Glasses and crystals play different roles in technology. When precise control of local structure is required, as in silicon-based electronics, crystals are used. On the other hand, when macroscopic homogeneity is required, as in optical fibers, glasses are used. The pairing of local disorder and macroscopic homogeneity in glasses is not a coincidence. The range of local packing arrangements present in glassy materials allows “defects,” such as impurities, to be locally accommodated in contrast to crystals for which disruptive grain boundaries are often the consequence. The flexible local packing of glasses also provides an opportunity for materials engineering. Many glasses with different structures and properties can be prepared with exactly the same composition. This last feature is a key element in the experiments of Pérez-Castañeda et al. (1). Pérez-Castañeda et al. report that two different glasses made from the same organic molecule can have dramatically different heat capacities below 1 K.
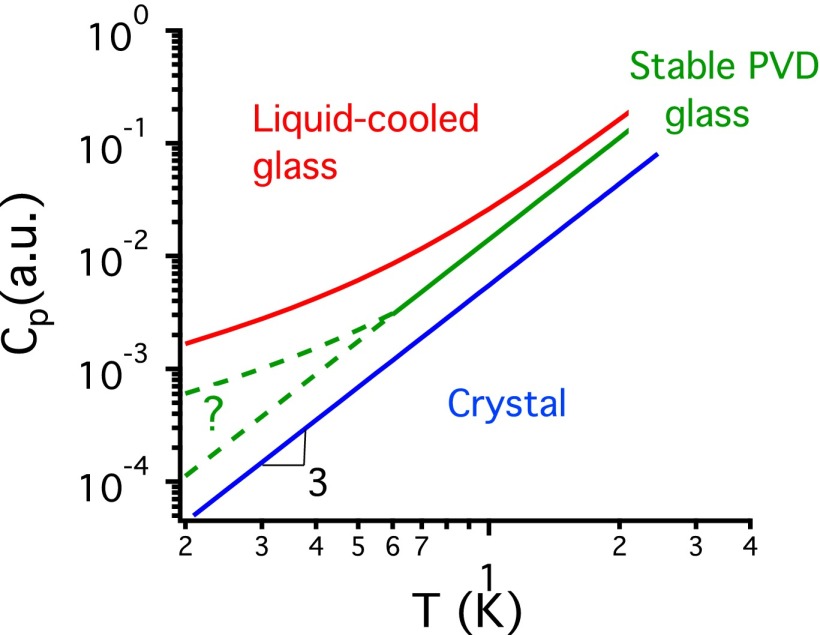
Many of us were first introduced to heat capacity in high school chemistry. The heat capacity was the boring part of the experiment that one used to interpret the temperature rise associated with a chemical reaction. For low-temperature solids, however, the heat capacity can provide important insights into structure and the interactions between atoms and molecules. The difference between the heat capacities of glasses and crystals below 1 K illustrates this point, as shown in Fig. 1. Crystals follow the Debye law, with the constant pressure heat capacity Cp proportional to T 3, as a result of their 3D periodicity. The heat capacity of glasses is much larger than crystals below 1 K and also has a different temperature dependence, with Cp roughly linear in T (2). The behavior of the heat capacity of glasses in this regime was unexpected and resulted in extensive research, starting in the 1970s. Other properties of glasses also differ from those of crystals in the low-temperature regime, with glasses having much lower thermal conductivities and distinct phonon propagation characteristics. Most remarkably, this set of properties is apparently universal for glasses, including silica, germania, selenium, and organic polymers (2). These properties of low-temperature glasses have been collectively interpreted in terms of quantum-mechanical tunneling between two-level systems (TLS) (3–5). Although some independent support for the existence of TLS comes from single-molecule spectroscopy experiments (6), the structural origins of the TLS are unclear. In addition, there is no accepted explanation as to why the right number of TLS with the correct distribution of barrier heights arises in all these different types of glassy materials.
Fig. 1.
Schematic representation of heat capacities of indomethacin in three solid forms. The T 3 dependence of Cp below 1 K for the glass prepared by physical vapor deposition (PVD) is unprecedented.
Against this backdrop of the apparently universal properties of glasses in the low-temperature regime, the results reported by Pérez-Castañeda et al. (1) are quite surprising. The authors studied the heat capacity of glasses of an organic molecule (indomethacin) produced by physical vapor deposition. The group produced glasses for which Cp maintains a T 3 dependence down to 0.6 K. As a control experiment, Pérez-Castañeda et al. heated the vapor-deposited glass into the supercooled liquid and then cooled to produce an ordinary glass, which exhibited a nearly linear temperature dependence for Cp below 1 K, as expected for glassy materials. At the lowest temperature at which both indomethacin glasses were measured, the heat capacity of the vapor-deposited glass was half that of the ordinary glass, indicating very significant differences in the density of TLS, according to the standard interpretation. To my knowledge, this is the first example of a glassy material for which the heat capacity is proportional to T 3 at temperatures below 1 K. As such, these results appear to challenge the standard interpretation that TLS are intrinsic to the amorphous state and responsible for the low-temperature heat capacity behavior of glasses.
To appreciate these new results from Pérez-Castañeda et al. (1), it is important to understand other properties of the indomethacin glasses used in these experiments. In the last 7 years, it has been established that physical vapor deposition can produce glasses that are remarkably different from liquid-cooled glasses of the same composition (7). For many molecules, including indomethacin, it has been established that deposition onto substrates near 0.85 Tg produces glasses with particularly striking properties (8, 9). (Here, Tg is the temperature at which a supercooled liquid falls out of equilibrium upon cooling and becomes a glassy solid.) Because organic glasses exhibit substantial surface mobility even below Tg, molecules near the surface can partially equilibrate during deposition, allowing the formation of better-packed glasses with higher density. Although higher-density glasses can also be produced by very slowly cooling a liquid, it has been estimated that cooling over more than 1,000 y would be required to match the density of the vapor-deposited glasses (10). Many of the properties of these vapor-deposited glasses can be qualitatively understood as a result of their better packing, including high kinetic stability and low enthalpy. Previous work on indomethacin has established two additional properties relevant for the interpretation of these experiments of Pérez-Castañeda et al. (1). High-density glasses produced by vapor deposition have slightly lower heat capacities than the ordinary liquid-cooled glass, even near room temperature, consistent with more efficiently packed local structures (11). In addition, highly stable glasses of indomethacin are anisotropic as measured by optical birefringence and X-ray scattering (9, 12).
Pérez-Castañeda et al. (1) consider two possible interpretations of their striking results. The first possibility is that the better packing of the vapor-deposited glasses (indicated by higher density and lower room-temperature heat capacity) eliminates a large number of TLS and pushes the transition to a linear temperature dependence to below 0.6 K. This view would be qualitatively consistent with recent work on amorphous silicon that reported that higher-density silicon samples had much lower heat capacities (at 2 K), indicating a much lower density of TLS (13). However, recent work on hyperaged natural amber did not show any change in the density of TLS relative to a freshly cooled sample (14). A second possible interpretation, the one favored by Pérez-Castañeda et al. (1), is that the anisotropy of the vapor-deposited glass interferes with interactions between the TLS in such a way as to disrupt the typical distribution of barrier heights. This explanation builds upon work arguing that strongly interacting TLS provide the most natural explanation for their universal presence in amorphous materials (15, 16). Some readers will consider a third possibility, that the vapor-deposited indomethacin is not really glassy but rather a disordered crystal. Arguing against this is the absence of sharp diffraction peaks and the observation that heating yields the supercooled liquid and not the crystal. In addition, highly stable vapor-deposited glasses have been formed from molecular mixtures, which would be unlikely if their local packing requires crystalline order (17).
Pérez-Castañeda et al. report that two different glasses made from the same organic molecule can have dramatically different heat capacities below 1 K.
By identifying a new exception to the “universal” low-temperature thermal properties of glasses, the work of Pérez-Castañeda et al. (1) opens new and important paths for understanding their origin. These authors exploit the ability of physical vapor deposition to produce glasses with structures and properties significantly different from liquid-cooled glasses, without altering chemical composition. As Pérez-Castañeda point out, future experiments on highly stable vapor-deposited glasses with more isotropic packing would be particularly useful for testing their proposed explanation for the suppression of TLS. More generally, the ability of vapor deposition to produce a large range of local packing arrangements provides an opportunity to systematically tune the low-temperature heat capacity, thus enabling a powerful new approach for investigating the low-temperature thermal properties of glasses and the role of two-level systems.
Supplementary Material
Footnotes
The author declares no conflict of interest.
See companion article on page 11275.
References
- 1.Pérez-Castañeda T, Rodríguez-Tinoco C, Rodríguez-Viejo J, Ramos MA. Supression of tunneling two-level systems in ultrastable glasses of indomethacin. Proc Natl Acad Sci USA. 2014;111:11275–11280. doi: 10.1073/pnas.1405545111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeller RC, Pohl RO. Thermal conductivity and specific heat of noncrystalline solids. Phys Rev B. 1971;4(6):2029. [Google Scholar]
- 3.Phillips WA. Structure and the low-temperature properties of amorphous s. J Non-Cryst Solids. 1978;31(1-2):267–283. [Google Scholar]
- 4.Anderson PW, Halperin BI, Varma CM. Anomalous low-temperature thermal properties of glasses and spin glasses. Philos Mag. 1972;25(1):1. [Google Scholar]
- 5.Jackle J. Ultrasonic attenuation in glasses at low-temperatures. Z Phys. 1972;257(3):212–223. [Google Scholar]
- 6.Boiron AM, Tamarat P, Lounis B, Brown R, Orrit M. Are the spectral trails of single molecules consistent with the standard two-level system model of glasses at low temperatures? Chem Phys. 1999;247(1):119–132. [Google Scholar]
- 7.Swallen SF, et al. Organic glasses with exceptional thermodynamic and kinetic stability. Science. 2007;315(5810):353–356. doi: 10.1126/science.1135795. [DOI] [PubMed] [Google Scholar]
- 8.Ramos SL, Oguni M, Ishii K, Nakayama H. Character of devitrification, viewed from enthalpic paths, of the vapor-deposited ethylbenzene glasses. J Phys Chem B. 2011;115(49):14327–14332. doi: 10.1021/jp203612s. [DOI] [PubMed] [Google Scholar]
- 9.Dalal SS, Fakhraai Z, Ediger MD. High-throughput ellipsometric characterization of vapor-deposited indomethacin glasses. J Phys Chem B. 2013;117(49):15415–15425. doi: 10.1021/jp405005n. [DOI] [PubMed] [Google Scholar]
- 10.Kearns KL, et al. Hiking down the energy landscape: Progress toward the Kauzmann temperature via vapor deposition. J Phys Chem B. 2008;112(16):4934–4942. doi: 10.1021/jp7113384. [DOI] [PubMed] [Google Scholar]
- 11.Kearns KL, Whitaker KR, Ediger MD, Huth H, Schick C. Observation of low heat capacities for vapor-deposited glasses of indomethacin as determined by AC nanocalorimetry. J Chem Phys. 2010;133(1):014702. doi: 10.1063/1.3442416. [DOI] [PubMed] [Google Scholar]
- 12.Dawson KJ, Zhu L, Yu L, Ediger MD. Anisotropic structure and transformation kinetics of vapor-deposited indomethacin glasses. J Phys Chem B. 2011;115(3):455–463. doi: 10.1021/jp1092916. [DOI] [PubMed] [Google Scholar]
- 13.Queen DR, Liu X, Karel J, Metcalf TH, Hellman F. Excess specific heat in evaporated amorphous silicon. Phys Rev Lett. 2013;110(13):135901. doi: 10.1103/PhysRevLett.110.135901. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Castañeda T, Jiménez-Riobóo RJ, Ramos MA. Two-level systems and boson peak remain stable in 110-million-year-old amber glass. Phys Rev Lett. 2014;112(16):165901. doi: 10.1103/PhysRevLett.112.165901. [DOI] [PubMed] [Google Scholar]
- 15.Leggett AJ, Vural DC. “Tunneling two-level systems” model of the low-temperature properties of glasses: Are “smoking-gun” tests possible? J Phys Chem B. 2013;117(42):12966–12971. doi: 10.1021/jp402222g. [DOI] [PubMed] [Google Scholar]
- 16.Coppersmith SN. Frustrated interactions and tunneling: Two-level systems in glasses. Phys Rev Lett. 1991;67(17):2315–2318. doi: 10.1103/PhysRevLett.67.2315. [DOI] [PubMed] [Google Scholar]
- 17.Whitaker KR, Scifo DJ, Ediger MD, Ahrenberg M, Schick C. Highly stable glasses of cis-decalin and cis/trans-decalin mixtures. J Phys Chem B. 2013;117(42):12724–12733. doi: 10.1021/jp400960g. [DOI] [PubMed] [Google Scholar]