Significance
Plasma cells (PCs) are terminally differentiated B cells that secret large amounts of antibodies to protect the host from infectious pathogens. Our study describes an important role for the adaptor protein Downstream-of-Kinase (DOK) 3 in promoting the differentiation of PCs by regulating the expression of programmed cell death 1 (PD-1) ligands PDL1 and PDL2. Moreover, we discover an unexpected role of calcium signalling in inhibiting the expressions of PD-1 ligands, and this inhibition is attenuated by DOK3. Knowing the signalling pathways that govern PC differentiation is critical for the optimal design of vaccines and adjuvants.
Abstract
The adaptor Downstream-of-Kinase (DOK) 3 functions as a negative regulator and attenuates B-cell receptor-mediated calcium signaling. Although DOK3 is dispensable for early B-cell development, its role in plasma cell (PC) differentiation is unknown. Here, we show that Dok3−/− mice have increased populations of T follicular-helper (Tfh) and germinal center (GC) B cells upon immunization with a T-cell–dependent antigen. However, interestingly, they generate significantly fewer PCs. Bone marrow reconstitution experiments show that the PC defect is B-cell intrinsic and due to the inability of Dok3−/− B cells to sustain programmed cell death 1 (PD-1) ligand 1 (PDL1) and up-regulate PD-1 ligand 2 (PDL2) expressions that are critical for PC differentiation. Overexpression of PDL2 rectifies the PC differentiation defect in Dok3−/− B cells. We further demonstrate that calcium signaling suppresses the transcription of PD-1 ligands. Abrogation of calcium signaling in B cells by deleting BTK or PLCγ2 or inhibiting calcineurin with cyclosporine A leads to increased expression of PD-1 ligands. Thus, our study reveals DOK3 as a nonredundant regulator of PC differentiation by up-regulating PD-1 ligand expression through the attenuation of calcium signaling.
Antibody-secreting plasma cells (PCs) with high affinity against antigens are generated during germinal center (GC) reactions (1, 2). Within GC, antigen-activated B cells receive help from a specialized subset of CD4+ T cells called T follicular-helper (Tfh) cells, undergo proliferation, Ig variable gene somatic hypermutation, and heavy chain isotype class switching and subsequently, differentiate into memory B cells and long-lived PCs (3). The cooperation between GC B and Tfh cells is tightly regulated and depends on cognate interactions involving a number of cell surface receptor-ligand pairs such as CD40-CD40L, CD80/86-CD28, ICOSL-ICOS, and many others (3). Interruptions of any of these molecular interactions will affect GC formation and compromise the antibody response.
Programmed cell death 1 (PD-1) and its interacting ligands, PDL1 and PDL2, are inhibitory molecules that regulate T-cell activation and tolerance (4, 5). Recently, PD-1 signaling was demonstrated to be critical for antibody production and diversification through regulating the generation and maintenance of PCs (6–8). PD-1 is not expressed on resting T cells but is inducibly expressed on activated T-cell subsets including Tfh cells (3). By contrast, the expression patterns of PDL1 and PDL2 are quite different. PDL1 is constitutively expressed on numerous immune cell types including B and T cells, whereas PDL2 expression is more restricted and is up-regulated upon activation on macrophages and GC B and dendritic cells (6, 9). Although the role of PD-1/PD-1 ligands interaction in driving PC formation is now beginning to be defined, it is still unclear how PDL1 and PDL2 expressions are being regulated in B cells and, in particular, activated B and GC B cells. Specifically, it is not known what signaling molecule and pathway would regulate the expression of PDL1 and PDL2 on activated B cells and affect PC differentiation.
Engagement of antigen by the B-cell receptor (BCR) induces a number of signaling pathways that culminate in the regulation of gene expression that drive the differentiation of activated B cells toward GC B and ultimately, memory B cells and PCs (10). One of the critical BCR-activated pathways is that of calcium signaling. This signaling pathway is initiated when the adaptor B-cell linker (BLNK) recruits Bruton’s tyrosine kinase (BTK) to activate phospholipase Cγ2 (PLCγ2) that together lead to Ca2+ flux in B cells (11, 12). After activation, another adaptor Downstream-of-kinase (Dok)-3 recruits Grb2 that together sequester away BTK to diminish PLCγ2 activation and, thereby, attenuate calcium signaling (12–15). Calcium signaling is known to induce the cell cycle entry of activated B lymphocytes, but it is not known whether it regulates the expression of any key molecules that might be critical for PC differentiation.
We had studied DOK3 in B cells and shown that it was not required for early B-cell development (14). DOK3 belongs to a family of seven related adaptors. DOK1, 2, and 3 are preferentially expressed in the immune system (13). DOK1 and 2 are found in T cells, whereas DOK1 and 3 are expressed in B lymphocytes. DOK1-deficient B cells have increased ERK activation (16). We and others had demonstrated that DOK3 deficiency resulted in elevated calcium signaling in B cells and is consistent with the phenotype of Dok3−/− mice that had enhanced T-cell–independent immune response (14, 15). Earlier, we documented no significant difference in T-cell–dependent antibody response in Dok3−/− mice although in that study, the antibody titers were quite varied, which perhaps reflected the not-so-pure genetic background of the newly generated mutant mice. We had also not studied in detail whether there was a role for DOK3 in GC B-cell and PC differentiation.
Here, we examine the role of DOK3 in B-cell terminal differentiation and show that DOK3 deficiency leads to the expansion of GC B and Tfh cells but impairs the formation of PCs. We further demonstrate that DOK3 promotes PC differentiation by maintaining an optimal level of PDL1 and up-regulating PDL2 expression on GC B cells. We also show that calcium signaling represses PDL1 and PDL2 gene expression. Taken together, our findings establish DOK3 as a key signaling molecule important for PC differentiation by attenuating calcium signaling to up-regulate PD-1 ligand expression.
Results
DOK3 Deficiency Leads to Increased Formation of Tfh and GC B Cells.
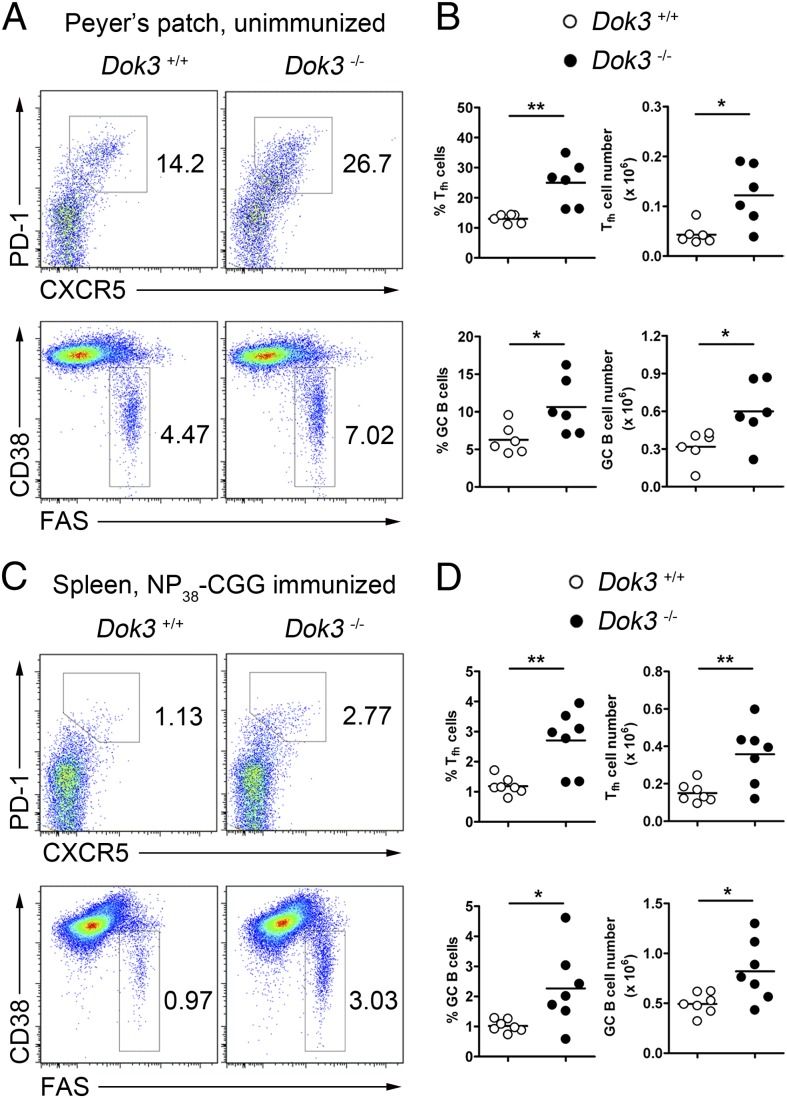
We generated Dok3−/− mice and showed that DOK3 was not required for early B lymphopoiesis (14). However, DOK3 is known to attenuate BCR signaling (14, 15), which should affect B-cell activation and terminal differentiation. So far, whether DOK3 has an effect on terminal B-cell differentiation in vivo after antigen recognition has not been fully explored. To address this possibility, we first examined the Peyer’s patches (PPs) of wild-type (WT) and Dok3−/− mice where chronic immune responses occurred. Interestingly, Dok3−/− mice have significantly expanded populations of GC B (CD19+CD38−Fas+) and Tfh (CD4+TCRβ+PD-1+CXCR5+) cells compared with WT controls (Fig. 1 A and B). To determine whether the same phenomenon could be recapitulated in an acute immune response, we immunized WT and Dok3−/− mice with alum-precipitated T-cell–dependent antigen, NP38-CGG [4-hydroxy-3-nitrophenyl-acetyl (NP) coupled to chicken gamma-globulin (CGG)]. We also found an approximately twofold enhancement in the fractions of GC B and Tfh cells in the spleen of Dok3−/− compared with WT mice at day 10 after immunization (Fig. 1C). Enumeration of GC B and Tfh cells confirmed that these cells were significantly increased in antigen-challenged mutant animals (Fig. 1D). These data indicated that DOK3 deficiency leads to the expansion of GC B and Tfh cells in mice.
Fig. 1.
Expansion of Tfh and GC B cells in Dok3−/− mice. Flow cytometry analysis (A) and quantification of the percentage and numbers (B) of Tfh (CD4+TCRβ+CXCR5+PD-1+) and GC B (CD19+CD38−Fas+) cells in the Peyer’s patches of unimmunized WT and Dok3−/− mice. (C) Representative flow cytometry analysis of Tfh and GC B cells in spleens of WT and Dok3−/− mice at day 10 after immunization. (D) Quantification of Tfh and GC B cells in immunized WT and Dok3−/− mice as shown in C. Numbers indicate percentage of CD4+ cells for Tfh cells and CD19+ cells for GC B cells. Each symbol represents one mouse analyzed. *P < 0.05; **P < 0.01.
Impaired T-Cell–Dependent Antibody Response in Dok3−/− Mice.
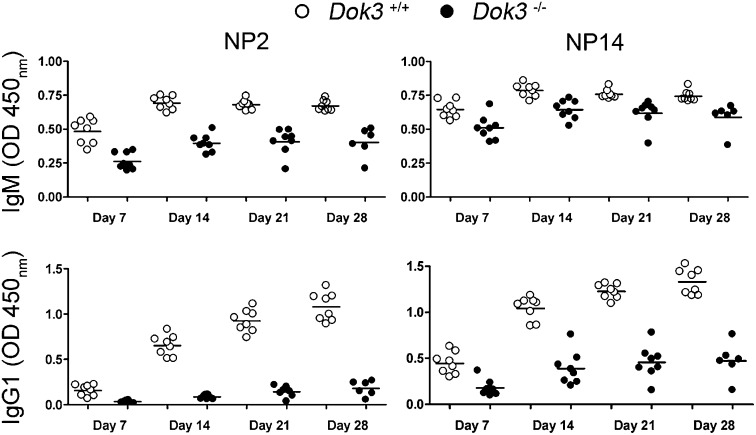
Given that both Tfh and GC B cells were significantly expanded in Dok3−/− mice and that GC B cells give rise to high-affinity long-lived PCs, we postulated that the mutant mice would have enhanced T-cell–dependent antibody response. To test this hypothesis, we measured antigen-specific antibody production by ELISA using NP2- and NP14-BSA to detect high-affinity and total antigen-specific antibodies in WT and Dok3−/− mice at various time-points after immunization. The humoral immune response to NP in mice is dominated by IgM and IgG1 antibodies. Surprisingly, Dok3−/− mice exhibited much lower anti-NP IgM and IgG1 antibody titers compared with WT controls (Fig. 2). Antigen-specific antibodies of other isotypes were also drastically reduced in the mutant mice suggesting that the defect was not due to skewed generation of antibodies of other isotypes (Fig. S1). These results indicated that DOK3 signaling is required for optimal T-cell–dependent antibody responses in mice.
Fig. 2.
Defective T-cell–dependent antibody responses in Dok3−/− mice. ELISA measurement of NP2- and NP14-binding IgM and IgG1 antibodies in the sera of WT and Dok3−/− mice at day 7, 14, 21, and 28 after immunization with NP38-CGG. Each symbol represents one animal analyzed.
DOK3 Is Required for the Differentiation of Antigen-Specific PCs.
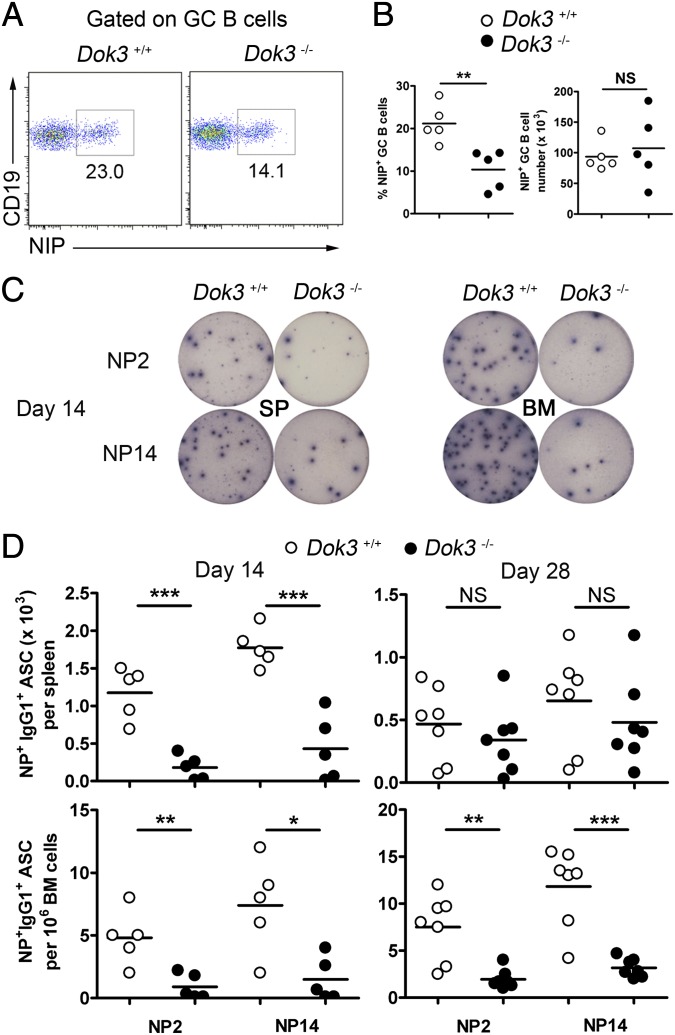
During T-cell–dependent antibody response, GC B cells differentiate into antibody-secreting PCs (1, 2, 10). Because Dok3−/− mice have expanded GC population but yet have impaired antigen-specific antibody response, we evaluated whether the absence of DOK3 would affect the generation of PCs. First, we examined the population of antigen-specific GC B (CD19+CD38−Fas+NIP+) cells in the spleens of WT and Dok3−/− mice at day 10 after immunization. Although the percentage of NP-specific GC B cells was reduced by ∼50% in Dok3−/− mice (Fig. 3A), the absolute number of NP-specific GC B cells was comparable between WT and Dok3−/− mice (Fig. 3B), and this phenomenon was due to the overall expansion of GC B cells in the mutants (Fig. 1 C and D). Thus, DOK3 deficiency does not affect the generation of antigen-specific GC B cells.
Fig. 3.
Impaired PC formation in Dok3−/− mice. Flow cytometry analysis (A) and quantification of the percentage and numbers (B) of NP-specific GC B cells in the spleens of WT and Dok3−/− mice at day 10 after immunization. (C) ELISPOT analyses of NP2- and NP14-binding IgG1 secreting PCs in the spleen (SP) and bone marrow (BM) of WT and Dok3−/− mice at day 14 after immunization. (D) Quantification of NP2- and NP14-binding IgG1 secreting cells in the spleen and bone marrow of WT and Dok3−/− cells at day 14 and 28 after immunizations. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Next, we investigated the differentiation of antigen-specific PCs. We used ELISPOT to quantitate both anti-NP2 and anti-NP14 antibody-secreting PCs in the spleens and bone marrow of NP38-CGG–immunized mice. At day 14 after immunization, we found that Dok3−/− mice generated significantly fewer NP-specific IgG1 PCs in their spleens and bone marrow compared with WT mice (Fig. 3 C and D). At day 28 after immunization, the pool of long-lived PCs in the bone marrow remained drastically reduced in the mutants (Fig. 3D). Thus, the absence of DOK3 in antigen-activated B cells compromises the generation of the long-lived PC pool.
DOK3 Deficiency Affects PD-1 Ligand Expression.
Previous studies have shown that interruptions of B-cell–T-cell cognate interactions involving various cell surface receptor-ligand pairs could affect GC formation and PC differentiation (2, 3, 10). These receptor-ligand pairs include CD40-CD40L, ICOS-ICOSL, CD80/86-CD28, Fas-FasL, and OX40-OX40L. To determine whether DOK3 deficiency could affect the expression of these molecules, we examined their cell surface protein expression on B and T cells of immunized WT and Dok3−/− mice. We found the expression levels of Fas, CD80, CD86, Ly108, ICOSL, OX40L, and CD40 to be comparable on CD19+ B cells from WT and Dok3−/− mice (Fig. S2A). There was also no difference in the expression of these molecules between GC B cells from WT and Dok3−/− mice at day 10 after immunization. Analyses of ICOS, OX40, and Ly108 also revealed no defective expression of these molecules on CD4+ T cells or Tfh cells in immunized WT and Dok3−/− mice (Fig. S2B).
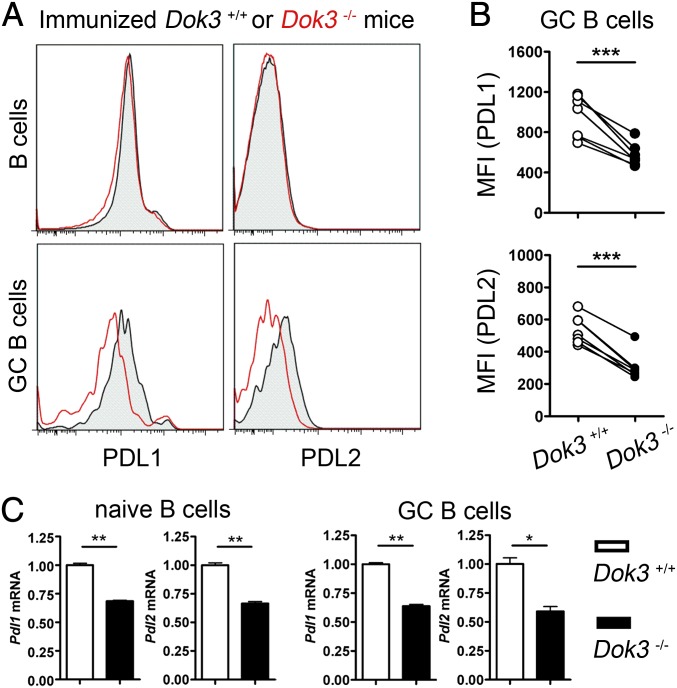
Recently, PD-1 and its ligands and, in particular PDL2, were shown to regulate the formation of long-lived PCs (6). Hence, we analyzed the expression of PD-1 on T cells, and PDL1 and PDL2 on B cells in WT and Dok3−/− mice. We found PD-1 expression and regulation to be normal on CD4+ T or Tfh cells in the absence of DOK3 (Fig. S2B). Interestingly, we consistently found PDL1 expressions to be lower on GC B cells in Dok3−/− mice compared with WT controls (Fig. 4 A and B). As for PDL2, it failed to be up-regulated on Dok3−/− GC B cells compared with WT GC B cells (Fig. 4 A and B). Quantitative real-time PCR analyses further indicated that DOK3 deficiency affected the expression of PDL1 and PDL2 at the transcriptional level because their mRNAs were significantly decreased in Dok3−/− naïve B and GC B cells compared with the respective WT controls (Fig. 4C). Thus, taken together, our data suggested that DOK3 deficiency affects the expression of PDL1 and PDL2 on B cells and, thereby, perturbs PC differentiation.
Fig. 4.
DOK3 deficiency leads to impaired PDL1 and PDL2 expression in B cells. (A) Representative histogram of protein expression levels of PDL1 and PDL2 on splenic B and GC B cells in WT and Dok3−/− mice at day 10 after immunization. (B) Cumulative flow cytometry analyses of mean fluorescence intensity (MFI) for PDL1 and PDL2 protein expression on GC B cells as shown in A. (C) Quantitative real-time PCR analyses of PDL1 and PDL2 mRNA levels in naïve B cells from unimmunized or GC B cells from immunized WT and Dok3−/− mice. mRNA level is normalized to that of GAPDH. *P < 0.05; **P < 0.01; ***P < 0.001.
B-Cell–Intrinsic DOK3 Signaling Regulates PD-1 Ligand Expression and PC Differentiation.
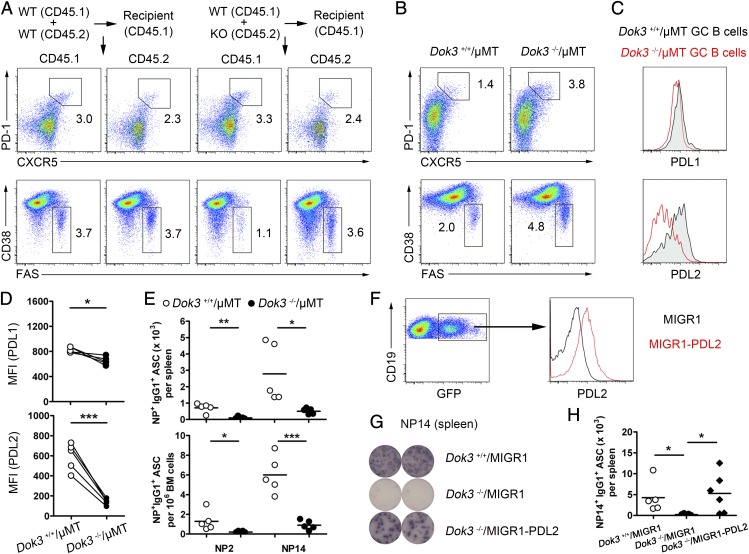
Because DOK3 is expressed in other cell types apart from B cells, it remains possible that DOK3 deficiency in non-B cells indirectly affects Tfh and GC B cells and the expression of PDL1 and PDL2 on GC B cells, leading to defective PC formation. To address whether the defects are due to a B-cell intrinsic lack of DOK3, we constructed bone marrow chimeras by using CD45.1 and CD45.2 WT cells (50:50), or CD45.1 WT and CD45.2 Dok3−/− cells (50:50). Reconstituted mice were left for 6 wk before NP38-CGG immunization. Splenic Tfh and GC B-cell populations were then analyzed by flow cytometry at day 10 after immunization. We found no difference in Tfh cell population arising from reconstituted CD45.1 WT/CD45.2 WT and CD45.1 WT/CD45.2 Dok3−/− cells (Fig. 5A and Fig. S3A), thus ruling out Tfh cell expansion in Dok3−/− mice is the causal event for the expansion of Dok3−/− GC B cells. However, interestingly we observed a significant increase in CD45.2 Dok3−/− GC B cells compared with CD45.1 WT GC B cells (Fig. 5A and Fig. S3A). The expression levels of PDL1 and PDL2 on CD45.2 Dok3−/− GC B cells were also lower (Fig. S3B). These data suggested that DOK3 regulates GC B cells and the expression of PDL1 and PDL2 in a B-cell–intrinsic manner.
Fig. 5.
B-cell–intrinsic DOK3 signaling regulates Tfh and GC B-cell compartments, PD-1 ligand expression, and PC formation. (A) Representative flow cytometry analysis of chimeras reconstituted with either WT (CD45.1)/WT (CD45.2) or WT (CD45.1)/KO (CD45.2) bone marrow cells to show Tfh and GC B cells at day 10 after immunization. (B) Flow cytometry analyses of Tfh and GC B cells in the spleen of recipient µMT mice reconstituted with Dok3−/−/µMT or Dok3+/+/µMT bone marrow cells at day 10 after immunization. (C) Histogram depiction of PDL1 and PDL2 expression on GC B cells from the immunized reconstituted mice as shown in B. (D) Cumulative flow cytometry analyses of MFI for PDL1 and PDL2 protein expression as shown in B. (E) Enumeration of NP2- and NP14-binding IgG1-secreting PCs from the reconstituted mice as shown in B. (F) Representative flow cytometry analysis of PDL2 expression on splenic B cells from recipient µMT mice reconstituted with µMT and retroviral transduced WT or Dok3−/− bone marrow cells. (G) ELISPOT analyses of NP14-binding IgG1 secreting PCs in the spleen of reconstituted mice at day 10 after immunization as shown in F. (H) Quantification of NP14-binding IgG1 secreting cells as shown in F. *P < 0.05; **P < 0.01; ***P < 0.001.
To further confirm that defective PC formation in Dok3−/− mice is B-cell intrinsic, we constructed another set of bone marrow chimeras that have only B cells lacking DOK3. This experiment was accomplished by transferring bone marrow cells in 20:80 ratio from Dok3−/− and B-cell–deficient µMT mice into irradiated µMT recipients. After reconstitution, the B-cell compartment in the chimera would be wholly DOK3 deficient, whereas the other hematopoietic cells present would mainly be WT with respect to DOK3. Mice were again immunized and, consistent with the data in Fig. 1, antigen-challenged Dok3−/−/µMT mice (µMT mice reconstituted with Dok3−/− and µMT bone marrow cells) have more Tfh and GC B cells compared with immunized Dok3+/+/µMT mice (µMT mice reconstituted with Dok3+/+ and µMT bone marrow cells) at day 10 after immunization (Fig. 5B and Fig. S3C). Similarly, both PDL1 and PDL2 were significantly down-regulated on GC B cells from Dok3−/−/µMT mice compared with Dok3+/+/µMT mice (Fig. 5 C and D). Furthermore, the formation of NP-specific IgG1 producing PCs was impaired in the spleens and bone marrow of Dok3−/−/µMT mice (Fig. 5E). These data further confirmed the B-cell intrinsic role of DOK3 in PC formation and also suggested that the expansion of Tfh cells in Dok3−/− mice depends on Dok3−/− B cells, possibly due to the impaired expression of PDL1 and PDL2.
PDL2 was reported to be important for PC differentiation (6). Because Dok3−/− GC B cells could not up-regulate PDL2 expression, we ask whether PDL2 overexpression in Dok3−/− B cells could rescue the defective PC formation in Dok3−/− mice. Hence, we constructed chimeric mice overexpressing PDL2 in Dok3−/− B cells. This set of experiments was achieved by transferring µMT bone marrow cells and Dok3−/− bone marrow progenitor cells transduced with retrovirus encoding PDL2 and GFP in 70:30 ratio into irradiated µMT recipients. After reconstitution, approximately 20–40% of the B cells had higher level of PDL2 expression (Fig. 5F). We were able to detect anti-NP14 IgG1 producing PCs in the spleens of chimeras at day 10 after immunization and, consistent with our hypothesis, PDL2 overexpression in Dok3−/− B cells significantly enhanced the formation of PCs in reconstituted µMT mice (Fig. 5 G and H). Taken together, these data indicated that DOK3 signaling in B cells is required to up-regulate PDL2 expression and drive PC differentiation.
DOK3 Regulates PD-1 Ligand Expression via Attenuating Calcium Signaling.
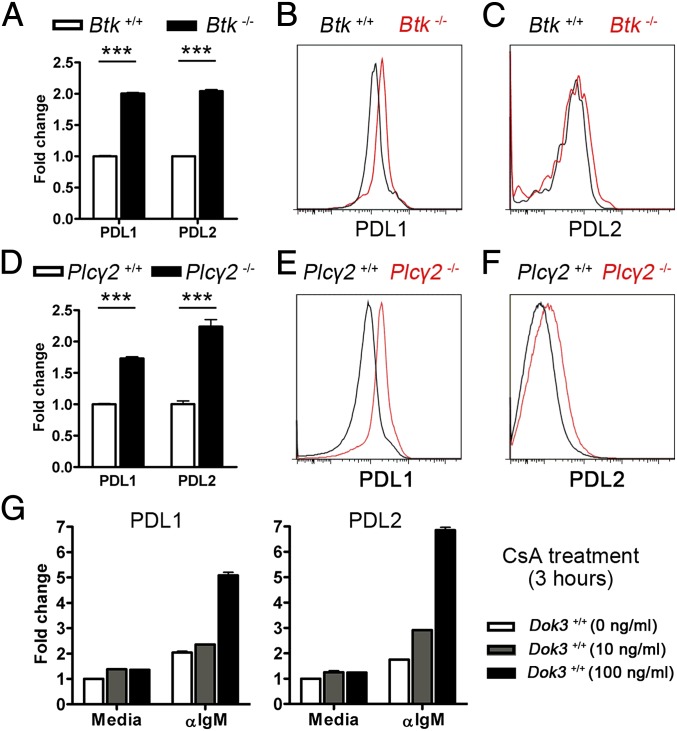
At the biochemical level, DOK3 is known to restrict the intensity of calcium signaling in B cells (14, 15). Hence, we hypothesized that Ca2+ signaling might regulate the expression of PD-1 ligands. Because DOK3 deficiency led to higher intensity of calcium signaling but lower expression of PDL1 and PDL2 (Fig. 4), we further speculated that Ca2+ signaling suppressed the transcriptional up-regulation of PDL1 and PDL2. If this hypothesis is correct, then inhibition of Ca2+ signaling could lead to up-regulation of PD-1 ligand expressions. At the molecular level, DOK3 had been shown to recruit Grb2, and together they negatively regulate BTK and PLCγ2, the two key enzymes involved in signaling Ca2+ mobilization in B cells (12, 14, 15). To test the hypothesis that calcium signaling negatively regulates PD-1 ligand expression, we first examined PDL1 and PDL2 gene expression in B cells lacking BTK or PLCγ2. Indeed, we were able to show that Btk−/− (Fig. 6 A and B and Fig. S4) and Plcγ2−/− (Fig. 6 D and E and Fig. S4) B cells had higher mRNA and protein levels of PDL1. PDL2 protein level was also found to be higher on Plcγ2−/− B cells compared with control (Fig. 6F and Fig. S4), although its level was unchanged on naive Btk−/− B cells but slightly higher on Btk−/− GC B cells (Fig. 6C and Fig. S4). These data supported the view that calcium signaling inhibits PDL1 and PDL2 expression in B cells.
Fig. 6.
Calcium signaling represses the transcript levels of PDL1 and PDL2. Quantitative real-time PCR analyses (A and D) and representative histograms of PDL1 (B and E) and PDL2 (C and F) expression levels in naïve B cells (A, B, and D–F) and GC B cells (C) from WT, Btk−/−, and Plcγ2−/− mice. mRNA level is normalized to that of GAPDH. ***P < 0.001. (G) Quantitative real-time PCR analyses of PDL1 and PDL2 mRNA in WT B cells that were pretreated with various doses of CsA for 1 h and subsequently left unstimulated (media) or stimulated with 10 µg/mL anti-IgM antibodies for 3 h. Figure shown is representative of three independent experiments.
To further confirm the role of calcium signaling in the negative regulation of PD-1 ligand expression, we used cyclosporin A (CsA), a commonly used inhibitor that specifically targets calcineurin, to block calcium-activated signaling pathways and transcription factors/repressors in WT B cells. Consistent with the data obtained so far, we were able to observe the transcriptional up-regulation of PDL1 and PDL2 gene expression in anti-IgM–activated B cells that were concurrently treated with either 10 or 100 ng/mL of CsA (Fig. 6G). Taken together, the data indicated that inhibition of calcium signaling up-regulates PDL1 and PDL2 expression and further suggested that DOK3 up-regulates PD-1 ligand expression in part through the attenuation of calcium signaling in B cells.
Discussion
We report here that DOK3 plays an unexpected role in regulating the antigen-driven phase of B-cell differentiation. We show that the formation of PCs was severely curtailed although GC B and Tfh cells were present in greater fractions in mice lacking DOK3. We further demonstrate that DOK3-deficient GC B cells have defective PD-1 ligand expressions, and overexpression of PDL2 in Dok3−/− B cells could rescue the defect of PC formation in the mutant mice (Figs. 4 and 5). Hence, our present study suggests that DOK3 plays an important role in PC differentiation through its regulation of PD-1 ligand expressions.
Tfh cells are important for GC B-cell differentiation and antibody response. The critical role of PD-1 signaling in Tfh cell differentiation had been examined recently (6–8). Blocking PD-1 signaling by deleting either PD-1 or PD-1 ligands resulted in the expansion of Tfh cells with altered cytokine production and impaired antibody production and IgA diversification (6, 8). It was also reported that PD-1 pathway inhibits the function of Tfr cells, and PD-1–deficient Tfr cells have enhanced suppressive ability to restrict antibody production (7). In our study, the expression of PD-1 on T cells was not changed, whereas the expressions of PD-1 ligands on GC B cells were significantly impaired in Dok3−/− mice (Fig. 4 and Fig. S2). By constructing different bone marrow chimeras, we confirmed that the expansion of Tfh cells in Dok3−/− mice was not T-cell intrinsic but rather dependent on Dok3−/− B cells (Fig. 5 and Fig. S3). Taken together, our data strongly suggest that the expansion of Tfh cell in Dok3−/− mice is due to the down-regulation of PDL1 and PDL2 on Dok3−/− GC B cells.
Although the role of PD-1 ligands in PC differentiation is beginning to be appreciated, what is not known is the signaling events or pathways that regulate the expression of these molecules in activated B cells. Our current study directly implicates DOK3 and its associated signaling pathways as critical regulators of PD-1 ligand expressions. DOK3 is an adaptor molecule involved in the negative regulation of BCR signaling. At the biochemical level, it recruits another adaptor, Grb2, and together, they sequester BTK and diminish PLCγ2 activation and, thereby, attenuate calcium signaling (14, 15). Thus, DOK3 might regulate PD-1 ligand expression through restricting the intensity of calcium signaling. In support of this model, we first show that DOK3 signaling directly regulates PD-1 ligand expression at the transcriptional level (Fig. 4). In the absence of DOK3 with elevated calcium signaling, the transcript levels of PDL1 and PDL2 were decreased. In the absence of BTK or PLCγ2 where calcium signaling is blocked, the transcript levels of PDL1 and PDL2 were increased (Fig. 6). Hence, these data suggest that calcium signaling inhibits PDL1 and PDL2 expression.
That calcium signaling directly negatively regulates PD-1 ligand gene expression is further corroborated and extended by our calcineurin inhibition study. We found that calcineurin inhibition up-regulated the expressions of PDL1 and PDL2 (Fig. 6G). Calcineurin is a serine-threonine phosphatase that is activated by calcium signaling and dephosphorylates NFAT and MEF2 families of transcription factors and, thereby, allowing them to translocate to the nucleus to drive or inhibit gene expressions (12, 17–21). It has been reported that different NFATs have different roles in B cells. NFAT2 deletion was found to impair BCR-mediated proliferation and facilitate activation-induced cell death, whereas NFAT1 deficiency rendered B cells resistant to BCR-mediated apoptosis (18, 19, 22, 23). We have attempted to identify whether any of the NFAT transcription factors could be involved in repressing PD-1 ligand expression, but we were not successful. Thus, a combination of NFAT factors and or other yet-to-be identified transcription factors could mediate the repression of PD-1 ligand gene expression.
Our findings in this study also provide more detailed insights to the cellular model of GC B–Tfh cell interaction, leading to the generation of long-lived PCs. In this model, DOK3 is activated in GC B cells after antigen recognition and, subsequently, attenuates BCR-mediated calcium signaling to constrain the size of GC B cells and to maintain optimal PDL1, and up-regulates PDL2 expression to allow for the generation of long-lived PCs. PDL1 and PDL2 on GC B cells also engage PD-1 on Tfh cells and limit the size of this compartment. Thus, in the absence of DOK3 in B cells, the reduced PDL1 and PDL2 expression on GC B cells lead to Tfh cell expansion. That PDL2 is important for PC formation also suggests that there is likely PDL2 reverse signaling in GC B cells, and this possibility needs to be further studied.
Materials and Methods
Mice.
C57BL/6, CD45.1, Btk−/−, and µMT mice were obtained from The Jackson Laboratory. Plcγ2−/− and Dok3−/− mice were described (14, 24) and bred to C57BL/6 background for more than 10 generations. All mice were housed under specific pathogen-free conditions in accordance with the protocols approved by the A*STAR Biological Resource Centre Institutional Animal Care and Use Committee. Mice were immunized i.p. with 100 µg of NP38-CGG (Biosearch Technologies) precipitated in Imject Alum (Pierce).
Flow Cytometry.
Single-cell suspensions from spleens, bone marrow, and Peyer’s patches were prepared and cells were stained with various combinations of fluorochrome-conjugated antibodies to CD4, CD19, PDL1, CD38, B220, CD45.1, CD45.2 (BioLegend), CD40, TCRβ, ICOS, PD-1, CXCR5, Fas, Ly108, ICOSL, OX40L, PDL2, OX40, CD80, and CD86 (BD Biosciences). Antigen-specific GC B cells were detected with anti-CD19, anti-CD38, and anti-Fas antibodies and NIP. Samples were acquired on LSRII cytometer (BD Biosciences) and analyzed with FlowJo (Tree Star).
Bone Marrow Chimeras.
Bone marrow reconstitution studies ware performed as described with minor modifications (25). Briefly, 3 × 106 mix bone marrow cells of µMT and WT (80%:20%), µMT and Dok3−/− (80%:20%), WT CD45.1 and WT CD45.2 (50%:50%), or WT CD45.1 and Dok3−/− CD45.2 (50%:50%) origins were injected i.v. into lightly irradiated µMT mice (800 rads) or WT CD45.1 mice (900 rads). Recipient mice were left for 6 wk before NP38-CGG immunization.
To overexpress PDL2 in Dok3−/− B cells, 5-fluorouracil–treated WT and Dok3−/− bone marrow cells were harvested and cultured in DMEM media supplemented with IL-3 (20 ng/mL), IL-6 (50 ng/mL), and stem cell factor (50 ng/mL) for 24 h. Cells were then transduced twice with retroviruses packaged from either MIGR1 vector or PDL2-overexpressing MIGR1-PDL2 vector over 2 d. Transduction efficiency was ∼30% as demonstrated by GFP-positive cells. After transduction, cells were mixed with µMT bone marrow cells (30%:70%) and injected i.v. into lightly irradiated µMT mice (800 rads).
ELISA and ELISPOT.
NP-specific antibodies and PCs were detected via ELISA and ELISPOT, respectively, as described (26).
Cell Isolation and Culture.
Total B cells were isolated from the spleens by using anti-CD43 magnetic beads (Miltenyi) according to the manufacturer’s instructions. For inhibitor studies, purified B cells were pretreated for 1 h with CsA (10 ng/mL or 100 ng/mL) and stimulated with anti-IgM for 3 h in the presence of CsA.
Quantitative Real-Time PCR.
Total RNA was isolated and real-time PCR was performed by using SYBR Green reagents. The primers are as follows: Gapdh forward, 5′ TGTGTCCGTCGTGGATCTGA 3′, reverse, 5′ TTGCTGTTGAAGTCGCAGGAG 3′; Pdl1 forward, 5′ TCAGCTACGGTGGTGCGGACT 3′, reverse, 5′ AGCTTCTGGATAACCCTCGGCCT 3′; Pdl2 forward, 5′ CCGGCCTGCACCATCGCTTT 3′, reverse, 5′ TCCCAAGACCGCAGGTCCAGAT 3′.
Statistical Analysis.
Two-tailed unpaired and paired t tests were performed by using Prism software.
Supplementary Material
Acknowledgments
We thank members of the K.-P.L. laboratory for insightful discussion and A*STAR Biomedical Research Council for grant support.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400539111/-/DCSupplemental.
References
- 1.Klein U, Dalla-Favera R. Germinal centres: Role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 2.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 3.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 6.Good-Jacobson KL, et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11(6):535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14(2):152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamoto S, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336(6080):485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol Rev. 2010;237(1):140–159. doi: 10.1111/j.1600-065X.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 11.Xu S, et al. B cell development and activation defects resulting in xid-like immunodeficiency in BLNK/SLP-65-deficient mice. Int Immunol. 2000;12(3):397–404. doi: 10.1093/intimm/12.3.397. [DOI] [PubMed] [Google Scholar]
- 12.Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol. 2007;7(10):778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashima R, Hishida Y, Tezuka T, Yamanashi Y. The roles of Dok family adapters in immunoreceptor signaling. Immunol Rev. 2009;232(1):273–285. doi: 10.1111/j.1600-065X.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 14.Ng CH, Xu S, Lam KP. Dok-3 plays a nonredundant role in negative regulation of B-cell activation. Blood. 2007;110(1):259–266. doi: 10.1182/blood-2006-10-055194. [DOI] [PubMed] [Google Scholar]
- 15.Stork B, et al. Subcellular localization of Grb2 by the adaptor protein Dok-3 restricts the intensity of Ca2+ signaling in B cells. EMBO J. 2007;26(4):1140–1149. doi: 10.1038/sj.emboj.7601557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanashi Y, et al. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes Dev. 2000;14(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews SF, et al. Developmentally regulated expression of MEF2C limits the response to BCR engagement in transitional B cells. Eur J Immunol. 2012;42(5):1327–1336. doi: 10.1002/eji.201142226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baksh S, et al. NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol Cell. 2002;10(5):1071–1081. doi: 10.1016/s1097-2765(02)00701-3. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharyya S, et al. NFATc1 affects mouse splenic B cell function by controlling the calcineurin—NFAT signaling network. J Exp Med. 2011;208(4):823–839. doi: 10.1084/jem.20100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancini M, Toker A. NFAT proteins: Emerging roles in cancer progression. Nat Rev Cancer. 2009;9(11):810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilker PR, et al. Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nat Immunol. 2008;9(6):603–612. doi: 10.1038/ni.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14(1):13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 23.Samanta DN, et al. B cell hyperresponsiveness and expansion of mature follicular B cells but not of marginal zone B cells in NFATc2/c3 double-deficient mice. J Immunol. 2005;174(8):4797–4802. doi: 10.4049/jimmunol.174.8.4797. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto A, et al. Cutting edge: Essential role of phospholipase C-gamma 2 in B cell development and function. J Immunol. 2000;165(4):1738–1742. doi: 10.4049/jimmunol.165.4.1738. [DOI] [PubMed] [Google Scholar]
- 25.Ou X, Xu S, Lam KP. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci USA. 2012;109(38):15401–15406. doi: 10.1073/pnas.1200386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S, Guo K, Zeng Q, Huo J, Lam KP. The RNase III enzyme Dicer is essential for germinal center B-cell formation. Blood. 2012;119(3):767–776. doi: 10.1182/blood-2011-05-355412. [DOI] [PubMed] [Google Scholar]