Duarte, CA) and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent was obtained from all patients before study entry.
Plasma siRNA Concentration, Complement Factors, and Cytokine Measurements.
Plasma concentrations of the siRNA were determined by using a modified hybridization-ligation assay (23). Plasma Bb levels were determined by ELISA. Serum CH50 values were determined by a standard sheep erythryocyte lysis-based assay. Serum cytokines levels were determined by using commercially available kits: for IL-6 and IL-12, ELISA; and for IFN-γ, IL-10, IL-4, and TNF-α, multiplex kits and a Luminex instrument (Invitrogen).
Supplementary Material
Acknowledgments
We thank the patients who participated in the clinical trials. The clinical trial was sponsored by Calando Pharmaceuticals. J.E.Z. is supported by National Institutes of Health National Institute of General Medical Sciences Training Grant GM08042 and the University of California at Los Angeles–Caltech Medical Scientist Training Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411393111/-/DCSupplemental.
References
- 1.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 2.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabernero J, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 4.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 5.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues: Their distribution and possible clinical relevance. J Clin Pathol. 1983;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuckerman JE, Ribas A, Davis ME. Reply to Perris, Borghese, and Magro. Pigment Cell Melanoma Res. 2011;24:983–985. doi: 10.1111/j.1755-148X.2011.00888.x. [DOI] [PubMed] [Google Scholar]
- 7.Shao J, Zhou B, Chu B, Yen Y. Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets. 2006;6(5):409–431. doi: 10.2174/156800906777723949. [DOI] [PubMed] [Google Scholar]
- 8.Heidel JD, et al. Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clin Cancer Res. 2007;13(7):2207–2215. doi: 10.1158/1078-0432.CCR-06-2218. [DOI] [PubMed] [Google Scholar]
- 9.Rahman MA, et al. Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses head and neck tumor growth. J Control Release. 2012;159(3):384–392. doi: 10.1016/j.jconrel.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug Chem. 2007;18(2):456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreher MR, et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98(5):335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 12.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuckerman JE, Choi CHJ, Han H, Davis ME. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci USA. 2012;109(8):3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 15.Henry SP, Monteith D, Kornbrust DJ, Levin Aa. Effects of intravenous infusion of phosphorothioate oligonucleotides on coagulation, complement activation and hemodynamics. Nucleosides Nucleotides. 1997;16(7-9):1673–1676. [Google Scholar]
- 16.Robbins M, et al. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther. 2008;19(10):991–999. doi: 10.1089/hum.2008.131. [DOI] [PubMed] [Google Scholar]
- 17.Heidel JD, Hu S, Liu XF, Triche TJ, Davis ME. Lack of interferon response in animals to naked siRNAs. Nat Biotechnol. 2004;22(12):1579–1582. doi: 10.1038/nbt1038. [DOI] [PubMed] [Google Scholar]
- 18.Inaba S, et al. Atelocollagen-mediated systemic delivery prevents immunostimulatory adverse effects of siRNA in mammals. Mol Ther. 2012;20(2):356–366. doi: 10.1038/mt.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidel JD, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci USA. 2007;104(14):5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caron WP, et al. Allometric scaling of pegylated liposomal anticancer drugs. J Pharmacokinet Pharmacodyn. 2011;38(5):653–669. doi: 10.1007/s10928-011-9213-5. [DOI] [PubMed] [Google Scholar]
- 21.Eliasof S, et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc Natl Acad Sci USA. 2013;110(37):15127–15132. doi: 10.1073/pnas.1309566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino Y, Nagaya K, Sekino H, Uchida MK, Suzuki-Nishimura T. Comparison of histamine release induced by synthetic polycations with that by compound 48/80 from rat mast cells. Jpn J Pharmacol. 1990;52(3):387–395. doi: 10.1254/jjp.52.387. [DOI] [PubMed] [Google Scholar]
- 23.Yu RZ, et al. Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Anal Biochem. 2002;304(1):19–25. doi: 10.1006/abio.2002.5576. [DOI] [PubMed] [Google Scholar]