Abstract
NAD(P)H: Quinone Oxidoreductase 1 (NQO1) is a ubiquitous flavo-enzyme that catalyzes two-electron reduction of various quinones by utilizing NAD(P)H as an electron donor. Our previous study found that progesterone (PG) can protect cardiomyocytes from apoptosis induced by Doxorubicin (Dox). Microarray analyses of genes induced by PG had let to the discovery of induction of NQO1 mRNA. We report here that PG induces NQO1 protein and activity in a dose dependent manner. Whereas NQO1 is well known as a target gene of Nrf2 transcription factor due to the presence of Antioxidant Response Element (ARE) in the promoter, PG did not activate the ARE, suggesting Nrf2 independent induction of NQO1. To address the role of NQO1 induction in PG induced cytoprotection, we tested the effect of NQO1 inducer β-naphthoflavone and inhibitor dicoumarol. Induction of NQO1 by β-naphthoflavone decreased Dox induced apoptosis and potentiated the protective effect of PG as measured by caspase-3 activity. PG induced NQO1 activity was inhibited with dicoumarol, which did not affect PG induced cytoprotection. Dicoumarol treatment alone potentiated Dox induced caspase-3 activity. These data suggest that while NQO1 plays a role in PG induced cytoprotection, there are additional components contributing to PG induced cytoprotection.
Introduction
Doxorubicin (Dox), an anthracycline quinone, is used as an antineoplastic agent. While Dox has been substantially prescribed for chemotherapy against hematological cancers such as acute leukemia and lymphomas as well as a wide range of solid tumors including breast, lung and thyroid carcinoma, the use of this drug is limited due to cardiac toxicity (1–3). The clinical manifestations of acute Dox toxicity include hypotension, tachycardia, and electrocardiogram abnormalities. Chronic cardiac toxicity can develop years after Dox therapy, including dilated cardiomyopathy and heart failure. Dox induced cardiomyopathy often leads to congestive heart failure with poor prognosis. Ultrastructural analyses of biopsy samples obtained from patients with cardiomyopathy after Dox treatment revealed loss of myofibrils, dilation of sarcoplasmic reticulum, cytoplasmic vacuolization, mitochondrial swelling and increased number of lysosomes (4, 5). In human endocardial biopsies, contraction and ring formation of nuclei suggestive of apoptosis have been documented within 24 hours after Dox injection (6). When exposed to different concentrations of Dox, cardiomyocytes in culture show a significant increase in the number of apoptotic cells (7). Our recent work has shown that progesterone (PG) can protect cardiomyocytes from Dox induced apoptosis (8).
A substantial amount of evidence suggests that reactive oxygen species (ROS) are intimately involved in the pathogenesis of Dox induced cardiomyopathy (9, 10). Dox causes an increased generation of ROS (11, 12). Experimental studies have shown that oxidative stress impairs contractile function and can result in myocyte hypertrophy, apoptosis and necrosis. The heart possesses antioxidant enzymes to detoxify ROS, including superoxide dismutases, catalase, glutathione peroxidases and NADPH quinone oxidoreductase 1 (NQO1). Studies have indicated that there is a decrease in antioxidant function with menopause (13, 14), in parallel with an increased incidence of cardiovascular diseasae. While many studies have investigated the cardiovascular effects of estrogen on antioxidant enzyme function, very little is known about the cardiovascular effects of PG.
PG secreted by the corpus luteum is responsible for the development of the secretory endometrium. The abrupt drop in PG secretion at the end of the menstrual cycle is the main determinant of the onset of menstruation. The effects of PG are mediated through an intracellular progesterone receptor (PR). The highest concentration of PR appears in the uterus, however, PR has also been found in most tissues including the heart (8, 15). Our recent work has shown that PG induces the expression of about 180 genes in cardiomyocytes, among which is NQO1 (8).
NQO1, also known as DT-diaphorase, is ubiquitously expressed among various tissues (16). As a flavo-enzyme that catalyzes two-electron reduction of various quinones utilizing NAD(P)H as an electron donor, NQO1-mediated reduction of quinones into hydroquinones is an important cellular defense mechanism against oxidative stress. NQO1 activity can be inhibited by dicoumarol [3-methylene-bis (4-hydroxycoumarin)], a potent and specific inhibitor that competes with NAD(P)H for binding to NQO1. NQO1 is induced in response to a number of agents including beta-naphthoflavone (βNF). The gene contains Antioxidant Response Element (ARE) in the promoter, which is a target of Nrf2 transcription factor that can be activated by a variety of xenobiotics, from oxidants to natural products such as isothiocyanates (17, 18). There is evidence that induction of NQO1 protects cells against reactive oxygen species (19). This study tests whether NOQ1 gene induction by PG in cardiomyocytes plays a role in PG induced cytoprotection.
MATERIALS AND METHODS
Cell Culture and Treatment of Drugs
Cardiomyocytes were prepared from 1 to 2 days old neonatal Sprague-Dawley rats (Harland, Indianapolis, IN) as previously described (7). The myocytes were seeded at a density of 2×106 cells per 100 mm dish, 0.3×106 cells per well of 6-well plates or 7.5×104 cells per well of 24-well plates. Cells were cultured in DMEM with 1 mM pyruvate, 10% fetal bovine serum, 100 units/ml penicillin and 100 units/ml streptomyocin for 3–4 days before experiments. For PG treatment, cells were cultured in DMEM containing 0.5% FBS to reduce experimental variations. At 1–3 days after culture with PG, media were changed to fresh DMEM containing 0.5% FBS for Dox treatment.
RT-PCR
Total RNA extracted using Trizol was used as a template for RT-PCR. Superscript II was used for reverse transcription at 37–50°C for 1 hr. The denaturation and primer extension temperature were 94°C and 72°C. The annealing temperature varied depending on the GC content of the primers as suggested by the oligonucleotide synthesis company (Biosynthesis, Lewisville, TX). The products were detected by agarose gel electrophoresis and ethidium bromide staining.
For real-time PCR, total RNA was isolated using the RNeasy Mini kit from Qiagen (Valencia, CA) for generation of cDNA using random hexamer as a primer and Omniscript Reverse Transcription Kit (Qiagen, Carlsbad, CA). Primers and Taqman® probes for rat β-glucosidase were purchased from Assay-on-Demand (Applied Biosystems, Foster City, CA). The real-time PCR was performed using the Taqman® Universal PCR Master Mix and the ABI PRISM® 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). The sequence for forward NQO1 primer was 5'CTCGCCTCATGCGTTTTTG and reverse NQO1 primer was 5'CCCCTAATCTGACCTCGTTCAT. Real-time PCR for NQO1 was performed using SYBR green according to the standard curve method.
Western Blot
Cells in 100 mm dishes were lysed by scraping in EB buffer (1% Triton X-100, 10 mM Tris pH 7.4, 5 mM EDTA pH 8.0, 50 mM NaCl, 50 mM NaF, 2 mM Na3VO3) with freshly added protease inhibitors: 10 µg/ml aprotinin and 1 mM phenylmethanesulfonyl fluoride. Protein concentration was measured by the Bradford method (Bio-Rad, Richmond, CA). Proteins were separated by SDS polyacrylamide gel electrophoresis using a mini-Protean II electrophoresis apparatus (Bio-Rad, Richmond, CA) run at 60 volts. The separated proteins were transferred to immobilon-P membranes (Millipore, Bedford, MA) by electrophoresis. The membrane was incubated with antibodies against NQO1 (a generous gift from Dr. David Ross) and then secondary antibodies conjugated with horseradish peroxidase. The bound antibodies were detected using an enhanced chemiluminescent reaction.
NQO1 Activity Assay
The assay was performed by modified procedures of Ernster according to published protocols (20). Briefly, cells were washed with PBS and harvested in a lysis buffer (25 mM Tris pH 7.4 and 125 mM sucrose) and sonicated for 30 seconds then centrifuged at 9000×g for 20 minutes. The supernatant was transferred to a fresh tube and mixed with 0.2 v/v 0.1 M CaCl2 in 0.25 M sucrose. The mixture was centrifuged at 27,000×g for 20 minutes. The supernatant was transferred to a fresh tube. Protein concentration of the samples was determined by the Bradford method (Bio-Rad, Richmond, CA) and 5 mg of proteins for each sample was added to the cuvette containing 1 ml assay buffer (25 mM Tris pH 7.4, 6 mg/100 ml BSA, 10 µl/100 ml Tween-20, 5 µM FAD, 0.2 mM NADH). NQO1 activity was determined using 100 µM 2, 6-dichloroindophenol as the substrate for absorbance at 600 nm. NQO1 activity was calculated as the decrease in absorbance per minute per mg total protein of the sample after addition of 10−5M dicoumarol.
ARE Reporter Assay
A 40 base pairs sequence from NQO1 gene containing ARE sequence was cloned to drive the expression of Firefly luciferase. The plasmid (0.2 µg, kindly provided by Dr. Jeffery Johnson) was cotransfected with a plasmid of Renilla-luciferase under the control of Thymidine Kinase promoter (0.04 µg) using Fugene 6 transfection reagent (Roche). At 48 hrs after transfection, cells were treated with PG or H2O2 for measurements of Firefly versus Renilla luciferase using a Dual Luciferase Assay Kit (Promega) and a Luminometer (Turner Designs).
Caspase Activity Assay
After 16 hrs of Dox treatment, detached cells were collected by centrifugation and were combined with attached cells from the same well in 6-well plates for caspase assay as described (7). The cells were dissolved in 250 µl of lysis buffer (0.5% Nonidet P-40, 0.5 mM EDTA, 150 mM NaCl, and 50 mM Tris pH 7.5). An equal volume (50 µl) of cell lysates were incubated for 1 hr at 37°C with 40 µM of N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC, Alexis Biochemicals, San Diego, CA) in 100 µl reaction buffer (10 mM HEPES, pH 7.5, 0.05 M NaCl and 2.5 mM DTT). The released AMC was measured using a 96-well fluorescence plate reader (Cambridge Bioresearch Model 7620) with an excitation wavelength of 365 nm and an emission wavelength of 450 nm.
Statistics
Student's t-test was used to compare the means of treated samples to that of control samples. Groups of three or more means were compared using ANOVA with post hoc Bonferroni test.
Results
PG Induces NQO1
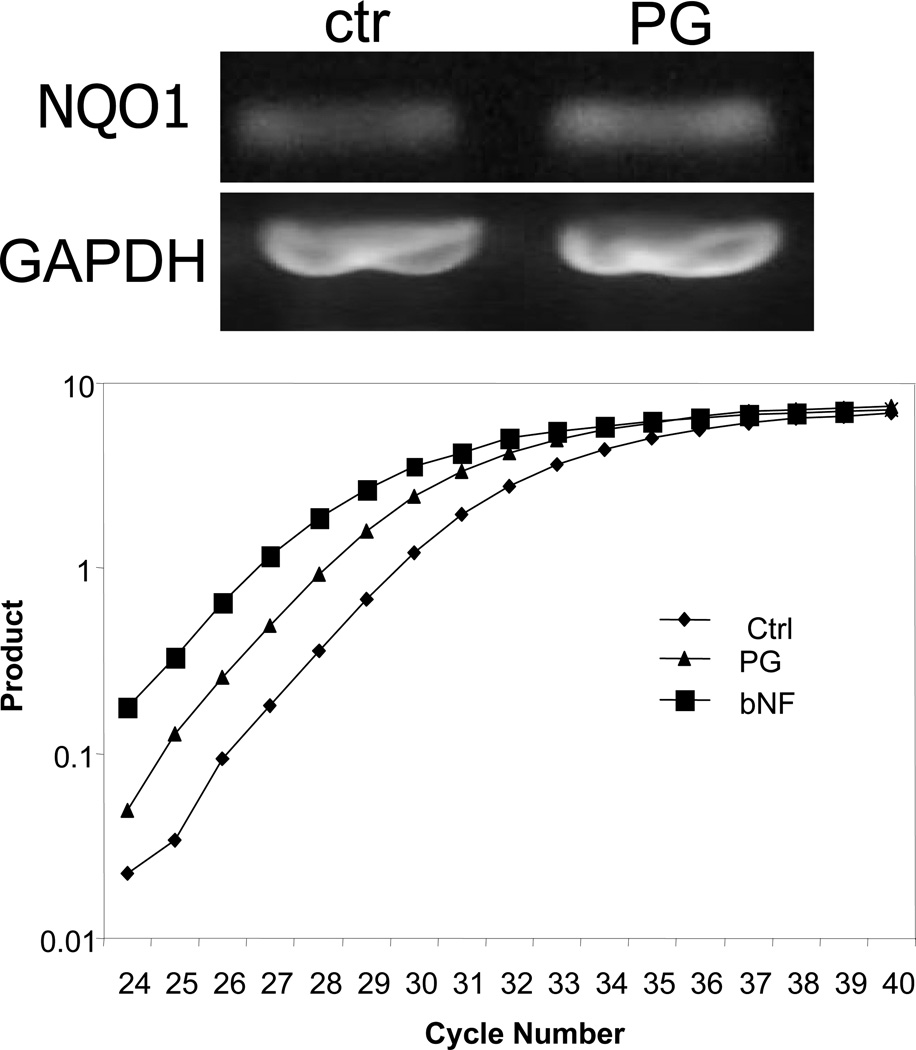
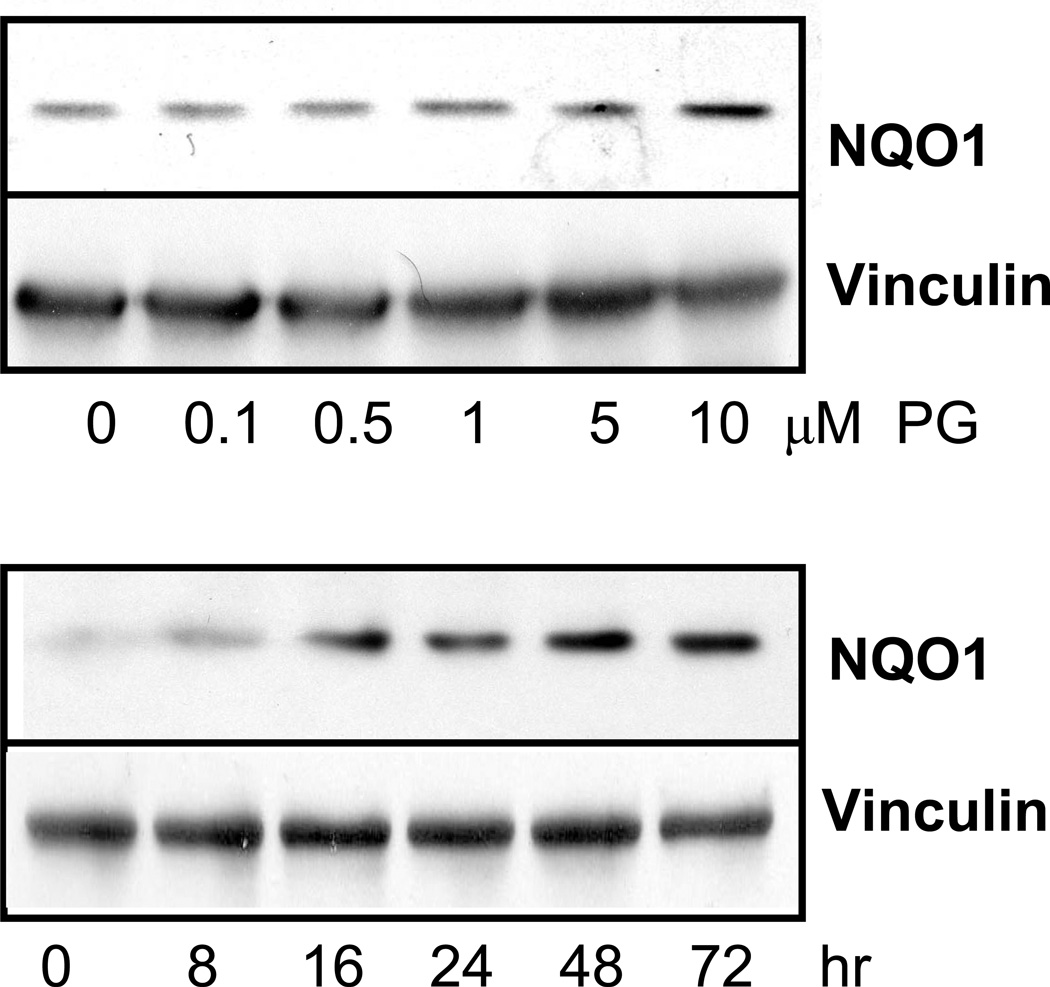
Our microarray experiments indicated that PG induces NQO1 mRNA by 2 fold in primary cultured cardiomyocytes (8). RT-PCR analyses confirmed that 24 hrs treatment of 10 µM PG induced elevation of NQO1 mRNA (Fig 1A). Using real-time PCR analyses, we found that PG induced NQO1 mRNA expression by 3.5 fold at 48 hrs (Fig 1B). beta-Naphthoflavone (βNF), a known NQO1 agonist used as a positive control, induced NQO1 mRNA by 6.1 fold at 48 hrs (Fig 1B). There was a dose dependent elevation of NQO1 protein with PG treatment that was significant at 5 to 10 µM PG (Fig 2A). The induction of NQO1 protein with 10 µM PG was detectable within 8 hrs of treatment and reached the highest at 48 hrs (Fig 2B).
Fig 1. PG Induces NQO1 mRNA.
Primary cultured cardiomyocytes in 6-well plates were treated with 10 µM PG or 1 µM βNF for 24 (A) or 48 hrs (B). RNA was extracted from the cells for RT-PCR (A) or real-time RT-PCR (B) as described in the Methods.
Fig 2. PG Dose Response and Time Course for Induction of NQO1 Protein.
Primary cultured cardiomyocytes in 6-well plates were treated with PG for 24 hrs at indicated doses (A), or 10 µM for indicate time (B). The level of NQO1 protein was measured using Western blot with vinculin as a loading control.
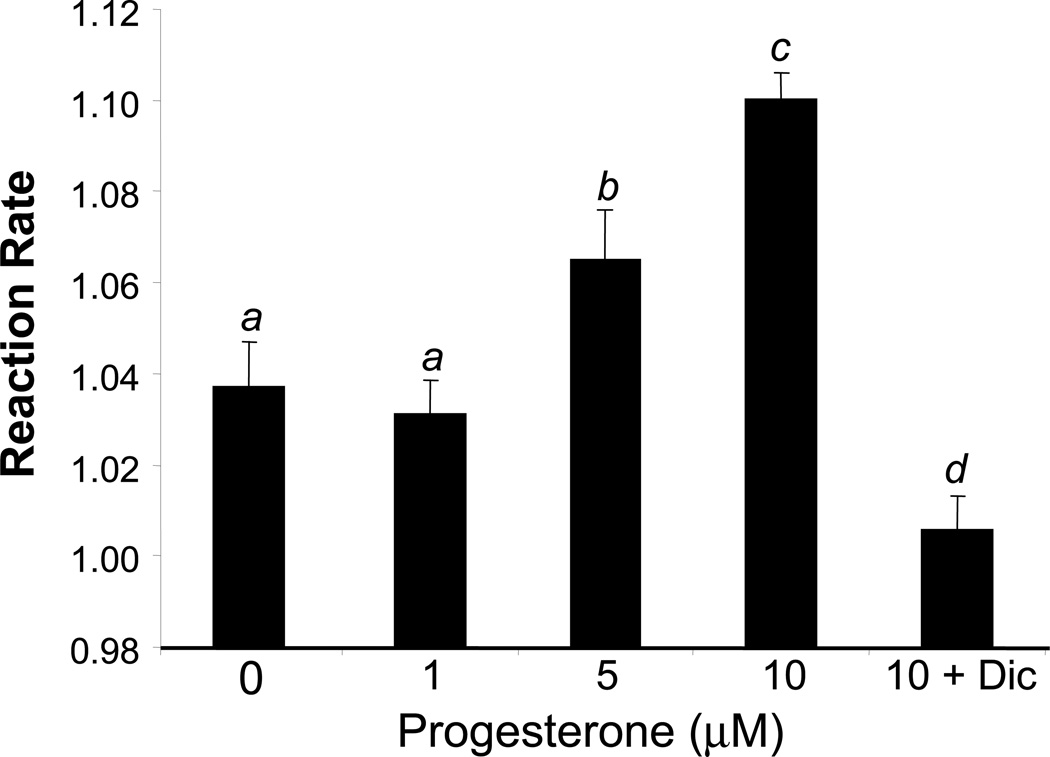
Induction of NQO1 gene expression resulted in an increase in the activity of NQO1 enzyme. With PG treatment from 0.5 to 10 µM for 24 hours, cardiomyocytes were harvested for measurements of NQO1 activity in the absence or presence of 10 µM Dicoumarol. PG induced NQO1 activity at 5 to 10 µM. Dicoumarol inhibited the activity of NQO1 induced by PG as expected (Fig 3).
Fig 3. PG Induces Elevation of NQO1 Enzyme Activity.
Primary cultured cardiomyocytes were treated with 1 to 10 µM PG without or with 10 µM dicoumerol for 24 hrs before harvesting for measurements of NQO1 enzyme activity as described in the Method. The means labeled with different letters indicate statistic significance using ANOVA with post hoc Bonferroni test. The means that are not significantly different from each other are labeled with a common letter.
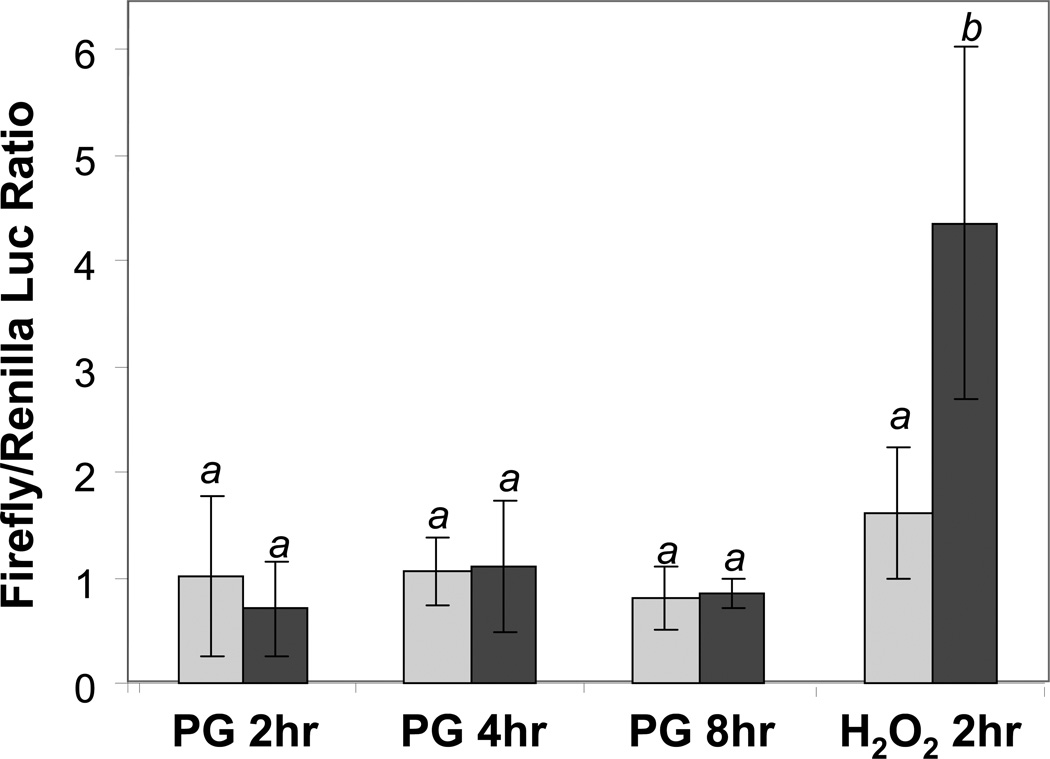
NQO1 gene contains ARE in the promoter and has often been used for measurement for activation of Nrf2 transcription factor, which binds to ARE. Using 40 base pairs NQO1 promoter sequence containing ARE, we measured ARE activation by PG. While the positive control H2O2 caused 3 fold induction of ARE activity as shown in luciferase reporter assay, PG did not activate ARE within 8 hrs (Fig 4). Measurements of ARE at later time points up to 24 hrs did not reveal activation of ARE by PG (data not shown). These data lead us to exclude activation of ARE as the mechanism of NQO1 gene induction by PG.
Fig 4. Inability of PG to Activate ARE in the Promoter of NQO1 Gene.
Primary cultured cardiomyocytes were transfected with ARE Firefly luciferase reporter along with Renilla luciferase for correction for transfection efficience. At 48 hrs after transfection, cells were treated with 10 µM PG for indicated time, or with 10 mins of 100 µM H2O2 for harvesting 2 hr after to measure for luciferase activities. The means labeled with different letters indicate statistic significance as described in Fig 3.
Induction of NQO1 Is Necessary but Not Sufficient for Cytoprotection
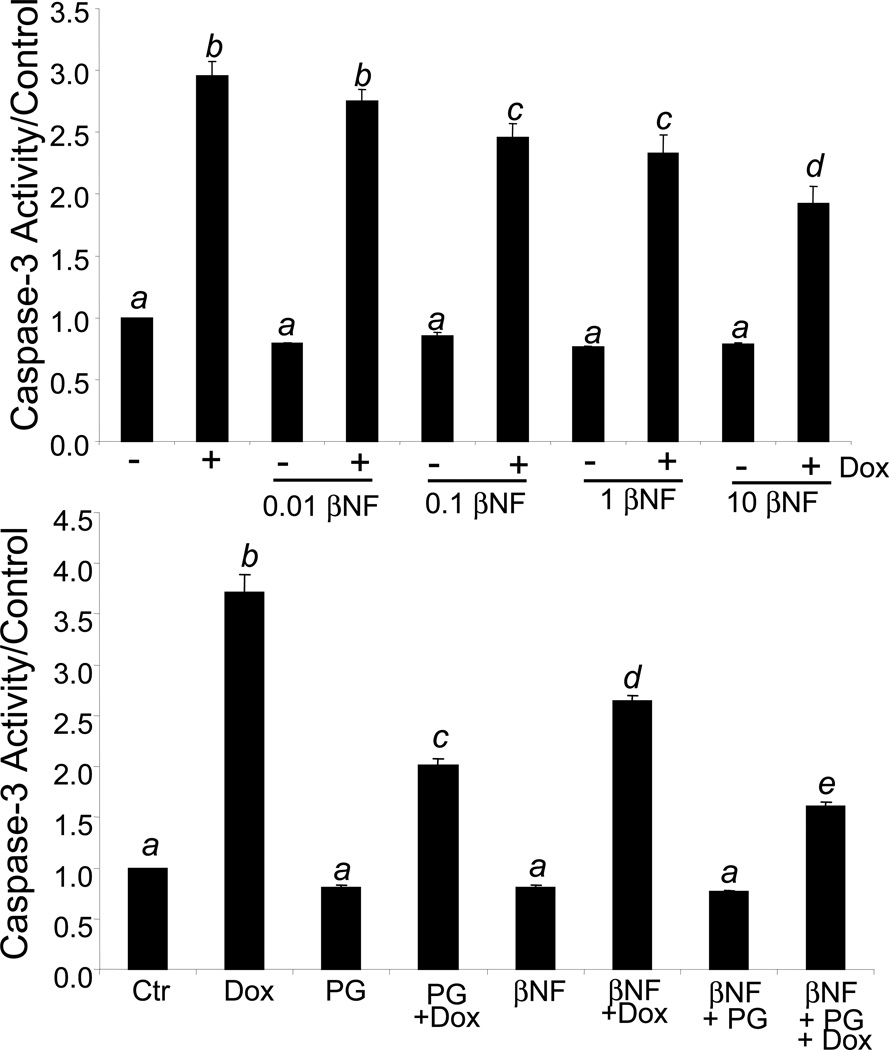
Cardiomyocytes are adherent in cell culture, but undergo cell death when exposed to Dox. We have previously shown that this cell death involves an apoptosis-like mechanism as observed with Annexin V staining, DNA ladder fragmentation, cytochrome C release, and increased caspase-3 activity (7). PG can attenuate Dox induced apoptosis (8). Caspase-3 provides a quantitative measurement of apoptosis. To test whether NQO1 mediates cytoprotection, we pretreated primary cardiomyocytes with various doses of βNF from 0.01 to 20 µM followed by Dox treatment to induce apoptosis. βNF treatment alone attenuated Dox induced Caspase-3 activity in a dose dependent fashion from 0.1 to 10 µM (Fig 5A). When cardiomyocytes were treated with PG in the presence of βNF, a slight enhancement of cytoprotection was observed (Fig 5B).
Fig 5. NQO1 Inducer βNF Decreases Dox Induced Caspase Activity.
Primary cultured cardiomyocytes were treated with βNF from 0.01 to 10 µM with or without 0.8 µM Dox for 16 hrs (A). Cells were pretreated with 10 µM PG in the presence or absence of 1 µM βNF before 16 hr treatment with 0.8 µM Dox (B). Cells were harvested for measurements of caspase activity using DEVD-AMC as a substrate as described in the Method. The means labeled with different letters indicate statistic significance as described in Fig 3.
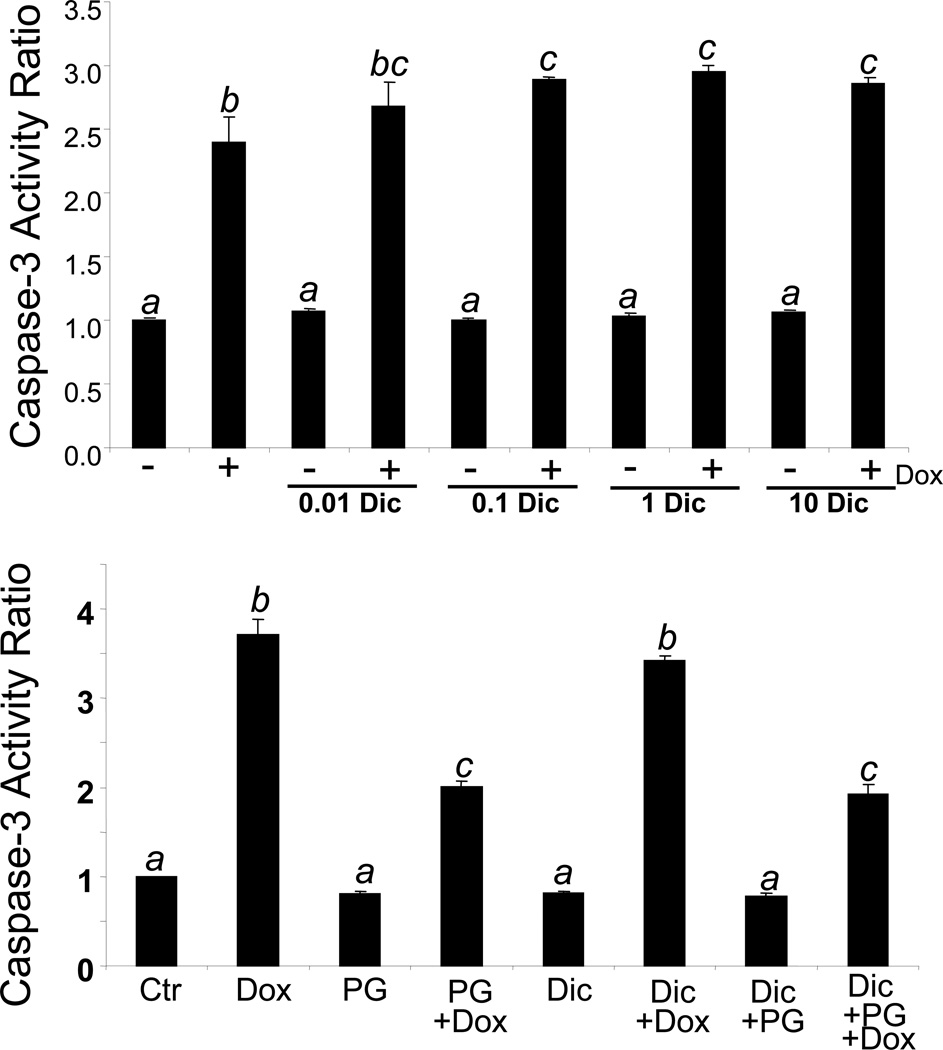
In contrast, incubation of cardiomyocytes with an NQO1 antagonist, dicoumarol, resulted in a modest increase of Dox induced caspase-3 activity (Fig 6A). To determine the importance of NQO1 induction by PG, we tested whether dicoumarol blocks PG induced cytoprotection. The results show that PG was still able to attenuate Dox induced caspase-3 activity in the presence of dicoumarol (Fig 6B). These data suggest that although inducing NQO1 enhances the cytoprotective effect of PG, inhibiting NQO1 does not block Dox induced apoptosis.
Fig 6. NQO1 Inhibitor Dicoumarol Potentiats Dox Induced Caspase.
Primary cultured cardiomyocytes were treated with dicoumarol (Dic) from 0.01 to 10 µM with or without 0.8 µM Dox for 16 hrs (A). Cells were pretreated with 10 µM PG in the presence or absence of 1 µM dicoumarol before 16 hrs treatment with 0.8 µM Dox (B). Cells were harvested for measurements of caspase activity using DEVD-AMC as a substrate as described in the Method. The means labeled with different letters indicate statistic significance as described in Fig 3.
Discussion
We have previously shown that PG can attenuate Dox induced apoptosis in cardiomyocytes (8). The present study characterizes induction of NQO1 expression by PG and address the role of NQO1 in PG induced cytoprotection. While PG causes a dose and time dependent induction of NQO1 protein and activity, PG did not activate ARE, a well known cis-element in the promoter of NQO1 gene and mediating NQO1 gene transcription by oxidants and xenobiotics. We found that increasing NQO1 activity by βNF showed some protective effect against Dox induced apoptosis, whereas inhibiting NQO1 activity by dicoumarol did not affect PG induced cytoprotection. These data indicate that induction of NQO1 is not the only mechanism underlying the cytoprotective effect of PG.
NQO1 has been previously shown to prevent Dox toxicity by reducing Dox to a more stable hydroquinone (21). The traditional view of Dox toxicity is that Dox induces a state of oxidative stress through a free radical mechanism. Dox has been shown to be able to produce superoxide anion through redox cycling (11, 12). Floreani, et al. (22) noted that induction of NQO1 by Resveratrol in isolated atria resulted in a decrease in toxicity of menadione, a quinone containing molecule similar to Dox. In our study, induction of NQO1 by βNF resulted in a dose dependent decrease in Dox induced apoptosis as measured with caspase-3 activity, supporting NQO1 as a cytoprotective gene.
In addition to detoxifying Dox, NQO1 has been found to be involved in apoptosis through an interaction with p53. Induction of p53 by DNA damaging agents mediates growth arrest or apoptosis. NQO1 physically interacts with and stabilizes p53 protein (23, 24). Inhibitors of NQO1, such as dicoumarol and agents disrupting the interaction resulted in an increase in p53 turnover and reduced p53 protein (23, 24). NQO1 knockout mice have a reduced sensitivity to chemical induced apoptosis in association with lack of p53 induction (25). In cardiomyocytes, inhibition of NQO1 with dicoumarol potentiates Dox induced apoptosis, suggesting that NQO1 may protect the cells through a p53 independent mechanism.
In menopausal or post-menopausal women, there is a decrease in the activity of various antioxidant enzymes that can be reversed with estrogen and progestin supplementation (26–28). The antioxidant potential of estrogen results in part from the hydroxyphenolic structure of its molecule (29) and also from its stimulatory effect on the activity of antioxidant enzymes (28, 30–33). PG has an anti-apoptotic effect in the ovary, uterus, breast and anterior pituitary. PR knockout animals are infertile and only PR containing cells are prevented from undergoing apoptosis by PG (34), suggesting PR plays a critical role in the antiapoptotic effect of PG.
In cardiomyocytes, induction of bcl-xL is important for PG induced cytoprotection (8). The fact that bcl-xL is a prosurvival factor explains the observation that inhibiting NQO1 did not block Dox induced apoptosis, since the effect of NQO1 relys on events upstream of bcl-xL and mitochondrial release of cytochrome c. PG treatment of bovine luteal cells was associated with an increase in the expression of anti-apoptotic protein bcl-2, a decrease in the pro-apoptotic protein bax and a decrease in caspase-3 activity that could be reversed with PR antagonists (35, 36). With the newly identified plasma membrane associated progesterone receptor (PGRMC1), Peluso and colleagues found its association with plasminogen activator inhibitor RNA binding protein-1 (PAIRBP1), mediating the anti-apoptotic actions of PG in granulocytes (37).
Our study is consistent with a few reports in the literature indicating a protective effect of PG. Administration of PG in a trauma-hemorrhage model was shown to increase cardiac output, heart performance and circulating blood volume (38). PG has also been shown to induce the expression of heat shock proteins, which are protective against cardiac injury (39). In considering the benefit of PG, PG receptors have also been discovered in vascular smooth muscle cells (40, 41) and PG administration has been found to lower the blood pressure in humans (42, 43). Most importantly, PG treatment has been found to exhibit protective affects against brain injury (44–46). In an acute global cerebral ischemia model of stroke, PG treatment for 7 days after cardiorespiratory arrest was found to completely prevent neuronal loss (44). Analysis of post infarcted regions of the cortex revealed decreased cerebral edema, and increased cell survival (44). PG has been shown to protect against lipid peroxidation in this model (47). The ongoing Phase III clinical trials with PG for treatment of traumatic brain injury (http://clinicaltrials.gov/ct2/show/NCT00822900?term=progesterone&rank=14) pave the foundation for future clinical use of PG in preveting myocardial injury.
Acknowledgements
This work was supported by NIH R01 HL 076530, T32 ES007091, Arizona Disease Control Research Commission (QMC) and Mark and Mary Anne Fay Investigator Awards (SM). We thank Ms. Yan Lin for technical assistance, Dr. David Ross at University of Colorado for NQO1 antibody, and Dr. Jeff A. Johnson at University of Wisconsin for NQO1 ARE-luciferase construct.
References
- 1.Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther. 1990;47:219–231. doi: 10.1016/0163-7258(90)90088-j. [DOI] [PubMed] [Google Scholar]
- 2.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 3.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000;207:77–86. doi: 10.1023/a:1007094214460. [DOI] [PubMed] [Google Scholar]
- 4.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med. 1978;65:823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- 6.Unverferth BJ, Magorien RD, Balcerzak SP, Leier CV, Unverferth DV. Early changes in human myocardial nuclei after doxorubicin. Cancer. 1983;52:215–221. doi: 10.1002/1097-0142(19830715)52:2<215::aid-cncr2820520206>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Chen QM, Alexander D, Sun H, Xie L, Lin Y, Terrand J, Morrissy S, Purdom S. Corticosteroids inhibit cell death induced by doxorubicin in cardiomyocytes: induction of antiapoptosis, antioxidant, and detoxification genes. Mol Pharmacol. 2005;67:1861–1873. doi: 10.1124/mol.104.003814. [DOI] [PubMed] [Google Scholar]
- 8.Morrissy S, Xu B, Aguilar D, Zhang J, Chen QM. Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell. 2010;9:799–809. doi: 10.1111/j.1474-9726.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest. 1997;100:1501–1506. doi: 10.1172/JCI119672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- 11.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261:3060–3067. [PubMed] [Google Scholar]
- 12.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–3074. [PubMed] [Google Scholar]
- 13.Demirbag R, Yilmaz R, Erel O. The association of total antioxidant capacity with sex hormones. Scand Cardiovasc J. 2005;39:172–176. doi: 10.1080/14017430510035862. [DOI] [PubMed] [Google Scholar]
- 14.Bednarek-Tupikowska G, Tupikowski K, Bidzinska B, Bohdanowicz-Pawlak A, Antonowicz-Juchniewicz J, Kosowska B, Milewicz A. Serum lipid peroxides and total antioxidant status in postmenopausal women on hormone replacement therapy. Gynecol Endocrinol. 2004;19:57–63. doi: 10.1080/09513590412331272328. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein J, Sites CK, Toth MJ. Progesterone stimulates cardiac muscle protein synthesis via receptor-dependent pathway. Fertil Steril. 2004;82:430–436. doi: 10.1016/j.fertnstert.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Lind C, Hochstein P, Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982;216:178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- 17.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinkova-Kostova AT, Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 20.Benson AM, Hunkeler MJ, Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez PL. The role of NAD(P)H oxidoreductase (DT-Diaphorase) in the bioactivation of quinone-containing antitumor agents: a review. Free Radic Biol Med. 2000;29:263–275. doi: 10.1016/s0891-5849(00)00314-2. [DOI] [PubMed] [Google Scholar]
- 22.Floreani M, Napoli E, Quintieri L, Palatini P. Oral administration of trans-resveratrol to guinea pigs increases cardiac DT-diaphorase and catalase activities, and protects isolated atria from menadione toxicity. Life Sci. 2003;72:2741–2750. doi: 10.1016/s0024-3205(03)00179-6. [DOI] [PubMed] [Google Scholar]
- 23.Asher G, Lotem J, Kama R, Sachs L, Shaul Y. NQO1 stabilizes p53 through a distinct pathway. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3099–3104. doi: 10.1073/pnas.052706799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anwar A, Dehn D, Siegel D, Kepa JK, Tang LJ, Pietenpol JA, Ross D. Interaction of human NAD(P)H:quinone oxidoreductase 1 (NQO1) with the tumor suppressor protein p53 in cells and cell-free systems. J Biol Chem. 2003;278:10368–10373. doi: 10.1074/jbc.M211981200. [DOI] [PubMed] [Google Scholar]
- 25.Iskander K, Gaikwad A, Paquet M, Long DJ, 2nd, Brayton C, Barrios R, Jaiswal AK. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005;65:2054–2058. doi: 10.1158/0008-5472.CAN-04-3157. [DOI] [PubMed] [Google Scholar]
- 26.Jain SK, Rogier K, Prouty L. Protective effects of 17beta-estradiol and trivalent chromium on interleukin-6 secretion, oxidative stress, and adhesion of monocytes: relevance to heart disease in postmenopausal women. Free Radic Biol Med. 2004;37:1730–1735. doi: 10.1016/j.freeradbiomed.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Moorthy K, Sharma D, Basir SF, Baquer NZ. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp Gerontol. 2005;40:295–302. doi: 10.1016/j.exger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Capel ID, Jenner M, Williams DC, Donaldson D, Nath A. The effect of prolonged oral contraceptive steroid use on erythrocyte glutathione peroxidase activity. J Steroid Biochem. 1981;14:729–732. doi: 10.1016/0022-4731(81)90008-x. [DOI] [PubMed] [Google Scholar]
- 29.Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987;210:37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- 30.Bednarek-Tupikowska G, Bohdanowicz-Pawlak A, Bidzinska B, Milewicz A, Antonowicz-Juchniewicz J, Andrzejak R. Serum lipid peroxide levels and erythrocyte glutathione peroxidase and superoxide dismutase activity in premenopausal and postmenopausal women. Gynecol Endocrinol. 2001;15:298–303. [PubMed] [Google Scholar]
- 31.Massafra C, Buonocore G, Gioia D, Sargentini I, Farina G. Effects of estradiol and medroxyprogesterone-acetate treatment on erythrocyte antioxidant enzyme activities and malondialdehyde plasma levels in amenorrhoic women. J Clin Endocrinol Metab. 1997;82:173–175. doi: 10.1210/jcem.82.1.3688. [DOI] [PubMed] [Google Scholar]
- 32.Ozden S, Dildar K, Kadir YH, Gulizar K. The effects of hormone replacement therapy on lipid peroxidation and antioxidant status. Maturitas. 2001;38:165–170. doi: 10.1016/s0378-5122(00)00216-4. [DOI] [PubMed] [Google Scholar]
- 33.Tranquilli AL, Mazzanti L, Cugini AM, Cester N, Garzetti GG, Romanini C. Transdermal estradiol and medroxyprogesterone acetate in hormone replacement therapy are both antioxidants. Gynecol Endocrinol. 1995;9:137–141. doi: 10.3109/09513599509160203. [DOI] [PubMed] [Google Scholar]
- 34.Kurita T, Wang YZ, Donjacour AA, Zhao C, Lydon JP, O'Malley BW, Isaacs JT, Dahiya R, Cunha GR. Paracrine regulation of apoptosis by steroid hormones in the male and female reproductive system. Cell Death Differ. 2001;8:192–200. doi: 10.1038/sj.cdd.4400797. [DOI] [PubMed] [Google Scholar]
- 35.Liszewska E, Rekawiecki R, Kotwica J. Effect of progesterone on the expression of bax and bcl-2 and on caspase activity in bovine luteal cells. Prostaglandins Other Lipid Mediat. 2005;78:67–81. doi: 10.1016/j.prostaglandins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Okuda K, Korzekwa A, Shibaya M, Murakami S, Nishimura R, Tsubouchi M, Woclawek-Potocka I, Skarzynski DJ. Progesterone is a suppressor of apoptosis in bovine luteal cells. Biol Reprod. 2004;71:2065–2071. doi: 10.1095/biolreprod.104.028076. [DOI] [PubMed] [Google Scholar]
- 37.Peluso JJ. Multiplicity of progesterone's actions and receptors in the mammalian ovary. Biol Reprod. 2006;75:2–8. doi: 10.1095/biolreprod.105.049924. [DOI] [PubMed] [Google Scholar]
- 38.Kuebler JF, Jarrar D, Bland KI, Rue L, 3rd, Wang P, Chaudry IH. Progesterone administration after trauma and hemorrhagic shock improves cardiovascular responses. Crit Care Med. 2003;31:1786–1793. doi: 10.1097/01.CCM.0000063441.41446.23. [DOI] [PubMed] [Google Scholar]
- 39.Knowlton AA, Sun L. Heat-shock factor-1, steroid hormones, and regulation of heat-shock protein expression in the heart. Am J Physiol Heart Circ Physiol. 2001;280:H455–H464. doi: 10.1152/ajpheart.2001.280.1.H455. [DOI] [PubMed] [Google Scholar]
- 40.Lin AL, McGill HC, Jr, Shain SA. Hormone receptors of the baboon cardiovascular system. Biochemical characterization of aortic and myocardial cytoplasmic progesterone receptors. Circ Res. 1982;50:610–616. doi: 10.1161/01.res.50.5.610. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz KBHL. Canine vascular tissues are targets for androgens, estrogens, progestins and glucocorticoids. J Clin Invest. 1982:750–758. doi: 10.1172/JCI110513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rylance PB, Brincat M, Lafferty K, De Trafford JC, Brincat S, Parsons V, Studd JW. Natural progesterone and antihypertensive action. Br Med J (Clin Res Ed) 1985;290:13–14. doi: 10.1136/bmj.290.6461.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regensteiner JG, Hiatt WR, Byyny RL, Pickett CK, Woodard WD, Moore LG. Short-term effects of estrogen and progestin on blood pressure of normotensive postmenopausal women. J Clin Pharmacol. 1991;31:543–548. doi: 10.1002/j.1552-4604.1991.tb03735.x. [DOI] [PubMed] [Google Scholar]
- 44.Gibson CL, Coomber B, Rathbone J. Is progesterone a candidate neuroprotective factor for treatment following ischemic stroke? Neuroscientist. 2009;15:324–332. doi: 10.1177/1073858409333069. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez Deniselle MC, Lopez Costa JJ, Gonzalez SL, Labombarda F, Garay L, Guennoun R, Schumacher M, De Nicola AF. Basis of progesterone protection in spinal cord neurodegeneration. J Steroid Biochem Mol Biol. 2002;83:199–209. doi: 10.1016/s0960-0760(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 46.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 47.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]