Significance
This study presents a direct visualization of the microtubule-p150glued(CAP-Gly) complex by cryo-EM and seeks to describe the molecular mechanism of the control of tubulin dynamics by p150 CAP-Gly. It highlights the neutralization of the acidic tubulin surface by the basic extensions of CAP-Gly, resulting in the activation of tubulin polymerization. In the condition where the lateral association is impeded (i.e., at low temperature), the extended CAP-Gly domain induces tubulin dimers to connect longitudinally. The two directional modes of self-association of tubulin suggest a foundation for its dynamic behavior at the tip of microtubules and its regulation.
Keywords: dynamic instability, dynactin, cytoskeleton, electron microscopy
Abstract
p150glued belongs to a group of proteins accumulating at microtubule plus ends (+TIPs). It plays a key role in initiating retrograde transport by recruiting and tethering endosomes and dynein to microtubules. p150glued contains an N-terminal microtubule-binding cytoskeleton-associated protein glycine-rich (CAP-Gly) domain that accelerates tubulin polymerization. Although this copolymerization is well-studied using light microscopic techniques, structural consequences of this interaction are elusive. Here, using electron-microscopic and spectroscopic approaches, we provide a detailed structural view of p150glued CAP-Gly binding to microtubules and tubulin. Cryo-EM 3D reconstructions of p150glued-CAP-Gly complexed with microtubules revealed the recognition of the microtubule surface, including tubulin C-terminal tails by CAP-Gly. These binding surfaces differ from other retrograde initiation proteins like EB1 or dynein, which could facilitate the simultaneous attachment of all accessory components. Furthermore, the CAP-Gly domain, with its basic extensions, facilitates lateral and longitudinal interactions of tubulin molecules by covering the tubulin acidic tails. This shielding effect of CAP-Gly and its basic extensions may provide a molecular basis of the roles of p150glued in microtubule dynamics.
The great variety of microtubule-associated proteins and the complexity of their interactions (1) are beginning to rival that of the actin-associated proteins. This complexity reflects the many different roles that actin and microtubules play in cellular cytoskeletal organization and activity. Our study focuses on the structural interactions of tubulin/microtubules with protein fragments corresponding to N-terminal portions of the p150glued subunit of the megadalton dynactin complex. Of particular interest is the 80-amino acid cytoskeleton-associated protein glycine-rich (CAP-Gly) domain (p150glued residues 25–105), which, when harboring critical mutations in neuronal dynactin, leads to devastating neurological disorders (2).
Several studies have characterized the interactions of p150glued with various +end-binding proteins (+TIPs), including end-binding protein 1 (EB1) and CLIP-170 (3–5), and the role of the negatively charged, C-terminal tubulin tails in binding positively charged domains of these proteins to the tubulin surface (6–9). Biophysical observations showed the ability of the +TIPs to promote microtubule polymerization (5, 6, 10). Recently, Lazarus et al. (11) have demonstrated that the N-terminal dimeric portion of p150glued is a neuron-specific anticatastrophe factor acting at the microtubule +end; a mutation in the CAP-Gly domain, which causes the lethal Perry syndrome, when introduced in their recombinant dimeric construct, abolishes the protective anticatastrophic depolymerization activity.
Here, by focusing on a single CAP-Gly domain of p150glued, intrinsic interactions with tubulin and microtubules were identified. The basic p150 fragments cause the lateral association of microtubules by neutralizing their repulsive negative surface charge. By limiting the extent of bundling, cryo-electron microscopic (cryo-EM) 3D reconstructions were obtained for CAP-Gly plus basic extensions connected to the negatively charged, flexible tails of tubulin. These reconstructions indicated a flexible connection of the CAP-Gly domain. Furthermore, this domain facilitated the assembly of tubulins into longitudinally connected oligomers at low temperatures and initiation of polymerization, likely through activating lateral associations of tubulin. The lateral interaction is likely due to the masking of the acidic charge of the tubulin surface. These two observed interactions provide a basis for the microtubule recovery following catastrophes. The properties of the +TIPs are much more than the sum of their parts. However, the identification of intrinsic properties of the components builds a foundation for exploring their cooperative interactions.
Results
EM Observation Shows That the Microtubule Lateral Association Is Induced by p150glued.
To understand the interaction between p150glued and microtubules in a structural context, we observed the p150glued–microtubule complex using cryo-EM. We generated several p150glued fragments containing the microtubule-binding CAP-Gly domain: namely, the CAP-Gly core [p150(25–105)]; 25 additional N-terminal residues [p150(1–105)]; 40 additional, unstructured C-terminal residues [p150(25–144)]; and both N- and C-terminal extensions [p150(1–144)] (Fig. S1) (12, 13). Both extensions contain several basic residues with predicted pI values of 12.0 and 12.6, respectively, as opposed to rather mild basic pI of 8.9 for CAP-Gly. A microtubule-pelleting assay showed that the binding of CAP-Gly alone to microtubules appears to be fairly weak (Fig. S1B, marked with an *), but addition of the upstream/downstream basic patches increased the binding affinity. Quantitative pelleting assays showed that CAP-Gly can recognize both alpha and beta tubulin at saturating levels (Fig. S2A). In the presence of 2 µM microtubules with 20 µM p150glued fragments, saturation of the proteins on microtubules was achieved, except for p150(25–105) (SI Discussion and Figs. S1B and S2).
The corresponding complexes observed under cryo-EM showed that microtubules associate with each other to form a lateral assembly (Fig. S1C). This assembly was not observed for p150(25–105). These microtubules sometimes opened up showing a sheet-like structure. We selected p150(25–105), p150(1–105), and p150(25–144) for further analysis because they yielded complexes in which microtubules were separated enough for image processing. In contrast, p150(1–144) caused strong microtubule bundling, which hampered structure analysis.
Cryo-EM Reconstruction Shows a Neck Formation of Tubulin E-Hooks and the CAP-Gly Basic Patch.
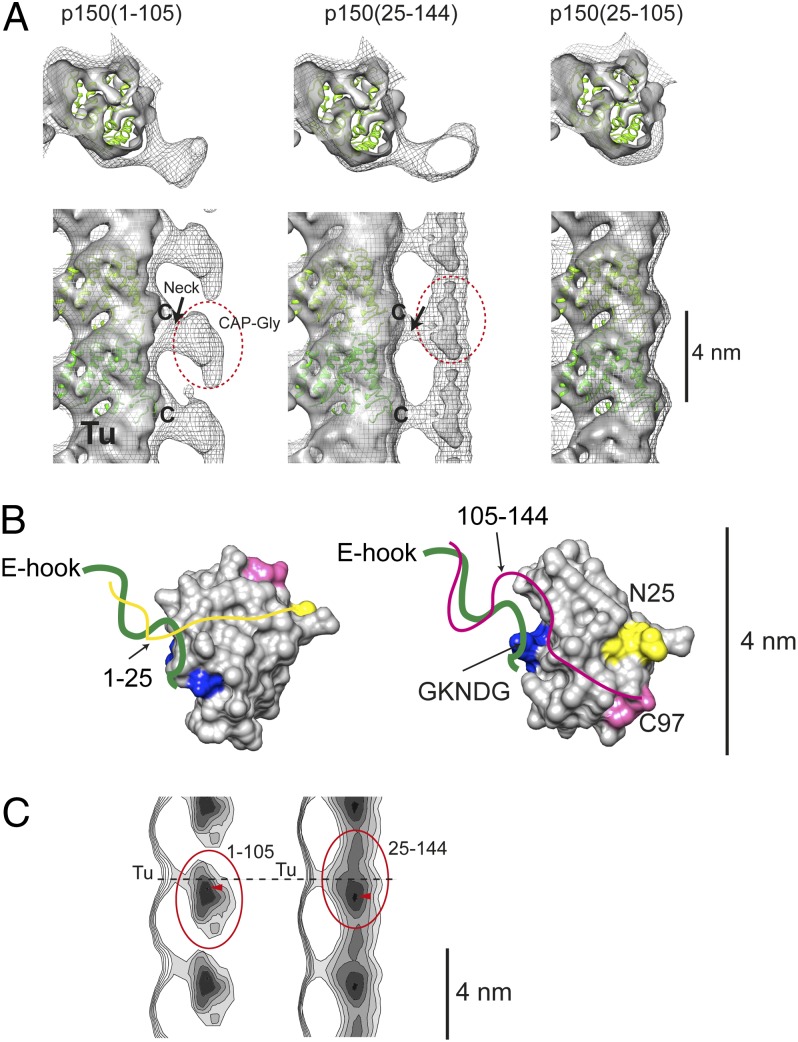
The reconstruction of microtubules bound to the CAP-Gly core p150(25–105) showed nondetectable protein decoration (Fig. 1A, Right) reflecting the weak interaction. p150(1–105) and p150(25–144) gave enough density to visualize the spatial relation of the CAP-Gly domains bound to the microtubules (Fig. 1A, Left and Center). The CAP-Gly density is located ∼2 nm away from the microtubule surface, connected through a neck protruding out from the microtubule surface. The reconstruction of p150(1–105) (chicken wire displayed density) gave an interpretable density corresponding to the CAP-Gly core, which has comparable size to the crystal structure of the CAP-Gly (PDB ID code 1TXQ) (Fig. 1B). We chose not to fit the crystal structure to the reconstruction due to the flexibility of the CAP-Gly binding.
Fig. 1.
(A) Shown are 3D reconstructions of microtubules with p150(1–105) (Left), p150(25–144) (Center), and p150(25–105) (Right). The sample was prepared by mixing 20-µM protein fragments with 2-µM microtubules in 80 mM Pipes-Na (pH 6.8), 1 mM MgCl2, and 1 mM EGTA. Chicken-wire densities show the reconstructions without amplifying the high-resolution density whereas the amplitude-corrected reconstruction is presented as a solid density, resulting in weakening the density of the decoration. A fitted tubulin atomic model is shown in green (PDB ID code 1TUB). Tubulin E-hooks are missing from the atomic model. The last visible residue from the atomic model is labeled “C.” Tu, tubulin protofilament. (B) Schematics of the interaction between E-hook and p150glued CAP-Gly. The N- and C-terminal parts of the atomic model of CAP-Gly (PDB ID code 1TXQ) are marked in yellow (N25) and pink (C97), respectively. The N- and C-terminal extensions of the CAP-Gly core are depicted as yellow and pink lines, respectively. These extensions are predicted to be disordered, but the experiments confirm the interactions with tubulin E-hooks (green line). The GKNDG motif interacting with tyrosine at the C terminus of the alpha tubulin E-hook is colored in blue. The mean orientations of the flexibly connected p150(1–105) (Left) and p150(25–144) in the reconstructions in A are suggested. (C) Lateral projection of one protofilament decorated with p150(1–105) (Left) and p150(25–144) (Right). Circles, CAP-Gly core; arrowheads, the highest density (CAP-Gly core). The dashed line indicates the position of the neck.
There is no apparent interaction between the core of the microtubules and the CAP-Gly. By adjusting the amplitude of relatively high frequency signals (SI Materials and Methods), the microtubule density started revealing secondary structure elements (Fig. 1A, gray solid density), which made it feasible to fit the atomic structure of a tubulin dimer. The molecular fitting showed that the C terminus of tubulin is connected to the neck (Fig. 1A, labeled with “C”). The ∼15 aa, negatively charged C-terminal tubulin tail (termed the E-hook) was not resolved in the atomic model (PDB ID code 1TUB) because of its flexible nature (14). Judging from the connection of the E-hook containing neck and the tubulin monomer, E-hook is leaning toward the minus end of the microtubule in the p150(1–105)-microtubule reconstruction. As the CAP-Gly core itself attaches to the neck, the neck density should also consist of the mass of the CAP-Gly basic extensions. Proteolytic treatment of the tubulin E-hooks by subtilisin abolished the binding of the CAP-Gly fragments (Fig. S2), also confirming the exclusive binding of the CAP-Gly fragments to E-hooks. On the other hand, the reconstruction of p150(25–144)-microtubule showed a decoration connected along the microtubule axis although the general flat bean-like shapes of CAP-Gly were recognizable by changing the density threshold (Fig. 1A, Center and 1C, Right). This density is connected through the neck, indicating that the essential interaction happens through the E-hook.
Based on the pelleting assay (Fig. S1B), the binding of the p150 fragments to the microtubules was greatly increased upon the addition of the basic patches (amino acids 1–25 and 106–144). Therefore, the densities connecting CAP-Gly and tubulin core (Fig. 1A, neck) likely correspond to E-hooks and the 1–25 basic patch for p150(1–105), and E-hooks and the 106–144 basic patch for p150(25–144) (Table 1). This observation indicates that the binding of CAP-Gly–containing fragments depends on oppositely charged surface interactions rather than a set of specific/conserved interactions. Moreover, the binding affinity of the CAP-Gly core to the tyrosinated E-hook was 11 µM whereas virtually no detectable binding was observed for the detyrosinated E-hook (>130 µM) (Table 1). This increase of the affinity agrees with previous reports (7) showing the recognition of the CAP-Gly core GKNDG motif by tyrosine at the end of the alpha E-hook. It suggests that the GKNDG motif is interacting with the C terminus of tubulin whereas the basic patches 1–25 and 106–144 wrap around the rest of the E-hook to secure the binding (Fig. 1B).
Table 1.
Dissociation constants (Kd, µM) of p150glued fragments to E-hook peptides using fluorescence correlation spectroscopy
| p150 fragments | αY | αE | βC |
| 1–210 dimer | 0.8 ± 0.3 | 3 ± 1 | 0.8 ± 0.3 |
| 1–105 | 6 ± 3 | 35 ± 20 | 9 ± 5 |
| 25–105 | 11 ± 5 | > 130 | 46 ± 20 |
| 25–144 | 5.4 ± 1.5 | 28 ± 30 | 10 ± 4 |
| 1–144 | 7 ± 3 | 13 ± 4 | 7 ± 2 |
αY, tyrosinated alpha tubulin E-hook; αE, detyrosinated alpha tubulin E-hook, βC; beta tubulin E-hook.
p150glued CAP-Gly Fragments Flexibly Recognize the Microtubule Surface.
The computational amplitude adjustment of the reconstruction weakened the density of the CAP-Gly to a close-to-noise level. This faint density suggests either that the occupancy of the protein fragments is low or that their attachment to the microtubule surface is very flexible. However, the pelleting assay in the corresponding conditions showed the saturation of p150 fragments on the microtubules, supporting a possibility that the blurred density is due to the flexible binding of the p150 fragments.
To explore this aspect in detail, density contours of the reconstructions were calculated. The lateral projections of the reconstructions from radius ∼130–180 Å (Fig. 1C) showed the strongest density of the p150(1–105) decoration to be 4 Å lower than the neck position (Fig. 1C, Left, red arrowhead), shifted toward the minus end, but 8 Å for p150(25–144) (Fig. 1C, Right, red arrowhead). This position corresponds to the core of CAP-Gly, and it suggests a flexible binding mode for the interaction of CAP-Gly fragments with E-hooks. Comparing the overall shapes of the CAP-Gly core densities raises the possibility that the orientation of the CAP-Gly may be flipped between p150(1–105) and p150(25–144) reconstructions (direction shown in Fig. 1B).
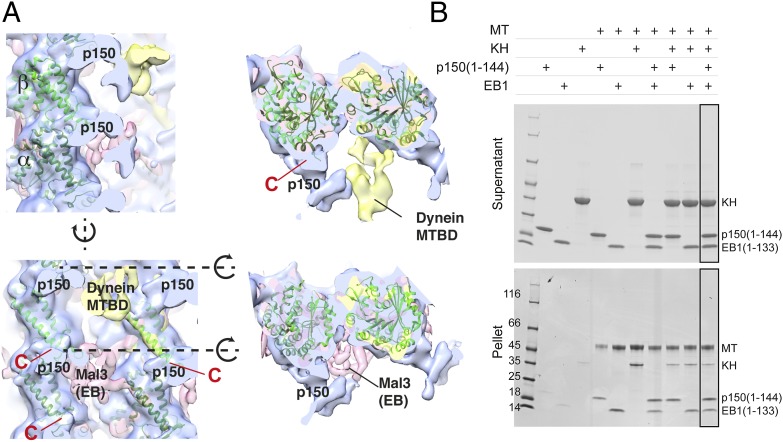
Further, the recognition site of CAP-Gly was mapped onto microtubules (Fig. 2, blue) and compared with the ones of dynein (Fig. 2A, yellow) (15) as well as the yeast EB protein Mal3 (Fig. 2A, pink) (16), a binding partner of p150glued during endosomal recruitment. Interestingly, all binding surfaces differ from each other, and there is enough space for all proteins to bind simultaneously to the same microtubule unit. p150glued binds to tubulin where the C-terminal E-hook is located (Fig. 2A, marked “C” in red) at the “neck” of the reconstruction. This position is well-separated from the binding sites for dynein/kinesin or EBs. The pelleting assay of the p150(1–144), EB1 CH domain and kinesin head to the microtubule also showed that they all bind to the microtubule surface (Fig. 2B, rightmost lane).
Fig. 2.
(A) Comparison of the binding surfaces on tubulins for p150glued neck, dynein (MTBD), and Mal3 (EB). The recognition sites of these proteins differ from each other. Red C, the C-terminal end of the tubulin atomic model, which precedes the flexible 15–16 residue E-hooks. Cross-sections of the surface of the side view (dotted line) are shown Right. (B) Pelleting assay of p150(1–144), kinesin head (KH), EB1 CH domain, and GTPgammaS-stabilized microtubules. The 2-µM microtubules and 20-µM proteins were mixed. Added proteins are indicated as “+.” The three proteins bind to microtubules simultaneously.
Microtubule Lateral Association Is Caused by Shielding of E-Hooks.
The saturated decoration of p150glued fragments on the microtubule surface caused microtubules to laterally associate with each other, which made the structural analysis particularly challenging. However, this observation drew our interest and led us to investigate the cause of the bundling triggered by CAP-Gly fragments.
We hypothesized that the lateral association of the microtubules may occur by the fact that CAP-Gly fragments cover the E-hooks. The negatively charged, flexible E-hooks could serve as an electrostatic shield that repels a close approach of neighboring microtubules. Binding of the positively charged p150glued segments to the negatively charged tubulin E-hooks may collapse the mobile barrier that keeps the microtubules apart. To test this hypothesis, we measured the change of turbidity at 400 nm by adding several p150glued fragments to taxol-stabilized microtubules (Fig. S3). The scattering increased as highly positively charged protein fragments were added, from 0.29 for microtubules alone up to 1.7 for p150(1–144) (net charge, +12.7). The degree of the increase in scattering correlates with the lateral association of microtubules observed in the corresponding electron micrographs (Fig. S1C). The turbidity was not increased for p150(25–105) (net charge, +1.6), which does not contain any basic patches. Interestingly, the addition of the construct p150(106–144), which has a net charge of +7 but does not contain CAP-Gly, also caused an increase of turbidity to 0.76 and the bundling of the microtubules. Consistent with this finding, we also observed the increase of turbidity and the lateral association of microtubules when tubulin E-hooks were removed by subtilisin (Fig. S3B). Further, we tested the change of turbidity with a control protein that has a high basic charge but is not derived from p150glued. For this purpose, we used histone H2A (15 kDa, net charge, +12.6) as a test case and measured the turbidity changing to 0.81, an increase similar to p150(106–144).
These results altogether suggest that the E-hooks of tubulin form a negative shield on the microtubule surface. The neutralization of the charge by basic proteins repels the shielding and leads to the lateral association of microtubules. The degree of the turbidity increase generally correlates well with the net charge of added protein fragments. This phenomenon does not require any specific protein interaction with microtubules as shown for the case of histone H2A or p150(106–144).
Longitudinal Tubulin Oligomerization Is Induced by CAP-Gly Plus Basic Patch at Low Temperatures.
The masking of the acidic tails of tubulin by CAP-Gly plus basic patches causes the lateral association of tubulin. Moreover, previous reports established the ability of p150 fragments to promote the polymerization of microtubules (5, 11). Therefore, we sought to correlate polymerization activity with the charge effects of various CAP-Gly fragments. First, the light scattering of tubulin below the critical concentration for spontaneous polymerization (2 µM) was monitored during a temperature shift from 4 °C to 37 °C (Fig. S4A, Tu). Consistent with previous reports, light scattering was elevated: i.e., tubulin polymerization, in the presence of the dimerized CAP-Gly, with its basic extensions [p150(1–210) dimer] (Fig. S4A) (11). We also observed assisted tubulin polymerization with p150(1–144) fragments (Fig. S4B) although the effect was much less, compared with the dimerized CAP-Gly. The stepwise change of ionic strength in the assay buffer confirmed the sensitivity of the process to the salt concentration, as expected from the electrostatic binding properties of the proteins. The pronounced tubulin copolymerization with p150(1–144) was observed when the tubulin concentration was high enough to self-promote polymerization (10 µM) (Fig. S4C, red lines).
To connect the tested protein fragments in the context of tubulin polymerization, we used the latter experimental condition to further examine the behavior of tubulins. The light-scattering profiles revealed an elevation of scattering with CAP-Gly, which included the C terminus basic extensions [p150(1–144), p150(25–144)] (Fig. S4C, red and purple) whereas the fragments without the extension [p150(1–105), p150(25–105)] did not have a significant influence. Interestingly, the profiles also showed that the presence of the C-terminal extension alone (residues 106–144) increased the final saturation level, agreeing with the increase of the turbidity seen with already polymerized, taxol-stabilized microtubules (Fig. S4C, pink). We further detected a striking increase in scattering for tubulin-CAP-Gly mixtures at the stage before the initiation of polymerization at 4 °C (Fig. S5A).
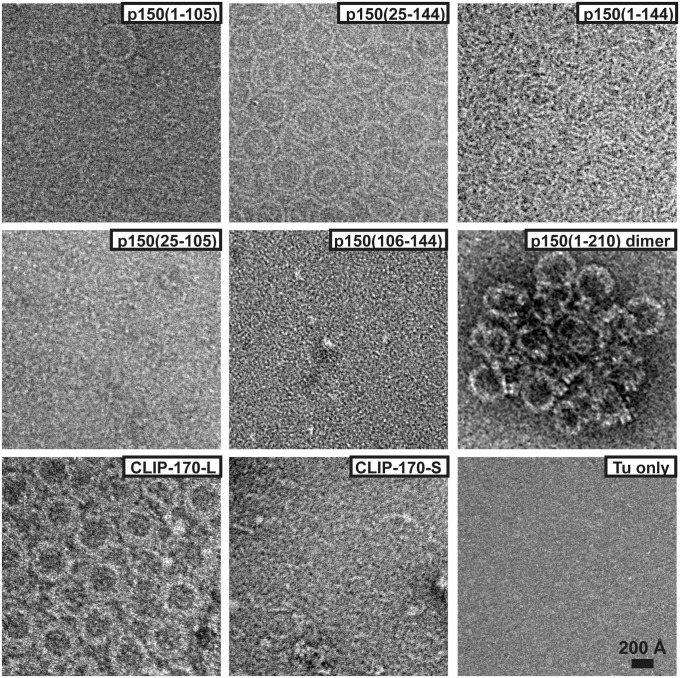
To visualize this rise, we analyzed the reaction mixtures using electron microscopy. The protein fragments were confirmed to bind to unpolymerized tubulins (Fig. S5B) and the CAP-Gly fragments containing the basic patches 1–25 or 106–144 induced tubulins to form linear, curved oligomers (Fig. 3) with a radius of curvature of ∼17 nm. These oligomers have the same morphology as the separated, curled protofilaments observed in vitro (17).
Fig. 3.
Negative stain EM observation of tubulin oligomers (10 µM) induced by p150glued fragments (10 µM) at 4 °C. Oligomer formation happens with p150(1–144) and p150(25–144), but there is very little or no oligomerization for p150(1–105), p150(25–105), and p150(106–144). p150(1–210) dimer causes tubulin oligomers and further clustering of the oligomers. CLIP-170L with two tandem CAP-Gly shows similar oligomer formation whereas CLIP-170S with one CAP-Gly shows no oligomeric formation.
This similarity indicates that the interaction of the CAP-Gly fragments to tubulin dimers at 4 °C increases tubulin’s longitudinal self-affinity.
The degree of oligomerization increased when more positively charged residues were present (Fig. 3 and Fig. S5C). When tubulin oligomer formation was induced by the p150(1–210) dimer, which has the most prominent effect on tubulin polymerization (Fig. S4A) (11), large agglomerates of tubulin oligomers were visible in EM (Fig. 3). Therefore, it is not feasible to quantify the degree of oligomerization using EM, even though light-scattering experiments showed comparable values with p150(1–144)-induced oligomers (Fig. S5A). In contrast, the longitudinal tubulin association did not occur in the presence of only CAP-Gly core, p150(25–105), or the basic patch without CAP-Gly, p150(106–144). These observations suggest that the activation of the longitudinal assembly of tubulin is facilitated by the bridging of both CAP-Gly and the basic extension, possibly like the way shown in the reconstruction of p150(25–144) (Fig. 1A, Center).
In comparison, CLIP-170 is a member of +TIPs that contains tandem-connected CAP-Gly domains. It has been reported that a CLIP-170 fragment containing only one CAP-Gly (CLIP-170S) does not enhance the polymerization of tubulin whereas a variant containing both CAP-Gly domains (CLIP-170L) increases the polymerization rate (18, 19). We extended this analysis to test whether oligomerization would occur with a CLIP-170-tubulin mixture using EM (Fig. 3). We detected that CLIP-170L induces tubulin oligomerization strongly whereas there was only sporadic oligomer formation with CLIP-170S. These results are consistent with the indication that the observed oligomers have a direct effect on microtubule polymerization.
Tubulin Oligomers Function as Intermediates During Polymerization.
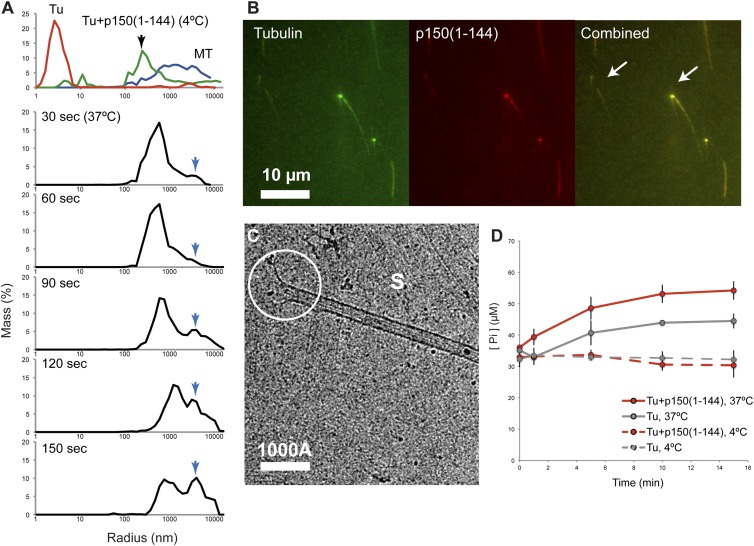
To understand the copolymerization of longitudinally connected tubulin oligomers induced by CAP-Gly fragments, we followed the process by dynamic light scattering (DLS). Although tubulin control showed a population of <10 nm (Fig. 4A, Top, red), the mixture of tubulin with p150(1–144) exhibited the population only at a size distribution of ∼100–1,000 nm (Fig. 4A, Top, green) at 4 °C. When tubulin polymerization was initiated by a temperature shift to 37 °C, a growing population of an ∼1,000–10,000 nm species was observed after 60–90 s (Fig. 4A, 90 s, blue arrow). At the same time, the population of the 100–1,000 nm decreased. After 150 s, the size distribution became comparable with microtubules alone (Fig. 4A, Top, blue). During this polymerization, the signal of tubulin dimers (1–10 nm) was not detected, strongly indicating that the oligomeric formation of tubulin and p150(1–144) directly transitions to tubulin polymers without disassembling into dimer units.
Fig. 4.
Intermediates of tubulin polymerization induced by p150(1–144). (A) DLS measurements of the mixture of tubulin and p150(1–144). (First row) The control scattering profile of tubulin at 4 °C (red), tubulin with p150(1–144) (green), and microtubule at 37 °C (blue). (second to sixth row) Scattering profiles of the mixture of tubulin-p150(1–144) after incubation at the indicated time points at 37 °C. The blue arrow traces the growth of the microtubules and sheets. (B) Copolymerization of p150(1–144) and tubulin into microtubules at 5 min observed by fluorescence microscopy. Two examples of the colocalization of CAP-Gly and tubulin are indicated by arrows. (C) Cryo-EM observation of tubulin and p150(1–144) copolymerization 5 min after mixing. S, sheet together with thin fibrils, which are precursors of protofilaments. The active end of a microtubule is indicated with a white circle. (D) GTPase activity measurements of tubulin alone (gray, dotted line), tubulin with p150(1–144) (red, dotted line) at 4 °C, and the corresponding curves at 37 °C (solid lines, respectively). Error bars (SD) are calculated from three independent measurements.
Further, snapshots of the copolymerization at 300 s were taken by cryo-EM and epifluorescence light microscopy. Consistent with the observations by dynamic light scattering, we could readily detect formations of microtubules. The striking population of laterally connected, sheet-like tubulin polymers was observed as well (Fig. 4C, marked as “S” and Fig. S6). The lateral interaction is promoted upon the temperature change to 37 °C. The ends of the closed microtubules often showed a flared morphology, sometimes with fragments of straightened oligomers attached to each other (Fig. S6, guided in magenta). The corresponding experiment using light microscopy revealed a strong colocalization of p150(1–144) at the end of microtubules as well as a weak colocalization on the surface of growing microtubules (Fig. 4B, arrows).
Taken together, the preformed, longitudinally connected tubulin oligomers (before tubulin polymerization) activate their lateral interactions upon temperature change, resulting in sheet-like formations. The oligomers tend to cluster at the end of the closed growing microtubule, likely incorporating into the microtubule structure.
GTP Hydrolysis Is Not Required for Tubulin Oligomer Formation.
The tubulin oligomers that are induced by p150glued have a linear, curved formation that could act as a building block for tubulin polymerization. Similar observations have been made for CLIP-170 (19). This curved morphology of tubulin oligomers can also be observed in GDP-tubulin rings (20), which is actually the preferred nucleotide state for depolymerized tubulin (21). Furthermore, curved protofilaments are observed at the depolymerizing ends of microtubules (17, 22, 23). Therefore, we asked whether the hydrolysis of GTP occurs coincidently with tubulin oligomerization. For this purpose, we measured the concentration of inorganic phosphate (Pi) released in the presence of tubulin and p150(1–144) during the formation of curved oligomers. Surprisingly, we did not observe any significant differences in [Pi] at any time of incubation (Fig. 4D, dotted lines): 32.7 µM (SD 2.4) (Tu only) vs. 32.1 µM (SD 2.0) [Tu plus p150(1–144)]. It indicates that GTP hydrolysis is not coupled to tubulin oligomerization. We also observed that tubulin-p150(1–144) oligomerizes in the presence of GMPCPP (a nonhydrolyzable analog of GTP) and that the oligomers retained their curved shape (Fig. S7). Thus, the observed curved tubulin oligomers are not due to GTP hydrolysis. On the contrary, the GTPase activity of tubulin was activated in the presence of p150(1–144) more than the tubulin control (Fig. 4D, red solid line) after the initiation of tubulin polymerization, suggesting that GTP hydrolysis is required only when the incorporation of the tubulin oligomers into the microtubule lattice occurs.
Deletion of Tubulin E-Hook Activates Tubulin Polymerization.
Our assays showed that the binding to CAP-Gly is mediated by tubulin E-hooks, in agreement with previous biochemical reports (6–9, 13, 24), and that the coverage of E-hooks by the basic extension activates tubulin polymerization. A similar activation of tubulin polymerization was observed by subtilisin-treated tubulin alone (Fig. S8) (25, 26). The resulting polymerized products showed bundling of the microtubules or open and connected sheets under EM (Fig. S8B), supporting the notion that the E-hooks shield tubulins from the lateral connection. Altogether, these findings suggest the importance of E-hooks to control tubulin polymerization kinetics using a negatively charged patch as a shield. The positively charged CAP-Gly fragments may modulate the tubulin polymerization by neutralizing the electrostatic shield of the E-hooks.
Discussion
Our study describes the interaction of tubulin E-hooks with p150glued from a structural point of view and seeks to describe the relationship of the molecular interaction with tubulin polymerization activity. p150glued CAP-Gly plus its adjacent basic patches recognizes the negative electrostatic surface of the tubulin acidic E-hooks. Its binding surface differs from other microtubule-binding proteins in the endosomal recruitment pathway. The basic patches affect tubulin self-assembly through interactions with tubulin's E-hooks. From our observations, we surmise a possible role of tubulin E-hooks in the context of CAP-Gly interaction. Namely, CAP-Gly and its basic patches may work as a cross-linker of tubulins. This crosslinking effect could be seen as oligomeric association when tubulin is not spontaneously associable (4 °C). Upon the change of the temperature to 37 °C, lateral association of protofilaments may be immediately activated. These two directional associations may lead to the acceleration of the polymerization. The effect is likely more efficient when CAP-Gly forms dimers (11), presumably due to the increase of the local concentration. We in fact observed a stronger local clustering of tubulin oligomers in the presence of the dimeric protein (Fig. 3). This bridging could serve as a stabilizer for tubulin to adopt a polymerizable conformation. In a cellular environment, this nucleation mechanism of p150glued may be facilitated when microtubules undergo the phase change from rapid shrinkage to growth. Efficient growth of the microtubules at the plus ends can be achieved if tubulin oligomers are used as the basic building block. Similar models were suggested for other +TIP proteins (18), and a comprehensive analysis combining these components is necessary.
Implications of the Nonoverlapping Recognition of p150glued, EB, and Dynein and of the Surfing Activity of p150glued.
Our structural analysis directly identified the interaction interface between p150glued CAP-Gly and tubulin E-hooks. Interestingly, this binding surface on tubulin is distinct from those of EBs and dynein (Fig. 2). CAP-Gly recognizes tubulin E-hooks protruding from the outer surface of the microtubule whereas EBs recognize the nucleotide-binding pocket at the groove between the microtubule protofilaments. Dynein binds to an area closer to helices H11/H12 of tubulin, which is also the binding site for the other major microtubule-motor protein kinesin (27) (Fig. 2). Moreover, p150glued has been reported to form a complex with EB1 and CLIP-170, and this +TIPs complex would track the plus ends of microtubules (1). Our finding that the binding sites of these three microtubule-associated proteins do not sterically hinder each other may facilitate a smooth bridging of the plus-end tracking and subsequent vesicle tethering to the dynein motor.
p150glued has been reported to diffuse one-dimensionally along the microtubule surface, termed surfing/skating (12, 28). Considering the main function of p150glued as an anchor for vesicles at the plus end of microtubules (29–32), it is conceivable that the surfing activity is a way of maximizing the chance of encountering EB proteins that directly recognize GTP-tubulin at the end of microtubules. Our study provides a molecular basis for this surfing mechanism. E-hooks, which are incorporated into the microtubule surface, provide a periodic array of negative electrostatic charges. CAP-Gly, with its basic patches, recognizes this surface. CAP-Gly also has a specific interaction with E-hooks, which can ensure efficient binding to microtubules. E-hooks give an opportunity for CAP-Gly to diffuse laterally to the next binding site by providing the continuous charged surface.
The Role of Tyrosinated Tubulin for p150glued.
It has been reported that p150glued CAP-Gly domains bind preferably to alpha tubulin that is tyrosinated at the C-terminal E-hook (7). Tubulin tyrosination occurs in its depolymerized form (33) and so freshly incorporated alpha tubulin harbors the modification at microtubule plus ends. This biased distribution of tyrosinated tubulin is likely the key for the microtubule plus-end recognition by CAP-Gly (7, 34). In our assays, p150glued CAP-Gly core has a higher affinity to the tyrosinated alpha E-hook compared with the detryrosinated E-hook and beta E-hook (Table 1), but the basic extensions increase the binding affinity of CAP-Gly fragments to other E-hooks. CAP-Gly prefers tyrosinated tubulin to bias itself toward the plus ends of microtubules. The situation in a cell, however, might be more complex. E-hooks are variable in isoforms in addition to the diverse decoration by posttranslational modifications. The weak interaction through basic extensions might play a role for reinforcing the affinity of p150glued to microtubules. Further studies are needed to understand the functions of different tubulin isoforms in the context of posttranslational modifications.
Materials and Methods
Recombinant proteins of p150glued fragments, CLIP-170, EB1, and KH are expressed in E. coli. Protein purifications and subsequent biochemical analyses were carried out as described in SI Material and Methods. Details of electron microscopic analyses are also found in SI Material and Methods, as well as in Fig. S9.
Supplementary Material
Acknowledgments
We thank Dr. Elena Conti and Dr. Wolfgang Baumeister for resources and support and the Core Facility of the Max Planck Institute of Biochemistry for MGC clones and peptide synthesis; Dr. Jürg Müller for a generously sharing Histone H2A protein; and Dr. Tanvir Shaikh, Dr. Yoko Y. Toyoshima, Dr. Christian Biertümpfel, Dr. Petra Schwille, and Dr. Sven Vogel for insightful discussion and careful readings of the manuscript. We also thank Dr. Giovanni Cardone for valuable discussions about image processing and Dr. Charles Sindelar for sharing his script for the conversion of parameters between different software. This study was supported by the Max Planck Society for the Advancement of Science and the Deutsche Forschungsgemeinshaft DFG through a grant within the SPP1464, GRK1721, and MI 1745/1.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Electron Microscopy Data Bank (accession nos. 2673, 2674, and 2675).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403135111/-/DCSupplemental.
References
- 1.Akhmanova A, Steinmetz MO. Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9(4):309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 2.Cronin MA, Schwarz TL. The CAP-Gly of p150: One domain, two diseases, and a function at the end. Neuron. 2012;74(2):211–213. doi: 10.1016/j.neuron.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson P, Stephens DJ. Microtubule plus-end loading of p150(Glued) is mediated by EB1 and CLIP-170 but is not required for intracellular membrane traffic in mammalian cells. J Cell Sci. 2006;119(Pt 13):2758–2767. doi: 10.1242/jcs.02999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lansbergen G, et al. Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J Cell Biol. 2004;166(7):1003–1014. doi: 10.1083/jcb.200402082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligon LA, Shelly SS, Tokito M, Holzbaur ELF. The microtubule plus-end proteins EB1 and dynactin have differential effects on microtubule polymerization. Mol Biol Cell. 2003;14(4):1405–1417. doi: 10.1091/mbc.E02-03-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi I, Wilde A, Mal TK, Ikura M. Structural basis for the activation of microtubule assembly by the EB1 and p150Glued complex. Mol Cell. 2005;19(4):449–460. doi: 10.1016/j.molcel.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Peris L, et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol. 2006;174(6):839–849. doi: 10.1083/jcb.200512058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honnappa S, et al. Key interaction modes of dynamic +TIP networks. Mol Cell. 2006;23(5):663–671. doi: 10.1016/j.molcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Weisbrich A, et al. Structure-function relationship of CAP-Gly domains. Nat Struct Mol Biol. 2007;14(10):959–967. doi: 10.1038/nsmb1291. [DOI] [PubMed] [Google Scholar]
- 10.Bieling P, et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J Cell Biol. 2008;183(7):1223–1233. doi: 10.1083/jcb.200809190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus JE, Moughamian AJ, Tokito MK, Holzbaur ELF. Dynactin subunit p150(Glued) is a neuron-specific anti-catastrophe factor. PLoS Biol. 2013;11(7):e1001611. doi: 10.1371/journal.pbio.1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8(3):264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Shiroguchi K, Edamatsu M, Toyoshima YY. Microtubule-binding properties of dynactin p150 expedient for dynein motility. Biochem Biophys Res Commun. 2006;340(1):23–28. doi: 10.1016/j.bbrc.2005.11.145. [DOI] [PubMed] [Google Scholar]
- 14.Sui H, Downing KH. 2010. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18:1022–1031. [DOI] [PMC free article] [PubMed]
- 15.Redwine WB, et al. Structural basis for microtubule binding and release by dynein. Science. 2012;337(6101):1532–1536. doi: 10.1126/science.1224151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer SP, Fourniol FJ, Bohner G, Moores CA, Surrey T. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell. 2012;149(2):371–382. doi: 10.1016/j.cell.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelkow EM, Mandelkow E, Milligan RA. Microtubule dynamics and microtubule caps: A time-resolved cryo-electron microscopy study. J Cell Biol. 1991;114(5):977–991. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slep KC, Vale RD. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol Cell. 2007;27(6):976–991. doi: 10.1016/j.molcel.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnal I, Heichette C, Diamantopoulos GS, Chrétien D. CLIP-170/tubulin-curved oligomers coassemble at microtubule ends and promote rescues. Curr Biol. 2004;14(23):2086–2095. doi: 10.1016/j.cub.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson WV, Lee M, Downing KH, Nogales E. Cryo-electron microscopy of GDP-tubulin rings. Cell Biochem Biophys. 1999;31(2):175–183. doi: 10.1007/BF02738171. [DOI] [PubMed] [Google Scholar]
- 21.Tran PT, Joshi P, Salmon ED. How tubulin subunits are lost from the shortening ends of microtubules. J Struct Biol. 1997;118(2):107–118. doi: 10.1006/jsbi.1997.3844. [DOI] [PubMed] [Google Scholar]
- 22.Moores CA, et al. A mechanism for microtubule depolymerization by KinI kinesins. Mol Cell. 2002;9(4):903–909. doi: 10.1016/s1097-2765(02)00503-8. [DOI] [PubMed] [Google Scholar]
- 23.Asenjo AB, et al. Structural model for tubulin recognition and deformation by kinesin-13 microtubule depolymerases. Cell Reports. 2013;3(3):759–768. doi: 10.1016/j.celrep.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Bu W, Su L-K. Characterization of functional domains of human EB1 family proteins. J Biol Chem. 2003;278(50):49721–49731. doi: 10.1074/jbc.M306194200. [DOI] [PubMed] [Google Scholar]
- 25.Sackett DL, Bhattacharyya B, Wolff J. Tubulin subunit carboxyl termini determine polymerization efficiency. J Biol Chem. 1985;260(1):43–45. [PubMed] [Google Scholar]
- 26.Peyrot V, Briand C, Andreu JM. C-terminal cleavage of tubulin by subtilisin enhances ring formation. Arch Biochem Biophys. 1990;279(2):328–337. doi: 10.1016/0003-9861(90)90499-o. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno N, et al. Dynein and kinesin share an overlapping microtubule-binding site. EMBO J. 2004;23(13):2459–2467. doi: 10.1038/sj.emboj.7600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2(1):20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, et al. Microtubule binding by dynactin is required for microtubule organization but not cargo transport. J Cell Biol. 2007;176(5):641–651. doi: 10.1083/jcb.200608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier-Larsen ES, Wallace KE, Pennise CR, Holzbaur ELF. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin. Hum Mol Genet. 2008;17(13):1946–1955. doi: 10.1093/hmg/ddn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moughamian AJ, Holzbaur ELF. Dynactin is required for transport initiation from the distal axon. Neuron. 2012;74(2):331–343. doi: 10.1016/j.neuron.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kardon JR, Reck-Peterson SL, Vale RD. Regulation of the processivity and intracellular localization of Saccharomyces cerevisiae dynein by dynactin. Proc Natl Acad Sci USA. 2009;106(14):5669–5674. doi: 10.1073/pnas.0900976106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 34.Mishima M, et al. Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition. Proc Natl Acad Sci USA. 2007;104(25):10346–10351. doi: 10.1073/pnas.0703876104. [DOI] [PMC free article] [PubMed] [Google Scholar]