Significance
The telomerase enzyme is essential for maintaining the replicative capacity of highly prolific cells, such as stem cells and cancer, by synthesizing telomeric DNA onto chromosome ends. Telomerase functions as an RNA–protein complex with an integral telomerase RNA (TR) component. While the templates from all other reverse transcriptases (RTs) merely specify the sequence for nucleotide addition, we found that the human TR template is embedded with a single-residue pausing signal for regulating DNA synthesis. Mutation of this pausing signal alters the fundamental function of telomerase for synthesizing exact telomeric DNA repeats. This is the first instance, to our knowledge, of a single-residue pausing signal found in the RNA template of an RT.
Keywords: telomeres, ribonucleoprotein, polymerase
Abstract
Telomerase is a specialized reverse transcriptase (RT) containing an intrinsic telomerase RNA (TR) component. It synthesizes telomeric DNA repeats, (GGTTAG)n in humans, by reiteratively copying a precisely defined, short template sequence from the integral TR. The specific mechanism of how the telomerase active site uses this short template region accurately and efficiently during processive DNA repeat synthesis has remained elusive. Here we report that the human TR template, in addition to specifying the DNA sequence, is embedded with a single-nucleotide signal to pause DNA synthesis. After the addition of a dT residue to the DNA primer, which is specified by the 49 rA residue in the template, telomerase extends the DNA primer with three additional nucleotides and then pauses DNA synthesis. This sequence-defined pause site coincides precisely with the helix paired region 1 (P1)-defined physical template boundary and precludes the incorporation of nontelomeric nucleotides from residues outside the template region. Furthermore, this sequence-defined pausing mechanism is a key determinant, in addition to the P1-defined template boundary, for generating the characteristic 6-nt ladder banding pattern of telomeric DNA products in vitro. In the absence of the pausing signal, telomerase stalls nucleotide addition at multiple sites along the template, generating DNA products with heterogeneous terminal repeat registers. Our findings demonstrate that this unique self-regulating mechanism of the human TR template is essential for high-fidelity synthesis of DNA repeats.
The ends of human chromosomes consist of precise repetitions of a 6-nucleotide (nt) sequence synthesized by the specialized reverse transcriptase (RT), telomerase (1). The telomerase core enzyme is minimally composed of the catalytic telomerase reverse transcriptase (TERT) and the integral telomerase RNA (TR) components (2). Human TR (hTR) is a 451-nt noncoding RNA containing an exceedingly short 11-nt template, which encodes specifically for the telomeric DNA repeat GGTTAG (Fig. 1A, Left). The resulting highly repetitive tract of DNA is bound in a sequence-specific manner by the shelterin complex, which protects natural chromosome termini from end-to-end fusions and other DNA damage responses (3, 4). High-fidelity synthesis of telomeric DNA repeats by telomerase is crucial for maintaining telomere function and chromosome stability. Appending the termini of telomeres with even single-nucleotide variations in the telomeric DNA repeat sequence is sufficient for compromising the protective function of the shelterin complex, culminating in deleterious genome instability and cell death (5–8).
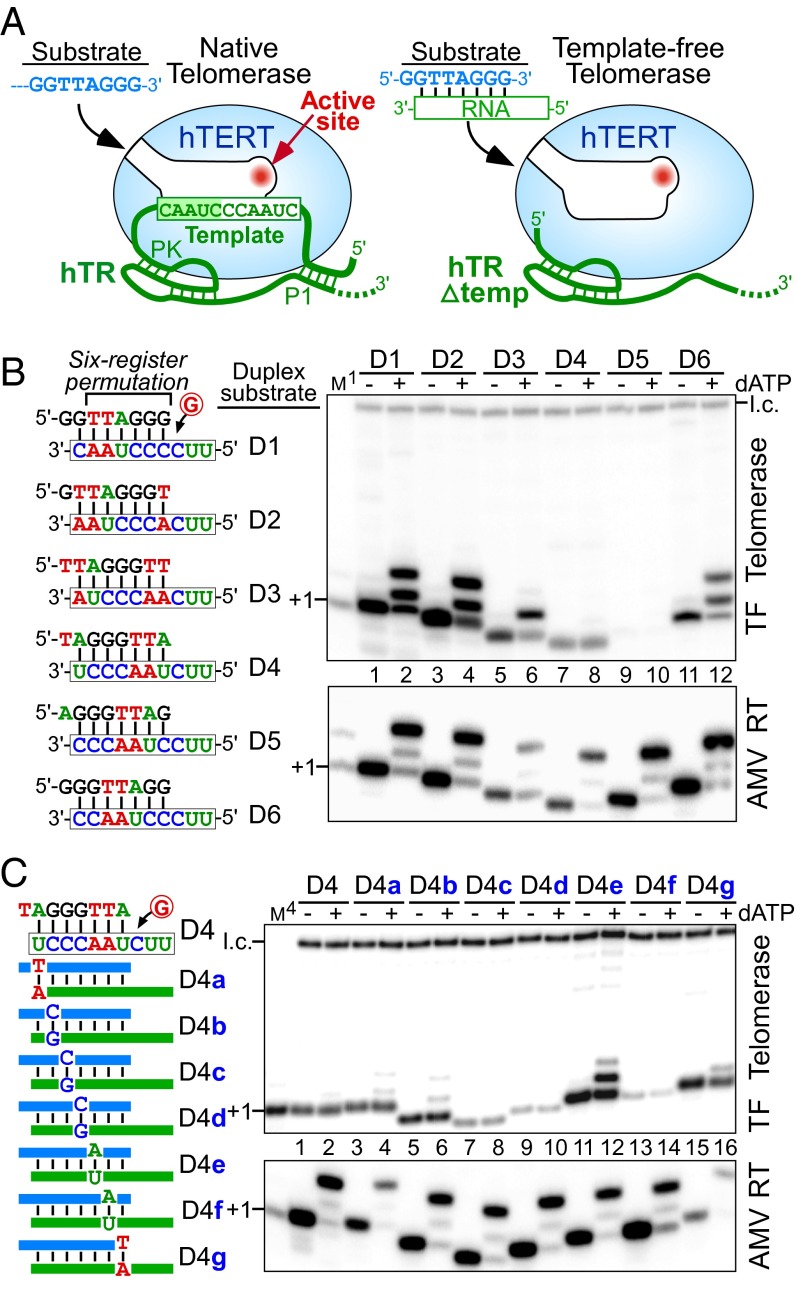
Fig. 1.
A single nucleotide in the RNA/DNA duplex signals a pause in nucleotide addition with template-free (TF) telomerase. (A) Schematic comparison of native (Left) and TF (Δtemp, Right) human telomerases. In the native telomerase, the RNA template is tethered to the 5′ P1 helix and the 3′ pseudoknot (PK) structures. TF telomerase was reconstituted in vitro with hTERT and a 5′ truncated hTR (Δtemp) that lacks the template and the P1 helix. Substrates for the activity assay are single-stranded DNA for native telomerase or a preannealed RNA/DNA duplex for TF telomerase. (B and C, Left) Sequences of permuted telomeric duplexes D1–D6 or D4 substitution variants. (B and C, Right) Activity assay of in vitro reconstituted TF telomerase (Upper) and AMV RT (Lower) with various duplex substrates. Substrates were extended by the enzyme with [α-32P]dGTP in the presence (+) or absence (−) of 0.5 mM dATP as denoted above the gel. A 32P end-labeled 18-mer oligonucleotide was included as a loading control (l.c.). The DNA primers GGTTAGGG (M1) or TAGGGTTA (M4) extended by one [α-32P]dGTP with terminal deoxynucleotidyl transferase (TdT) were included as size markers.
Whereas TR sequences are highly divergent across taxa, the TR template itself is highly conserved (9, 10). Within vertebrates, the template sequence is conserved with the 5′ boundary defined by a long-range base-paired region known as helix paired region 1 (P1), which constrains and restricts the region that functions as the template for DNA synthesis (Fig. 1A). This P1-defined template boundary relies on the physical base pairing of helix P1 as well as the length of the flanking linker to the template, functioning as a physical template boundary element (11). Extensive evidence has demonstrated the importance of the specific TR template sequence for telomerase enzymatic function, whereby alterations in the template sequence alone change the rate and processivity of telomeric DNA repeat synthesis (12–15). Additionally, telomerase exhibited differential activity toward telomeric DNA primers with permuted sequences (16).
During telomeric DNA repeat synthesis, telomerase catalyzes nucleotide addition to the DNA primer, which forms a duplex with the RNA template within the active site. Each nucleotide addition creates a discrete RNA/DNA duplex sequence inside the binding pocket of the catalytic TERT subunit. It has remained elusive how the telomerase active site handles this growing and dynamically changing duplex during processive nucleotide addition. In this study, we investigated how the active site of human telomerase utilizes its specific RNA template during nucleotide polymerization. By using a template-free telomerase system and specific assays, we discovered that the hTR template is embedded with a single-residue signal to pause nucleotide addition at an exact position, safeguarding the 5′ boundary of the template region. This sequence-defined pause signal represents a unique self-regulating mechanism of the human telomerase template for the precise synthesis of the GGTTAG repeats.
Results
The Duplex Sequence Specifies Nucleotide Addition Pausing.
To investigate how the telomerase active site interacts with different duplexes, we used a human telomerase lacking the template region from hTR (13) and examined telomerase activity with preannealed RNA/DNA duplexes as substrates (Fig. 1A, Right). This template-free (TF) telomerase was assayed with six permuted RNA/DNA duplexes, D1–D6, that represent the six distinct sequence registers formed during nucleotide addition for the synthesis of a GGTTAG repeat (Fig. 1B, Left). Interestingly, the TF telomerase exhibited distinct extension patterns and diverse activities with each permuted duplex. In the presence of [α-32P]dGTP, the DNA primers from all duplex substrates were extended by only one nucleotide (Fig. 1B, lanes 1, 3, 5, 7, and 11), aside from D5, which was nearly inactive (Fig. 1B, lane 9). With the addition of dATP to the reaction, D1, D2, and D6 were extended by three nucleotides, reaching the end of the 3-nt RNA template as expected of a conventional RT (Fig. 1B, lanes 2, 4, and 12). Unexpectedly, TF telomerase only extended D3 by two nucleotides and D4 by a single nucleotide (Fig. 1B, lanes 6 and 8). The differences in the extension pattern between these permuted duplexes suggested that the duplex sequence alone determined the pausing site during nucleotide addition. A recent study with TF telomerase reported a similar result (17). Furthermore, this sequence-defined pausing appears to be a telomerase-specific attribute, as avian myeloblastosis virus (AMV) RT extended the DNA primers to the end of each RNA template regardless of the duplex sequence (Fig. 1B, AMV RT).
A Single Base Pair in the Duplex Defines the Nucleotide Addition Pause Site.
We further examined which base pair(s) in the duplex signals for this unanticipated nucleotide addition pausing. Transversion substitutions were introduced for each base pair in the D4 duplex and each variant was assayed with TF telomerase (Fig. 1C, Left). Each of the D4 substitutions retained the pause signal with the exception of D4e, which had the first rA:dT base pair converted to rU:dA. D4e permitted the addition of a second nucleotide, shifting the pause site from +1 to +2 (Fig. 1C, lane 12). However, TF telomerase failed to extend the D4e duplex with a third nucleotide, pausing nucleotide addition before reaching the end of the RNA template. We suspected that the second rA:dT base pair, with the first rA:dT base pair mutated, gained the function as a pause signal. Indeed, transversion substitutions at both rA:dT base pairs completely abolished the sequence-defined pausing (Fig. S1A), whereas substitutions at the second rA:dT base pair alone failed to significantly alter the pause signal of the first rA:dT base pair (Fig. S1B). Thus, the first rA:dT base pair formed in the duplex induced a pause in DNA synthesis following the incorporation of three additional base pairs.
To discern the functional group(s) in the rA:dT base pair necessary and sufficient for inducing the sequence-defined pause in nucleotide addition, we designed variants of D4 with specific functional groups modified in the first rA:dT base pair (Fig. S2 A and B). The TF telomerase assays with these D4 variants revealed that the transition substitutions, rA:dU, rG:dC, and 7-deaza-rA:dT, which altered individual functional groups in the major and minor grooves of the duplex, had no significant effect on the position of the pause (Fig. S2C, lanes 3–8). However, the transversion substitution of the first rA:dT base pair in D4 to rU:dA effectively shifted the pause site (Fig. S2C, lanes 9 and 10). This result suggests that any ribonucleotide purine:deoxyribonucleotide pyrimidine base pair, rR:dY, is sufficient as signal to pause nucleotide addition. Thus, it appears that the telomerase active site does not recognize specific functional groups in the signaling rA:dT base pair. The rR:dY requirement for the signaling base pair suggests an indirect readout of the pause signal by the TERT protein.
A DNA Overhang Alleviates Nucleotide Addition Pausing.
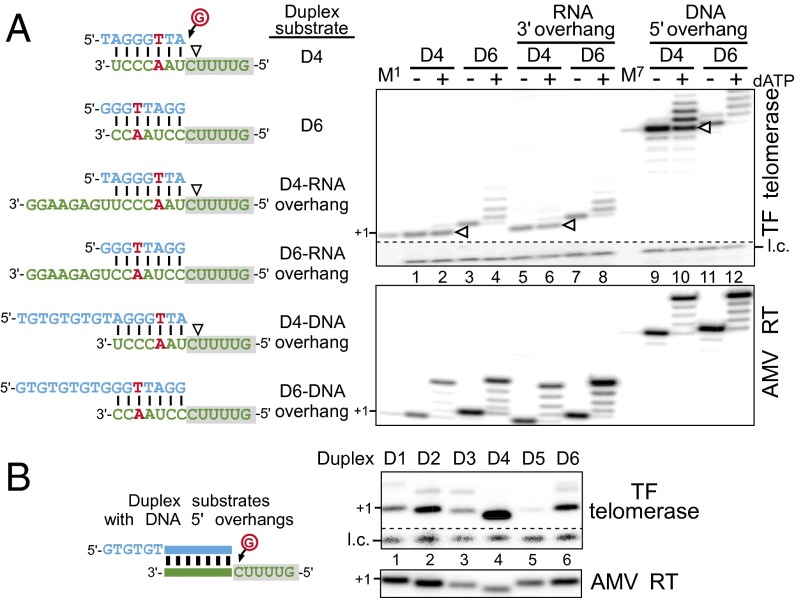
In the native telomerase, interactions between the DNA primer, TR template, and TERT anchor sites collectively contribute to nucleotide addition processivity (13, 18). Thus, we assayed the D4 and D6 duplexes appended with either DNA or RNA overhangs to better imitate the intermediate products of a native telomerase reaction (Fig. 2A, Left). Whereas D4 harbors an effective pausing signal, D6 represents a duplex following template translocation with nucleotide addition reinitiated. Our results demonstrate that a DNA 5′ overhang, and not an RNA 3′ overhang, permitted partial bypass of the sequence-defined pause site for the D4 duplex (Fig. 2A, lanes 2, 6, and 10). Additional D4 substrates with various DNA overhangs or duplex lengths also displayed a similar partial bypass of the pause signal with some variation in activity (Fig. S3). The D6 duplex, which lacks the pause signal, appeared unaffected by the presence of either an RNA 3′ or DNA 5′ overhang (Fig. 2A, lanes 4, 8, and 12). The protein–DNA interactions between the TERT anchor sites and the DNA 5′ overhang presumably facilitated duplex translocation and increased nucleotide addition processivity, thus alleviating the sequence-defined pause. We further explored the RNA 5′ flanking sequence on the sequence-defined pause with two RNA sequences, UGUU and CCAg (Fig. S4A). These two sequences represent the RNA 5′ regions flanking the duplex before or following template translocation. Regardless of the flanking sequence, the pause site was retained within each of these duplexes (Fig. S4B). Thus, TERT binding the DNA 5′ overhang, and not the single-stranded RNA template region, appears to promote nucleotide addition processivity and potentially regulates the sequence-defined pause.
Fig. 2.
Functional assays of RNA/DNA duplex overhangs for sequence-defined pausing. (A) Activity assay of duplex substrates with various overhangs. Sequences of duplexes D4 and D6 appended either RNA 3′ or DNA 5′ overhangs (Left). The sequence-defined pausing site is denoted (white triangles). Activity assay of duplex substrates with various overhangs (Right). In vitro reconstituted TF telomerase or AMV RT were assayed with RNA/DNA duplexes and [α-32P]dGTP in the presence (+) or absence (−) of 0.5 mM dATP as denoted above the gel. A 32P end-labeled 7-mer oligonucleotide is included as a loading control (l.c.). The DNA primers TAGGGTTA (M1) and (TG)4TAGGGTTA (M7) extended by one [α-32P]dGTP with TdT were included as size markers. (B) TF telomerase is inactive with the D5 duplex that contains a DNA 5′ overhang. Activity assay of in vitro reconstituted TF telomerase and AMV RT with duplex substrate variants (D1–D6) appended with DNA 5′ overhangs. Substrates were extended by the enzyme with [α-32P]dGTP. A 32P end-labeled 7-mer oligonucleotide was included as a loading control (l.c.).
In addition to the distinct extension patterns, the six permuted RNA/DNA duplexes also exhibited markedly different activities with TF telomerase (Fig. 1B). The inactivity of D5 with TF telomerase is particularly striking and possibly results from either low binding affinity to the telomerase active site or inefficient nucleotide addition catalysis onto this substrate (Fig. 1B, lanes 9 and 10). We thus examined whether increasing the TERT binding affinity to the duplex by extending the DNA 5′ overhang would increase TF telomerase activity with the D5 substrate. However, in the presence of a DNA 5′ overhang, D5 remained virtually inactive (Fig. 2B, lane 5). A competitive inhibition assay suggested that the six duplexes, D1–D6, with identical DNA 5′ overhangs, had similar apparent binding affinities to the TERT active site (Fig. S5). Thus, it seems that D5 inactivity is likely the result of inefficient catalysis, rather than low binding affinity to the active site.
Native Telomerase Exhibits Sequence-Defined Pausing.
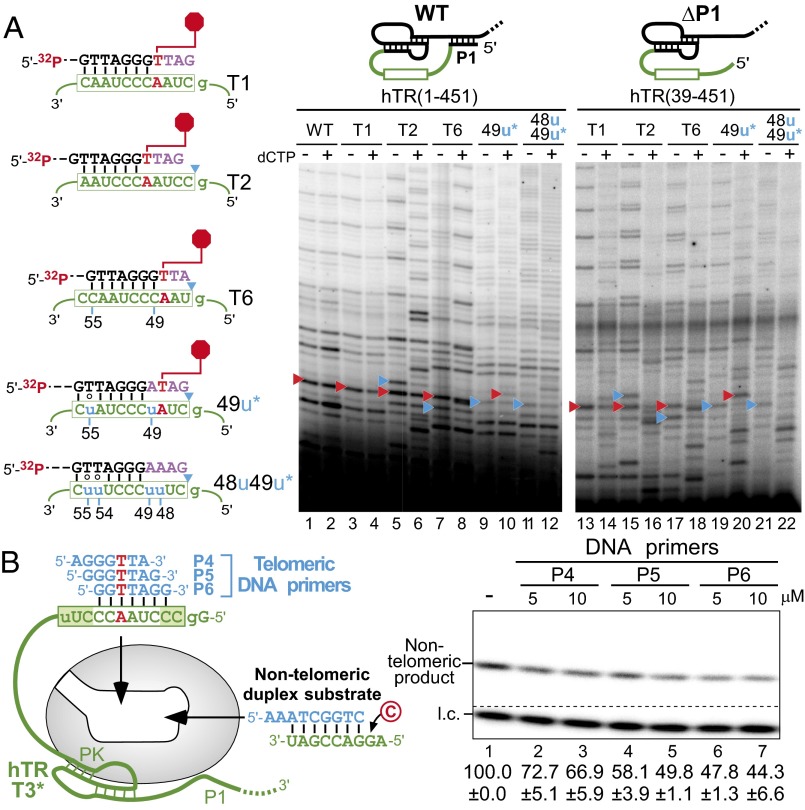
Within the full-length native hTR, the sequence-defined pause site coincides precisely with the helix P1-defined template boundary (Fig. 1A, Left). To uncouple this putative sequence-defined pause from the P1-defined template boundary in the native telomerase, we reconstituted telomerase in 293FT cells with full-length hTR that harbored permuted template sequences, T1–T6. Telomerases containing hTR T1–T6 were immunopurified from 293FT cells and assayed for activity with correspondingly permuted 7-mer DNA primers, which anneal to the same position on the template relative to the P1-defined boundary (Fig. 3A, Left). The T1–T6 templates were each flanked at 5′ and 3′ ends by guanosine residues to further define each template region and prevent incorporation of nontelomeric nucleotides from outside the template with the omission of dCTP from the reaction. In accordance with the TF telomerase results, each template-permuted telomerase demonstrated major sequence-defined pausing at a position three nucleotides following the first rA:dT base pair (Fig. 3A, white and red triangles). In T2–T6, minor bands corresponding to pausing at the physical boundary were visible (Fig. 3 and Fig. S6, blue triangles). In the presence of dCTP, minor bands corresponding to products derived from the incorporation of nontelomeric sequences from outside the template were visible (Fig. 3A, compare even and odd lanes). This result indicated that the P1-defined boundary, when uncoupled from the sequence-defined pause, is insufficient in preventing template boundary bypass. The inadequacy of the P1-defined boundary and the predominate pause at the sequence-defined pause site were consistently observed, regardless of the length or permutation of the DNA primer assayed with each TR template permutation (Fig. S6). Thus, sequence-defined pausing effectively regulates nucleotide addition in the native telomerase.
Fig. 3.
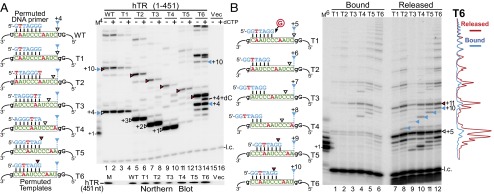
In vivo reconstituted telomerases with permutated templates exhibit sequence-defined nucleotide addition pausing. (A and B, Left) Sequences of the DNA primer and the hTR template variants for telomerase direct assay. The sequence-defined pausing sites before (white triangles) or after (red triangles) template translocation are denoted. The pausing sites at the P1-defined physical template boundary are also denoted (blue triangles). (A, Right) Activity assay for telomerase with template permutations. Telomerases reconstituted in 293FT cells with full-length hTR containing permuted template sequences were assayed with similarly permuted DNA primers in the presence (+) or absence (−) of 0.5 mM dCTP. The DNA primer AGGGTTA extended by one [α-32P]dGTP with TdT was included as a size marker (M4). The vector-only transfected cells (Vec, lanes 15 and 16) were included as a negative control. Northern blot for hTR coimmunopurified with FLAG-tagged TERT is shown under the activity gel. T7-transcribed hTR (451 nt) was included as a size marker (M). (B, Right) Analysis of bound and released DNA products. In vivo reconstituted telomerases (T1–T6) were immobilized on anti-FLAG beads and assayed with a 7-mer DNA primer. DNA products released were separated from those bound to the immobilized enzyme and analyzed by gel electrophoresis. A 6-mer 32P end-labeled oligonucleotide was added to the reaction mix before separation from the beads and a secondary 7-mer 32P end-labeled oligonucleotide was added during phenol/chloroform extraction. Intensity traces of released (red) and bound (blue) products from the T6 template mutant are shown to the right of the gel. The DNA primer GGTTAGG extended by one [α-32P]dGTP with TdT was included as a size marker (M6).
It has been presumed that telomerase generates the characteristic 6-nt ladder banding pattern of DNA products by releasing these products at the P1-defined physical boundary during processive DNA repeat synthesis. In light of the sequence-defined pause position coinciding precisely with the P1-defined physical boundary, we investigated which mechanism is principally responsible for DNA product release. Telomerases reconstituted in vivo with hTR template permuted variants, T1–T6, and FLAG-tagged hTERT were immobilized on beads and assayed with a 7-mer DNA primer, 5′-GGTTAGG-3′ (Fig. 3B, Left). Following the telomerase extension reaction, DNA products that remained bound to the immobilized telomerases were separated from the released products and analyzed. The results show that telomerase released DNA products at the sequenced-defined pause site and, to a slightly lesser level, at the P1-defined boundary (Fig. 3B, lanes 7–12). Therefore, nucleotide addition arrest induced by either mechanism was sufficient for product release. Notably, there was a higher accumulation of short DNA products in the released fraction (Fig. 3B, Right, T6 trace lines), likely resulting from insufficient interactions between the short DNA product and TERT DNA anchor sites (19, 20).
Processive Repeat Synthesis Does Not Require Sequence-Defined Pausing.
Precise product release is an important component of processive telomeric DNA repeat addition. Because sequence-defined pausing precedes product release, we explored whether this contributes to the repeat addition processivity, a unique attribute of telomerase. A telomerase template mutant 48u49u, which had the pausing signal completely eliminated, was analyzed for repeat addition processivity and DNA extension patterns (Fig. 4A, Left). When boundary bypass was prevented by the absence of dCTP, template variants T2 and T6 arrested nucleotide addition at the sequence-defined pause site (Fig. 4A, lanes 5 and 7). In contrast, the 48u49u mutant paused at multiple sites along the template, producing a more evenly distributed banding pattern (Fig. 4A, lanes 11 and 12) (12). This suggests that the sequence-defined pausing mechanism prevents premature stalling during nucleotide addition and promotes pausing specifically at the sequence-defined site. Thus, sequence-defined pausing is a key determinant for generating the characteristic 6-nt ladder banding pattern of telomerase products. Surprisingly, the 48u49u mutant retained repeat addition processivity, suggesting that the sequence-defined pause is not essential for telomerase processive repeat addition (Fig. 4A, lane 11).
Fig. 4.
Functional assays of hTR template variants for sequence-defined pausing and binding affinity of the DNA/RNA duplex to the active site. (A, Left) Sequences of the DNA primer and the hTR template variants T1, T2, T6, 49u, and 48u49u. The sequence-defined pausing site (red hexagon) and the P1-defined boundary (blue triangles) are denoted. The asterisk (*) denotes that the 49u and 48u49u mutants contain additional mutations (55u and 54u55u, respectively) to permit processive repeat addition. (A, Right) Telomerase reconstituted in vivo with hTR template variants, T1, T2, T6, 49u, and 48u49u, were assayed with the 18-mer 32P end-labeled DNA primer (TTAGGG)3 in the presence (+) or absence (−) of 0.5 mM dCTP. The sequence-defined (red triangles) and P1-defined (blue triangles) pause sites are denoted. The secondary structure of full-length hTR (1–451) with helix P1 and truncated hTR (39–451) without helix P1 are shown above the gel. (B) Competitive inhibition assay of DNA primer competitors against a nontelomeric duplex substrate. Telomerase was reconstituted in vivo with the hTR template permuted variant T3* that lacks helix P1, the linker poly(U) tract, and contains a 56u mutation. The T3* telomerase was preannealed with excess P4, P5, or P6 telomeric DNA primers at 5 and 10 µM as competitors when assayed against 10 µM nontelomeric substrate with [α-32P]dCTP. A 32P end-labeled 7-mer oligonucleotide was included as a loading control (l.c.). Quantitation of the relative activity in the presence of competitor is displayed below the gel with the SE (±) derived from two independent experiments.
The complete removal of the P1-defined boundary by the deletion of helix P1 did not impair telomerase processivity, when boundary bypass was prevented by the omission of dCTP from the reaction. In this system, the T2 and T6 template variants paused nucleotide addition at the expected positions (Fig. 4A, lanes 13, 15, 17, 19, and 21). However, in the presence of dCTP, which permitted template boundary bypass, the addition of nontelomeric nucleotides from the region outside the template boundary drastically impaired telomerase processivity (Fig. 4A, lanes 14, 16, 18, 20, and 22). Therefore, although important for defining the template boundary, tethering the template to helix P1 is dispensable for telomerase repeat addition processivity as long as the nontelomeric nucleotide incorporation is prevented.
Sequence-Defined Pausing Potentially Results from Catalytic Deficiency.
We further investigated the mechanism of sequence-defined pausing within the context of in vivo reconstituted telomerase. Telomerase may pause nucleotide addition either by (i) triggering a rapid release of the D5 duplex or (ii) preventing catalysis with this duplex substrate. To distinguish between these two possibilities, we examined whether the telomerase active site has a lower binding affinity for the D5 duplex. A competitive inhibition assay was performed to discern the extent that each DNA primer/hTR template duplex competes for the telomerase active site against a nontelomeric duplex substrate (Fig. 4B, Left). Telomerase was reconstituted in vivo with the hTR template permuted variant T3*, which lacks helix P1 and the linker poly(U) tract and contains an A56-to-U mutation to prevent DNA primer misalignment. The T3* telomerase was preannealed with three different single-stranded DNA primers, P4, P5, and P6, and then assayed for competitive inhibition against an exogenous nontelomeric duplex substrate (Fig. 4B, Left). The reaction was performed in the presence of [α-32P]dCTP to permit the selective extension of the nontelomeric duplex substrate. The three duplexes formed with the P4, P5, or P6 DNA primers had similar levels of nontelomeric substrate activity inhibition, indicating comparable binding affinities to the active site (Fig. 4B, lanes 2–7). As a control, excess single-stranded DNA primers did not exhibit significant inhibition with TF telomerase that lacks any RNA template for forming a DNA/RNA duplex (Fig. S7). Thus, the inactivity of the D5 duplex observed with TF telomerase and the lower activity of the P5 DNA primer with native telomerase are likely the result of inefficient catalysis, rather than poor binding of the duplex to the telomerase activity site.
Discussion
Telomerase synthesizes telomeric DNA repeats by iteratively copying the intended template sequence from the integral RNA component. The short template region from the vastly larger TR is precisely defined to avert incorporation of nontelomeric nucleotides from the template-flanking region. The sequence-defined pausing mechanism we have discovered in human telomerase provides insights into the template boundary definition for vertebrate telomerase. Our results show that the sequence-defined pause signal functions synergistically with the P1-defined physical boundary during the telomerase catalytic cycle. Following the processive addition of six nucleotides to the DNA primer, the first rA:dT base pair formed in the duplex signals a pause in DNA synthesis, which coincides with the P1-defined boundary (Fig. 5, step A). This pausing, although not actively promoting, permits duplex dissociation from the active site (Fig. 5, step B), which is followed by complete product release (Fig. 5, step C) or template translocation (Fig. 5, step D). Moreover, this sequence-defined mechanism stimulates high nucleotide addition processivity before reaching the pause site, with the loss of this mechanism resulting in a myriad of weaker pause sites throughout the template and products with heterogeneous terminal registers. Thus, this sequence-defined pausing is a key, yet previously overlooked, determinate for generating 6-nt DNA repeats with identical GGTTAG terminal sequences.
Fig. 5.
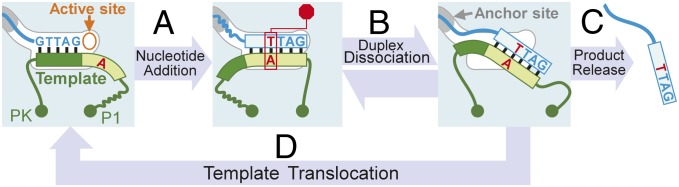
A model of the telomerase sequence-defined catalytic cycle. A duplex formed from the RNA template and DNA primer is bound to the telomerase active site. Nucleotide addition (step A) proceeds, specified by the template sequence. After addition of three nucleotides past the pause signal (red), nucleotide addition is arrested. Duplex disassociation (step B) leads to an unbound duplex with DNA 5′ overhang bound to the TERT anchor site. The strand separation of RNA/DNA duplex results in either complete DNA product release (step C) or template translocation (step D) that aligns and regenerates the duplex bound by the active site, which is ready for further nucleotide addition (return to step A).
We propose that the incorporation of the first nucleotide following template translocation onto the D5 duplex is the rate limiting step for processive telomeric DNA repeat synthesis. In a processive human telomerase reaction, RNA/DNA duplexes formed immediately before and following template translocation have the sequence register GGTTAG, which is identical to the D5 duplex. In the context of TF telomerase, the D5 duplex is inactive despite the presence of a DNA 5′ overhang (Fig. 2B, lane 5). Additionally, the telomerase active site itself is impartial to the duplex formed before and posttemplate translocation, evidenced by the sequence of the single-stranded RNA 5′ flanking the D5 duplex failing to alter sequence-defined pausing with TF telomerase (Fig. S4). However, the processive addition of DNA repeats by native telomerase requires extension of the D5 duplex formed posttemplate translocation. Thus, inefficient nucleotide addition onto the D5 duplex posttemplate translocation provides an explanation for the low repeat addition rate and processivity of human telomerase core enzyme.
We cannot exclude the possibility that template-containing telomerase employs additional mechanisms to facilitate extending the unfavorable D5 duplex posttemplate translocation. Telomerase reconstituted with full-length template-permuted hTR T5 retained a low level of activity with the DNA primer GGGTTAG, which forms a D5-like duplex with the hTR template, whereas TF telomerase is inactive with the D5 duplex (Fig. 3A, lanes 11–12 and Fig. S6B). The principle difference between TF and template-containing telomerases is the tethering of the RNA template to the core enzyme. This tethering potentially promotes nucleotide addition to the D5 duplex posttemplate translocation (Fig. 1A, Left). It has been previously proposed for Tetrahymena telomerase that changes in the tension of the template-flanking RNA regions during nucleotide addition facilitates proper template translocation (18). This would seemingly explain the differences between the TF and the template-containing telomerases for extending the D5 duplex. However, a human telomerase lacking the P1 helix, removing the potential tension on the 5′ end of the template, retains processive repeat addition as long as template boundary bypass is precluded (Fig. 4A, lane 13). The 3′ template-flanking RNA linker constrained by the pseudoknot may facilitate nucleotide addition to the D5 duplex. Future studies are necessary to explore the precise mechanism by which native telomerase extends the D5 duplex posttemplate translocation to permit processive telomeric DNA repeat addition.
Our comparative study of purple sea urchin, Neurospora, and Tetrahymena telomerases showed that sequence-defined pausing is unique to vertebrates and purple sea urchin, and not found in either ciliate or fungal telomerases (Fig. S8) (21). Interestingly, purple sea urchin telomerase appears to have a weaker sequence-defined pause than that found in human, suggesting the sequence-defined pausing has recently evolved along the invertebrate/vertebrate lineage. The lack of the sequence-defined pausing mechanism in fungal and ciliate telomerases is presumably offset by their distinct and more robust physical template boundaries. Ciliate and fungal TR templates have boundaries rigidly defined by protein binding and immediately adjacent helical structures, respectively (21–25). In contrast, vertebrate and purple sea urchin TR templates are tethered by a flexible linker to the distal P1 helix (11, 26). This P1-defined physical boundary is relatively less stringent and thus necessitates an ancillary mechanism, such as sequence-defined pausing, to facilitate precise synthesis of telomeric DNA repeats. Furthermore, the sequence-defined pausing mechanism correlates well with the high degree of TR template sequence conservation in vertebrate species (9, 23, 27).
The sequence-defined pause contributes to the synthesis of the exact GGTTAG register at the chromosome termini, evident by the characteristic 6-nt ladder banding pattern of telomerase-generated DNA products in vitro. Consistent with this finding, a previous study reported that telomerase-positive cells exhibit a markedly higher frequency of the GGTTAG register at chromosome termini (28), implying that telomerase is responsible for generating the terminal GGTTAG sequence in vivo rather than the result of nuclease resectioning. Interestingly, the telomeric DNA motif bound and protected by the single-stranded telomeric DNA binding protein, POT1, ends with the same GGTTAG register (29). The conservation of the terminal GGTTAG register as the product of telomerase DNA synthesis and as the moiety bound by POT1 implies a biologically functional connection and potential coevolution between telomerase and POT1. Telomerase would synthesizes DNA with a specific terminal repeat for precise binding and protection by POT1.
The fidelity of telomeric DNA repeat synthesis by telomerase is crucial for telomere function and genome stability in germ-line and stem cells as well as in cancer. Template mutations will generate mutant telomeric DNA products that will negatively affect binding by the shelterin complex (8) and impair the sequence-defined pausing and synthesis of precise telomeric DNA products by telomerase (5, 12). The self-regulating attributes of the human telomerase RNA template ensures the synthesis of exact GGTTAG repeats with exquisitely high fidelity. This unprecedented property of human telomerase demonstrates a unique means for an RT to use its RNA template for regulating DNA repeat synthesis.
Materials and Methods
In Vitro Reconstitution of Human Telomerase.
The hTERT protein was expressed in rabbit reticulocyte lysate (RRL) from pNFLAG-hTERT plasmid DNA using the TnT T7 Quick Coupled kit (Promega). The hTR pseudoknot (residues 32–195 or 64–184) and CR4/5 (residues 239–328) fragments were in vitro transcribed, gel purified, and assembled with hTERT in RRL at a final concentration of 1.0 µM for 30 min at 30 °C.
In Vivo Reconstitution of Human Telomerase.
293FT cells were grown in DMEM (Corning) supplemented with 10% (vol/vol) FBS (Atlanta Biologicals), 1× penicillin-streptomycin-amphotericin B mix (Lonza) and 5% (vol/vol) CO2 at 37 °C to 80–90% confluency. Cells in a six-well plate were transfected with 0.4 μg of pcDNA-NFLAG-human telomerase reverse transcriptase (hTERT), 1.6 μg of pBS-U1-human telomerase RNA (hTR) (30), and 6 μL of Fugene HD transfection reagent (Promega). Cells were harvested 48 h posttransfection and homogenized in lysis buffer [10 mM Tris⋅HCl pH 7.5, 200 mM NaCl, 1 mM EGTA, 0.5% CHAPS, 1 mM MgCl2, 10% (vol/vol) glycerol, 5 mM β-mercaptoethanol, and 1× complete protease inhibitor mixture (Roche)]. Fifty microliters of cell lysate was combined with 5 μL anti-FLAG M2 magnetic beads (Sigma) prewashed with 1× TBS buffer (50 mM Tris⋅HCl pH 7.4 and 150 mM NaCl) and incubated at 4 °C with gentle rotation for 1 h. The beads were washed three times with 1× TBS buffer and once with 1× telomerase reaction buffer (50 mM Tris⋅HCl, pH 8.0, 50 mM KCl, 3 mM MgCl2, 2 mM DTT, and 1 mM spermidine).
Additional materials and methods for telomerase activity and competitive inhibition assays are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Heather Upton and Dr. Kathy Collins for their generous gifts of reagents and protocols for Tetrahymena telomerase reconstitution. We also thank Drs. Vicki Lundblad, Nancy Horton, and Jim Allen for critical reading of the manuscript. This work was supported by National Institutes of Health Grant R01GM094450 (to J.J.-L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11234.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402531111/-/DCSupplemental.
References
- 1.Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci USA. 1989;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podlevsky JD, Chen JJ-L. It all comes together at the ends: Telomerase structure, function, and biogenesis. Mutat Res. 2012;730(1–2):3–11. doi: 10.1016/j.mrfmmm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336(6081):593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 5.Stohr BA, Xu L, Blackburn EH. The terminal telomeric DNA sequence determines the mechanism of dysfunctional telomere fusion. Mol Cell. 2010;39(2):307–314. doi: 10.1016/j.molcel.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guiducci C, Cerone MA, Bacchetti S. Expression of mutant telomerase in immortal telomerase-negative human cells results in cell cycle deregulation, nuclear and chromosomal abnormalities and rapid loss of viability. Oncogene. 2001;20(6):714–725. doi: 10.1038/sj.onc.1204145. [DOI] [PubMed] [Google Scholar]
- 7.Li S, et al. Rapid inhibition of cancer cell growth induced by lentiviral delivery and expression of mutant-template telomerase RNA and anti-telomerase short-interfering RNA. Cancer Res. 2004;64(14):4833–4840. doi: 10.1158/0008-5472.CAN-04-0953. [DOI] [PubMed] [Google Scholar]
- 8.Hanish JP, Yanowitz JL, de Lange T. Stringent sequence requirements for the formation of human telomeres. Proc Natl Acad Sci USA. 1994;91(19):8861–8865. doi: 10.1073/pnas.91.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J-L, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100(5):503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 10.Xie M, et al. Structure and function of the smallest vertebrate telomerase RNA from teleost fish. J Biol Chem. 2008;283(4):2049–2059. doi: 10.1074/jbc.M708032200. [DOI] [PubMed] [Google Scholar]
- 11.Chen J-L, Greider CW. Template boundary definition in mammalian telomerase. Genes Dev. 2003;17(22):2747–2752. doi: 10.1101/gad.1140303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drosopoulos WC, Direnzo R, Prasad VR. Human telomerase RNA template sequence is a determinant of telomere repeat extension rate. J Biol Chem. 2005;280(38):32801–32810. doi: 10.1074/jbc.M506319200. [DOI] [PubMed] [Google Scholar]
- 13.Qi X, et al. RNA/DNA hybrid binding affinity determines telomerase template-translocation efficiency. EMBO J. 2012;31(1):150–161. doi: 10.1038/emboj.2011.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilley D, Lee MS, Blackburn EH. Altering specific telomerase RNA template residues affects active site function. Genes Dev. 1995;9(18):2214–2226. doi: 10.1101/gad.9.18.2214. [DOI] [PubMed] [Google Scholar]
- 15.Förstemann K, Zaug AJ, Cech TR, Lingner J. Yeast telomerase is specialized for C/A-rich RNA templates. Nucleic Acids Res. 2003;31(6):1646–1655. doi: 10.1093/nar/gkg261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maine IP, Chen SF, Windle B. Effect of dGTP concentration on human and CHO telomerase. Biochemistry. 1999;38(46):15325–15332. doi: 10.1021/bi991596+. [DOI] [PubMed] [Google Scholar]
- 17.Wu RA, Collins K. Human telomerase specialization for repeat synthesis by unique handling of primer-template duplex. EMBO J. 2014;33(8):921–935. doi: 10.1002/embj.201387205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berman AJ, Akiyama BM, Stone MD, Cech TR. The RNA accordion model for template positioning by telomerase RNA during telomeric DNA synthesis. Nat Struct Mol Biol. 2011;18(12):1371–1375. doi: 10.1038/nsmb.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt HDM, Lobb DA, Beattie TL. Characterization of physical and functional anchor site interactions in human telomerase. Mol Cell Biol. 2007;27(8):3226–3240. doi: 10.1128/MCB.02368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finger SN, Bryan TM. Multiple DNA-binding sites in Tetrahymena telomerase. Nucleic Acids Res. 2008;36(4):1260–1272. doi: 10.1093/nar/gkm866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Autexier C, Greider CW. Boundary elements of the Tetrahymena telomerase RNA template and alignment domains. Genes Dev. 1995;9(18):2227–2239. doi: 10.1101/gad.9.18.2227. [DOI] [PubMed] [Google Scholar]
- 22.Tzfati Y, Fulton TB, Roy J, Blackburn EH. Template boundary in a yeast telomerase specified by RNA structure. Science. 2000;288(5467):863–867. doi: 10.1126/science.288.5467.863. [DOI] [PubMed] [Google Scholar]
- 23.Qi X, et al. The common ancestral core of vertebrate and fungal telomerase RNAs. Nucleic Acids Res. 2013;41(1):450–462. doi: 10.1093/nar/gks980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai CK, Miller MC, Collins K. Template boundary definition in Tetrahymena telomerase. Genes Dev. 2002;16(4):415–420. doi: 10.1101/gad.962602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MC, Liu JK, Collins K. Template definition by Tetrahymena telomerase reverse transcriptase. EMBO J. 2000;19(16):4412–4422. doi: 10.1093/emboj/19.16.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, et al. Identification of purple sea urchin telomerase RNA using a next-generation sequencing based approach. RNA. 2013;19(6):852–860. doi: 10.1261/rna.039131.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic Acids Res. 2008;36(Database issue):D339–D343. doi: 10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sfeir AJ, Chai W, Shay JW, Wright WE. Telomere-end processing the terminal nucleotides of human chromosomes. Mol Cell. 2005;18(1):131–138. doi: 10.1016/j.molcel.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Lei M, Podell ER, Cech TR. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11(12):1223–1229. doi: 10.1038/nsmb867. [DOI] [PubMed] [Google Scholar]
- 30.Cristofari G, et al. Human telomerase RNA accumulation in Cajal bodies facilitates telomerase recruitment to telomeres and telomere elongation. Mol Cell. 2007;27(6):882–889. doi: 10.1016/j.molcel.2007.07.020. [DOI] [PubMed] [Google Scholar]