Significance
In 1901, when Robert Koch proposed that the bacilli causing human and bovine tuberculosis were not identical, this view caused much controversy. Now, 113 y later, we know that the bovine tuberculosis agent, Mycobacterium bovis, together with other animal strains, forms a separate phylogenetic lineage apart from the human Mycobacterium tuberculosis lineages, but the molecular reasons why bovine and animal strains only play minor roles in human tuberculosis epidemiology remain unknown. Herein, we show by genetic transfer and virulence experiments that specific mutations in a virulence regulator contribute to lower fitness and virulence of M. bovis and related strains for the human host, likely obstructing the capacity of causing overt disease needed for efficient human-to-human transmission.
Keywords: evolution, phylogeny, adaptation, zoonosis
Abstract
Although the bovine tuberculosis (TB) agent, Mycobacterium bovis, may infect humans and cause disease, long-term epidemiological data indicate that humans represent a spill-over host in which infection with M. bovis is not self-maintaining. Indeed, human-to-human transmission of M. bovis strains and other members of the animal lineage of the tubercle bacilli is very rare. Here, we report on three mutations affecting the two-component virulence regulation system PhoP/PhoR (PhoPR) in M. bovis and in the closely linked Mycobacterium africanum lineage 6 (L6) that likely account for this discrepancy. Genetic transfer of these mutations into the human TB agent, Mycobacterium tuberculosis, resulted in down-regulation of the PhoP regulon, with loss of biologically active lipids, reduced secretion of the 6-kDa early antigenic target (ESAT-6), and lower virulence. Remarkably, the deleterious effects of the phoPR mutations were partly compensated by a deletion, specific to the animal-adapted and M. africanum L6 lineages, that restores ESAT-6 secretion by a PhoPR-independent mechanism. Similarly, we also observed that insertion of an IS6110 element upstream of the phoPR locus may completely revert the phoPR-bovis–associated fitness loss, which is the case for an exceptional M. bovis human outbreak strain from Spain. Our findings ultimately explain the long-term epidemiological data, suggesting that M. bovis and related phoPR-mutated strains pose a lower risk for progression to overt human TB, with major impact on the evolutionary history of TB.
Tuberculosis (TB) is caused by bacilli from the genetically compact Mycobacterium tuberculosis complex (MTBC), which gathers eight defined phylogenetic lineages in addition to the more distantly related Mycobacterium canettii group (1–3): M. tuberculosis sensu-stricto from lineages L1–L4 and L7 form a large group of human-adapted strains responsible for the vast majority of global human TB cases, whereas Mycobacterium africanum lineages (L5, L6), which are restricted to humans from West Africa, are phylogenetically linked with the eighth lineage, comprising the various animal-adapted strains, with Mycobacterium bovis as the most downstream member in the phylogeny (Fig. 1A) (4, 5). Animal strains exhibit a wide host range that includes livestock animals in close contact with humans. Episodes of bovine TB in cattle herds have been reported in 128 of 155 countries during the period 2005–2008 (6). Although the bulk of these episodes is mainly found in developing countries (6), bovine TB remains a major problem even in some industrialized countries, best exemplified by the United Kingdom, which has experienced an important resurgence of bovine TB since the 1980s (7). Because M. bovis and other closely related animal-adapted strains are also capable of causing TB in humans, this situation raises concerns regarding the zoonotic risk. Indeed, human TB cases resulting from M. bovis are estimated to be around 2% worldwide (8), with higher incidence (18–30% of TB cases) in some developing countries, such as Tanzania (9). Despite this globally large number of cases, several lines of evidence suggest that M. bovis and phylogenetically related strains do not exhibit the same virulence and transmissibility for the human host as M. tuberculosis sensu-stricto. First, there are very few reports of human-to-human transmission of M. bovis. The infection occurs mostly through consumption of unpasteurized milk or close contact with infected animals (6, 10) suggesting that M. bovis exhibits a lower transmissibility among humans than M. tuberculosis sensu-stricto. Second, an important population study in Denmark showed that morbidity was much lower among patients infected with M. bovis than M. tuberculosis (11). Because human-to-human transmission is associated with overt disease, this conclusion is consistent with a lower transmissibility. Third, isolates from lineage L6) (also named West African 2 and corresponding to one group of M. africanum strains), which are very closely related phylogenetically to the strains of the animal lineage, exhibit features suggesting that they are also less virulent than M. tuberculosis sensu-stricto. M. africanum strains are restricted to West Africa and, despite major population movements between this geographic area and the rest of the world, they have failed to disseminate worldwide. This observation has fueled speculations about a potential local zoonotic reservoir (12). Moreover, an epidemiological study showed that household contacts of TB patients progress less frequently to active disease when they are infected with M. africanum L6 than with M. tuberculosis sensu-stricto (13, 14), a situation similar to that observed for M. bovis (11). Consistently, M. africanum L6 strains exhibit a lower fitness than M. tuberculosis sensu-stricto in the mouse model of TB (15). Collectively, these observations infer that animal-adapted strains, along with related M. africanum L6 members, have acquired mutations that affect their fitness for humans. However, the nature of the genetic differences explaining these specific phenotypic traits has remained unknown. Identifying these alterations and their molecular consequences is of tremendous importance to understanding the critically relevant factors determining transmission efficiency and host-specific adaptation. This information is also highly relevant to evaluate the risk associated with the various MTBC strains.
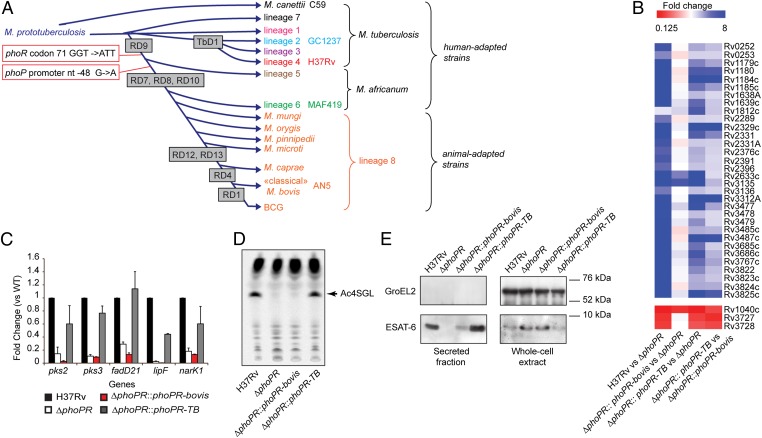
Fig. 1.
The phoPR allele from animal-adapted and M. africanum L6 strains is deficient. (A) Schematic global phylogenetic tree of the MTBC (1, 2). The length of the branches does not correlate with phylogenetic distance. Names of the strains used in this study are indicated. (B) Genome-wide transcriptional profiles of WT M. tuberculosis H37Rv, ΔphoPR mutant, and phoPR-bovis– or phoPR-TB–complemented strains. Fold-change values from individual probes for each gene were averaged. Those genes showing a statistically significant average fold-change > 2 or < 0.5 in the WT or the phoPR-TB–complemented strains relative to the ΔphoPR mutant were selected as positively or negatively regulated by PhoP, respectively. (C) qRT-PCR analysis of expression of main reporter genes of the PhoP regulon. (D) TLC analysis of lipids extracted from [14C] propionic acid-labeled cultures. The position of the major SL (the tetra-acylated sulfoglycolipids, Ac4SGL) is highlighted (arrow). (E) Immunoblot of secreted and whole-cell fractions probed with ESAT-6– or GroEL2- (used as a lysis control) specific antibodies.
In the present study, we combined comparative genomics, molecular genetics, and virulence tests to explore the impact of three SNPs evolutionarily conserved in animal-adapted and M. africanum L6 strains. These mutations affect a two-component regulation system known for its deep impact on the virulence of M. tuberculosis. Our findings establish that these SNPs impair the production and secretion of major protein- and lipid-virulence determinants and ultimately provide the likely genetic mechanism responsible for the lower prevalence and virulence of these lineages in humans.
Results and Discussion
SNPs Specific to the Animal and M. africanum Lineages Impair the Function of PhoPR.
Through genome comparison of 30 MTBC strains, we identified three SNPs affecting the phoPR genes of members of the animal-adapted and M. africanum L6 lineages that were not seen in the M. tuberculosis sensu-stricto genomes (Fig. 1A and Fig. S1). The phoPR genes encode a two-component regulatory system that is known for its strong impact on virulence and immunogenicity of M. tuberculosis (16, 17) because of its key role in the regulation of lipid synthesis and secretion of the 6-kDa secreted antigenic target ESAT-6 (18–22). To explore whether these SNPs affect the expression of the PhoP regulon, we first compared the transcriptome of M. tuberculosis strains lacking the endogenous phoPR genes (ΔphoPR) and their complemented derivatives expressing either the M. bovis or M. tuberculosis allele of phoPR (phoPR-bovis and phoPR-TB, respectively). These comparisons were performed in parallel in M. tuberculosis strains from two distinct genetic backgrounds: strain GC1237 from L2 (also named East Asia or Beijing cluster) and the reference strain H37Rv from L4 (also named Euro-American cluster) (Fig. 1A). In both genetic backgrounds, most genes of the PhoP regulon (i.e., genes differentially expressed between the WT and corresponding ΔphoPR variant) exhibited clear expression differences between the phoPR-bovis and phoPR-TB complemented strains (Fig. 1B and Fig. S2A), a finding confirmed by quantitative RT-PCR (qRT-PCR) (Fig. 1C and Fig. S2B). This observation is highly relevant for pathogenicity because PhoPR controls the synthesis and export of many M. tuberculosis virulence factors, such as LipF, ESAT-6, and lipids of the polyacyltrehalose (PAT) and sulfolipid (SL) families (18, 19, 23–25). Consistently, the M. tuberculosis-specific lipids, PAT and SL, were barely detectable in the ΔphoPR and the phoPR-bovis–complemented mutants relative to WT or phoPR-TB–complemented strains (Fig. 1D and Fig. S2C). Similarly, export of ESAT-6 was strongly dependent on the presence of phoPR-TB allele in the M. tuberculosis WT and recombinant strains (Fig. 1E and Fig. S2D).
To define which mutation impairs the function of the phoPR-bovis allele, we first compared the production of PhoP in WT, ΔphoPR, or complemented strains carrying the phoPR-bovis or phoPR-TB allele (Fig. S3). A similar amount of the PhoP protein was detected in the WT and the complemented strains, suggesting that the SNP located in the promoter region of the phoPR-bovis allele does not impair the expression of the phoPR operon. We next produced two phoPR allele chimeras combining phoP-bovis, carrying the promoter SNP, with phoR-TB (phoP-bovis+phoR-TB) or phoR-bovis, harboring the missense mutation, with phoP-TB (phoP-TB+phoR-bovis). These chimeras were used to complement the ΔphoPR mutant. Both the qRT-PCR and lipid analyses demonstrated that the phoR-bovis allele is defective, whereas the promoter mutation seems to have no impact on its own (Fig. S3).
SNPs in the phoPR-bovis Allele Impact Virulence in M. tuberculosis.
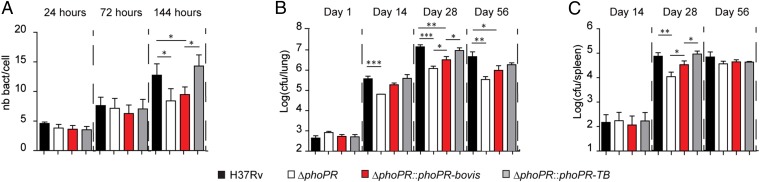
Next, we assessed whether the modulation of the PhoP regulon, caused by the SNPs in the phoPR-bovis allele, affects host–pathogen interaction. M. tuberculosis is an intracellular pathogen that thrives inside macrophages and other phagocytes in infected individuals. Therefore, we first infected human primary macrophages with the different recombinant strains and monitored the intracellular bacterial load at various time points postinfection. This experiment revealed a lower bacterial load at 6 d postinfection for the M. tuberculosis mutants expressing the phoPR-bovis allele relative to its phoPR-TB counterpart (Fig. 2A), a defect similar to that of the ΔphoPR mutant. We also conducted low-dose intranasal infection of immunocompetent BALB/c mice (200 cfu per mouse) using the M. tuberculosis H37Rv strains carrying the phoPR-TB or phoPR-bovis allele, as well as WT and ΔphoPR strains as controls. We found that complementation with the phoPR-bovis allele only partially restored the capacity of the recombinant H37Rv strain to multiply in infected animals, in contrast to the strain with the phoPR-TB allele (Fig. 2 B and C). Thus, the SNPs in the phoPR-bovis allele transferred to M. tuberculosis attenuate its virulence both in human cells and in the mouse infection model.
Fig. 2.
The phoPR-bovis allele impacts the interaction of M. tuberculosis with cellular or animal hosts. (A) Quantification of intracellular bacteria at 24-, 72-, or 144-h postinfection. Human-monocyte-derived macrophages (hMDM) were infected (at a multiplicity of infection of 2) for 2 h with indicated strains. Data are mean ± SD of three independent experiments performed in duplicate. (B and C) Seven-week-old BALB/c mice were infected intranasally with ∼200 cfu of the indicated strains. At the indicated time points, lungs (B) or spleens (C) were homogenized and plated for colony-forming unit determination. Data are means ± SD of four mice at days 14 and 56 and eight mice at days 1 and 28, from two independent experiments. The difference between experimental groups was evaluated by the two-tailed Student t test: P values, *P < 0.05, **P < 0.01, ***P < 0.005.
The PhoPR System Is Defective in Animal-Adapted and M. africanum L6 Strains But Compensatory Evolution Has Restored ESAT-6 Secretion.
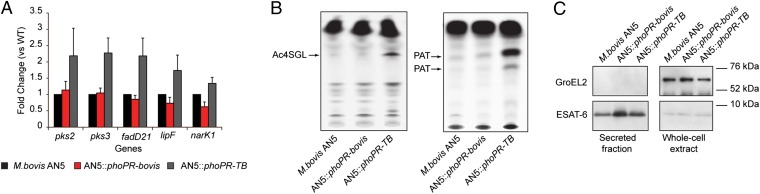
Given the strong phenotype of the phoPR-bovis allele in M. tuberculosis sensu-stricto, we sought to determine whether the phoPR-bovis allele is indeed responsible for an impaired PhoPR regulation in the M. bovis and M. africanum L6 genetic backgrounds. To this end, representative strains of these lineages were transformed with the phoPR-TB allele and assessed for the expression levels of genes from the PhoP regulon by qRT-PCR. Expression of indicator genes pks3, pks2, lipF, fadD21, and narK1 was much stronger in M. bovis and M. africanum recombinants expressing phoPR-TB compared with strains expressing phoPR-bovis only (Fig. 3A and Fig. S4A). Consistently, lipids (i.e., SL and PAT) described as specific for M. tuberculosis sensu-stricto (26) were now detectable in the recombinant M. bovis and M. africanum strains carrying the phoPR-TB allele, as demonstrated by thin-layer chromatography (TLC) and mass spectrometry of the purified compounds (Fig. 3B and Figs. S4 and S5). These findings indicate the direct link between the phoPR mutations and the lipid profile of various tubercle bacilli. In contrast, ESAT-6 was secreted at comparable levels in WT and recombinant strains (Fig. 3C and Fig. S6), suggesting that the animal-adapted and M. africanum L6 lineages have evolved to retain ESAT-6 secretion despite the mutations in phoPR.
Fig. 3.
The phoPR-bovis allele is deficient but ESAT-6 is secreted in animal-adapted strains. (A) qRT-PCR analysis of expression of main reporter genes of the PhoP regulon in WT and recombinant M. bovis AN5 grown liquid culture. (B) TLC analysis of lipids extracted from [14C] propionic acid-labeled cultures. (C) Immunoblot of secreted and whole-cell fractions from WT or recombinant M. bovis strains probed with ESAT-6– or GroEL2-specific antibodies.
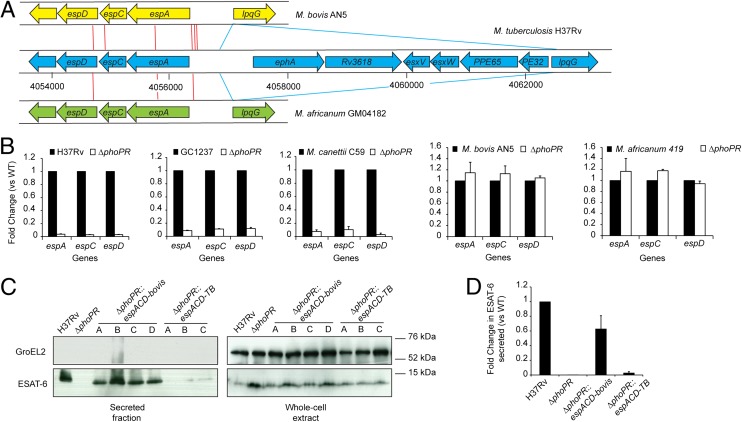
Secretion of ESAT-6 requires the espACD operon (27, 28), which is part of the PhoP regulon (18, 20–22). In addition to PhoPR, several regulators, such as the nucleoid-associated proteins Lsr2 (29) and EspR (30–32) and the two-component system MprAB (33), were found to modulate the transcription of the espACD operon in response to environmental signals and stresses. Interestingly, both animal-adapted and M. africanum L6 strains harbor a specific deletion, named region of difference 8 (RD8) (34), and several SNPs just upstream of espACD relative to M. tuberculosis sensu-stricto strains (Fig. 4A). The RD8 deletion removes binding sites for EspR and MprAB, and therefore may impact the expression of espACD. These observations led us to assess espACD gene expression in a collection of animal-adapted or M. africanum L6 strains and compare it to M. tuberculosis sensu-stricto WT or recombinant strains. We found unexpected high levels of espACD expression in all animal-adapted or M. africanum L6 strains, in contrast to the situation in M. tuberculosis ΔphoPR or recombinant strains expressing phoPR-bovis (Fig. S7), suggesting that espACD expression escaped the PhoPR control in these strains. This exceptional result was confirmed in ΔphoPR mutants of representatives of the animal-adapted and M. africanum L6 strains showing no reduction of the transcript level relative to WT, in contrast to M. tuberculosis or M. canettii (Fig. 4B). The fact that M. canettii, which is an early-branching representative in the MTBC phylogenetic tree (3), exhibits a similar PhoPR control on espACD expression as M. tuberculosis strongly supports the hypothesis that animal-adapted and M. africanum L6 strains specifically acquired this property during evolution. In line with these observations, transfer of the espACD allele from M. bovis (espACD-bovis) into M. tuberculosis ΔphoPR increases espACD expression and restores ESAT-6 secretion, whereas transfer of a second copy of the corresponding region from M. tuberculosis (espACD-TB) has only marginal impact (Fig. 4 C and D and Fig. S8). Overall, our results demonstrate that mutations of the PhoPR system impair the production of molecules, such as SL, PAT, and LipF, important for pathogenicity in the animal-adapted and M. africanum L6 lineages. Nevertheless, these strains acquired compensatory mutations that short-cut the regulation loop controlling espACD expression in M. tuberculosis.
Fig. 4.
RD8 deletion allows ESAT-6 secretion independently from PhoPR. (A) Schematic representation of the genetic structure at the espACD locus in the M. tuberculosis sensu-stricto and animal-adapted or M. africanum L6 strains. Red lines indicate individual SNPs identified between pairwise-compared genomes. Blue lines indicate the boundaries of the RD8 deletion. (B) qRT-PCR analysis of espACD genes expression in five strains of the MTBC harboring a ΔphoPR deletion. (C) Immunoblot of secreted ESAT-6 from H37Rv WT, ΔphoPR mutant, and ΔphoPR mutant carrying the espACD fragment from M. bovis (espACD-bovis) or the control similar fragment from H37Rv (espACD-TB). Several transformants were analyzed. The bacterial supernatants and cell pellets were probed for ESAT-6 or GroEL2. (D) Quantification of the ESAT-6 signals in secreted protein fractions from the indicated strains (relative to H37Rv).
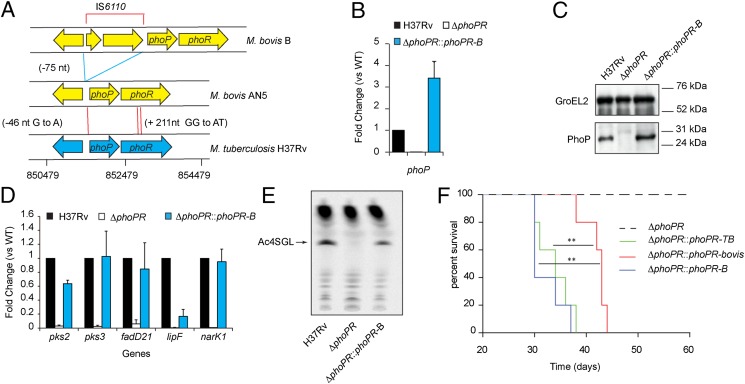
IS6110 Insertion Upstream phoPR in the Hypervirulent M. bovis B Compensates the phoPR-bovis Deficiency.
Despite the compensatory genetic events affecting ESAT-6 secretion, epidemiological data support the hypothesis that animal-adapted and M. africanum L6 strains have not recovered the same virulence for humans as M. tuberculosis sensu-stricto strains (likely because of the above-mentioned expression differences in other members of the PhoPR regulon). Thus, documented outbreaks of human TB caused by strains from the animal-adapted lineage are extremely rare. Of note, a unique multidrug-resistant M. bovis isolate, M. bovis B, was responsible for 36 TB cases in Spain (35), where this specific strain successfully spread via human-to-human transmission. Interestingly, this M. bovis B isolate harbors a mobile element, IS6110, inserted 75-bp upstream of the phoPR operon (36) (Fig. 5A). When we transferred this particular phoPR allele from M. bovis B (phoPR-B) into M. tuberculosis ΔphoPR and M. bovis ΔphoPR, we found increased phoP transcription and PhoP production in comparison with WT (Fig. 5 B and C and Fig. S9). Consistently, we detected increased transcription of PhoP regulon genes (Fig. 5D), production of SL and PAT (Fig. 5E and Fig. S9), and ESAT-6 secretion in the recombinant M. tuberculosis or M. bovis AN5 strains harboring the phoPR-B allele. Thus, the occurrence of IS6110 upstream phoPR globally compensates the deleterious effect of the SNPs found in the phoPR-bovis allele, and this suppressive effect is likely a result of increased expression of phoPR driven by a strong IS6110-contained promoter (36, 37).
Fig. 5.
Insertion of IS6110 upstream phoPR in M. bovis B strain suppresses the phoPR-bovis deficiency. (A) Schematic representation of the genetic structure at the phoPR locus in the M. bovis B strain relative to “classic” M. bovis and M. tuberculosis strains. The positions of SNPs specific to the animal-adapted and M. africanum L6 and of the IS6110 insertion site are indicated in brackets. Red lines indicate individual SNPs identified between pairwise-compared genomes. Blue lines indicate the boundaries of IS6110. (B) qRT-PCR analysis of phoPR expression in M. tuberculosis H37Rv strain, the ΔphoPR mutant, and the recombinant M. tuberculosis strain expressing the phoPR-B allele. (C) Western blot analysis of PhoP from WT and phoPR-B expressing strains. (D) qRT-PCR analysis of expression of PhoP regulon genes in the indicated strains. (E) TLC analysis of lipids extracted from [14C] propionic acid-labeled cultures. (F) Survival of SCID mice infected with ∼4 × 103 cfu of the indicated strains (five mice per group). Curves are significantly different between the phoPR-bovis expressing strain and the phoPR-TB– and phoPR-B–expressing strains (P < 0.005, according to the log-rank (Mantel– Cox) test).
Finally, we sought to determine the impact of the IS6110 insertion on virulence. Because we observed in our initial experiments (Fig. 2 B and C) that the attenuation phenotype associated with the phoPR-bovis allele was clearly seen in the acute phase of infection in mice, we used for these experiments severe combined immunodeficient (SCID) mice, which reproduce this initial phase (38). Consistent with the phenotype in vitro, the phoPR-B allele provides enhanced in vivo virulence in mice. Indeed, infection of SCID mice showed the median survival times were similar for M. tuberculosis strains expressing phoPR-TB (34 d) or phoPR-B (30 d) and significantly higher for the recombinant strain expressing phoPR-bovis (43 d) or the ΔphoPR mutant (Fig. 5F).
Taking these data together, it seems very likely that the highly virulent, human-to-human transmission-associated phenotype of the M. bovis B strain is caused by the specific IS6110 insertion, which suppresses the various phoPR-bovis–associated deficiencies.
Conclusions
In this study we demonstrated that the PhoPR regulation system is deficient in animal-adapted and M. africanum strains primarily because of a mutation within the phoR gene. The key role of PhoPR for the M. tuberculosis pathogenicity was previously established: a spontaneous punctual mutation altering the DNA-binding capacity of PhoP in the H37Ra strain or insertional inactivation of the phoP gene in two M. tuberculosis strains significantly impaired the multiplication in mice or macrophages (17, 18, 20, 21). Here, we found that specific mutations present within the phoPR-bovis allele, when transferred into M. tuberculosis, also strongly impact the virulence of the recombinant strain, raising important questions about the consequences of these mutations for the evolutionary history of the concerned lineages of tubercle bacilli.
Overall, our results provide a molecular explanation for the intriguing epidemiological data showing that M. bovis and other animal-adapted strains are only rarely found in human TB outbreaks, despite their close genetic relationship with M. tuberculosis sensu-stricto and their ability to infect humans through contaminated milk. Our findings may also be extended to the closely related M. africanum L6 strains, which exhibit a similar impaired fitness relative to M. tuberculosis sensu-stricto (13). Our results suggest an evolutionary scenario (Fig. S10) in which mutations at the phoPR locus occurred in the common ancestor of the animal-adapted and M. africanum strains. These mutations impacted the synthesis and secretion of lipid- and protein- pathogenicity factors, with the likely consequence of dramatically reduced fitness for the human host. The strains, which successfully got through this low-virulence bottleneck, acquired compensatory mutations, such as the RD8 deletion, that restored the secretion of ESAT-6 via EspACD independently of phoPR but still failed to provide the same virulence for humans as the ancestral phoPR-TB allele. The animal-adapted strains may have acquired later additional mutations, allowing them to persist in various animal species that might exhibit a different susceptibility than humans, such as Mycobacterium microti in voles and cats (39), the Dassie bacillus in dassies (40), Mycobacterium mungi in the banded mongooses (41), Mycobacterium pinnipedii in seals (42), or M. bovis in cattle, deer, and badgers (6). Of note, specific adaptation of M. tuberculosis sensu-stricto to the human host seems to be conversely associated with lower virulence than M. bovis strains in certain animal species, such as rabbits, goats, or cattle (43–45). Finally, in some exceptional animal-adapted isolates, such as the M. bovis B strain, additional fitness for the human host was gained through insertion of a mobile element upstream phoPR, leading to overexpression of this operon, favoring aerosol transmission of bacilli via patients who have developed active pulmonary TB. This succession of genetic events is fully compatible with previously proposed phylogenic schemes (1, 2, 46) and underlines how a few point mutations in important genes, combined with selected compensatory mutations, can have a long-lasting and powerful impact on the evolution and adaptation of a pathogen to specific hosts.
Materials and Methods
Mutations in the various M. tuberculosis sensu-stricto, M. bovis, M. africanum, or M. canettii strains were performed by allelic exchange. The resulting mutants were characterized by PCR using specific primers. The complemented strains were produced by inserting a single copy of the indicated genes on the mycobacterial chromosome. Lipid analysis was performed on exponentially growing strains labeled for 24 h with [14C] propionic acid or on stationary-phase bacteria cultured as pellicles for mass spectrometry. RNA levels were determined by quantitative real-time PCR using SYBR green and specific primers. Transcriptome analyses were performed using Agilent manufactured customized microarrays with RNA extracted from exponentially growing bacteria, labeled with Cy5 or Cy3, and hybridized competitively. Western-blot of proteins secreted or associated with bacterial cells were performed on 3-wk-old cultures fractionated by centrifugation before protein extraction, separation by SDS/PAGE, membrane transfer, and hybridization with ESAT-6–, PhoP-, or GroEL2-specific antibodies. Animal studies were conducted on 7-wk-old mice infected intranasally and euthanized at various days postinfection for bacterial load evaluation in lung and spleens. Human macrophages infection assays were carried out in human monocyte-derived macrophages obtained from anonymous nontuberculous donors and infected at multiplicity of infection 2 for 2 h with GFP-expressing bacteria. Statistical analyses were performed using GraphPad Prism software.
A detailed materials and methods section is provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Geanncarlo Lugo-Villarino and Dr. Stephen V. Gordon for critical reading of the manuscript. We thank the members of the Genotoul platforms TRI and ANEXPLO (Institut de Pharmacologie et de Biologie Structurale) for epifluorescence microscopy imaging and for animal experiments, respectively; and Dr. Marie-Agnès Dillies and Dr. Jean-Yves Coppée from the Transcriptome Platform PF2 at the Institut Pasteur for advice. This work was supported in part by the Juan de la Cierva Programme JCI-2009-03799 from the Spanish Ministry of Science and Innovation (to J.G.-A.); the Centre National de la Recherche Scientifique; the Fondation pour la Recherche Médicale (“Equipe FRM” DEQ20090515399 and DEQ20130326471); the European Union 7th Framework Programme (Contracts NEWTBVAC 241745, MM4TB 260872, and FEDER/POCTEFA/REFBIO EFA237/11); the Spanish Ministry of Economy and Competitiveness (Contracts BIO2011-23555 and FIS12/1970); and the Région Midi-Pyrénées (CPER 2007-2013) for the scientific project and for the ASB3 animal facility.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.M.S. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE54421: samples GSM1314812–GSM1314819).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406693111/-/DCSupplemental.
References
- 1.Brosch R, et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci USA. 2002;99(6):3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comas I, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45(10):1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Supply P, et al. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat Genet. 2013;45(2):172–179. doi: 10.1038/ng.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostowy S, Cousins D, Brinkman J, Aranaz A, Behr MA. Genomic deletions suggest a phylogeny for the Mycobacterium tuberculosis complex. J Infect Dis. 2002;186(1):74–80. doi: 10.1086/341068. [DOI] [PubMed] [Google Scholar]
- 5.Smith NH, Hewinson RG, Kremer K, Brosch R, Gordon SV. Myths and misconceptions: The origin and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2009;7(7):537–544. doi: 10.1038/nrmicro2165. [DOI] [PubMed] [Google Scholar]
- 6.Michel AL, Müller B, van Helden PD. Mycobacterium bovis at the animal-human interface: A problem, or not? Vet Microbiol. 2010;140(3–4):371–381. doi: 10.1016/j.vetmic.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Jalava K, et al. No increase in human cases of Mycobacterium bovis disease despite resurgence of infections in cattle in the United Kingdom. Epidemiol Infect. 2007;135(1):40–45. doi: 10.1017/S0950268806006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller B, et al. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis. 2013;19(6):899–908. doi: 10.3201/eid1906.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleaveland S, et al. Mycobacterium bovis in rural Tanzania: Risk factors for infection in human and cattle populations. Tuberculosis (Edinb) 2007;87(1):30–43. doi: 10.1016/j.tube.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Mandal S, et al. Investigating transmission of Mycobacterium bovis in the United Kingdom in 2005 to 2008. J Clin Microbiol. 2011;49(5):1943–1950. doi: 10.1128/JCM.02299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnus K. Epidemiological basis of tuberculosis eradication. 3. Risk of pulmonary tuberculosis after human and bovine infection. Bull World Health Organ. 1966;35(4):483–508. [PMC free article] [PubMed] [Google Scholar]
- 12.Demers A-M, et al. Mycobacterium africanum is not a major cause of human tuberculosis in Cape Town, South Africa. Tuberculosis (Edinb) 2010;90(2):143–144. doi: 10.1016/j.tube.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 13.de Jong BC, et al. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis. 2008;198(7):1037–1043. doi: 10.1086/591504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentley SD, et al. The genome of Mycobacterium africanum West African 2 reveals a lineage-specific locus and genome erosion common to the M. tuberculosis complex. PLOS Neglect Trop Dis. 2012;6(2):e1552. doi: 10.1371/journal.pntd.0001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bold TD, et al. Impaired fitness of Mycobacterium africanum despite secretion of ESAT-6. J Infect Dis. 2012;205(6):984–990. doi: 10.1093/infdis/jir883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez E, et al. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol Microbiol. 2001;41(1):179–187. doi: 10.1046/j.1365-2958.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, et al. Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe. 2008;3(2):97–103. doi: 10.1016/j.chom.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Walters SB, et al. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol. 2006;60(2):312–330. doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalo-Asensio J, et al. The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem. 2006;281(3):1313–1316. doi: 10.1074/jbc.C500388200. [DOI] [PubMed] [Google Scholar]
- 20.Frigui W, et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008;4(2):e33. doi: 10.1371/journal.ppat.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesne-Seck ML, et al. A point mutation in the two-component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. J Bacteriol. 2008;190(4):1329–1334. doi: 10.1128/JB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galagan JE, et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature. 2013;499(7457):178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. 1999;34(2):257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 24.Passemar C, et al. Multiple deletions in the polyketide synthase gene repertoire of Mycobacterium tuberculosis reveal functional overlap of cell envelope lipids in host-pathogen interactions. Cell Microbiol. 2014;16(2):195–213. doi: 10.1111/cmi.12214. [DOI] [PubMed] [Google Scholar]
- 25.Goyal R, et al. Phosphorylation of PhoP protein plays direct regulatory role in lipid biosynthesis of Mycobacterium tuberculosis. J Biol Chem. 2011;286(52):45197–45208. doi: 10.1074/jbc.M111.307447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asselineau J. Branched-chain fatty acids of mycobacteria. Indian J Chest Dis Allied Sci. 1982;24(2-3):143–157. [PubMed] [Google Scholar]
- 27.Fortune SM, et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA. 2005;102(30):10676–10681. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacGurn JA, Raghavan S, Stanley SA, Cox JS. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol Microbiol. 2005;57(6):1653–1663. doi: 10.1111/j.1365-2958.2005.04800.x. [DOI] [PubMed] [Google Scholar]
- 29.Gordon BRG, et al. Lsr2 is a nucleoid-associated protein that targets AT-rich sequences and virulence genes in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2010;107(11):5154–5159. doi: 10.1073/pnas.0913551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghavan S, Manzanillo P, Chan K, Dovey C, Cox JS. Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature. 2008;454(7205):717–721. doi: 10.1038/nature07219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blasco B, et al. Virulence regulator EspR of Mycobacterium tuberculosis is a nucleoid-associated protein. PLoS Pathog. 2012;8(3):e1002621. doi: 10.1371/journal.ppat.1002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt DM, et al. Long-range transcriptional control of an operon necessary for virulence-critical ESX-1 secretion in Mycobacterium tuberculosis. J Bacteriol. 2012;194(9):2307–2320. doi: 10.1128/JB.00142-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang X, et al. MprAB regulates the espA operon in Mycobacterium tuberculosis and modulates ESX-1 function and host cytokine response. J Bacteriol. 2013;195(1):66–75. doi: 10.1128/JB.01067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon SV, et al. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol. 1999;32(3):643–655. doi: 10.1046/j.1365-2958.1999.01383.x. [DOI] [PubMed] [Google Scholar]
- 35.Rivero A, et al. High rate of tuberculosis reinfection during a nosocomial outbreak of multidrug-resistant tuberculosis caused by Mycobacterium bovis strain B. Clin Infect Dis. 2001;32(1):159–161. doi: 10.1086/317547. [DOI] [PubMed] [Google Scholar]
- 36.Soto CY, et al. IS6110 mediates increased transcription of the phoP virulence gene in a multidrug-resistant clinical isolate responsible for tuberculosis outbreaks. J Clin Microbiol. 2004;42(1):212–219. doi: 10.1128/JCM.42.1.212-219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safi H, et al. IS6110 functions as a mobile, monocyte-activated promoter in Mycobacterium tuberculosis. Mol Microbiol. 2004;52(4):999–1012. doi: 10.1111/j.1365-2958.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 38.North RJ, Izzo AA. Mycobacterial virulence. Virulent strains of Mycobacteria tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177(6):1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith NH, Crawshaw T, Parry J, Birtles RJ. Mycobacterium microti: More diverse than previously thought. J Clin Microbiol. 2009;47(8):2551–2559. doi: 10.1128/JCM.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mostowy S, Cousins D, Behr MA. Genomic interrogation of the dassie bacillus reveals it as a unique RD1 mutant within the Mycobacterium tuberculosis complex. J Bacteriol. 2004;186(1):104–109. doi: 10.1128/JB.186.1.104-109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander KA, et al. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg Infect Dis. 2010;16(8):1296–1299. doi: 10.3201/eid1608.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cousins DV, et al. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int J Syst Evol Microbiol. 2003;53(Pt 5):1305–1314. doi: 10.1099/ijs.0.02401-0. [DOI] [PubMed] [Google Scholar]
- 43.Dunn PL, North RJ. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect Immun. 1995;63(9):3428–3437. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whelan AO, et al. Revisiting host preference in the Mycobacterium tuberculosis complex: experimental infection shows M. tuberculosis H37Rv to be avirulent in cattle. PLoS ONE. 2010;5(1):e8527. doi: 10.1371/journal.pone.0008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nedeltchev GG, et al. Extrapulmonary dissemination of Mycobacterium bovis but not Mycobacterium tuberculosis in a bronchoscopic rabbit model of cavitary tuberculosis. Infect Immun. 2009;77(2):598–603. doi: 10.1128/IAI.01132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith NH, Gordon SV, de la Rua-Domenech R, Clifton-Hadley RS, Hewinson RG. Bottlenecks and broomsticks: The molecular evolution of Mycobacterium bovis. Nat Rev Microbiol. 2006;4(9):670–681. doi: 10.1038/nrmicro1472. [DOI] [PubMed] [Google Scholar]