Abstract
Background
Bacteremia is a significant cause of morbidity and mortality in critically ill children. Our objective was to assess whether daily chlorhexidine gluconate (CHG) bathing compared with standard bathing practices would reduce bacteremia in critically ill children.
Methods
In an unmasked, cluster-randomized, two-period crossover trial (Pediatric SCRUB), 10 pediatric intensive care units (ICUs) at 5 hospitals in the United States were randomly assigned to bathe patients > 2 months of age daily with a 2% CHG-impregnated cloth or with standard bathing practices for a six-month period. Units switched to the alternative bathing method during the second six-month period. Among 6,482 eligible patient admissions, 1521 were excluded due to a length of stay less than 2 days and 14 refused to participate. The primary outcome was an episode of bacteremia. This study is registered with ClinicalTrials.gov (Identifier: NCT00549393).
Findings
4·947 patient admissions were eligible for analysis. In the intent to treat population, there was a non-statistically significant reduction in incidence of bacteremia among patients receiving daily CHG bathing (3·52 per 1,000 days, 95%CI 2·64–4·61) compared with patients receiving standard bathing practices (4·93 per 1,000 days, 95%CI 3·91–6·15) [adjusted incidence rate ratio (aIRR) 0·71, 95% CI 0·42–1·20]. In the per protocol population, the incidence of bacteremia was 36% lower among patients receiving daily CHG bathing (3·28 per 1,000 days, 95%CI 2·27–4·58)) compared with patients receiving standard bathing practices (4·93 per 1,000 days, 95%CI 3·91–6·15) [aIRR 0·64, 95% CI 0·42–0·98]. There were no serious study related adverse events, and the incidence of CHG-associated skin reactions was 1·2 per 1,000 days (95% CI 0·60–2·02).
Interpretation
Critically ill children receiving daily CHG bathing had a lower incidence of bacteremia, and the treatment was well tolerated.
Funding
Primarily by Sage Products, Inc.. Additional funding from U.S. National Institutes of Health.
Keywords: bacteremia, pediatric intensive care units, chlorhexidine, randomized controlled trial, catheter related infections
Bloodstream infections (BSI) are associated with significant morbidity, mortality and healthcare costs in adults.1 Hospitalized children often have higher BSI rates than adults.2 In critically ill children, primary BSIs have an estimated attributable cost of $39,000 per episode 3 and an associated mortality of 11–18%.4,5 Additionally, all positive blood cultures, including those due to commensal skin organism such as coagulase negative Staphylococci , lead to increased antibiotic use, laboratory charges, and hospital length of stays.6–10 While national collaboratives work to reduce central line-associated bloodstream infections (CLABSIs) and BSIs 11–13, data are needed to address the efficacy and tolerability of novel BSI prevention strategies in children. Chlorhexidine gluconate (CHG) is a topical antiseptic that inhibits organism growth and reduces skin colonization. CHG is used for infection prevention in many hospital settings.14 Because BSIs are often caused by a patient’s bacterial flora, reducing bacteria on the skin may reduce the risk of contaminating the patient’s catheter insertion site, catheter hub, or site of peripheral blood cultures. At the time this study was designed in 2006–2007, two studies in hospitalized adults suggested that daily CHG baths could decrease BSIs; one a single-center randomized study and the other a multicenter before-and-after intervention study.15–16 No data were available on whether daily CHG bathing was tolerated and effective in hospitalized children. A collaborative of Children’s Hospitals with large pediatric intensive care units (ICUs) was assembled to assess whether daily CHG bathing compared with standard bathing practices would reduce bacteremia in critically ill children. Because CHG bathing could change the local ecologic environment, unit instead of individual randomization was chosen to prevent contamination between treated and untreated patients.
Patients and Methods
Study Design
The Pediatric Scrubbing with Chlorhexidine Reduces Unwanted Bacteria (SCRUB) Trial was an investigator-initiated, unmasked, cluster-randomized, two period crossover trial in ten intensive care units (ICU) at five hospitals in the United States (The Johns Hopkins Hospital, Children’s Hospital of Philadelphia, St. Louis Children’s Hospital, Seattle Children’s Hospital, and Children’s National Medical Center) between February 2008 and September 2010 (supplemental Table 1). The ICU was the unit of randomization. Each unit used the randomized bathing procedure for all consented patients for two six-month study periods that were separated by a two-week washout period before switching to the alternative bathing procedure. The study was initially designed to have two five-month study periods, but due to fewer eligible patients than anticipated, the study periods were extended to six-months. A staggered study initiation was planned. Patient characteristics and microbiology data were entered at each site into an Access Database (Microsoft Access, 2007, Bellevue, WA) and sent to the coordinating center. Each site obtained Institutional Review Board approval. Informed consent was obtained (see supplemental Table 2 regarding how caregivers were informed at each site). This study is registered with ClinicalTrials.gov (Identifier: NCT00549393).
Randomization and masking
During study periods, each hospital had one control and one intervention unit. ICUs were randomized with stratification by hospital and ICU type (cardiac and medical/surgical) to balance the type of ICUs in the intervention group during each period. We used a random number generator (Microsoft Excel, 2007, Bellevue, WA) to select assignment. Assignment was concealed from the units until they agreed to participate. During the study period, the investigators and caregivers were not masked. After study completion when the primary outcome was assigned from submitted laboratory data, outcome assessors were masked to the allocation.
Eligibility
All ICU patients were eligible for inclusion, but enrollment targeted those with an anticipated unit stay of >2 days. Patients were not eligible to receive CHG bathing if they were <2 months of age, had an indwelling epidural or lumbar drain, had severe skin disease or burns, or had a CHG allergy. Children <2 months of age were ineligible, because the U.S. Food and Drug Administration (FDA) has not approved CHG for use in this age group.
Intervention
In the control arm patients received daily bathing with standard bathing practices (either soap and water or Comfort Bath [Sage Products, Inc., Cary, IL] based on each ICU’s routine practice). In the intervention arm, patients received daily bathing with a 2% CHG-impregnated cloth [Sage Products, Inc., Cary, IL] (supplemental Table 3). The 2% CHG-impregnated cloth is not FDA approved for daily bathing, so this study was performed under an investigator held investigational new drug (IND) license (IND 77,954). Patients in the intervention unit who were not eligible to be bathed with CHG received standard bathing practices, as did all patients during the washout period. Bedside nurses were educated on the study bathing procedures. Bathing compliance was measured at each site by medical record review, review of a study bathing log, and periodic auditing of caregivers.
Measurements
The primary outcome was bacteremia defined as any single positive blood culture, including those that grew commensal skin organisms (e.g. coagulase negative staphylococci). Any positive blood culture during the at-risk period (supplemental Table 4) qualified as a first event. At the start of the trial, a second event was defined in the same subject to occur when the culture grew the same organism and was determined to be an independent event (generally greater than 7 days between isolates) or a different organism isolated from a subsequent blood culture. As sites finalized data collection and prior to unmasking the data, it became apparent that the sites were having difficulty objectively applying the initial protocol definition of a second episode of bacteremia; therefore, our definition of a second event was redefined as a different organism cultured at least 7 days after the first event or the same organism cultured at least 14 days after the first event. Study team members, who were masked to treatment assignment, defined distinct events of bacteremia. The primary outcome measurement was the incidence of bacteremia per 1000 patient-days at risk. Bacteremia was chosen as the primary outcome given the significant morbidity of bacteremia in critically ill children, the more common occurrence of bacteremia compared with CLABSI, and the recognition that the rare occurrence of CLABSI and the available funding might not allow capture of enough events to power CLABSI as the primary outcome. Additionally, commensal skin organisms were included as these bacteria have significant impact on clinical care of children.6,8 Recognizing that antibiotic treatment of any bacteria cultured from the blood is common practice in children with catheters but not always in patients without catheters, exploratory analyses were performed after removing commensal skin organisms in all patients and in those patients without catheters.
The secondary outcome of primary CLABSI was defined using Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) surveillance criteria for CLABSI.17 Each hospital’s infection preventionists monitored bacteremia in patients with indwelling catheters, prospectively applied NHSN criteria to identify CLABSIs, and provided a list of primary CLABSIs to the study team. Infection preventionists were not masked to study assignment. Study team members used standard NHSN methodology to collect catheter days . The secondary outcome measurement was the incidence of CLABSI per 1000 catheter-days. Additional secondary outcomes, including rates of surgical site infections and incidence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, will be reported in detail in subsequent manuscripts. Adverse events were ascertained as described in supplemental Table 5. Clinical care teams determined whether rashes were related or unrelated to study treatment.
Study Interruption
On June 28th, 2008 the 2% CHG-impregnated washcloths were recalled due to product contamination with Burkholderia cepacia. All IRBs and the FDA were notified and two hospitals (two treatment and two control units) that had begun enrollment in Period 1 were placed on study hold. Following product remediation and IRB approval, the study was restarted. The four units that were placed on study hold extended their Period 1 end-date to complete 6 months. Patients enrolled in all four units at the time of the recall were administratively censored on the recall date in the final analysis.
Statistical Analysis
The sample size was estimated using baseline incidence of bacteremia and CLABSI from each site. Detailed data were not available for each ICU, to estimate the expected effect of patient exclusions (e.g. age <2 months), analytic exclusions (e.g. patients with a length of stay ≤2 days), and the variance within units. A 40% reduction in bacteremia was estimated from available data in critically ill adults.15,18 Because there were no previous data on which to base a design effect for clustering, a design effect of 1·2 was included. It was estimated that 148 bacteremias and 62 CLABSIs would be captured with 10 ICUs enrolling for 12 months and the study would have >80% power to detect an incidence rate ratio of 0.6 for bacteremia and 0.45 for CLABSI comparing treatment and control groups with a two-sided alpha of 0·05. An unplanned interim analysis was performed by The Johns Hopkins Biostatistics CenTeR at the request of the site 5 IRB prior to this site starting enrollment; no correction of the reported P value was performed for this interim test.
Two populations were constructed to assess the effect of CHG bathing. The intention-to-treat (ITT) population included all eligible patients ≥2 months of age except those who refused to participate and did not consent to have protected health information collected (Figure 1 – shaded in gray). The per protocol (PP) population included all eligible patients ≥2 months of age who received any treatment and select patients who were not given treatment due to defined exclusions (Figure 1 – shaded in yellow). Patients with defined exclusions were included in the PP population because similar patients were not identified and excluded in the control group. Our original study design presumed that post-randomization informed consent for CHG bathing would be obtained from most critically ill children. However, due to unanticipated challenges in obtaining informed consent, we reassessed and revised our statistical plan after final collection of data but before unmasking the dataset. The baseline characteristics of each unit were compared between the first and second periods to assess for distribution of potential confounders, using Wilcoxon rank-sum and Chi square tests [Table 1].19,20 Within the treatment units, children either not consented to treatment or whose guardian refused to consent had similar baseline characteristics to those that were treated [Supplemental Table 6]. Therefore, we specified that the estimated effect of daily CHG bathing would be better reflected by the PP population, which was identified as the primary population of analysis.
Figure 1.
Study profile. Ten intensive care units were randomized to either CHG bathing or standard bathing practices during the First Period and switched to the alternative bathing procedure during Period 2.
Table 1.
Period assignment of each intensive care unit (ICU) and clinical and demographic characteristics comparing each ICU during period 2 and period 1a
| Site | Period 1 assignment |
Patient admissions |
Median Age | Raceb | Any Complex Chronic Conditionc |
Central Venous Catheterd |
Median PRISM IIIe |
|---|---|---|---|---|---|---|---|
| Children’s Hospital of Philadelphia | |||||||
| Unit 1 | Treatment | 306 / 417 | 5•57 / 7•64f | 0•45 / 0•39 | 0•78 / 0•78 | 0•40 / 0•33 | 2 / 3 |
| Unit 2 | Control | 429 / 342 | 6•50 / 5•85 | 0•32 / 0•46f | 0•79 / 0•82 | 0•34 / 0•31 | 2 / 3f |
| Children’s National Medical Center | |||||||
| Unit 1 | Treatment | 388 / 325 | 4•61 / 2•89f | 0•82 / 0•91f | 0•78 / 0•64f | 0•40 / 0•37 | 3 / 2f |
| Unit 2 | Control | 68 / 120 | 0•55 / 0•73 | 0•79 / 0•71 | 1.00 / 0•97 | 0•83 / 0•67f | 6 / 5 |
| Johns Hopkins Hospital | |||||||
| Unit 1 | Treatment | 264 / 206 | 4•49 / 3•84 | 0•49 / 0•47 | 0•83 / 0•88 | 0•59 / 0•63 | 8 / 8 |
| Unit 2 | Control | 208 / 206 | 4•08 / 7•10 | 0•48 / 0•53 | 0•75 / 0•78 | 0•50 / 0•50 | 5 / 5 |
| St• Louis Children’s Hospital | |||||||
| Unit 1 | Control | 310 / 413 | 6•37 / 6.99 | 0•21 / 0•32f | 0•74 / 0•69 | 0•42 / 0•43 | 4 / 3f |
| Unit 2 | Treatment | 152 / 117 | 2•59 / 2•73 | 0•22 / 0•15 | 0•92 / 0•90 | 0•82 / 0•78 | 9 / 8 |
| Seattle Children’s Hospital | |||||||
| Unit 1 | Treatment | 216 / 254 | 5•92 / 6•12 | 0•46 / 0•46 | 0•79 / 0•84 | 0•50 / 0•51 | 3 / 3 |
| Unit 2 | Control | 88 / 118 | 0•71 / 0•76 | 0•51 / 0•48 | 0•98 / 0•95 | 0•85 / 0•83 | 5 / 6 |
All results shown as period 2 / period 1. Patient admissions represent Intent to Treat population. Patient characteristics were compared for Per Protocol population using two sample proportion z-test or Wilcoxon rank-sum test.
Proportion non-Caucasian
ICD9 codes were collected for each patient’s hospitalization and categorized into underlying complex chronic medical conditions.19 For comparison, patients were grouped as having any or no complex chronic condition.
Proportion of patients with a central venous catheter during their ICU admission
Pediatric Risk of Mortality (PRISM III) Score grades severity of illness and predicts mortality in pediatric ICU patient20
P<0•05
In the primary analysis, we compared incidence rates between each unit’s intervention and control periods. Poisson regression models produced estimates of adjusted incidence rate ratios (aIRR) that adjusted for unit, secular trends in infection rates over time, and patient characteristics. The period effect was not significant in any of the analyses. A robust variance estimator was used to account for hospital-level clustering. We used multiple imputations to impute missing data that primarily occurred in PRISM scores at one site. Sensitivity analyses confirmed that excluding patients with missing data or including imputed results did not impact out estimate of the treatment effect. Similar models were used to evaluate both the primary and secondary outcomes. A sub-group analysis was performed to assess rates of bacteremia in patients with central venous catheters (CVCs). An exploratory analysis was performed to compare crude ICU-mortality in treatment and control groups. All tests were 2-sided with a type-1 error rate set at 0·05. Data were managed and analyzed using R version 2·12 21 and Stata, version 11·0 (Stata Corp., College Station, TX).
Role of the Funding Source
The study was designed, conducted, and analyzed by the authors. The commercial sponsor had no role in the design of the study, collection or analysis of the data, manuscript preparation or decision regarding publication. The sponsor was permitted to review the manuscript, but the authors had full access to all the data in the study and the final responsibility for the decision to submit for publication.
RESULTS
Study Population
Of the 6,945 patient admissions screened, 4,947 admissions (71%) were eligible and had outcome data collected - 2,525 in control and 2,422 in treatment units (Figure 1). Of the 2,422 admissions to treatment units, 354 guardians refused consent to treatment, 521 guardians were not available to consent to treatment, and 32 patients were excluded, leaving 1,515 on treatment and 1,547 included in the PP population. Overall, key characteristics were balanced between periods (Table 1). The median time at-risk in the treatment and control groups was 3 days (range 1–119 days and 1–183 days, respectively). There were 161 deaths (crude mortality 3·25%, 95% CI 2·79–3·28).
Primary Outcome (Bacteremia)
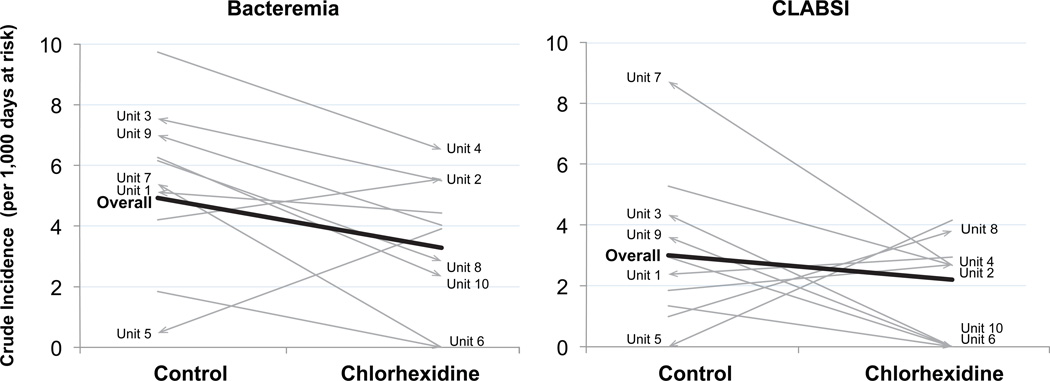
In the PP group, 113 episodes of bacteremia were identified; 34 in intervention units and 79 in control units. The crude overall incidence of bacteremia was 3·28 (range 0–6·53, 95%CI 2·27–4·58) in intervention units and 4·93 (range 0·47–9·74, 95%CI 3·91–6·15) in control units (supplemental Table 7). Eight of 10 units had a lower crude incidence of bacteremia during the treatment period compared with the control period regardless of whether assigned to CHG bathing in period 1 or period 2 and regardless of whether baseline rates were above or below the median baseline incidence (Figure 2). A child bathed with CHG had a 36% lower risk of bacteremia than a child bathed with standard practices (aIRR 0·64, 95% CI 0·42–0·98) (Table 2). The majority of bacteremias (101 of 113, 89%) occurred in children with CVCs. Children with CVCs that were bathed with CHG had a 34% lower risk of bacteremia (aIRR 0·66, 95% CI 0·47–0·94). After removing commensal skin organisms from the outcome in those without catheters, exploratory analyses found that a child bathed with CHG had a lower risk of bacteremia than a child bathed with standard practices (aIRR 0·65, 95% CI 0·44–·95) Similarly, after removing commensal skin organisms from the outcome of all patients, a child bathed with CHG had a lower risk of bacteremia than a child bathed with standard practices (aIRR 0·68, 95% CI 0·39–1·22). Therefore, all treatment subgroups had reductions in risk of bacteremia similar to the primary PP treatment group. In the 103 patient admissions during which a bacteremia occurred there were 17 deaths (crude ICU mortality in patients with bacteremia was 16.5%, 95%CI 10·56–24·85). Overall, the crude ICU mortality was 3.49% (95%CI 2·82–4·30) in patients not bathed with chlorhexidine and 2•59% (95%CI 2•82–4•30) in those bathed with chlorhexidine (ICU mortality absolute difference 0•90%, 95% CI −0•17 – 1•97, p=0•1104).
Figure 2.
Change in crude incidence of bacteremia and CLABSI for per protocol population. Each line represents one unit and the slope of the line represents the change in incidence of bacteremia or CLABSI observed between control and treatment periods. Arrows represent the assignment change from period 1 to period 2 (e.g. an arrow pointing to CHG side of the graph implies that the unit in question was assigned to the CHG arm in Period 2 and the control arm in Period 1 – the assignment started as control and moved to CHG). The thick line represents the overall crude incidence rates comparing the control and CHG units. Units are identified for easy reference to supplemental table 7.
Table 2.
Rates of bacteremia and central-line associated bloodstream infections (CLABSI) comparing treatment and control groups
| Per Protocol | Events in Control Units |
Events in Treatment Units |
Crude Control IR per 1000 at- risk days (95%CI) |
Crude Treatment IR per 1000 at- risk days (95%CI) |
Crude Absolute difference per 1000 at-risk days (95%CI) |
Crude IRR (95% CI) |
Adjusted IRR (95% CI)a |
p- value |
|---|---|---|---|---|---|---|---|---|
| 10 Outcome (Bacteremia) | 79 | 34 | 4.93 (3.91 to 6.15) |
3.28 (2.27 to 4.58) |
−1•66 (−3•21 to −0•11) |
0•66 (0•43 to 1•00) |
0•64 (0•42 to 0•98) |
0•044 |
| Bacteremia in patients with CVCs | 70 | 31 | 6•31 (4•92 to 7•97) |
4•37 (2•97 to 6•21) |
−1•94 (−4•07 to 0•19) |
0•69 (0•44 to 1•07) |
0•66 (0•47−0•94) |
0•021 |
| 20 Outcome (CLABSI) | 28 | 13 | 3•00 (2•00 to 4•33) |
2•20 (1•17 to 3•76) |
−0•80 (−2•43 to 0•83) |
0•73 (0•35 to 1•46) |
0•68 (0•35 to 1•31) |
0•249 |
| Intention to Treat | ||||||||
| 10 Outcome (Bacteremia) | 79 | 53 | 4•93 (3•91 to 6•15) |
3•52 (2•64 to 4•61) |
−1•41 (−2•86 to 0•03) |
0•71 (0•49 to 1•02) |
0•71 (0•42 to 1•20) |
0•199 |
| Bacteremia in patients with CVCs | 70 | 43 | 6•31 (4•92 to 7•97) |
4•36 (3•16 to 5•88) |
−1•95 (−3•91 to 0•03) |
0•69 (0•46 to 1•03) |
0•65 (0•44 to 0•97) |
0•034 |
| 20 Outcome (CLABSI) | 28 | 13 | 3•00 (2•00 to 4•33) |
1•63 (0•87 to 2•79) |
−1•37 (−2•79 to 0•05) |
0•54 (0•26 to 1•08) |
0•52 (0•25 to 1•08) |
0•081 |
IR – Incidence rate; IRR – Incidence rate ratio
CVC – central venous catheter
adjusted for presence of a central venous catheter, PRISM III score, presence of any complex chronic condition, age, period, unit, and hospital-level clustering
In the ITT group, 132 episodes of bacteremia were identified; 53 in intervention units and 79 in control units. In this group, a child admitted to the CHG bathed unit had a non-statistically significant reduction in the risk of bacteremia when compared with a child admitted to be the standard bathing practice unit (aIRR 0·71, 95% CI 0·42–1·20). A child with CVC admitted to the treatment unit had a 35% reduced risk of bacteremia when compared with a child admitted to be the standard bathing practice unit (aIRR 0·65, 95% CI 0·44–0·97). The crude ICU mortality was 3•49% (95%CI 2•82–4•30) in patients assigned to control units and 3•01% (95%CI 2•40–3•77) in those assigned to treatment units (ICU mortality absolute difference 0•48%, 95% CI −0•51 – 1•47, p=0•3416).
Secondary Outcome (CLABSI)
Among 2,302 patient admissions in the PP group, 41 CLABSIs were identified; 13 CLABSIs in intervention units and 28 in control units. The crude overall incidence of CLABSI was 2·20 per 1,000 catheter days (range 0–4·16, 95%CI 1·17–3·76) in intervention units and 3·00 per 1,000 catheter days (range 0–5·28, 95%CI 2·00–4·34) in control units. The incidence of CLABSI was lower in a child on treatment compared with control (aIRR 0·68, 95% CI 0·35–1·31), although this did not reach statistical significance. In the 38 patient admissions during which a CLABSI occurred there were 7 deaths (crude ICU mortality in patients with CLABSI was 18·4%, 95%CI 9·22 – 33·42).
In the ITT group, 41 CLABSIs were identified; 13 in intervention units and 28 in control units. In this group, there was a non-statistically significant reduction in the risk of CLABSI in a child admitted to a treatment unit compared with a child admitted to a control unit (aIRR 0·52, 95% CI 0·25–1·08).
Microbiology of outcomes
In the PP population, 77 of 113 episodes of bacteremia (68%) were caused by Gram-positive organisms (Table 3 and supplemental Table 8), including 53 (47%) due to coagulase-negative staphylococcus and 10 (9%) due to Enterococcus species. The crude incidence of bacteremia caused by Gram-positive organisms was 46% lower in treatment compared to control groups (IR 1·93 and 3·56 per 1,000 patient days at risk, respectively; IRR 0·54, 95%CI 0·31–0·91). No differences in crude incidence rates of bacteremia caused by Gram-negative organisms or yeast were noted. There was a 47% reduction that was not statistically significant in crude incidence of CLABSIs due to Gram-positive organisms comparing treatment and control groups (IR 0·85 and 1·60 per 1,000 patient days at risk, respectively; IRR 0·53, 95%CI 0·15–1·52).
Table 3.
Microorganisms isolated from patients with bacteremia and central line-associated bloodstream infections (CLABSI) in the per protocol population
| Bacteremia | CLABSI | |||||
|---|---|---|---|---|---|---|
| Treatment | Control | p-valuea | Treatment | Control | p-valuea | |
| Microorganisms, number | 34 | 79 | 13 | 28 | ||
| Gram positive, number | 20 (59%) | 57 (72%) | 0•015 | 5 (38%) | 15 (54%) | 0•212 |
| Staphylococcus, coagulase negative | 15 | 38 | 2 | 7 | ||
| Enterococcus species | 1 | 9 | 2 | 6 | ||
| Staphylococcus aureus | 3 | 4 | 1 | 2 | ||
| Other | 1 | 6 | 0 | 0 | ||
| Gram negative, number | 10 (29%) | 15 (19%) | 0•936 | 4 (30%) | 9 (32%) | 0•578 |
| Enterobacter cloacae | 3 | 3 | 2 | 2 | ||
| Klebsiella species | 2 | 4 | 0 | 3 | ||
| Other | 5 | 8 | 2 | 4 | ||
| Yeast, number | 3 (9%) | 6 (8%) | 0•744 | 3 (23%) | 3 (11%) | 0•594 |
| Mixed Gram positive & Gram negative, number | 1 (3%) | 1 (1%) | 0•787 | 1 (8%) | 1 (4%) | 0•776 |
Based on crude incidence rate ratios
Adverse Events
There were no serious study-related adverse events. Skin reactions were reported during 69 (1·7%) patient admissions (supplemental Table 9). Skin reactions were reported in a greater proportion of admissions to intervention than to control units (43 [2·8%] vs 26 [1·0%], p<0.01); however, treating clinicians determined that only 12 of 43 skin reactions in treatment units were related to CHG bathing. Reactions included faint macular erythema (6), maculopapular erythema (5) and dermatitis (1). The crude incidence of CHG-related skin reactions was 1·12 per 1000 days exposed (95% CI 0·06–2·02).
Thirty-nine of 1547 (2·6%) patients withdrew from treatment and cited as reasons: skin irritation due to CHG (12), skin irritation due to underlying condition (e.g. graft versus host disease) or other medication reaction (10), no reason provided (8), did not like smell or feel (3), allergic reaction (2), did not tolerate bathing procedure (2), concern about chemical exposure (1), and preferred to use a lotion not compatible with CHG (1).
Discussion
The results of a national multicenter study including over 4900 ICU admissions show a 36% reduction in the incidence of bacteremia in critically ill children receiving daily CHG bathing. In addition, daily CHG bathing was well tolerated in this population and could be quickly and widely implemented to prevent bacteremia.
Large scale interventions to decrease healthcare associated infections commonly have not included children. During planning for this trial, early studies in adult ICU patients suggested that CHG bathing reduced BSIs 15,16, however, no data were available to evaluate the safety and efficacy in children. Two recent systematic reviews have summarized additional studies and found a reduction or possible reduction in BSIs with daily CHG bathing. 22,23These reviews highlighted that only one completed study was a randomized trial and none included children. Our findings support and are consistent with studies evaluating CHG bathing in critically ill adults.
In the SCRUB trial, the primary outcome of bacteremia encompassed any positive blood culture, including those growing commensal Gram-positive skin organisms. Gram-positive commensal skin organisms cause a large proportion of bacteremias in children, including 21% of CLABSIs in a recent national sampling, and they also frequently contaminate blood cultures.24–26 National guidelines have recognized that confirming bacteremia or CLABSI in children is challenging, especially because most blood cultures in pediatric patients with central venous catheters are drawn from the catheter and often only one culture is obtained; therefore, many physicians empirically treat children presuming an infection exists.27 The vast majority of bacteremias in this study occurred in patients with CVCs, a population that is usually treated for all positive blood cultures, including those due to commensal skin organisms. The incidence of bacteremias due to all Gram positive organisms was lower in children receiving CHG bathing compared to those receiving standard bathing practices. The decrease in blood cultures growing coagulase-negative staphylococci, a common commensal skin organism, was dramatic, but so was the decrease in blood cultures growing enterococci. Whether these reductions were due to Gram-positive CLABSIs, bacteremias, or contaminated blood cultures, reducing Gram positive bacteremias should have a significant impact on patient outcomes. Furthermore, our findings of a similar crude ICU mortality in patients with CLABSI (18.4%) and bacteremia (16.5%) support bacteremia as an important outcome in critically ill children.
The observed reduction in CLABSI rates in those receiving daily CHG bathing was consistent with what has previously been reported in association with daily CHG bathing.16 Although the reduction was not statistically significant in this study, all analyses consistently showed a treatment effect of lower incidence of bacteremia and CLABSI in patients receiving CHG bathing compared with control. Between the planning and execution of the study, the predicted versus observed number of CLABSI decreased by 34% whereas those of bacteremia decreased only 11%. Over the last few years, several statewide and national collaboratives have launched dramatic efforts and successfully reduced CLABSI rates, including a national pediatric ICU collaborative.11–13 The sample size estimates in this study relied on CLABSI rates that preceded these national efforts. The results of this study showed a consistent treatment effect, but the study was not sufficiently powered to detect a significant rate reduction for these infrequent events or for mortality. Larger studies would be required to confirm our observed lower CLABSI rate and crude mortality in CHG bathed patients.
Routine CHG bathing has not been studied previously in a pediatric population and the use of CHG has been more controversial among pediatricians who remember safety issues associated with hexachlorophene.28 CHG can cause severe skin irritation, delayed hypersensitivity, and anaphylaxis.29 Establishing safety and tolerability data was an important component of this trial as skin sensitivity is common in this population.30 Only 12 (0·8%) patients bathed with CHG in this study withdrew due to CHG-related skin irritation. There were no severe adverse reactions.
Though relatively underutilized, especially in pediatrics, the cluster randomized crossover design is a strength of this study. Incorporating a crossover into the design enabled us to estimate the treatment effect by comparing each unit to itself during treatment and control periods, recognizing that cardiac ICUs are more similar to themselves during two periods than cardiac ICUs are to medical ICUs during the same period. This trial included hospitals from across the U.S. serving diverse patient populations. Over 4,900 admissions were included making this one of the largest clinical trials in critically ill children. The treatment effect was compared within and across units, and there was remarkable consistency in the findings. This analytic approach enabled minimal adjustment and provided robust estimates of the reduction in bacteremia.
A few limitations should be considered. First, only 64% of patient admissions in treatment units were bathed with CHG. Analysis of patients who did and did not receive CHG bathing in treatment units revealed similar characteristics. Therefore, we believe that our challenges obtaining informed consent for a population–based intervention did not result in biased populations. Thus although the results in the ITT population were not statistically significant, the treatment effect estimate (aIRR 0.71) was similar to that of the PP population (aIRR 0.66), suggesting a clinically significant and relevant result. Second, each institutional IRB dictated how caregivers should be informed, so in some cases, different methods were performed in control and treatment units. Although this raises concern for selective enrollment bias, the crossover design and analysis controlled for potential unit-specific enrollment differences, and findings of reduced crude incidence of bacteremia in the ITT analyses were consistent across sites. Third, there was no central committee to adjudicate CLABSIs but each site’s IP was relied on to consistently and impartially apply NHSN definitions. Although definitions can be applied differently across institutions, the crossover design dictates comparison of each unit to itself, so inter-institutional variability should not have impacted our findings. Fourth, we did not capture and adjust for other possible variables associated with bacteremia, such as presence of a peripheral intravenous catheter. Finally, the included ICUs represent academically-affiliated tertiary care ICUs that serve especially sick populations. The generalizability of these findings to other settings requires further investigation.
CONCLUSION
This is an important large multicenter study that showed a simple and easily implementable intervention decreased bacteremia among critically ill children. In this setting, nosocomial bacteremia costs lives and increases healthcare resource utilization. The data in this study support CHG bathing of critically ill children. While the observed results would be further strengthened by replication in other similar studies, broad use of this intervention could reduce morbidity and costs from bacteremia in this vulnerable and understudied population.
Supplementary Material
Acknowledgements
We acknowledge members of the Pediatric S.C.R.U.B. Study Group: The Johns Hopkins Hospital (Ilana Alezra BA, Andrew Lee MHS, Claire Beers MSN RN, Judy Ascenzi MSN RN, Dr. Ivor Berkowitz MD), Children’s Hospital of Philadelphia (Priya Prasad MPH, Sarah Smathers MPH, Mark Helfaer MD, Larissa Hutchins, RN and Pattie Hubbs, RN), St. Louis Children’s Hospital (Mary Pat Darnell BA, Nikoleta Kolovos MD, Paul Checchia MD, Maria Fernandez RN, Dana Jewell RN, Kym Galbraith RN), Seattle Children’s Hospital (Amanda Adler BA, Jerry Zimmerman MD PhD, Kelly Merrill RN), and Children’s National Medical Center (Nalini Singh MD, John Berger MD, Donna Donovan RN, Dory Walczak RN,). We also acknowledge Evan Anderson MD (Northwestern University), Howard Lederman MD PhD (Johns Hopkins University), The Johns Hopkins Biostatistics CenTeR, Michael Climo MD (Medical College of Virginia), the ICU nurses who ensured successful implementation of the study at each site, and the families and patients for their participation.
Funding/Support: This study was funded primarily by grants from Sage Products, Inc. (to Drs. Elward, Song, Zerr, Coffin, and Perl). Additional funding came from NIH/NIAID 1 K23 AI081752 (to Dr. Milstone) and UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Data were presented in part at the Annual Scientific Meetings of the Society of Healthcare Epidemiology of America, Dallas, April 2011 (abstract #LB-14), and the Infectious Diseases Society of America, Boston, October 2011 (abstract #LB-36).
Author contributions: Study concept and design: Milstone, Perl, Song, Speck. Acquisition of data: Milstone, Coffin, Elward, Zerr, Song, Orscheln, Speck. Analysis and interpretation of data: Milstone, Reich, Obeng, Coffin, Elward, Zerr, Song, Perl, Orscheln, Speck. Critical revision of the manuscript for important intellectual content: Milstone, Reich, Obeng, Coffin, Elward, Zerr, Song, Perl, Orscheln, Speck. Statistical analysis: Milstone, Reich, Obeng. Study supervision: Milstone, Coffin, Elward, Zerr, Song, Perl, Orscheln, Speck. Dr. Milstone had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of Interest Statement: Drs. Milstone, Perl, Zerr, Coffin, Song, Elward received grant support from Sage Products, Inc.. Dr. Milstone has received grant support from BioMerieux Inc.. Dr. Zerr has received grant support from Vioguard, Ltd. Dr. Perl has received grant support from Merck and is on an advisory board for Pfizer and Hospira. Dr. Song has received grant support from Optimer Pharmaceuticals. No other authors reported any conflicts.
REFERENCES
- 1.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271(20):1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37(10):783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Elward AM, Hollenbeak CS, Warren DK, Fraser VJ. Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2005;115(4):868–872. doi: 10.1542/peds.2004-0256. [DOI] [PubMed] [Google Scholar]
- 4.Armenian SH, Singh J, Arrieta AC. Risk factors for mortality resulting from bloodstream infections in a pediatric intensive care unit. Pediatr Infect Dis J. 2005;24(4):309–314. doi: 10.1097/01.inf.0000157086.97503.bd. [DOI] [PubMed] [Google Scholar]
- 5.Gray J, Gossain S, Morris K. Three-year survey of bacteremia and fungemia in a pediatric intensive care unit. Pediatr Infect Dis J. 2001;20(4):416–421. doi: 10.1097/00006454-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Segal GS, Chamberlain JM. Resource utilization and contaminated blood cultures in children at risk for occult bacteremia. Arch Pediatr Adol Med. 2000;154(5):469–473. doi: 10.1001/archpedi.154.5.469. [DOI] [PubMed] [Google Scholar]
- 7.Favre B, Hugonnet S, Correa L, Sax H, Rohner P, Pittet D. Nosocomial bacteremia: clinical significance of a single blood culture positive for coagulase-negative staphylococci. Infect Control Hosp Epidemiol. 2005;26(8):697–702. doi: 10.1086/502605. [DOI] [PubMed] [Google Scholar]
- 8.van der Heijden YF, Miller G, Wright PW, Shepherd BE, Daniels TL, Talbot TR. Clinical impact of blood cultures contaminated with coagulase-negative staphylococci at an academic medical center. Infect Control Hosp Epidemiol. 2011;32(6):623–625. doi: 10.1086/660096. [DOI] [PubMed] [Google Scholar]
- 9.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265(3):365–369. [PubMed] [Google Scholar]
- 10.Waltzman ML, Harper M. Financial and clinical impact of false-positive blood culture results. Clin Infect Dis. 2001;33(3):296–299. doi: 10.1086/321881. [DOI] [PubMed] [Google Scholar]
- 11.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. New Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Griswold M, Harris JM, 2nd, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI's quality transformation efforts. Pediatrics. 2010;125(2):206–213. doi: 10.1542/peds.2009-1382. [DOI] [PubMed] [Google Scholar]
- 13.Schulman J, Stricof R, Stevens TP, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–444. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 14.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46(2):274–281. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
- 15.Climo MW, Bush A, Fraser VJ, et al. Daily Bathing with Chlorhexidine Reduces the Incidence of Methicillin Resistant Staphylococcus aureus (MRSA), Vancomycin Resistant Enterococci (VRE), and Healthcare-Associated Bloodstream Infections (HABSI): Results of a Multicenter Trial. 17th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Baltimore, MD. 2007. Apr, Abstract 297 at. [Google Scholar]
- 16.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Archives of internal medicine. 2007;167(19):2073–2079. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Bleasdale SC, Hayes RA, Trick WE, et al. Does Chlorhexidine Gluconate Bathing of Medical Intensive Care Unit Patients Prevent Blood Stream Infections?. Abstract LB2-28; Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. 2005. Sep, [Google Scholar]
- 19.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 Pt 2):205–209. [PubMed] [Google Scholar]
- 20.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Computational Graphical Stat. 1996;5:299–314. [Google Scholar]
- 22.Derde LP, Dautzenberg MJ, Bonten MJ. Chlorhexidine body washing to control antimicrobial-resistant bacteria in intensive care units: a systematic review. Intensive Care Med. 2012;38(6):931–939. doi: 10.1007/s00134-012-2542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Horo JC, Silva GL, Munoz-Price LS, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol. 2012;33(3):257–267. doi: 10.1086/664496. [DOI] [PubMed] [Google Scholar]
- 24.Advani S, Reich NG, Sengupta A, Gosey L, Milstone AM. Central line-associated bloodstream infection in hospitalized children with peripherally inserted central venous catheters: extending risk analyses outside the intensive care unit. Clin Infect Dis. 2011;52(9):1108–1115. doi: 10.1093/cid/cir145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niedner MF, Huskins WC, Colantuoni E, et al. Epidemiology of central line-associated bloodstream infections in the pediatric intensive care unit. Infect Control Hosp Epidemiol. 2011;32(12):1200–1208. doi: 10.1086/662621. [DOI] [PubMed] [Google Scholar]
- 26.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 27.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection, 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuman RM, Leech RW, Alvord EC., Jr Neurotoxicity of hexachlorophene in the human: I. A clinicopathologic study of 248 children. Pediatrics. 1974;54(6):689–695. [PubMed] [Google Scholar]
- 29.Krautheim AB, Jermann TH, Bircher AJ. Chlorhexidine anaphylaxis: case report and review of the literature. Contact Dermatitis. 2004;50(3):113–116. doi: 10.1111/j.0105-1873.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee A, Harlan R, Breaud AR, et al. Blood concentrations of chlorhexidine in hospitalized children undergoing daily chlorhexidine bathing. Infect Control Hosp Epidemiol. 2011;32(4):395–397. doi: 10.1086/659154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.