DNA replication machinery copies an entire genome with remarkable speed and accuracy in preparation for each cell division. However, DNA polymerase unidirectionality and requirement for a free 3′ hydroxyl group render these enzymes incapable of duplicating the extreme ends of eukaryotic linear chromosomes. This deficiency leads to a gradual loss of terminal sequence with each round of replication that threatens the integrity of essential genetic information. This calamity is avoided through the function of telomeres, typically structures of short repeating sequence added to chromosome 3′ ends by telomerase (1). Soon after its discovery as a polymerase, telomerase was found to reverse transcribe a template sequence contained within the telomerase integral RNA subunit TER (2). Telomerase biochemical activity has proven to be unique even among reverse transcriptases (RTs) in features including the release of single-stranded rather than duplex product DNA, which is necessary for regenerating the template for telomerase’s distinctive repeat synthesis processivity (3). How telomerase gained new types of nucleic acid handling specificity relative to other RTs has been the subject of intense research, much of which has focused on the structure and function of nontemplate specializations of TER and unique domains of the telomerase protein RT subunit TERT (4). In PNAS, Brown et al. present surprising evidence of a previously missed specialization of human telomerase (5). Using elegant and incisive biochemical assays, the authors demonstrate that this enzyme reads sequence cues within the product–template duplex to dramatically influence the specificity of product synthesis. The work reveals an unanticipated divergence of telomerase from other polymerases in active site adaptation for its cellular duty.
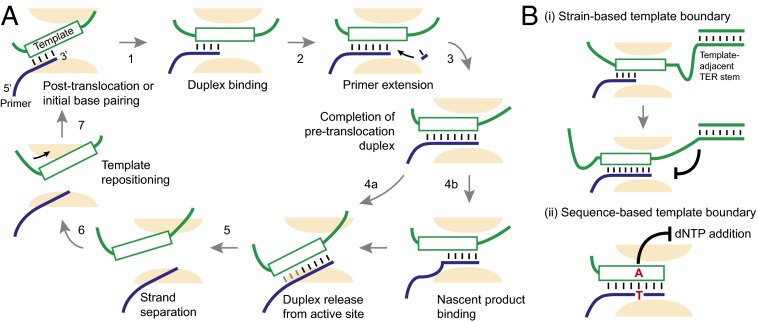
The diverse template and substrate maneuvers that occur during repeat synthesis constitute a telomerase catalytic cycle (Fig. 1A). A primer 3′ end base pairs at one end of the TER template and, on engagement of the duplex in the active site, is extended using familiar two-metal-ion chemistry (6). Synthesis proceeds to the end of the template region to complete a single telomeric repeat with a defined 3′ permutation. At this point, telomerase must perform its signature move, releasing product from the template, which can be followed by realigning the product 3′ end for repeat synthesis processivity (Fig. 1A). The base pairs between product and template are destabilized in a process that is mysterious, not least because it must accomplish a reaction that is thermodynamically unfavorable. Also curious is how the template is repositioned to recycle the active site to favor binding a short primer–template duplex rather than the longer duplex that was unpaired. Concurrent with these template rearrangements, one repeat of product DNA is extruded (7, 8). Central to the quest of understanding telomerase function is resolving how structures cooperate to orchestrate the nucleic acid handling required for the enzyme’s catalytic cycle. Limited high-resolution structural information on the enzyme has made this quest challenging, yet creative biochemical analyses have allowed the deduction of many mechanistic details.
Fig. 1.
Unique nucleic acid handling specificities adapt telomerase for repeat synthesis. (A) The telomerase catalytic cycle begins with formation of the initial primer–template duplex, which docks in the active site for repeat synthesis. Step 2 is proposed to be rate limiting by refs. 5 and 7, and steps 4–7 and 1 could be in reversible equilibrium (7). Step 3 has been shown to be duplex sequence dependent (5). After repeat completion, duplex is proposed to release from the active site (step 4a). We suggest this release could be mediated through single-stranded DNA filling of a nascent product binding site, with or without sliding of remaining duplex from the active site (step 4b). (B) A template-adjacent RNA stem (i) and duplex sequence-based signal (ii) define the boundaries of the region of TER used as template.
Among the best-characterized principles for telomerase specialization is the establishment of the internal template. Strict definition of template boundaries is essential for next repeat synthesis because imprecise products prevent formation of a new primer–template duplex. A template-adjacent TER stem was shown to function in template definition in the Tetrahymena thermophila telomerase and later in yeast and human enzymes (4). The stem structure and RNA–protein interactions constrain the amount of single-stranded RNA that can be pulled through the active site (Fig. 1B). This strain-based mode of template boundary definition seems particularly important in the T. thermophila telomerase, for which stretching and compression of RNA on either side of the template have been proposed to drive destabilization of product–template base pairing (9).
The findings of Brown et al. compel reconsideration of our understanding of template boundary definition. The authors start by reconstituting human telomerase with TER lacking the template region. The enzyme was supplied with a template in the form of an RNA oligonucleotide preannealed with a DNA primer. This preformed RNA/DNA hybrid substrate allows assay of primer extension in a context where template use is not influenced by physical contiguity with other structures. The authors make the unanticipated observation that telomerase did not always extend the primer to the end of the template oligonucleotide, even when the resulting duplex remained shorter than the active site is known to accommodate (5). A series of trans templates was used to identify a single nucleotide position responsible for the halting of synthesis. Notably, when the same series of template sequences was assayed in the context of telomerase with full-length TER, the sequence signal was similarly critical, strikingly able to induce synthesis pausing and subsequent translocation at locations independent of distance to template-flanking structures. In other words, human telomerase seems to use a sequence cue inherent to the product–template duplex to sense when it has made a full repeat (Fig. 1B). This surprising finding reveals a characteristic of human telomerase that is unprecedented among other polymerases and key to establishing the specificity of the telomerase catalytic cycle. In addition, it accounts for why the template-flanking TER stem could be deleted in rodents (10).
How might the sequence-based template boundary signal specify the pretranslocation duplex? Previous work led the Chen group to a model that translocation does not occur with either primer or template remaining in the active site, meaning that the active site binds and aligns duplex only (11). Brown et al. use a primer–template competition assay to provide evidence that the sequence-based template boundary signal stops synthesis without forcing duplex release from the active site. A recent study using single-molecule FRET observation of template and product movements through the catalytic cycle revealed a dynamic interconversion of FRET states at the end of repeat synthesis, when the template boundary signal would be active (7). Parks and Stone propose that FRET state switching is between the pretranslocation and posttranslocation registers of duplex bound in the active site (7). Because interconversion was fast relative to the next dNTP addition, none of the proposed steps of pretranslocation duplex release, strand separation, template repositioning, and posttranslocation duplex binding (Fig. 1A) would limit the rate of processive repeat synthesis. This model matches prediction from Brown et al. that
It will be exciting for future studies to test models that incorporate the new specificities of nucleic acid handling established by Brown et al.
the posttranslocation duplex undergoes a rate-limiting positioning within the active site after it is bound. Alternatively, the FRET dynamic could reflect duplex movement or partial dissociation occurring before translocation (Fig. 1A). In this case, the sequence-based template boundary signal would induce some change in product and template positioning before a rate-limiting conversion to full strand separation. It will be exciting for future studies to test models that incorporate the new specificities of nucleic acid handling established by Brown et al.
An important future objective is to identify the physical TERT and TER determinants responsible for reading the sequence-based template boundary signal and to determine whether they might contribute to the still-mysterious coordination between template and single-stranded DNA handling. Sequence-defined pausing combined with repeat-by-repeat enzyme extrusion of single-stranded DNA raises the provocative hypothesis that elongating telomerase presents a specific register of just-released product to other DNA binding factors. Brown et al. point out that sequence-defined pausing appears variably strict across telomerases from different species and is lacking entirely in T. thermophila (5). These differences may have important biological underpinnings: for example, giving ciliate telomerase improved ability to add telomeric repeats to the fragmented chromosomes produced during macronuclear development (4). How telomerases of different organisms carry out fundamental aspects of the catalytic cycle in mechanistically divergent ways may well be reflected in the structural details of their TERTs, TERs, and other telomerase holoenzyme components. Therefore, as the many modes by which telomerase has specialized as a polymerase for telomeric repeat synthesis are revealed, so too are the ways in which these specializations are optimized to the particular telomere biology of each organism.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
See companion article on page 11311.
References
- 1.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 3.Collins K. Single-stranded DNA repeat synthesis by telomerase. Curr Opin Chem Biol. 2011;15(5):643–648. doi: 10.1016/j.cbpa.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn EH, Collins K. Telomerase: An RNP enzyme synthesizes DNA. Cold Spring Harb Perspect Biol. 2011;3(5):a003558. doi: 10.1101/cshperspect.a003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown AF, et al. A self-regulating template in human telomerase. Proc Natl Acad Sci USA. 2014;111:11311–11316. doi: 10.1073/pnas.1402531111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci USA. 1993;90(14):6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parks JW, Stone MD. Coordinated DNA dynamics during the human telomerase catalytic cycle. Nat Commun. 2014;5:4146. doi: 10.1038/ncomms5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu RA, Collins K. Human telomerase specialization for repeat synthesis by unique handling of primer-template duplex. EMBO J. 2014;33(8):921–935. doi: 10.1002/embj.201387205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman AJ, Akiyama BM, Stone MD, Cech TR. The RNA accordion model for template positioning by telomerase RNA during telomeric DNA synthesis. Nat Struct Mol Biol. 2011;18(12):1371–1375. doi: 10.1038/nsmb.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic Acids Res. 2008;36(Database issue):D339–D343. doi: 10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi X, et al. RNA/DNA hybrid binding affinity determines telomerase template-translocation efficiency. EMBO J. 2012;31(1):150–161. doi: 10.1038/emboj.2011.363. [DOI] [PMC free article] [PubMed] [Google Scholar]