Significance
Children from families of low socioeconomic status (SES) are vulnerable to a variety of health problems. These risks begin in early childhood and persist across the lifecourse. Studies hint that nurturant parenting may offset these health risks, but it remains unclear whether these findings reflect a causal process and have clinical utility. Here we describe a randomized controlled trial, which sought to improve parenting and build youth competencies in low-SES African American families. The endpoint was low-grade inflammation, a process that underlies many health problems to which low-SES youth are vulnerable. Eight years after the intervention, youth who participated had significantly less inflammation than controls. If substantiated, these findings may provide a strategy for narrowing some of America’s social and racial disparities in health.
Abstract
Children of low socioeconomic status (SES) are at elevated risk for health problems across the lifespan. Observational studies suggest that nurturant parenting might offset some of these health risks, but their design precludes inferences about causal direction and clinical utility. Here we ask whether a psychosocial intervention, focused improving parenting, strengthening family relationships, and building youth competencies, can reduce inflammation in low-SES, African Americans from the rural South. The trial involved 272 mothers and their 11-y-old children from rural Georgia, half of whose annual household incomes were below the federal poverty line. Families were randomly assigned to a 7-wk psychosocial intervention or to a control condition. When youth reached age 19, peripheral blood was collected to quantify six cytokines that orchestrate inflammation, the dysregulation of which contributes to many of the health problems known to pattern by SES. Youth who participated in the intervention had significantly less inflammation on all six indicators relative to controls (all P values < 0.001; effect sizes in Cohen’s d units ranged from −0.69 to −0.91). Mediation analyses suggested that improved parenting was partially responsible for the intervention’s benefits. Inflammation was lowest among youth who received more nurturant-involved parenting, and less harsh-inconsistent parenting, as a consequence of the intervention. These findings have theoretical implications for research on resilience to adversity and the early origins of disease. If substantiated, they may also highlight a strategy for practitioners and policymakers to use in ameliorating social and racial health disparities.
Children of low socioeconomic status (SES) are at elevated risk for health problems across the lifespan (1, 2). These disparities begin in the earliest stages of the life course. The offspring of low-income families have disproportionately high rates of growth restriction, preterm birth, and neonatal mortality (3). As children from low-SES families mature, they continue to experience health problems at rates that are substantially higher than those of their more advantaged peers. Low-SES youth show increased prevalence of obesity, insulin resistance, and asthma (4–7). These conditions appear to set the stage for chronic diseases associated with aging. When they reach the later stages of life, those raised in low-SES families show excessive morbidity and mortality from stroke, coronary heart disease, some cancers, and chronic lung diseases (8–11). These associations are typically independent of SES in adulthood, suggesting that childhood disadvantage can leave a biological “residue” with long-term health consequences.
Despite these trends, not all low-SES children have, or go on to develop, health problems (12). Recent evidence suggests that a subset of youth develop “resilience” to the health consequences of low-SES environments if they receive high-quality parenting. One study followed rural adolescents across 3 y and found that disadvantage was associated with increasing allostatic load, a composite indicator of cardiometabolic risk. However, these trends were absent in low-SES youth whose mothers were rated as being highly responsive to their needs (13). Similar patterns have emerged in retrospective studies of adults. One such study found that low childhood SES was associated with higher prevalence of metabolic syndrome at midlife (14). Again, however, this effect was offset by nurturant parenting. Subjects who reported being raised in nurturant low-SES families had metabolic risks identical to those of subjects from higher-SES households. A third study explored how maternal nurturance relates to inflammation, a process central to the pathogenesis of many health problems that pattern by SES (15–17). Among subjects reared in low-SES families, maternal nurturance was associated with better regulation of inflammation, as reflected by the activity of transcription control pathways and cytokine responses to microbial stimulation (18).
These are provocative findings. To the extent that they reflect a causal process in which nurturant parenting offsets some of the health risks associated with childhood disadvantage, they have theoretical implications for a number of research domains, including those focused on social disparities, early origins of disease, and resilience to adversity. Such findings might also have implications for practitioners and policymakers seeking interventions to ameliorate health disparities (19). However, causal inferences cannot easily be gleaned from existing studies, because their observational designs are prone to residual confounding and reverse directionality errors. Here, we navigate around these interpretational problems by conducting secondary analyses of a randomized controlled trial, designed to improve parenting quality, strengthen familial relationships, and build youth competencies in low-SES African Americans from the rural South. Across the United States, there marked racial disparities in pediatric health (20). These disparities are prominently manifest in cardiometabolic risk, where African American youth are more likely to display central obesity, high blood pressure, insulin resistance, and subclinical atherosclerosis compared with their Caucasian peers (7, 20–22). Low-grade inflammation is a common pathogenic mechanism in all of these predisease states, and it also facilitates their progression into clinical entities such as diabetes, coronary heart disease, and stroke (23, 24), which are highly prevalent among African Americans residing in the Stroke Belt that stretches across the Southeastern United States, where our study takes place. Accordingly, we collected blood from youth whose families took part in the trial, and examined whether participating in this family-oriented intervention reduced expression of cytokines that orchestrate low-grade inflammation.
Results
The Strong African American Families (SAAF) program was designed to enhance parenting, strengthen family relationships, and foster youth competencies. The data presented here come from SAAF’s original clinical trial (25), which included 667 African American mothers and their 11-y-old children, all of whom resided in rural Georgia. At study entry, 46% of the families lived below federal poverty standards, and 90% fell into the federal low-income category. Of the families, 372 were randomly assigned to SAAF and 295 were randomly assigned to an assessment-only control condition. SAAF comprised seven weekly group meetings with separate activities for parents and children, as well as a family curriculum.
Eight years after the trial ended, we measured low-grade inflammation in 272 youth, who were 19 y of age at the time. These youth constitute the analytic sample here. At study entry, they were identical to the broader sample in terms of demographics, parenting quality, and mental health (Table S1; all P values > 0.15). However, these youth did participate more actively in SAAF, attending a mean of 5.11 sessions vs. 4.45 for those subjects without inflammation data, and their mothers showed greater improvements in parenting across the trial (Table S1; all P values > 0.02). Of the youth in the analytic sample, 173 had participated in SAAF and the other 99 had been assigned to the control group. Comparisons of these groups suggested that, despite attrition, the effects of random assignment were still evident. Specifically, SAAF and control subjects with inflammation data were identical in terms of sex, family and neighborhood SES, parenting quality, externalizing behavior, and cigarette smoking (all t values > 0.90, P values > 0.36; Table 1). Nevertheless, to minimize residual confounding, we adjusted for sex and family SES in all analyses presented.
Table 1.
Sample characteristics at study entry
| SAAF (n = 173) |
Control (n = 99) |
P | |||
| M | SD | M | SD | ||
| Sex, male | 0.43 | 0.50 | 0.41 | 0.50 | 0.757 |
| Parenting quality | 41.93 | 4.73 | 41.52 | 4.81 | 0.501 |
| Family SES risk | 2.42 | 1.45 | 2.36 | 1.48 | 0.776 |
| Neighborhood SES risk | 0.28 | 2.48 | −0.04 | 3.05 | 0.366 |
| Externalizing problems | 11.23 | 7.78 | 11.00 | 9.11 | 0.826 |
| Smoking frequency | 0.05 | 0.22 | 0.06 | 0.24 | 0.766 |
P values are based on independent-groups t tests.
Peripheral blood was collected from these youth and, using a multiplex assay from MesoScale Discovery, we measured circulating levels of six cytokines that orchestrate inflammation. The cytokines were interleukins (IL)-1β, -6, -8, and -10, plus tumor necrosis factor α and IFN-γ. Correlations showed that concentrations of these molecules were strongly interrelated. The average intercytokine correlation was r = 0.75 and the range was r = 0.53–0.95. Because of this clustering, we formed an inflammation composite for each subject by summing the six z-scored cytokine values. The composite showed high levels of internal consistency (Cronbach’s α = 0.93) and functioned as the primary endpoint in the analyses below.
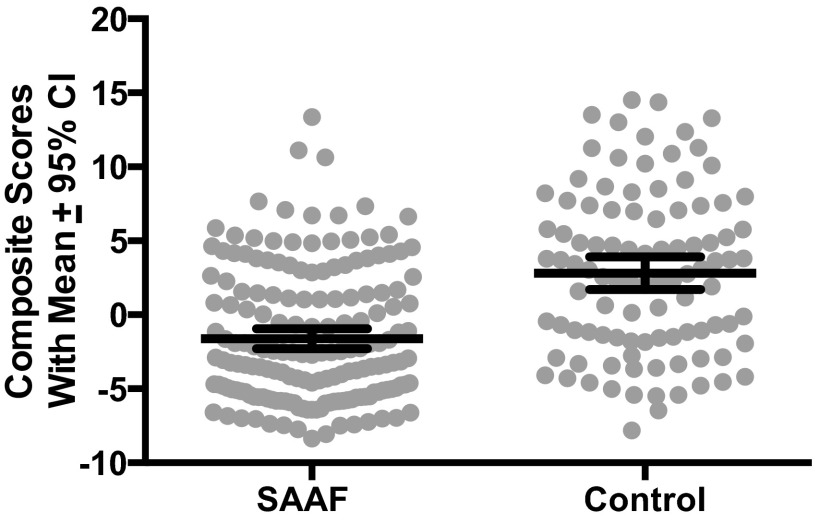
Our initial analysis was designed to determine whether SAAF affected inflammation. Adjusting for sex and family SES, subjects who participated in SAAF displayed significantly lower scores on the inflammation composite than did controls, F(1,268) = 51.63, P < 0.0001. Fig. 1 depicts this finding. To gauge the magnitude of this difference, we also computed the effect size indicator Cohen’s d. This value gives the difference between groups in pooled SD units; in the behavioral sciences, d values of 0.10, 0.30, and 0.50 (absolute values) are considered small, medium, and large-sized disparities, respectively (26). The d for this comparison was −0.90, indicating that, among SAAF subjects, inflammation composite values were almost 1 SD lower than those of controls.
Fig. 1.
Youth whose families participated in SAAF had less inflammation than did controls. The endpoint is a composite indicator of inflammation, formed by summing each subject’s z-scored values for interleukins-1β, 6, 8, and 10, plus tumor necrosis factor-α and IFN-γ. Dots represent individual data points. Within each group, the wide horizontal bar is the mean composite score, and the error bars reflect 95% confidence intervals.
Next, we disaggregated the composite to determine whether SAAF’s effects varied across cytokines. As Table 2 shows, they did not. Adjusting for sex and family SES, SAAF youth displayed significantly lower concentrations of all six cytokines measured, Fs (1,268) = 28.97–51.04, P values < 0.001, compared with control subjects. The d values for these comparisons ranged from −0.69 to −0.91, with an average of −0.79. Fig. 2 depicts these comparisons graphically.
Table 2.
SAAF effects on inflammation, separately by cytokine
| SAAF (n = 173) |
Control (n = 99) |
|||||
| M | SEM | M | SEM | F (1, 268) | Cohen’s d | |
| Composite | −1.60 | 0.37 | 2.80 | 0.489 | 51.63*** | −0.91 |
| IFN-γ | 0.64 | 0.03 | 0.99 | 0.04 | 40.40*** | −0.80 |
| IL-10 | 0.74 | 0.03 | 0.99 | 0.04 | 30.94*** | −0.70 |
| IL-1β | 0.08 | 0.06 | 1.45 | 0.07 | 51.04*** | −0.90 |
| IL-6 | 0.83 | 0.07 | 1.60 | 0.10 | 41.46*** | −0.81 |
| IL-8 | 2.76 | 0.05 | 3.24 | 0.07 | 28.97*** | −0.68 |
| TNF-α | 0.83 | 0.04 | 1.28 | 0.05 | 45.06*** | −0.85 |
F values are from between-group ANOVAs comparing SAAF and Control subjects, with Sex and Family SES as covariates. Cohen’s d is a measure of effect size, expressed in SD units. ***P < 0.001.
Fig. 2.
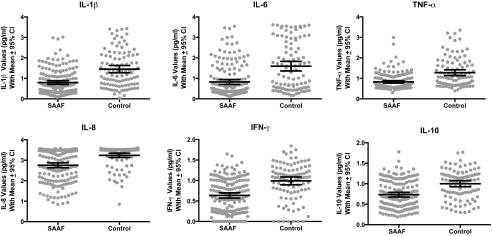
SAAF’s effects were consistent across the six cytokines measured. In each figure, dots represent individual data points, the wide horizontal bar is the group mean, and the error bars reflect 95% confidence intervals.
SAAF is a multifaceted intervention that could have resulted in lower inflammation through any of several pathways. Based on the findings described in the Introduction, we hypothesized that some of this effect was attributable to improved parenting. To test this hypothesis, we estimated a mediation model with latent difference scores (27). First, we calculated a latent difference score that reflected the degree to which parenting improved from before to after SAAF implementation. Second, we calculated regression coefficients reflecting the associations between SAAF status and improved parenting (Path A) and improved parenting and the inflammation composite (Path B). Third, the indirect or mediating effect of parenting was quantified as the product of these two regression coefficients (A × B). Nonparametric bootstrapping was used to obtain the bias-corrected and accelerated confidence intervals of the indirect effect (28). The indirect effect was calculated 5,000 times using random sampling with replacement to build a sampling distribution. Sex and family SES were statistically controlled in the models.
The results of this analysis suggested that lower inflammation among SAAF participants was partially attributable to improved parenting. Fig. 3 depicts these findings. The positive coefficient for Path A indicates that participation in SAAF was associated with statistically significant improvements in parenting from pre- to posttrial. The negative coefficient for Path B indicates that, the more a mother’s parenting improved from pre- to posttrial, the lower her child’s inflammation composite score was at age 19. Multiplying these coefficients yielded an indirect “mediated” effect of −0.19 with a bootstrapped 95% confidence interval of −0.52, −0.02. These values indicate that the indirect pathway from SAAF to improved parenting to lower inflammation was statistically significant. Nevertheless, SAAF remained associated with inflammation even after accounting for parenting, as the significant Path C′ coefficient indicates. This result suggests that SAAF reduced inflammation through a combination of improved parenting and additional pathways. Overall model fit was good (29), with χ2 (4) = 4.04, P = 0.40, comparative fit index = 1.00, and root mean square error of approximation = 0.006 (CI = 0, 0.09).
Fig. 3.
Results are consistent with the hypothesis that SAAF’s ability to reduce inflammation is partially attributable to improved parenting. The figure shows results from a mediation model with latent difference scores. Solid and dashed lines reflect significant and nonsignificant paths, respectively. Unstandardized coefficients are shown. *P < 0.05; **P < 0.01; ***P < 0.001.
To evaluate these patterns’ consistency, we reestimated mediation models separately for each cytokine. Table 3 displays the results. In each case, analyses supported a scenario wherein SAAF related to lower inflammation via improved parenting, as well as other pathways.
Table 3.
Results of mediation models with latent difference scores, separately by cytokine
| Parameter | IFN-γ | IL-10 | IL-1β | IL-6 | IL-8 | TNF-α |
| Main effect: SAAF → Cytokine | −0.35*** | −0.27*** | −0.65*** | −0.76*** | −0.48*** | −0.45*** |
| SAAF → Changes in parenting | 1.18* | 1.18* | 1.18* | 1.19* | 1.18* | 1.18* |
| Changes in parenting → Cytokine | −0.01* | −0.01** | −0.02* | −0.03** | −0.02+ | −0.02* |
| Direct effect: SAAF → Cytokine | −0.34*** | −0.25*** | −0.63*** | −0.73*** | −0.46*** | −0.43*** |
| Indirect (mediated) effect SAAF → Parenting → Cytokine | −0.01 | −0.02 | −0.02 | −0.04 | −0.02 | −0.02 |
| 95% CI for indirect (mediated) effect with 5,000 bootstraps | [−0.039, −0.001] | [−0.039, −0.002] | [−0.071, −0.002] | [−0.104, −0.005] | [−0.060, 0] | [−0.053, −0.002] |
Values are unstandardized coefficients derived from latent difference score mediation models. ***P < 0.001; *P < 0.05.
The questionnaire used in mediation analyses captures both nurturant-involved and harsh-inconsistent parenting (30). To clarify the aspects of parenting that were responsible for SAAF’s effects, we re-estimated the mediation models after separating these dimensions. However, the results suggested that uni-dimensional improvements were insufficient to modify inflammation: for nurturant-involved parenting, indirect effect = −0.11, 95% CI [−0.39, 0.01]; for harsh-inconsistent parenting, indirect effect = −0.10, 95% CI [−0.38, 0.00]. Thus, SAAF was associated with the lowest inflammation levels in youth when it both increased the frequency of nurturant-involved parenting and decreased the frequency of harsh-inconsistent parenting.
To clarify further SAAF’s actions, we considered the possibility that it reduced youth smoking or adiposity, both of which are potent inflammatory stimuli (31). In separate models, we included variables reflecting smoking (frequency of cigarette use) and adiposity (body mass index). These variables were treated as mediators that linked changes in parenting quality with the inflammation composite. However, these indirect/mediating pathways did not reach statistical significance in either circumstance: for smoking, indirect effect = 0.01, 95% CI [−0.01, 0.04]; for adiposity, indirect effect = 0.00, 95% CI [−0.01, 0.03]. We also tested models where SAAF worked through smoking and obesity to reduce inflammation, regardless of parenting changes. Again, there was no evidence to support such a scenario: for smoking, indirect effect = −0.03, 95% CI [−0.26, 0.08]; for adiposity, indirect effect = 0.03, 95% CI [−0.03, 0.33].
Last, we considered whether SAAF’s effects might vary depending on SES. Families who live in more disadvantaged circumstances face greater material, psychosocial, and medical challenges. As a result, they might derive larger benefits from the intervention. To test this hypothesis, we added a neighborhood SES × SAAF interaction term to the latent mediation model described earlier, and tested for mediated moderation (32). Overall model fit was good, with χ2 (8) = 6.99, P = 0.54, comparative fit index = 1.00, and root mean square error of approximation = 0.00 (CI = 0, 0.07). The indirect pathway linking the interaction term, changes in parenting, and inflammation scores was statistically significant (coefficient = −0.046; 95% CI [−0.113, −0.009] with 5,000 bootstraps). These findings indicate that the more disadvantaged a family was, the more SAAF improved parenting quality (coefficient = 0.33, P < 0.01), and the lower youth inflammation scores were at age 19 (coefficient = −0.14, P < 0.05).
Discussion
Children from low-SES families are at heightened risk for health problems across the lifespan (1, 2, 4, 11). Although recent studies suggest that nurturant parenting might offset some of these health risks (13, 14, 18), they were not designed to permit inferences about causality or clinical utility. We conducted secondary analyses of data from a family-oriented intervention to determine whether it related to inflammation among low-SES, African American youth from the rural South. The results indicated that youth who participated in SAAF had significantly less inflammation 8 y after the intervention than did controls. These benefits were evident across six different inflammatory cytokines, and were most pronounced for the families living in the most disadvantaged circumstances. Mediation analyses were consistent with a scenario in which SAAF reduced inflammation, in part, by improving the quality of parenting these youth received.
These results may have implications for both research and practice. Conceptually, they build upon the observational studies described in the Introduction (13, 14, 18) and suggest that the buffering influences of enhanced parenting on inflammatory and cardiometabolic outcomes are likely to be causal in nature. In that regard, our results converge with the “parental effects” commonly observed in animal models, wherein maternal caregiving tendencies exert lasting influences on offspring physiology, especially in the brain, endocrine, and immune systems (33–35). Clinically, the findings suggest that SAAF, and perhaps other interventions focused on strengthening parenting and families, could play a role in forestalling or ameliorating some of the health problems for which low-SES youth are at risk. Because we did not assess actual health outcomes, further studies are needed to determine SAAF’s relevance for practice. Low-grade inflammation contributes to multiple health problems that pattern by SES during childhood and adolescence, including obesity, insulin resistance, high blood pressure, the early stages of coronary heart disease, and psychiatric conditions like depression, posttraumatic stress disorder, and substance abuse (15, 16, 36–39). A trial with endpoints like these would help clarify the clinical significance of our findings. Studies that track endpoints like cardiovascular morbidity would also be informative, as inflammation plays a key pathogenic role in atherosclerosis (15). However, the lengthy follow-up period required would make such work logistically challenging.
How might participating in SAAF have led youth to show less inflammation 8 y later? Mediation analyses suggested that the intervention’s benefits were partially attributable to improved parenting, but that additional mechanisms of action were also responsible. We considered the possibility that SAAF rendered youth less vulnerable to initiating smoking or developing obesity, both of which are potent inflammatory stimuli. Although findings were inconsistent with this scenario, it will be important in follow-up studies to evaluate more thoroughly the role of lifestyle. We did not collect pretrial measures of adiposity, and smoking rates in our subjects were negligible before and after SAAF. Therefore, changes in these candidate mediators could not be evaluated in relation to the intervention or inflammation. Also of interest would be SAAF’s effects on other lifestyle factors, like dietary composition and physical activity, as well as deep abdominal fat, which is a major reservoir of inflammatory activity (24, 37, 40). Future research should also consider the hypothesis that SAAF helped to ameliorate the impact of stressors common in this population such as deprivation, conflict, violence, and discrimination. Indeed, SAAF may have instilled a “shift and persist” style in youth. This style entails a combination of shifting (accepting life for what it is and adapting oneself to it) and persisting (enduring life with strength by holding on to meaning and optimism), which together mitigate the health impact of stressors that many low-SES youth face. Indeed, research shows that low-SES youth who display shift and persist traits have inflammation profiles and health outcomes similar to their high-SES peers (41, 42). Consistent with our findings of partial mediation, SAAF could have instilled shift and persist tendencies via its emphasis on nurturant-involved parenting and/or its efforts to directly instill competencies in youth. Finally, SAAF’s parenting components may have reduced the frequency of conflict, violence, and neglect in the home, any of which can activate stress-related autonomic and endocrine pathways in youth, with downstream consequences of inflammation (43–49). In future intervention research, it will be important to obtain deeper and broader coverage of parenting behaviors, life stress, and strategies like shift and persist, so these mediator hypotheses can be tested formally.
Several limitations of this study must be noted. First, the SAAF trial was not designed with inflammation as an endpoint. As a result, we did not collect pretrial blood samples that could be used to determine whether the intervention and control groups’ inflammation profiles changed differentially over time. At study entry, the SAAF and control groups were similar in terms of SES, parenting quality, mental health, and lifestyle. These findings are consistent with the possibility that the groups began the trial with similar inflammation profiles. Nevertheless, until prepost data are available, conclusions about SAAF’s capacity to bring about changes in inflammation must be viewed as tentative. Second, we obtained blood for cytokine assessment from only a subset of participants. Before SAAF, these families were identical to the broader trial population in terms of SES, parenting quality, mental health, and lifestyle. With that said, these families were more actively engaged in the intervention, and perhaps as a consequence showed greater improvements in parenting. As a result, the disparities in inflammation we detected here may be larger in magnitude than would have been observed in the full sample. Lastly, the study did not assess SAAF’s effects on formal health outcomes, so the clinical relevance of the inflammation findings remains uncertain. All of these limitations can be addressed in a follow-up trial that is designed and executed with an a priori focus on assessing inflammation and its downstream clinical repercussions.
Despite these limitations, the study provides initial evidence suggesting that a family-oriented intervention can reduce inflammation in youth, in part by improving the quality of the parenting they receive. To the extent that they are substantiated in future research, these results may provide a strategy for narrowing some of the racial and social disparities in health apparent in the United States (1, 50), particularly those conditions originating in childhood (51, 52). Indeed, rates of childhood poverty have risen steadily in the United States in recent years, and this trend has been especially pronounced in rural, African American communities (53). When layered on top of existing social and racial disparities, these childhood poverty trends have the potential to worsen American’s population health in the coming decades (54) and to limit the country’s ability to develop its human capital (55). Thus, interventions with the capacity to ameliorate these disparities could pay long-term social, public health, and economic dividends (2, 56). Our findings suggest that such interventions have the potential to be efficacious across the lower end of the socioeconomic spectrum, but would benefit the most disadvantaged families to the greatest degree. If so, policymakers might embrace an approach to health equity that draws on the principle of “proportionate universalism,” where interventions are administered universally, but with intensity scaled to the level of family need (57).
Methods
Design.
Details of SAAF’s original clinical trial are provided elsewhere (25). Briefly, the trial enrolled 667 African American mothers and their 11-y-old children, all of whom resided in nine rural counties in Georgia. These counties are composed of small communities in which poverty rates are among the highest in the nation. Eight years after the trial, we measured inflammation in 272 of the youth, who were age 19 at the time. These youth constitute the analytic sample here. Of the youth, 156 were female and 116 were male (57% and 43%, respectively). Their families had an average of 2.64 children, and 63.0% lived in single-mother-headed households. A total of 4.1% of study caregivers had a college or university degree; 83.7% had completed high school or earned a GED. Economically, these households are best characterized as working poor. Caregivers worked an average of 26.4 h per week, and had a median household income of $1,608 per month. Of the families, 56.6% lived below federal poverty thresholds; 90.4% were low-income by federal standards, meaning their household income was ≤250% of poverty thresholds.
Families were assigned randomly to SAAF or to a control group. SAAF consisted of seven weekly group meetings held at community facilities, which included an average of 10 families. The meetings included separate, concurrent parent and youth skill-building sessions that lasted 1 h. Parents were taught nurturant-involved parenting techniques along with high levels of monitoring and control, adaptive racial socialization strategies, methods for communicating about sex, and establishment of clear expectations about alcohol use. Children learned the importance of having and abiding by household rules, adaptive behaviors to use when encountering racism, the importance of setting goals for the future and making plans to attain them, and strategies for resisting alcohol use. Each meeting also included a 1-h joint parent–child session during which families practiced the skills they had learned. Thus, families were offered a total of 14 h of training. During the same period, control families received three leaflets via postal mail that described child development and provided tips for stress management and exercise promotion.
Measures.
Parenting quality.
Before and after the trial, African American field researchers visited the families’ homes to administer psychosocial instruments. All assessments were conducted in private, with no other family members present. As part of these assessments, parents reported the frequency of nurturant-involved and harsh-inconsistent parenting using a questionnaire developed for research with rural African American families (30). This measure includes 13 questions answered on a Likert-type scale ranging from 1 (never) to 4 (always). Nurturant-involved parenting items assess the extent to which parents are aware of their children’s activities, talk with their children about issues that bother the children, listen to their children's perspectives during arguments, and consider the children’s opinions when making decisions on family matters. The harsh-inconsistent items assess parents’ use of slapping, hitting, and shouting to discipline their children. Follow-up assessments took place a mean of 3 mo after the end of the intervention, and the interval between pretesting and posttesting averaged 8 mo. To form a parenting quality composite, we reverse coded the harsh-inconsistent items and summed them with the nurturant-involved items. The parenting quality composite was internally consistent at both pre- and posttrial administrations (Cronbach’s αs = 0.73 and 0.77; respectively).
Inflammation.
When SAAF youth turned 19, a subsample of 500 were randomly selected to participate in a follow-up project on allostatic load. A total of 489 of them agreed, and a certified phlebotomist visited their home to collect venous blood into Serum Separator Tubes (Becton-Dickinson) through antecubital venipuncture. The specimens were couriered to the laboratory and, upon receipt, centrifuged according the manufacturer’s instructions. Serum was harvested, divided into aliquots, and then frozen at −80 °C. A total of 434 of 489 youth had adequate serum for inflammation analyses. To minimize the influence of cytokine degradation, we further limited analyses to specimens that had been processed within 24 h of venipuncture (272 of 434 samples). During this processing window, leukocytes are likely to have released some cytokines spontaneously, and we cannot differentiate this fraction from levels actually in circulation.
After samples had been thawed, low-grade inflammation was measured by electrochemiluminescence on a SECTOR Imager 2400A (MesoScale Discovery). Briefly, thawed samples were assayed in duplicate using a Human Pro-Inflammatory 7-Plex Ultra-Sensitive assay (MesoScale Discovery), following instructions provided by the manufacturer. This assay is optimized for assessment of low-grade inflammation in peripheral blood samples from healthy subjects (58). Its lower limits of detection range from 0.10 pg/mL (IL-8) to 0.80 pg/mL (IL-10). Across runs, the median intraassay coefficients of variation were 4.00% (IL-1β), 4.82% (IL-6), 1.78% (IL-8), 11.45% (IL-10), 4.34% (TNF-α), and 13.31% (IFN-γ).
Lifestyle variables.
To understand how SAAF affected inflammation, we considered adiposity and smoking as potential mediators. Adiposity was operationalized at the age 19 home visits as body mass index (BMI). Subjects’ weight and height were recorded by the field researcher and used to calculate BMI (weight in kilograms divided by the square of height in meters). At the same visit, frequency of cigarette smoking was measured via youth self report, on a 7-point scale ranging from “not at all” to “about two packs a day.” Because the distributions of smoking frequency were skewed, we applied a log transformation to normalize the ratings.
Family SES.
We formed a composite indicator of family SES consisting of six dichotomous variables measured at study entry. A score of 1 was assigned to each of the following characteristics: family poverty based on federal guidelines, primary caregiver unemployment, receipt of Temporary Assistance for Needy Families, primary caregiver single parenthood, primary caregiver education level less than high school graduation, and caregiver-reported inadequacy of family income. Higher scores indicate greater socioeconomic risk.
Neighborhood SES.
We formed a composite indicator of neighborhood SES at study entry. Using subjects’ addresses in conjunction with 2000 STF3A census tract data, we recorded neighborhood poverty, unemployment rates, and the proportion of individuals with less than a high school education. Standardized values of these indicators were summed to form the composite. Higher scores reflect greater neighborhood socioeconomic risk.
Externalizing problems.
Parents completed subscales of the school-age Child Behavior Checklist, reflecting delinquent and aggressive behaviors (59). Scores were summed to develop a composite indicator of externalizing problems (32 items, Cronbach’s α = 0.91).
Supplementary Material
Acknowledgments
This research described here was supported by National Institute of Child Health and Human Development Grant R01 HD058502; National Heart, Lung, and Blood Institute Grant R01 HL108723; and National Institute on Drug Abuse Grant P30 DA027827.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406578111/-/DCSupplemental.
References
- 1.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 3.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: A systematic review. Am J Prev Med. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychol Bull. 2002;128(2):295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 5.Singh GK, Siahpush M, Kogan MD. Rising social inequalities in US childhood obesity, 2003-2007. Ann Epidemiol. 2010;20(1):40–52. doi: 10.1016/j.annepidem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(5) Suppl:757S–769S. doi: 10.1378/chest.07-1904. [DOI] [PubMed] [Google Scholar]
- 7.Goodman E, Daniels SR, Dolan LM. Socioeconomic disparities in insulin resistance: Results from the Princeton School District Study. Psychosom Med. 2007;69(1):61–67. doi: 10.1097/01.psy.0000249732.96753.8f. [DOI] [PubMed] [Google Scholar]
- 8.Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: Systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 9.Galobardes B, Davey Smith G, Jeffreys M, McCarron P. Childhood socioeconomic circumstances predict specific causes of death in adulthood: The Glasgow student cohort study. J Epidemiol Community Health. 2006;60(6):527–529. doi: 10.1136/jech.2005.044727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 11.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 12.Chen E, Miller GE. Shift and persist strategies: Why being low in socioeconomic status isn’t always bad for your health. Perspect Psychol Sci. 2012;7(2):135–158. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Dev Psychol. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 14.Miller GE, et al. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22(12):1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on Atherothrombosis Inflammation in atherosclerosis: From pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health Committee on Early Childhood, Adoption, and Dependent Care Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 20.Flores G. Committee On Pediatric Research Technical report—racial and ethnic disparities in the health and health care of children. Pediatrics. 2010;125(4):e979–e1020. doi: 10.1542/peds.2010-0188. [DOI] [PubMed] [Google Scholar]
- 21.Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med. 2009;68(5):807–813. doi: 10.1016/j.socscimed.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh GK, Kogan MD, van Dyck PC. Changes in state-specific childhood obesity and overweight prevalence in the United States from 2003 to 2007. Arch Pediatr Adolesc Med. 2010;164(7):598–607. doi: 10.1001/archpediatrics.2010.84. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutr Rev. 2007;65(12 Pt 2):S253–S259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 24.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brody GH, et al. The Strong African American Families Program: Translating research into prevention programming. Child Dev. 2004;75(3):900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Malwah, NJ: Lawrence Erlbaum; 2003. [Google Scholar]
- 27.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 29.Babyak MA, Green SB. Confirmatory factor analysis: An introduction for psychosomatic medicine researchers. Psychosom Med. 2010;72(6):587–597. doi: 10.1097/PSY.0b013e3181de3f8a. [DOI] [PubMed] [Google Scholar]
- 30.Brody GH, et al. The influence of neighborhood disadvantage, collective socialization, and parenting on African American children’s affiliation with deviant peers. Child Dev. 2001;72(4):1231–1246. doi: 10.1111/1467-8624.00344. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor MF, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. New York: The Guilford Press; 2013. [Google Scholar]
- 33.Zhang TY, et al. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol. 2006;73(1):72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Hofer MA. Early social relationships: A psychobiologist’s view. Child Dev. 1987;58(3):633–647. [PubMed] [Google Scholar]
- 35.Coe CL, Lubach GR. In: Psychoneuroimmunology. 4th Ed. Ader R, editor. London: Elsevier Academic Press; 2007. pp. 455–474. [Google Scholar]
- 36.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 37.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 38.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchinson MR, et al. Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiecolt-Glaser JK. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med. 2010;72(4):365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen E, et al. Resilience in low-socioeconomic-status children with asthma: Adaptations to stress. J Allergy Clin Immunol. 2011;128(5):970–976. doi: 10.1016/j.jaci.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen E, Lee WK, Cavey L, Ho A. Role models and the psychological characteristics that buffer low-socioeconomic-status youth from cardiovascular risk. Child Dev. 2013;84(4):1241–1252. doi: 10.1111/cdev.12037. [DOI] [PubMed] [Google Scholar]
- 43.Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Dev Psychopathol. 2011;23(3):921–938. doi: 10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- 44.Brody GH, et al. Harsh parenting and adolescent health: A longitudinal analysis with genetic moderation. Health Psychol. 2014;33(5):401–409. doi: 10.1037/a0032686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troxel WM, Matthews KA. What are the costs of marital conflict and dissolution to children’s physical health? Clin Child Fam Psychol Rev. 2004;7(1):29–57. doi: 10.1023/b:ccfp.0000020191.73542.b0. [DOI] [PubMed] [Google Scholar]
- 47.Evans GW, Exner-Cortens D, Kim P, Bartholomew D. Childhood poverty and blood pressure reactivity to and recovery from an acute stressor in late adolescence: The mediating role of family conflict. Psychosom Med. 2013;75(7):691–700. doi: 10.1097/PSY.0b013e31829f9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danese A, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16(3):244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DR. Miles to go before we sleep: Racial inequities in health. J Health Soc Behav. 2012;53(3):279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berenson GS, Srnivasan SR. Bogalusa Heart Study Group Cardiovascular risk factors in youth with implications for aging: The Bogalusa Heart Study. Neurobiol Aging. 2005;26(3):303–307. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 53.Addy S, Engelhardt W, Skinner C. Basic facts about low-income children, 2011. New York: National Center for Children in Poverty, Mailman School of Public Health, Columbia University; 2013. [Google Scholar]
- 54.National Research Council . U.S. Health in International Perspective: Shorter Lives, Poorer Health. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 55.Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proc Natl Acad Sci USA. 2006;103(27):10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- 57.Marmot M, Bell R. Fair society, healthy lives. Public Health. 2012;126(Suppl 1):S4–S10. doi: 10.1016/j.puhe.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Fu Q, Zhu J, Van Eyk JE. Comparison of multiplex immunoassay platforms. Clin Chem. 2010;56(2):314–318. doi: 10.1373/clinchem.2009.135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]