Significance
5-Lipoxygenase (5LO) is a key enzyme in biosynthesis of leukotrienes (LTs), lipid mediators of inflammation. To study the roles of the 5LO accessory proteins coactosin-like protein (CLP) and 5LO-activating protein (FLAP), we knocked down these proteins in human monocytic cells. Our results show that expression of CLP was required for full cellular 5LO activity when cells were activated with Ca2+ ionophore, as well as with a physiological stimulus (lipopolysaccharide followed by N-formylmethionyl-leucyl-phenylalanine). During LT biosynthesis in stimulated cells, 5LO typically translocates to the nuclear membrane. This redistribution, from cytosolic to perinuclear, was clearly compromised in both CLP- and FLAP-deficient cells. Our results suggest that the CLP–5LO interaction may be a target for reduced LT production.
Keywords: inflammation, eicosanoid, oxylipin
Abstract
5-Lipoxygenase (5LO) is a key enzyme in leukotriene (LT) biosynthesis. Two accessory proteins, coactosin-like protein (CLP) and 5-lipoxygenase–activating protein (FLAP), can support 5LO activity. To study the roles of CLP and FLAP, we knocked down these proteins in the human monocytic cell line Mono Mac 6 (MM6). Expression of CLP increased MM6 cellular 5LO activity for all stimuli tested. CLP is not absolutely crucial, however; some 5LO activity remained in all incubations of CLP knockdown cells. FLAP knockdown had minor effects in the presence of exogenous arachidonic acid, but led to prominent reductions in 5LO product formation from endogenous substrate. Similar effects were observed after CLP and FLAP knockdown in human primary macrophages as well. In addition, FLAP knockdown reduced conversion of leukotriene A4 to leukotriene C4 (LTC4), suggesting a role for the activity of LTC4 synthase. After stimulation of MM6 cells by phorbol myristate acetate and ionophore A23187, a perinuclear ring pattern was observed for 5LO. This redistribution from cytosolic to perinuclear was clearly compromised in both CLP- and FLAP-deficient cells. In addition, association of CLP with the nucleus was almost absent after 5LO knockdown, and was clearly reduced in FLAP knockdown cells. Coimmunoprecipitation experiments indicated that 5LO-CLP complex formation in MM6 cells was increased by stimulation with ionophore, and that this complex was formed to the same extent in FLAP knockdown cells. A possible interpretation of our findings is that on cell stimulation, formation of the 5LO-CLP complex augments the translocation from cytosol to nucleus, whereas FLAP stabilizes association of this complex with the perinuclear membrane.
Leukotrienes (LTs) are lipid mediators of inflammation with roles in both normal host defense and pathophysiological conditions (1). 5-Lipoxygenase (5LO) is a key enzyme in LT biosynthesis, and its regulation is a determinant of LT formation (2). In addition, 5LO is involved in biosynthesis of lipoxins and resolvin E1 (3).
Cellular 5LO activity depends on several cofactors that regulate LT biosynthesis and prevent LT production in unstimulated situations. Increased intracellular Ca2+ levels activate and translocate 5LO to the perinuclear region, where it accesses its substrate arachidonic acid (AA) released by cytosolic phospholipase A2 (cPLA2) (1). Cellular formation of LTs from endogenous AA depends on the small membrane protein 5LO-activating protein (FLAP) (4). FLAP has been suggested to function as a membrane anchor scaffold for 5LO to facilitate the transfer of AA to 5LO (5, 6). In the cell, 5LO is subject to phosphorylation. MAP kinase pathways can activate 5LO during cell stress (7), whereas phosphorylation by protein kinase A inhibits cellular 5LO activity (8).
5LO has two domains, an N-terminal C2-like regulatory domain with a β-sandwich structure and a catalytic C-terminal domain composed predominantly of α-helices that harbors the prosthetic iron (9). Ca2+, phosphatidylcholine (PC), and coactosin-like protein (CLP) bind to the C2-like domain (10–12), and this domain is important in Ca2+-stimulated membrane association in intact cells (13). Human CLP was first identified as a 5LO-binding protein in yeast two-hybrid screening with 5LO as bait (14). Native PAGE and chemical cross-linking experiments showed that in vitro 5LO binds CLP with 1:1 molar stoichiometry (15). A member of the ADF/cofilin group of actin-binding proteins, CLP also binds F-actin (16). Binding of CLP has been shown to support Ca2+-induced 5LO activity in vitro and to increase leukotriene A4 (LTA4) formation when PC is included in incubation mixtures (12, 17).
In the present study, we knocked down the expression of CLP and FLAP in MM6 cells and human macrophages, respectively, and compared the effects on LT formation. Our results show that expression of CLP up-regulates cellular 5LO activity with all stimuli tested. FLAP is essential in the processing of endogenous AA and also plays an important role in the efficient formation of leukotriene C4 (LTC4).
Results
Generation of Stable CLP, FLAP, and 5LO Knockdown Cells.
To achieve stable knockdown for CLP, FLAP, and 5LO in Mono Mac 6 (MM6) cells, we used lentiviral vectors stably expressing shRNA. This resulted in successful knockdown, as determined for both mRNA and protein (Fig. S1). The expression levels of CLP and FLAP mRNAs were reduced to 10–13% after knockdown, and only weak protein bands remained in the Western blots. There was no significant difference in the expression level of CLP or FLAP before and after differentiation (Fig. S2). As expected, 5LO expression was strongly up-regulated during differentiation with vitamin D3 and TGF-β (18–20), and this up-regulation was repressed in cells previously subjected to 5LO knockdown. In the differentiated knockdown cells, 5LO mRNA was reduced to 15% of control levels, and 5LO protein was practically absent (Fig. S1C). The expression levels of 5LO, CLP, and FLAP in control cells (transfected with nontarget shRNA) were comparable with those in parental untransformed WT cells. Knockdowns were specific; 5LO did not change after knockdown of FLAP or CLP (Figs. 1C and 5 and Fig. S2), and CLP was stable after knockdown of 5LO or FLAP (Fig. 7). In addition, FLAP expression remained unaffected after knockdown of 5LO or CLP (Fig. S2).
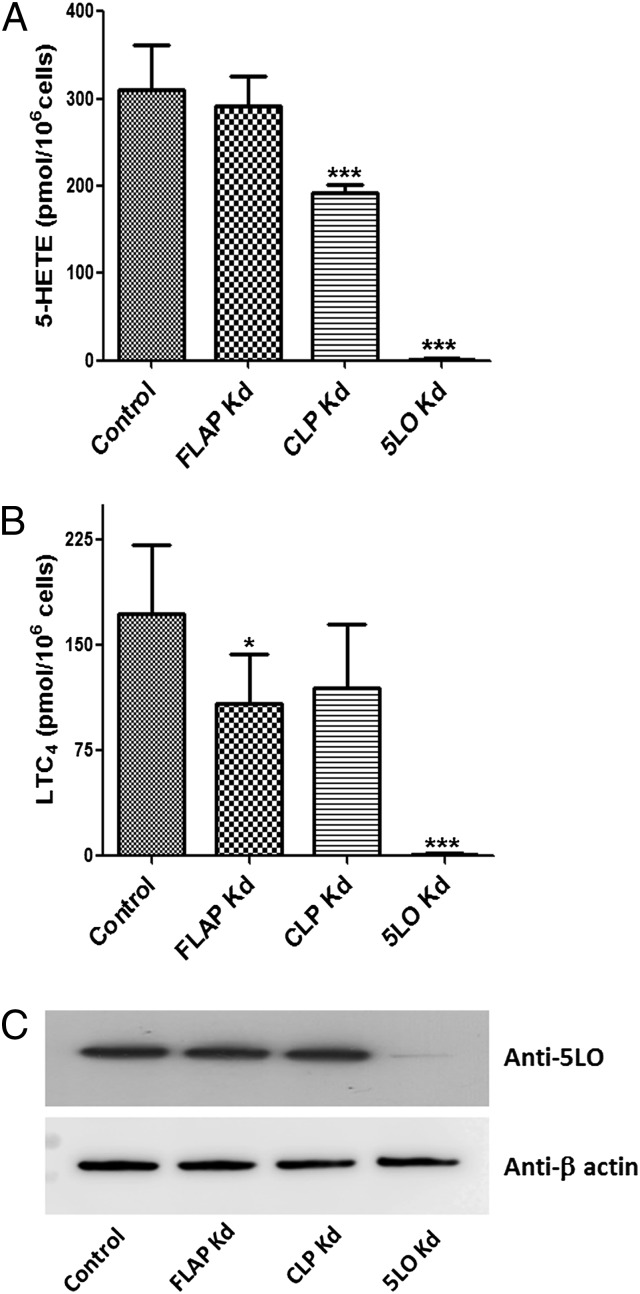
Fig. 1.
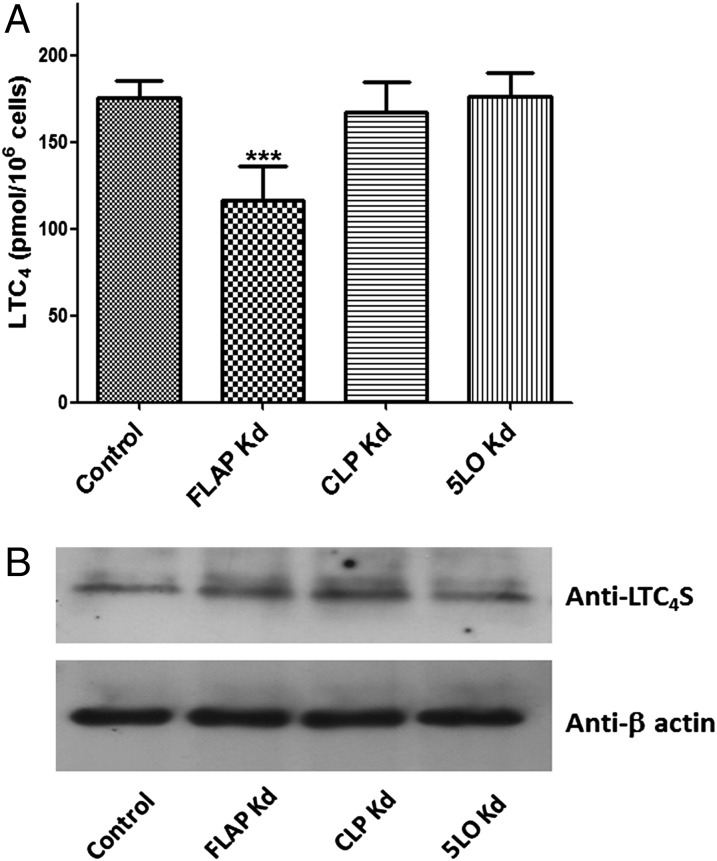
CLP increases the cellular activity of 5LO in MM6 cells. (A and B) Control and stable knockdown (Kd) cells were differentiated as described in Materials and Methods. Differentiated cells (∼1.2 × 106 cells in 1 mL of PGC buffer) were incubated with 5 µM ionophore A23187 and 40 µM AA for 10 min at 37 °C, after which the reaction was stopped by adding 1 mL of ice-cold methanol with internal standards. After solid-phase extraction, formation of 5-HETE (A) and LTC4 (B) was analyzed by HPLC. Data are mean ± SD of two independent experiments; n = 6. ***P < 0.001; *P < 0.05. (C) Western blot analysis of 5LO expression levels in control (nontarget shRNA) and Kd cells. The samples are supernatants of total cell lysates, and each sample contains 50 μg of protein.
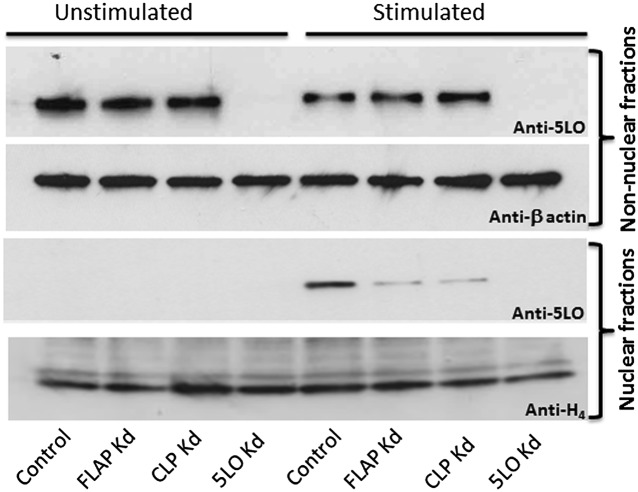
Fig. 5.
CLP and FLAP are required for association of 5LO with the nucleus during MM6 cell stimulation. Differentiated MM6 cells (control transfected with nontarget shRNA and knockdown cells) were primed with PMA (100 nM) at 37 °C for 10 min, followed by stimulation with ionophore A23187 (5 µM) for another 5 min. After cooling on ice, subcellular fractionation was performed by mild detergent (0.1% Nonidet P-40) lysis (Materials and Methods). Equal protein amounts (40 µg) of nonnuclear and nuclear fractions were analyzed by 5LO Western blot analysis. β-actin and histone H4 served as loading controls of nonnuclear and nuclear fractions, respectively. Similar results were obtained in two additional experiments.
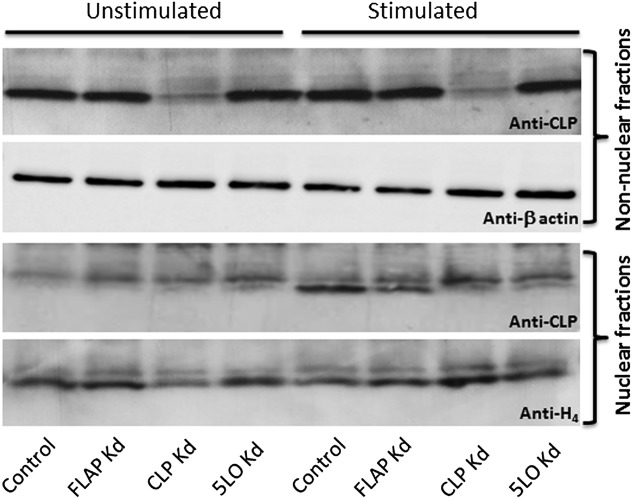
Fig. 7.
Association of CLP with the nucleus during MM6 cell stimulation is reduced in 5LO and FLAP knockdown cells. Subcellular fractions from stimulated MM6 cells were prepared as described in Fig. 5. Equal protein amounts (60 µg) of nonnuclear and nuclear fractions were analyzed by CLP Western blot analysis. Similar results were obtained in two additional independent experiments.
Cells were analyzed regularly before use in different experiments to ensure a stable extent of knockdown. Cell proliferation during the differentiation period (4 d) was always checked and compared for the various cell types (nontarget shRNA control, CLP, FLAP, and 5LO knockdowns) in a specific experiment. Typically, during differentiation, proliferation ceased similarly for all cell types, and the MM6 cells became slightly adherent. No obvious morphological changes were observed for these knockdown cells compared with controls.
CLP Increases Cellular 5LO Activity.
After differentiation, the major 5LO products in control and WT MM6 cells incubated with ionophore and exogenous AA were 5(S)-OH-eicosatetraenoic acid (5-HETE) and LTC4. Formation of leukotriene B4 (LTB4) was always minor (<10% of LTC4). Moreover, the yield of nonenzymatic hydrolysis products of LTA4 was <10% of that for these products of LTC4, indicating a high capacity for conversion of LTA4 to LTC4 in differentiated MM6 cells. In CLP knockdown cells, formation of 5-HETE and LTC4 was reduced by 39% and 31%, respectively, compared with control cells (Fig. 1). In FLAP knockdown cells, there was a significant decrease in the formation of LTC4 (37% reduction), but 5-HETE formation was hardly affected. These differences in 5LO activity were not related to differing 5LO expression levels (Fig. 1C); nearly complete depletion of both 5-HETE and LTC4 formation, as well as of 5LO protein, was observed in 5LO knockdown cells.
These results indicate that CLP is required in MM6 cells to obtain the maximum cellular 5LO activity in incubations with the nonphysiological combination of ionophore and exogenous AA. The findings with FLAP knockdown cells support the idea that processing of exogenous AA to 5-HETE does not depend on FLAP. The decreased formation of LTC4 in FLAP knockdown cells suggests that FLAP may influence LTC4 synthase activity.
Effects of CLP or FLAP Knockdown Are More Prominent in the Absence of Exogenous AA.
Formation of 5-HETE and LTC4 was considerably lower when MM6 cells were stimulated with ionophore A23187 alone compared with stimulation with ionophore A23187 plus AA. For this incubation condition, the absence of CLP resulted in a 53% reduction of 5-HETE formation and a 56% reduction in LTC4 formation. For FLAP knockdown cells, an approximate 78% decrease in both 5-HETE and LTC4 was observed (Fig. 2A).
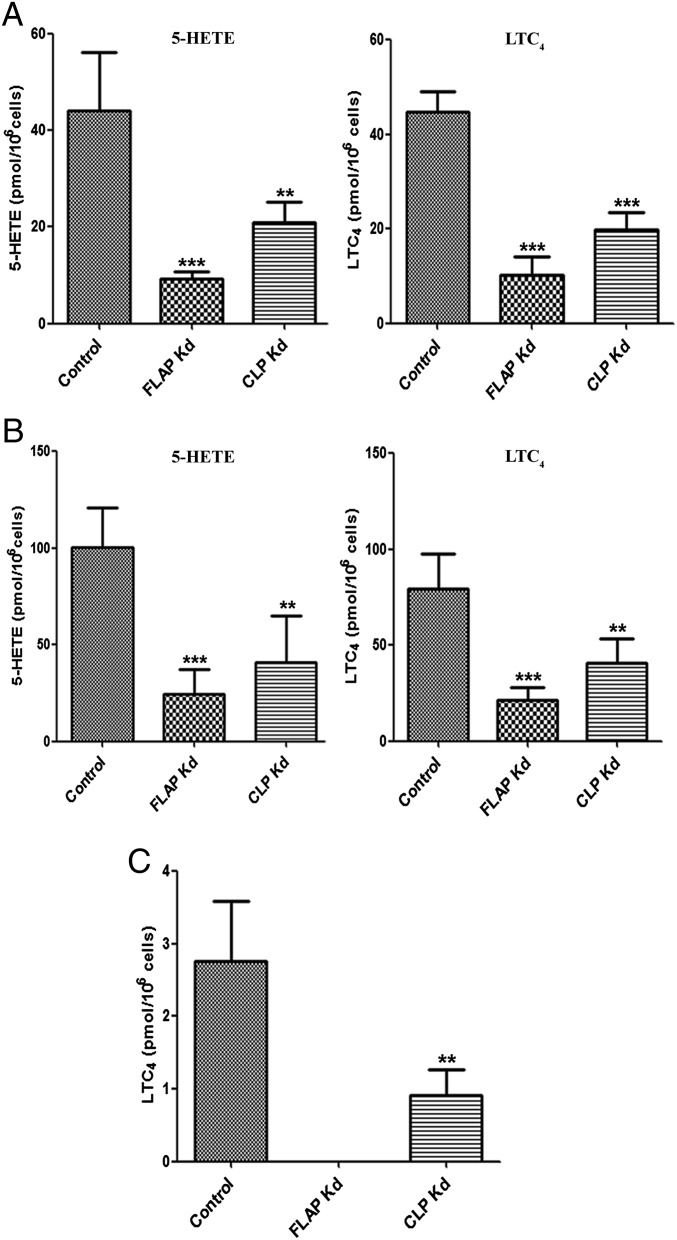
Fig. 2.
Effects of CLP or FLAP knockdown on 5LO product formation in the absence of exogenous AA. Control and stable knockdown cells were differentiated as described. (A) Differentiated cells (∼2 × 106 cells in 1 mL of PGC buffer) were incubated with 5 µM ionophore A23187 for 10 min at 37 °C. (B) Differentiated cells (∼2 × 106 cells in 1 mL of PGC buffer) were first primed with PMA 100 nM at 37 °C for 10 min and then stimulated with 5 µM ionophore (A23187) for another 10 min. (C) Differentiated cells (∼4 × 106 cells in 1 mL of PGC buffer) were first primed with LPS (1 µg/mL) for 20 min and then stimulated with fMLP (1 µM) for another 10 min. Data are mean ± SD of two independent experiments; n = 6 (A and B) or n = 5 (C). ***P < 0.001; **P < 0.005.
Priming of MM6 cells with phorbol myristate acetate (PMA) before ionophore stimulation has been shown to increase 5LO activity in parallel with increased 5LO phosphorylation and nuclear association (19). For this incubation condition, absence of CLP resulted in a 59% reduction in 5-HETE formation and a 50% reduction in LTC4 formation. For FLAP knockdown cells, an approximate 76% decrease in both 5-HETE and LTC4 was observed (Fig. 2B).
We finally subjected MM6 cells to a physiological stimulus, involving LPS priming followed by N-formylmethionyl-leucyl-phenylalanine (fMLP). With this stimulus, only LTC4 formation was detectable, with no 5-HETE formation (Fig. 2C). The absence of CLP resulted in an approximate 70% decrease in LTC4 formation, and no LTC4 was detectable after FLAP knockdown. The formation of 5-HETE and LTC4, and their ratios, in the various conditions are summarized in Table S1.
The incubation results show that the presence of CLP in MM6 cells increased 5LO activity for all stimuli tested (Table S1). At the same time, CLP was not crucial, given that some 5LO activity remained in all incubations of CLP knockdown cells. FLAP knockdown had minor effects in the presence of exogenous AA (Fig. 1), but showed prominent reductions of 5LO product formation from endogenous substrate. When MM6 cells were stimulated with LPS plus fMLP, expression of FLAP was crucial for 5LO activity (Fig. 2).
FLAP Has a Role in the Efficient Formation of Cys-LTs.
To investigate whether FLAP can influence LTC4 synthase activity, we incubated MM6 cells with LTA4. We found a significant reduction (by ∼34%) in LTC4 formation in FLAP knockdown cells, whereas CLP and 5LO knockdown cells had the same LTC4 yield as control cells (Fig. 3A). The expression levels of LTC4 synthase in all cells were comparable (Fig. 3B). Moreover, LTB4 formation was minor in these LTA4 incubations (<10% of LTC4). When conversion to LTC4 was decreased (FLAP knockdown cells), a significant increase in the LTB4 isomers formed nonenzymatically from LTA4 was observed. These results indicate that FLAP may modulate LTC4 synthase activity and/or might prevent hydrolysis of LTA4.
Fig. 3.
FLAP plays a role in the cellular formation of LTC4. (A) Differentiated control and knockdown MM6 cells (∼2 × 106 cells in 1 mL of PBS) were incubated with 5 µM LTA4 for 5 min at 25 °C. The formation of LTC4 was determined by HPLC. Data are mean ± SD of two independent experiments; n = 6. ***P < 0.001. (B) Cell lysates of control and knockdown cells were subjected to Western blot analysis with anti-LTC4 synthase antibody. The samples are supernatants of total lysates, and each sample contains 50 μg of protein. As a loading control, membranes were reprobed with β-actin to analyze the expression levels of LTC4 synthase.
CLP Coimmunoprecipitates with 5LO After Ionophore Stimulation.
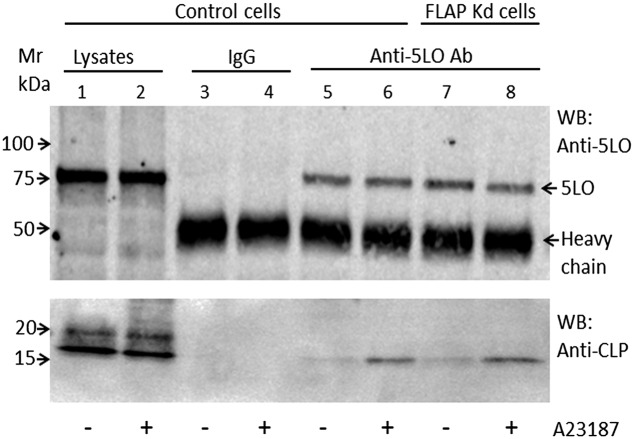
MM6 cells were stimulated with ionophore A23187 or vehicle for 5 min. Cells were lysed in presence of protease inhibitors, and the lysate was subjected to immunoprecipitation with an affinity-purified 5LO antibody. Cell lysates and immunoprecipitates were subjected to Western blot analysis for 5LO and CLP. The 5LO bands were similar in intensity regardless of cell stimulation in both the input lysates and in immunoprecipitates. The amounts of CLP were also similar in the input lysates, but the CLP band was considerably stronger in the immunoprecipitate from ionophore-treated cells compared with unstimulated control cells (Fig. 4). The same result was observed when PMA-primed cells were stimulated with ionophore (Fig. S3). This indicates that binding between 5LO and CLP in MM6 cells is increased during cell stimulation, leading to LT production. Interestingly, ionophore up-regulated coimmunoprecipitation (co-IP) of CLP with 5LO was observed in FLAP knockdown cells as well. This suggests that binding of CLP to 5LO occurs at nonnuclear sites, and that binding of CLP to 5LO at the perinuclear membrane may be independent of FLAP. When immunoprecipitates were analyzed by FLAP Western blot, no signal appeared, even though FLAP was present in the input lysates.
Fig. 4.
Increased CLP-5LO coimmunoprecipitation in ionophore-stimulated MM6 cells. Differentiated control and FLAP knockdown MM6 cells (∼2 × 106 cells) in PGC buffer were stimulated with ionophore A23187 (5 µM) as indicated for 5 min and then lysed (Materials and Methods). Equal amounts of protein lysates were subjected to immunoprecipitation with either normal rabbit IgG or anti-5LO antibody. 5LO and CLP in the lysates (inputs) and in immunoprecipitates were detected by immunoblot analysis with anti-5LO and anti-CLP antibodies. Similar results were obtained for control MM6 cells in four additional independent experiments and for FLAP knockdown cells in one additional experiment.
CLP and FLAP Are Required for Stable Nuclear Membrane Association of 5LO.
Intracellular localization of 5LO was assessed by subcellular fractionation using mild detergent (Nonidet P-40) lysis, followed by Western blot analysis of the resulting nonnuclear and nuclear fractions. In unstimulated cells, almost all 5LO resided in the nonnuclear fraction; no association of 5LO with the nuclear fraction was detectable in control or FLAP/CLP knockdown cells (Fig. 5). When control MM6 cells were primed with PMA and stimulated with ionophore, a substantial amount of 5LO appeared in the nuclear fraction, as reported previously (19). The outcome was different for CLP and FLAP knockdown cells. In both cases, association of 5LO with the nuclear fraction was clearly diminished (Fig. 5). β-actin served as an internal loading control for nonnuclear fractions, and histone H4 served as a loading control for nuclear fractions. As determined by densitometric analysis (Fig. S4), the 5LO/H4 ratio of the nuclear fraction from FLAP knockdown cells decreased to approximately 18–25% compared with control cells, and the 5LO/H4 ratio of the nuclear fraction from CLP knockdown cells decreased to approximately 24–32% compared with control cells (estimated from three independent experiments).
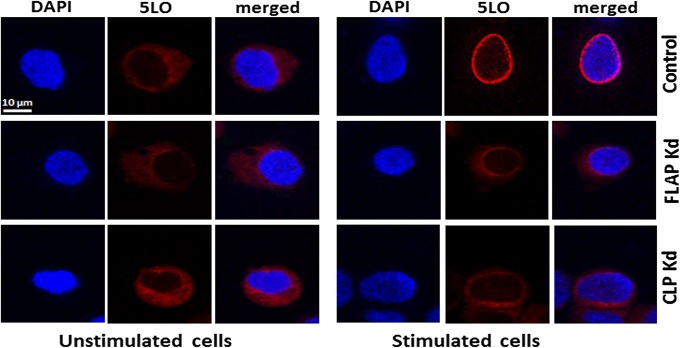
The subcellular localization of 5LO in differentiated MM6 cells was also evaluated by immunocytochemistry analysis (Fig. 6). In unstimulated nontarget shRNA control cells, 5LO was localized in the cytosol; no staining overlapped with the nuclear marker DAPI. The pattern was the same for unstimulated CLP and FLAP knockdown cells. After stimulation of control MM6 cells by PMA and ionophore A23187, a clear perinuclear ring pattern was seen for 5LO in the majority of cells. This redistribution from cytosolic to perinuclear was clearly compromised by CLP or FLAP knockdown. As shown in Fig. 6, in stimulated CLP and FLAP knockdown cells, 5LO staining remained diffusely cytosolic, rather similar to that seen in the unstimulated cells.
Fig. 6.
Immunocytochemistry of 5LO in unstimulated and stimulated MM6 cells. Control and FLAP/CLP knockdown cells were differentiated and subjected to stimulation with PMA and ionophore A23187 (Materials and Methods). Immunofluorescence images show single staining for 5LO (red; Cy3), nucleus (blue; DAPI), and merged pictures. (Scale bar: 10 µm.) Similar data were obtained in three additional independent analyses.
CLP Comigrates with 5LO on Cell Stimulation.
On stimulation of neutrophils or MM6 cells, an increased nuclear association has been demonstrated for CLP as well (12, 17, 21). Here we confirmed the pattern that nuclear fractions from MM6 cells stimulated with PMA followed by ionophore were enriched not only with 5LO, but also with CLP (Fig. 7). To determine whether this change in subcellular localization of CLP depended on 5LO binding, we tested the knockdown cells as well. After stimulation of 5LO knockdown cells, the CLP Western blot band of the nuclear fraction was clearly diminished (Fig. 7). In fact, the weak shadow band still present was similar to that seen for CLP knockdown cells, suggesting that it may be background. Moreover, in the nuclear fraction from FLAP knockdown cells, there was a reduction in the nuclear 5LO Western blot band (Fig. S4), as well as in the CLP band (Fig. S5). These observations support the idea that CLP may comigrate with 5LO on cell activation, leading to increased association with the nucleus.
Knockdown of CLP and FLAP in Human Macrophages Decreases LT Formation.
We evaluated the roles of FLAP and CLP in primary macrophages derived from peripheral blood monocytes. Control and knockdown cells for CLP and FLAP were prepared using the protocol described for MM6 cells, except for the puromycin selection. After stimulation with ionophore A23187, the major product formation was 5-HETE, and different levels of LTB4 and LTC4 were observed for different donors (Fig. S6). For the human macrophages, knockdown of CLP resulted in an approximate 60% reduction in 5-HETE, a 55% reduction in LTB4, and a 58% reduction in LTC4. Knockdown of FLAP resulted in a 60% reduction in 5-HETE and 65% reductions in LTB4 and LTC4 formation. The similar expression of 5LO in knockdown cells and the degree of knockdown for CLP and FLAP are shown in Fig. S6D. These results further confirm a role for CLP in cellular 5LO activity.
Discussion
To compare the roles of soluble CLP and membrane-bound FLAP in LT biosynthesis, we knocked down these proteins in the human monocytic cell line MM6. 5LO activity was determined in cells stimulated by four different protocols. Our results indicate that the presence of CLP in MM6 cells increased 5LO activity in all conditions (Figs. 1 and 2 and Table S1). Thus, CLP can support 5LO activity in intact cells as well, complementing previous in vitro data (12, 17). CLP is not absolutely crucial, however; some 5LO activity remained in all incubations of CLP knockdown cells. FLAP knockdown had only minor effects in incubations including exogenous AA, but 5LO product formation from endogenous substrate was greatly reduced. This finding is in accordance with the ideas that FLAP has a role in providing endogenous substrate to 5LO at the nuclear membrane (6) and that exogenous AA can be converted by 5LO in a FLAP-independent manner.
When cells are stimulated to produce LTs, 5LO typically translocates to the nuclear membrane. We confirmed this pattern for MM6 cells as well. In differentiated unstimulated MM6 cells, 5LO was located in the cytosol, and this was not changed by CLP or FLAP knockdown. Stimulation of control MM6 cells with PMA plus ionophore induced association of 5LO with the nuclear membrane, which was greatly diminished in both CLP and FLAP knockdown cells (Figs. 5 and 6). When FLAP knockdown MM6 cells were incubated with ionophore plus AA, the high rate of 5-HETE synthesis was almost unaffected, and LTC4 formation was also high (∼60% of control). This finding indicates that CLP, together with cellular membrane (perinuclear or other locations), can serve as an alternative support for 5LO activity leading to LT formation in ionophore stimulated MM6 cells, particularly from exogenous AA. On the other hand, when cells were stimulated by receptor-mediated signaling (LPS plus fMLP), FLAP was crucial for the low 5LO activity (3 pmol LTC4 per 106 cells, <1% of the maximum capacity of MM6; Fig. 2C), indicating that during this mimic of physiological conditions, all detectable 5LO activity occurred at the nuclear membrane. Furthermore, our finding that FLAP knockdown reduced the yield of LTC4 in LTA4 incubations indicates that FLAP may support the activity of LTC4 synthase, in line with these two proteins in the membrane-associated proteins in eicosanoid and glutathione metabolism family forming a cellular complex (5, 22).
A fraction of CLP associates with the nuclear fraction when cells are stimulated (12, 17). This inducible appearance of CLP in the nuclear fraction was practically absent in 5LO knockdown cells (Fig. 7 and Fig. S5). It also was clearly reduced in FLAP knockdown cells, seemingly in parallel with the compromised translocation of 5LO. Furthermore, in co-IP analyses, cellular binding of CLP to 5LO (native proteins) was weak but detectable in unstimulated cells, and increased considerably after ionophore stimulation (Fig. 4 and Fig. S3). We previously reported that binding of the purified proteins in vitro occurred in the absence of Ca2+, and that epitope-tagged constructs (e.g., FLAG-5LO, Myc-CLP) could be efficiently coimmunoprecipitated after overexpression in HEK 293 cells (no ionophore stimulation) (15). Those findings suggested a static association between CLP and 5LO, whereas the ionophore-inducible binding seen in the present study indicates a different situation for the native proteins in leukocytes with high capacity for LT biosynthesis. These observations suggest that CLP and 5LO migrate together when MM6 cells are stimulated, and that interference with the association of 5LO to the nuclear membrane (by FLAP knockdown) reduces the translocation of CLP as well. At the same time, knockdown of CLP hampered translocation of 5LO to the nuclear membrane. The foregoing findings, together with the effects on LTC4 and 5-HETE production, support an interplay of these proteins during cellular translocation and activation of 5LO.
The co-IP finding of increased binding of CLP to 5LO in cells stimulated with ionophore can be interpreted in different ways. It may indicate that CLP follows 5LO to the nuclear membrane and remains bound to the active LT-producing complex. CLP might contribute to the formation/stability of the active complex, or simply may be a remnant from the translocation mechanism. Not all 5LO associates with the nuclear fraction in stimulated cells, however; possibly, the pool of 5LO remaining in the cytosol is the fraction that binds CLP to an increased extent after cell stimulation. More intriguingly, it can be speculated that the increased CLP–5LO binding observed in ionophore-stimulated cells reflects the fraction of 5LO in transit, and that efficient transit requires binding to CLP. This possibility would seem to be compatible with the increased ionophore-induced CLP-5LO binding occurring in FLAP knockdown cells as well. At the same time, FLAP knockdown reduced the nucleus association for both 5LO and CLP. Apparently, the “safe arrival” and permanent association of the CLP-5LO complex to the perinuclear membrane is reduced by FLAP knockdown. Interestingly, in stimulated RBL-2H3 cells using a cell-permeable cross-linker, FLAP was found to bind 5LO, as well as a 10-kDa protein referred to as AP-10 (5).
Comparison of the product patterns in the MM6 cell incubations with previous in vitro data may provide a clue as to the role of CLP. When MM6 cells were incubated with ionophore or with PMA plus ionophore, the LTC4/5-HETE ratio was close to 1 for both control and CLP knockdown cells (Table S1). Previously, when purified recombinant 5LO was incubated in vitro, the formation of LTA4 increased between threefold and fivefold when CLP was present along with phosphatidylcholine and Ca2+, giving a LT/5-HETE ratio of 0.4 in one study (17) and 0.6 in another study (12). When purified 5LO was incubated with only Ca2+ and phosphatidylcholine (no CLP), the LT/5-HETE ratio was considerably lower, close to 0.1 (12, 17). If CLP were an important scaffold factor for 5LO activity (from endogenous substrate) at the nuclear membrane, then CLP knockdown would be expected to change the product pattern, particularly for MM6 cells stimulated with PMA plus ionophore, leading to prominent 5LO association with the nucleus; however, knockdown of CLP did not change the relative amounts of LT to 5-HETE (Table S1). Both LTC4 and 5-HETE were reduced to almost the same extent. Interestingly, FLAP knockdown did not affect the product pattern either (Table S1). It appears possible that in MM6 cells, these proteins may provide backup for each other. We confirmed these findings through knockdown of CLP and FLAP in primary human macrophages derived from three donors. When incubated with ionophore, both 5-HETE and LTs were significantly reduced compared with control cells (Fig. S6).
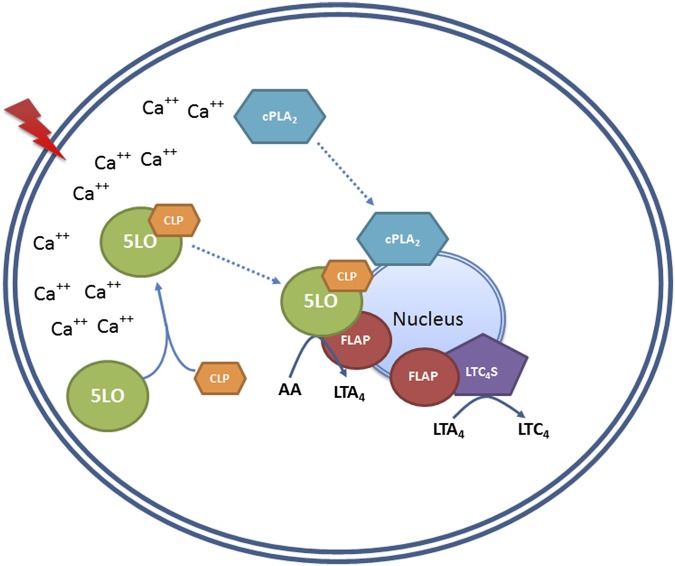
Several findings of this study have mechanistic implications for the generation of LTs. First, the presence of CLP leads to increased 5LO activity in intact cells (MM6 and primary macrophages). Moreover, both CLP and FLAP have roles in the translocation and association of 5LO to the nuclear membrane. Production of both LTC4 and 5-HETE from endogenous substrate was decreased, but LTC4/5-HETE ratios were maintained in CLP and FLAP knockdown cells. For cells provided with exogenous AA and stimulated with ionophore, it appears that CLP together with membrane can function as a scaffold for 5LO activity, as observed in vitro. A scenario for the interactions of 5LO, CLP, FLAP, and membrane is suggested in Fig. 8. Our results suggest that the CLP–5LO interaction can be a target for reduced LT production in the cell.
Fig. 8.
Putative scheme of LT biosynthesis in MM6 cells, showing possible functions of CLP and FLAP. Cell stimulation leading to increased intracellular Ca2+ may induce binding between 5LO and CLP in the cytosol. The 5LO-CLP complex translocates, and aided by FLAP, this complex associates with the nucleus. At the nuclear membrane, AA released from phospholipids by the action of cPLA2 (also known to translocate to the nuclear membrane on cell stimulation) is presented to 5LO with the help of FLAP, and LTA4 is produced. The association of FLAP with LTC4S also may also influence efficient conversion of LTA4 to LTC4.
Materials and Methods
Generation of Stable Knockdown Cells for CLP, FLAP, and 5LO.
MM6 cells were transfected with lentiviral constructs expressing shRNAs against CLP (NM_021149.2–784s1c1), FLAP (NM_001629.2–401s1c1), and 5LO (NM_000698.1–1039s1c1) obtained from Sigma-Aldrich. Human mononuclear cells were isolated from buffy coats (Karolinska Hospital Blood Bank). Monocytes were infected with lentivirus containing shRNAs. Virus-infected cells were cultured with media containing 50 ng/mL macrophage colony-stimulating factor for 7 d (without puromycin selection) and treated with 50 nM calcitriol for the final 24 h of this differentiation period.
Cell Incubation.
The control (nontarget shRNA) and different knockdown MM6 cells were grown in cell culture and differentiated with 5 ng/mL TGF-β and 50 nM calcitriol for 96 h. Differentiated cells were incubated, and LT and 5-HETE formation was determined by solid-phase extraction and reverse-phase HPLC.
Quantitative Real-Time PCR.
Total RNA was extracted with the Bioline RNA Mini Kit, and cDNA was synthesized using oligo(dT) primers.
Subcellular Fractionation by Detergent Lysis.
MM6 cells were stimulated with PMA followed by ionophore A23187, then suspended in Nonidet P-40 lysis buffer. Nuclear/nonnuclear fractions were prepared and subjected to Western blot analysis. Histone H4 and β-actin served as internal markers for nuclear and nonnuclear fractions, respectively.
Immunoprecipitation.
Differentiated MM6 control cells were stimulated with ionophore A23187 or with PMA followed by ionophore. Samples were immediately chilled on ice, and cell pellets were resuspended in lysis buffer. Cell lysates were precleared by incubation with protein A-Sepharose. The precleared samples were incubated with either normal rabbit IgG or purified anti-5LO antibody overnight at 4 °C. Protein A-Sepharose was added, and incubation was continued for another 1 h. The immunocomplexes were washed four times with lysis buffer and eluted by heating at 90 °C for 5 min in Laemmli sample buffer. After centrifugation, the supernatants were analyzed by immunoblotting with anti-5LO and anti-CLP antibodies.
Immunofluorescence Microscopy.
The differentiated MM6 control, FLAP, and CLP knockdown cells were stimulated with PMA followed by ionophore A23187. Samples were immediately chilled on ice for 2 min and centrifuged onto glass slides at 4 °C. The cells were then fixed with methanol at −20 °C for 4 min, washed, blocked with 10% nonimmune goat serum, and incubated with anti-5LO antibody overnight at 4 °C. The samples were washed, and then incubated with Cy3 goat anti-rabbit IgG (Invitrogen). After coating with aqueous antifade gel mounting medium (Vector Laboratories) with DAPI under coverslips, the fluorescent signal was observed with an Olympus Fluoview FV1000 microscope system.
More details on methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Patrick Provost (Centre Hospitalier de Universitaire de Québec Research Center/Centre Hospitalier de l'Université Laval) for helpful discussions. This work was supported by the Swedish Research Council, Else Kröner-Fresenius-Stiftung (Dr. Hans Kröner-Graduiertenkolleg), and Karolinska Institutet.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410983111/-/DCSupplemental.
References
- 1.Haeggström JZ, Funk CD. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem Rev. 2011;111(10):5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 2.Rådmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396(1):105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 3.Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clin Chest Med. 2012;33(3):559–570. doi: 10.1016/j.ccm.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans JF, Ferguson AD, Mosley RT, Hutchinson JH. What’s all the FLAP about?: 5-lipoxygenase–activating protein inhibitors for inflammatory diseases. Trends Pharmacol Sci. 2008;29(2):72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Mandal AK, et al. The nuclear membrane organization of leukotriene synthesis. Proc Natl Acad Sci USA. 2008;105(51):20434–20439. doi: 10.1073/pnas.0808211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson AD. Structure-based drug design on membrane protein targets: Human integral membrane protein 5-lipoxygenase–activating protein. Methods Mol Biol. 2012;841:267–290. doi: 10.1007/978-1-61779-520-6_12. [DOI] [PubMed] [Google Scholar]
- 7.Werz O, Bürkert E, Samuelsson B, Rådmark O, Steinhilber D. Activation of 5-lipoxygenase by cell stress is calcium independent in human polymorphonuclear leukocytes. Blood. 2002;99(3):1044–1052. doi: 10.1182/blood.v99.3.1044. [DOI] [PubMed] [Google Scholar]
- 8.Luo M, et al. Phosphorylation by protein kinase A inhibits nuclear import of 5-lipoxygenase. J Biol Chem. 2005;280(49):40609–40616. doi: 10.1074/jbc.M507045200. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert NC, et al. The structure of human 5-lipoxygenase. Science. 2011;331(6014):217–219. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammarberg T, Provost P, Persson B, Rådmark O. The N-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J Biol Chem. 2000;275(49):38787–38793. doi: 10.1074/jbc.M006136200. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni S, Das S, Funk CD, Murray D, Cho W. Molecular basis of the specific subcellular localization of the C2-like domain of 5-lipoxygenase. J Biol Chem. 2002;277(15):13167–13174. doi: 10.1074/jbc.M112393200. [DOI] [PubMed] [Google Scholar]
- 12.Esser J, et al. Coactosin-like protein functions as a stabilizing chaperone for 5-lipoxygenase: role of tryptophan 102. Biochem J. 2010;425(1):265–274. doi: 10.1042/BJ20090856. [DOI] [PubMed] [Google Scholar]
- 13.Chen XS, Funk CD. The N-terminal “beta-barrel” domain of 5-lipoxygenase is essential for nuclear membrane translocation. J Biol Chem. 2001;276(1):811–818. doi: 10.1074/jbc.M008203200. [DOI] [PubMed] [Google Scholar]
- 14.Provost P, Samuelsson B, Rådmark O. Interaction of 5-lipoxygenase with cellular proteins. Proc Natl Acad Sci USA. 1999;96(5):1881–1885. doi: 10.1073/pnas.96.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provost P, et al. 5-Lipoxygenase interacts with coactosin-like protein. J Biol Chem. 2001;276(19):16520–16527. doi: 10.1074/jbc.M011205200. [DOI] [PubMed] [Google Scholar]
- 16.Provost P, et al. Coactosin-like protein, a human F-actin–binding protein: Critical role of lysine-75. Biochem J. 2001;359(Pt 2):255–263. doi: 10.1042/0264-6021:3590255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakonjac M, et al. Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc Natl Acad Sci USA. 2006;103(35):13150–13155. doi: 10.1073/pnas.0605150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brungs M, Rådmark O, Samuelsson B, Steinhilber D. Sequential induction of 5-lipoxygenase gene expression and activity in Mono Mac 6 cells by transforming growth factor beta and 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1995;92(1):107–111. doi: 10.1073/pnas.92.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werz O, Klemm J, Samuelsson B, Radmark O. Phorbol ester up-regulates capacities for nuclear translocation and phosphorylation of 5-lipoxygenase in Mono Mac 6 cells and human polymorphonuclear leukocytes. Blood. 2001;97(8):2487–2495. doi: 10.1182/blood.v97.8.2487. [DOI] [PubMed] [Google Scholar]
- 20.Sorg BL, et al. Analysis of the 5-lipoxygenase promoter and detailed characterization of a vitamin D receptor binding site. Biochim Biophys Acta. 2006;176(7):686–697. doi: 10.1016/j.bbalip.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Pergola C, et al. ERK-mediated regulation of leukotriene biosynthesis by androgens: A molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci USA. 2008;105(50):19881–19886. doi: 10.1073/pnas.0809120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bair AM, Turman MV, Vaine CA, Panettieri RA, Jr, Soberman RJ. The nuclear membrane leukotriene synthetic complex is a signal integrator and transducer. Mol Biol Cell. 2012;23(22):4456–4464. doi: 10.1091/mbc.E12-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]