Abstract
Although glucocorticoids are well known for their capacity to suppress the immune response, glucocorticoids can also promote immune responsiveness. It was the purpose of this investigation to evaluate the molecular basis for this apparent dichotomous immunologic effect. Glucocorticoid treatment of natural killer cells (NK) was shown to reduce NK cell cytolytic activity by reduction of histone promoter acetylation for perforin and granzyme B, which corresponded with reduced mRNA and protein for each. In contrast, glucocorticoid treatment increased histone acetylation at regulatory regions for interferon gamma and IL-6, as well as chromatin accessibility for each. This increase in histone acetylation was associated with increased proinflammatory cytokine mRNA and protein production upon cellular stimulation. These immunologic effects were evident at the level of the individual cell and demonstrate glucocorticoids to epigenetically reduce NK cell cytolytic activity while at the same time to prime NK cells for proinflammatory cytokine production.
Keywords: Natural killer cells, Glucocorticoids, Epigenetic, Proinflammatory cytokines, Granule constituents
1.Introduction
Glucocorticoids (GC) are primary stress hormones necessary for life that are synthesized in the adrenal cortex and released into the blood stream homeostatically and in response to environmental and physiological stress. GC orchestrate various physiological processes (e.g. metabolism, energy production, vascular tone, bone mineralization, central nervous system and immune function) by regulation of gene transcription, both induction and repression, of nearly 25% of the genome [1; 2; 3]. Because of their potent anti-inflammatory and immunosuppressive effects, synthetic GC are widely used to treat inflammatory and autoimmune diseases, and are known to suppress both innate and adaptive immune responses. GC suppress cell mediated lytic activities [4; 5] as well as cytokine production [6; 7; 8; 9; 10; 11] by many immune cell populations; including NK [4; 12], T lymphocyte[13], monocyte [11; 14; 15] and macrophage [16]. Of particular note, natural killer cells and NK cell cytolytic activity (NKCA) are especially responsive to GC [4; 12; 17; 18; 19]. Even though GC are known to suppress immune function under some conditions, GC under other conditions can promote immune responsiveness [20; 21; 22; 23; 24]. Physiological doses of GCs have been shown to enhance immunoglobulin production by mitogen-stimulated human lymphocytes[25] and GC have been shown to stimulate B lymphocyte number and antibody production in vitro and in vivo [26]. Further, during the early stages of T-cell activation, low levels of GC enhance T-cell receptor induced lymphocyte proliferation increase T-cell responsiveness to IL-2 and enhance proliferation of memory T cells [27; 28]. GC have been shown to synergistically enhance the induction of IL-1 beta and IL-6 [29] and the biological effects of IL-2, interferon (IFN) gamma, granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, and oncostatin M [30]. These immune modulating effects of GC are concentration and time dependent [31; 32] and it is clear that in addition to well-known immunosuppressive effects, GC are also able to exert modulating and enhancing effects upon the immune system [33; 34; 35]. In experimental models, both suppressive and immune enhancing effects of GC have been demonstrated experimentally for inflammatory cytokine mRNA and protein production by monocytes [11; 14; 15], phagocytosis by macrophages[16], acute-phase protein gene expression by hepatocytes[36], delayed-type hypersensitivity reactions [37] and wound healing[38]. In those studies, immune enhancing effects were observed at lower GC concentration and immune suppressive effects at higher GC concentration.
The timing of GC administration also affects immune outcomes in that a 24 hour pre-treatment of experimental animals with GC potentiated the proinflammatory response to subsequent endotoxin challenge; whereas, the administration of GC 1 hour after endotoxin challenge resulted in suppression of the proinflammatory response [20]. In human volunteers, a 2 week administration of dexamethasone (a synthetic GC) resulted in an attenuation of GC mediated inhibition of IL-6 and TNF-alpha production in vitro [39]. Further, experimentally increased plasma cortisol levels (concentrations of 75 to 85 µg/dL for a 6-hr period) ending 12–144 hours before injection of endotoxin, resulted in an increased proinflammatory response to the bacterial product[13]. In that study, time as well as GC concentration were important in determining the effect of GC. More recent work has demonstrated exposure of humans to cortisol concentrations of 35 to 45 µg/dL total plasma cortisol (approximately 80 nM free cortisol) enhanced inflammatory responses to a subsequent, stimulus with endotoxin [40]. Those results demonstrated a “preparative” or “priming” effect of GC on the immune proinflammatory response. A GC concentration of 35 to 45 µg/dL is similar to concentrations commonly observed during human systemic stress responses [40].
The basis for these effects of GC are not well understood, although various possibilities have been proposed [41; 42; 43; 44] including; mechanisms upstream of the binding of GC to its receptor, modified intracellular GC concentrations or insufficient GR expression. Those studies also reported mechanisms downstream of the binding of GC to GR that involve GC signaling pathways [45; 46]. In addition to these, another possibility is that GC influence epigenetic processes that result in the observed immunological effects. Histone tail post translational modifications (e.g. acetylation, methylation) regulate gene transcription [47; 48; 49] and GC have been shown to modify NK cell function epigenetically [50; 51]. In those reports, GC at a high concentration reduced NKCA; global histone acetylation, the acetylation of histone (H) 4 lysine (K) position 8, and promoter accessibility for perforin, interferon gamma and granzyme B. These epigenetics effects corresponded with reduced production of granule constituents (perforin and granzyme B) as well as reduced constitutive and stimulated production of IL-6, TNF alpha and IFN gamma. Histone acetylation was fully recovered by treatment of the NK cells with a histone deacetylase inhibitor, which also restored NKCA and proinflammatory cytokine production levels. Those results demonstrated GC to dysregulate NK cell function through an epigenetic mechanism that reduced histone tail acetylation status, immune effector gene transcription and levels of immune effector proteins necessary to the full functional activity of NK cells [50]. Those results are consistent with the known immune suppressive effects of a high GC concentration on NK cell effector function. In the experiments described herein, the effect of a low GC concentration on NK cells was evaluated with the hypothesis that a dichotomous phenotype would be revealed by treatment of NK cells with a lower GC concentration.
2.Materials and methods
2.1. Cell Culture
IL-2 dependent NK92 cells (established from a patient with non-Hodgkin’s lymphoma with the capacity to lyse a broad range of leukemia, lymphoma and myeloma cell lines at low effector to target ratio in vitro) was obtained from the American Type Culture Collection, Rockville, MD and maintained in alpha MEM with 12.5% horse serum (Gibco Laboratories, Grand Island, NY), 12.5% fetal bovine serum (FBS) (Gibco Laboratories, Grand Island, NY), penicillin, streptomycin (Whittaker M. A. Bioproducts, Walkersville, MD), 0.2 mM inositol: (Sigma Aldrich, St. Louis, MO), 0.1 mM 2-mercaptoethanol; (Gibco Laboratories, Grand Island, NY) and 0.02 mM folic acid: (Sigma Aldrich, St. Louis, MO). NK92 cell cultures were also supplemented with IL-2 (100 units/ml). The human erythroleukemic like cell line, K562, was obtained from the American Type Culture Collection, Rockville, MD. K562 cells were maintained in suspension in RPMI 1640 (Gibco Laboratories, Grand Island, NY) supplemented with 10% FBS (Gibco Laboratories, Grand Island, NY), 100 units/ml penicillin, 100ug/ml streptomycin (Whittaker M. A. Bioproducts, Walkersville, MD), 0.1 mM non-essential amino acids and 2 mM L-glutamine (Gibco Laboratories, Grand Island, NY).
2.2. Cellular Treatment
NK92s, cultured at 2.5×105 cells/mL, were treated with or without dexamethasone (GC) (Sigma Aldrich, St. Louis, MO) (10−10 M) for 4 days in the presence of IL-2 (100 units/mL), every 48 hours cells were collected, washed, and resuspended in fresh media to a concentration of 2.5×105 cells/mL supplemented with or without dexamethasone and IL-2 (100 units/mL). For the final 24 hours of treatment cells were collected, washed, and resuspended to a concentration of 2.5×105 cells/mL with or without dexamethasone in the absence of IL-2. Following this treatment NK92s were washed with media and resuspended to 1 × 106 cells/ml and used for analysis. In all cases, cell number and viability were determined by exclusion of 0.1% Trypan blue. Viability was maintained between 85 and 95% in all cases. In some experiments, cell cultures described above were subjected to an additional 24 hour treatment in the presence of GC (10−7M) to determine the sensitivity of the cells to further GC treatment (switch to −7M).
2.3. Evaluation of cytokine production
Cytokines were measured in NK92 culture supernatants as described previously [50]. To assess stimulated cytokine production, NK92s (2.5× 105 cells/ml) were treated as described above and then stimulated with IL-2 (5,000 U) or IL-12 (40 pg/mL) for an additional 4 hours. Cell culture supernatants were harvested and aliquots of the supernatants were stored at −80 °C for subsequent cytokine determination by ELISA (R&D Systems, Minneapolis, MN).
2.4. Analysis of Gene Expression
Messenger RNA from NK92 cells was obtained using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). RevertAid First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON) was used to generate cDNA for quantitative real-time PCR from 500 ng total RNA. Negative reverse transcriptase samples confirmed absence of contaminating DNA. All reactions were performed in triplicate using SYBRgreen supermix (Biorad) with the following synthetic oligonucleotide primer pairs. IFN-gamma: forward 5’-TGG AAA GAG GAG AGT GAC AG-3’ and reverse 5’- ATT CAT GTC TTC CTT GAT GG-3’. IL-6: forward 5’- CAA CCT GAA CCT TCC AAA GAT G-3’ and reverse 5’- ACC TCA AAC TCC AAA AGA CCA G-3’. Perforin: forward 5’- GGA GTG CCG CTT CTA CAG-3’ and reverse 5’- CGT AGT TGG AGA TAA GCC TGA G-3’. Granzyme B: forward 5’- AAG ACG ACT TCG TGC TGA CA-3’ and reverse 5’- CCC CAA GGT GAC ATT TAT GG-3’. PCR cycles were denaturation at 94°C (30 s) and annealing at 55–61°C (30 s), no extension phase was necessary and performed using the Opticon 2 Real-Time Detector (Bio-Rad, Hercules, CA) and analyzed using the Opticon Monitor Software (Bio-Rad, Hercules, CA). The AAC(t) method was used to determine changes in transcript levels between untreated and chronic GC treated cells using beta-actin as a reference gene.
2.5.Natural killer cell cytolytic activity (NKCA)
K562 tumor cells were radioactively labeled with 100 uCi of [51Cr] (New England Nuclear, Boston, MA). Radiolabeled K562 cells were washed and then incubated for 4 hour with NK92 cells. Following incubation, the supernatants were removed using a Skatron harvesting press (Skatron Inc., Sterling, VA) and the associated radioactivity determined. Effector to target ratios for NKCA were 2, 1, 0.5 and 0.25:1 as described previously [50].
Results are expressed as % cytotoxicity and calculated by the formula:
All experimental means were calculated from triplicate values. Lytic units (LU) were calculated by a program written by David Coggins, FCRC, Frederick, MD and represents the number of cells per 107 effectors required to achieve 20% lysis of the targets. *DPM=disintegrations per minute.
2.6. Immunofluorescent Flow Cytometric Analysis of Intracellular and Surface Proteins
For detection of surface proteins, NK92 cells (1.0 × 105/assessment) were incubated with antibodies for 30 minutes on ice (agitated after 15 minutes). Cells were then washed twice with 0.1% BSA (Sigma Aldrich, St. Louis, MO) in PBS (Gibco, Grand Island, NY) and resuspended in 1% paraformaldehyde (PFA) (Sigma Aldrich, St. Louis, MO). Surface staining antibodies included anti-IL12Rβ2 (PerCP conjugated) (R&D Systems, Minneapolis, MN) and anti-IL-2Rα/CD25 (Alexa488 conjugated) (BD Biosciences, San Jose, CA). For intracellular protein analysis, cells were washed twice with 0.1%BSA following surface staining, then fixed and permeabilized with Cytofix/Cytoperm solution (BD Pharmingen, San Jose, CA) for 20 min at 4°C. The cells were then washed twice with Perm/Wash Buffer (BD Biosciences, San Jose, CA) and probed with antibodies specific for molecules of interest including anti-granzyme B (Alexa Fluor 647 conjugated) (BD Biosciences, San Jose, CA), anti-perforin (PE-conjugated) (BD Biosciences, San Jose, CA), and anti-glucocorticoid receptor (GR) (unconjugated) (Abcam, Cambridge, MA). Secondary anti-IgG (FITC conjugated) (Millipore, Temecula, CA) was added for 30 min at 4°C to unconjugated primary antibodies. For intracellular cytokine analysis of IFN-gamma (PE conjugated) (BD Biosciences, San Jose, CA) cells were incubated in leukocyte activation cocktail (BD Pharmigen, San Jose, CA) at 37°C for 4 hours prior to permeabilization and antibody staining. Samples were analyzed with a FACSCanto Fluorescence-Activated Cell Sorter in the Core Laboratory of the Cardinal Bernardin Loyola University Cancer Center equipped with a 15mW argon-ion laser and a red diode laser using FACSDiva software for data acquisition. After staining, cells were analyzed by flow cytometry. 10,000–30,000 events were recorded and analyzed with FlowJo v8.4.1. Flow cytometric analysis was confirmed by microscopy.
2.7. Western Blot
For Western blot analysis, nuclear and cytoplasmic compartments of NK92 cells were extracted from 1–3 × 106 cells via the Fermentas ProteoJET Cytoplasmic and Nuclear Protein Extraction protocol (Fermentas, Burlington, ON). Nuclei were lysed using Nuclear Lysis Buffer (Fermentas, Burlington, ON) and both lysed nuclei and cytoplasm were resuspended in Laemmlis SDS-Sample Buffer (4×) (Boston Bioproducts, Boston, MA). Samples were boiled for 10 minutes and proteins separated by electrophoresis with a 12% agarose gel and transferred to a nitrocellulose membrane for blotting. Proteins were visualized with anti-NF-κB p65 (Abcam, Cambridge, MA), anti-cJun (Abcam, Cambridge, MA), anti-STAT4 (Santa Cruz, Santa Cruz, CA), anti-GR (Abcam, Cambridge, MA) and HRP conjugated goat anti-rabbit IgG secondary antibody (Millipore, Temecula, CA) and chemiluminescence reagent (ThermoScientific, Rockford, IL). Quality of nuclear and cytoplasmic compartment extraction was determined by Laminin B and GAPDH, respectively (Abcam, Cambridge, MA). Blot density was quantified using Image J software.
2.8. Chromatin Immunoprecipitation (ChIP) Assay
NK92 cells (5 × 106) were cross-linked with 1% formaldehyde for 10 min and terminated by the addition of glycine to a final concentration of 125 mM for 5 min. The cells were washed twice with ice-cold PBS. Cross-linked nuclei were extracted using the Fermentas ProteoJET Cytoplasmic and Nuclear Protein Extraction protocol (Fermentas, Burlington, ON) and lysed with 500 µL of high salt lysis buffer (Santa Cruz, Santa Cruz, CA). The samples were sonicated using a Branson Sonifier 250 on ice for a total of four, 15 second cycles per sample. Sonicated samples were diluted and aliquoted into 100µl volumes. Pre-cleared supernatants with ChIP-Grade Protein-G Magnetic Beads (Cell Signaling, Danvers, MA) were collected and immunoprecipitated overnight at 4°C with the following antibodies: anti-acetyl-H4K8 (Millipore, Temecula, CA), anti-acetyl-H3K9 (Cell Signaling, Danvers, MA), anti-acetyl H3K27 (Abcam, Cambridge, MA), anti-trimethyl H3K4 (Cell Signaling, Danvers, MA), anti-trimethyl H3K9 (Cell Signaling, Danvers, MA), anti-trimethyl H3K27 (Cell Signaling, Danvers, MA) and control rabbit Ig (Cell Signaling, Danvers, MA). ChIP-Grade Protein-G Magnetic Beads (Cell Signaling, Danvers, MA) were added and incubated an additional 4 hours at 4°C. The immune complexes were collected, washed, and eluted. Cross-linking was reversed by heating at 65°C overnight and 10 minutes at 95°C in elution buffer (Santa Cruz, Santa Cruz, CA). DNA was recovered using the MiniElute Reaction Cleanup Kit (Qiagen, Valencia, CA) and resuspended in 50 µl of dH2O. Quantitative real-time PCR was performed using an Opticon 2 Real-Time Detector; PCR cycles were denaturation at 94°C (30 s) and annealing at 55–61°C (30 s), no extension phase was necessary. The DNA samples were amplified with primer pairs to the −22KB enhancer (F: 5’-GAA TTG GCT TGA CAC CTC TGT CCT-3’ and R: 5’-TTC CAT CTC TCG GCA AAG AGC AGT-3’) and proximal promoter of the IFNG locus (F: 5’-TCA TCG TCA AAG GAC CCA AGG AGT-3’ and R: 5’-ATG GTG ACA GAT AGG CAG GGA TGA-3’), the proximal promoters of PRF1 (F: 5’-GGC ACA GTT CCA AGC ACT TCA CAA-3’ and R: 5’-AGC CTC ACT GTG CCT CAG TTT CTT-3’), GZMB (F: 5’-AGC CTG TTG CCT CTG TGA GAA AGT-3’ and R: 5’-TGG GAT TTG CTG GCA ACC TAG ACA-3’), IL6 (F: 5’-CAG AAG AAC TCA GAT GAC TGG-3’ and R: 5’-GCT GGG CTC CTG GAG GGG-3’), and TNF (F: 5’-CGC TTC CTC CAG ATG AGC TC-3’ and R: 5’-TGC TCT CCT TGC TGA GGG A-3’). PCR data was analyzed using Opticon Monitor 3 Software (BioRad, Hercules, CA). Modification levels were calculated as percent input using the equation.
Percent Input = 100 x 2^(C(t)untreated – C(t)treated cells).
2.9.Chromatin Accessibility
The EpiQ Chromatin Analysis Kit (BioRad, Hercules, CA) was used to determine the accessibility of the IFNG enhancer, IL6 proximal promoter, GZMB proximal promoter, GAPDH gene, and RHO gene. The methodology supplied in the kit was as directed for cells in suspension. Briefly, 250,000 NK92 cells were collected from treatments and resuspended in the supplied EpiQ chromatin buffer and either left undigested or nuclease digested. DNA was then isolated and quantification of accessibility, a measure of intact genomic regions, was performed by q-RT-PCR. The EpiQ Chromatin Analysis Kit Data Analysis Tool was used to determine percent accessibility. The control primers for the genomic regions RHO (F: 5’-AGG TCA CTT TAT AAG GGT CTG GGG G-3’ and R: 5’-AGT TGA TGG GGA AGC CCA GCA CGA T-3’) and GAPDH (F: 5’- ACC TCC CAT CGG GCC AAT CTC AGT C-3’ and R: 5’-GGG TGA CTG TCG AAC AGG AGG AGG A-3’) were supplied by Bio-Rad and used as directed as 0% and >95% accessibility respectively. Analysis of chromatin accessibility at the IFNG enhancer, GZMB promoter, and IL6 promoter were performed using the primer sets listed in the ChIP methodology section.
2.10. Statistical methods
Data are expressed as means with the standard error of the mean (SEM) or standard deviation (SD) as noted. Main study variables were analyzed using Student’s t test, a two-sided alpha of 0.05 was set for significance. The Statistical Package for Social Sciences (SPSS: version 13.0) was used for data analysis.
3. Results
3.1. Effect of chronic glucocorticoid (GC) treatment on proinflammatory cytokine production
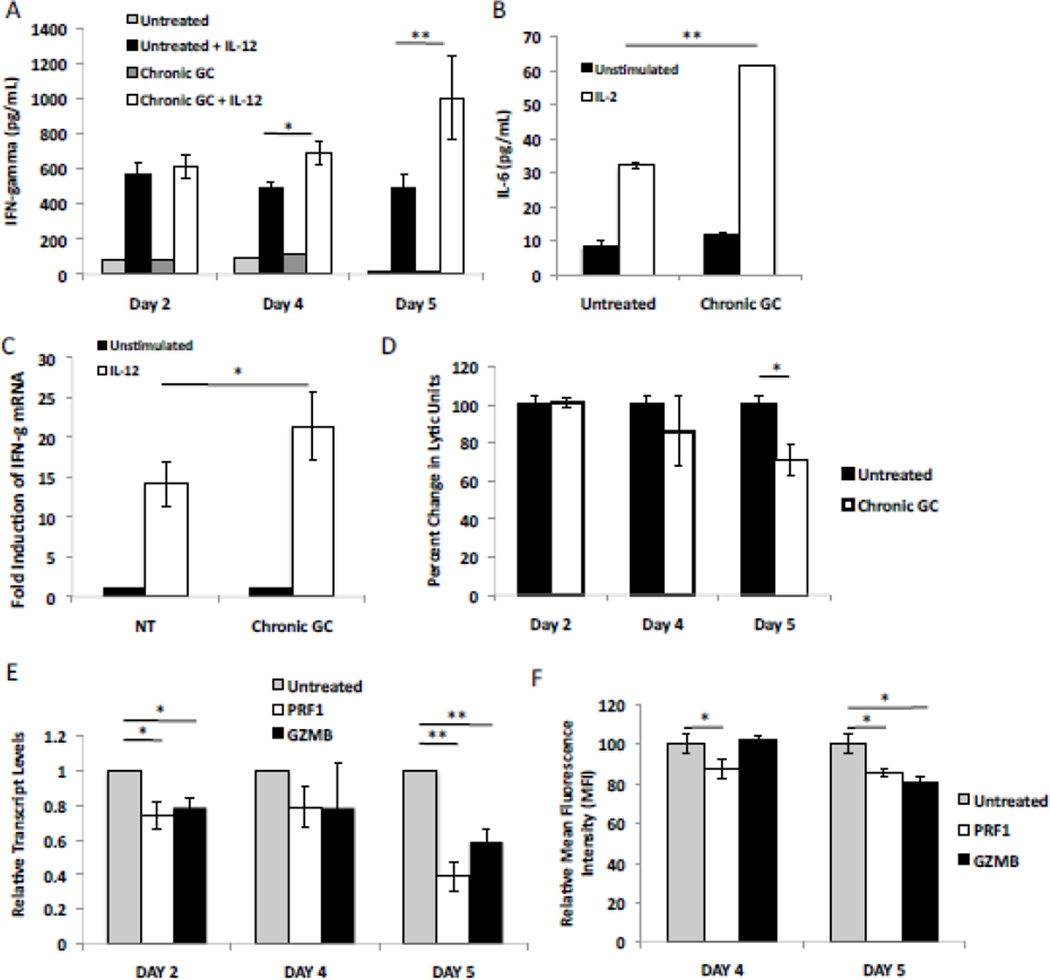
The human NK cell line, NK92, was cultured with dexamethasone (10−10M) for 5 days (termed chronic GC treatment) and the effect of GC determined by comparison to NK92 cells simply cultured in medium alone (untreated). When stimulated with IL-12, cells harvested after 4 and 5 days of GC treatment (Chronic GC + IL-12), exhibited an increased production of IFN gamma when compared to untreated cells stimulated with IL-12 (Untreated + IL-12) (Fig. 1A). GC treatment alone had no impact on basal IFN gamma production (Chronic GC vs. Untreated). The effect of chronic GC treatment was maximal at 5 days with 10−10M GC. 10−9M GC did enhance stimulated IFN gamma production after 5 days but the enhancement was less pronounced than with 10−10M GC. GC (10−7M and 10−8M) inhibited stimulated IFN gamma production at 2, 4 and 5 days of treatment. Data are not shown. Hence the effect of GC at 10−10M was further examined. Intracellular cytokine staining of NK cells confirmed these findings and demonstrated a 32.4±3.8% increase in intracellular IFN gamma mean fluorescence intensity (MFI) after 5 days of chronic GC treatment, when compared to untreated cells (Table 1). The shift in MFI was accompanied by a modest increase in the percentage of IFN gamma positive cells (Table I). This was not an IFN gamma specific phenomenon, as IL-2 stimulated IL-6 production increased from 32.2±1.8pg/mL to 61.6±2.4pg/mL after 5 days of GC treatment (Fig. 1B). mRNA isolated and quantified from NK cells after 5 days of GC treatment exhibited a 23-fold induction of IFN gamma mRNA after IL-12 stimulation, while untreated cells exhibited a 14-fold induction (Fig. 1C). These results demonstrate chronic GC treatment to prime NK cells for enhanced proinflammatory cytokine production via enhanced transcription of cytokine genes after cellular stimulation.
Fig. 1.
Effect of chronic glucocorticoid (GC) treatment on NK cell proinflammatory cytokine production and natural killer cell cytotoxic activity (NKCA). (A) IFN gamma production (as measured by ELISA) by NK92 cells, either untreated or treated with GC for various time periods, and then either stimulated or not (unstimulated) with IL-12 for 4 hours, n=3. (B) IL-6 production as measured by ELISA by NK92 cells harvested from 5 day cultures (either untreated or treated with GC) and either unstimulated or stimulated with IL-2(5,000 U), for 4 hours, n=3. (C) IFN gamma transcript levels for IL-12 (40 pg) stimulated and unstimulated cultures with and without chronic (5 day) GC exposure. IFN-gamma transcript levels from unstimulated, untreated cells were set to 1. (D) NKCA for target K562 cells as percent change in lytic units ± SEM of NK92 harvested at indicated days of treatment, n=3. (E) NK92 cells, either untreated or treated with GC for various time periods were harvested and relative transcript levels determined for two granule constituents, perforin (PRF1) and granzyme B (GZMB). Results were standardized to transcript levels in untreated NK92 cells (grey bars) set to 1. (F) Flow cytometric analysis of granule constituent protein levels after 4 and 5 day chronic GC treatment. Graphs demonstrate mean fluorescent intensity (MFI) for perforin and granzyme B. Untreated cells were set to 100%. *; p<0.05, **; p<0.01.
Table 1.
Effect of chronic glucocorticoid (GC) treatment on proinflammatory cytokine production and granule constituent levels in NK92 cells
| Mean Fluorescent Intensity (MFI) and/or Percentages of Cytokine and Intracellular Protein Positive Cells | ||||
|---|---|---|---|---|
| Cellular Protein | Untreated | Chronic GC Treatment (5 day) |
P value | (%) Change |
| IFN-gamma (MFI) |
1452 ± 71 | 1922 ± 91 | 0.0005 | 32.4 ± 3.8 |
| IFN-gamma Positive (%) |
85.0 ± 1.0 | 90.0 ± 1.7 | 0.001 | 5.7 ± 0.37 |
| Perforin (MFI) |
7421 ± 192 | 6371 ± 138 | 0.017 | −14.1 ± 1.86 |
| Perforin Positive (%) |
95.3 ± 0.29 | 94.3 ± 1.36 | 0.309 | −0.98 ± 1.42 |
| Granzyme B (MFI) |
11009 ± 150 | 10115 ± 188 | 0.017 | −8.13 ± 1.71 |
| Granzyme B Positive (%) |
98.2 ± 0.40 | 97.8 ± 0.31 | 0.318 | −0.34 ± 0.31 |
Values are mean fluorescent intensity (MFI) of surface and intracellular proteins ± S.E.M. after 4 hour stimulation with leukocyte activation cocktail stimulation, N= at least three independent experiments. Isotype control MFI was always < 150. % Change= (MFI Untreated- MFI Chronic GC treatment)/(MFI Untreated) × 100. P values, No Treatment vs. GC Treatment.
3.2. Effect of chronic GC treatment on natural killer cell cytolytic activity (NKCA)
Chronic GC treatment of NK cells produced a time dependent effect on NKCA, with an 18% decrease by day 4 and a 25% decrease by day 5 (Fig. 1D). Human NKCA is primarily mediated via granule dependent lysis of tumor cells; therefore, the mRNA level of two key NK cell granule constituents, perforin and granzyme B, was assessed. As early as day 2 of GC treatment, mRNA expression for both perforin (PRF1) and granzyme B (GZMB) was reduced when compared to untreated cells. By day 5 of GC treatment PRF1 transcripts were reduced by 60% and GZMB transcripts by 40% compared, (Fig. 1E). Flow cytometry confirmed reductions in both perforin and granzyme B MFI levels after 5 days of chronic GC treatment (Fig. 1F and Table 1). These data demonstrate chronic GC treatment to reduce perforin and granzyme B levels with concomitant reductions in NKCA. Furthermore, the percentages of perforin or granzyme B positive cells were essentially unchanged after chronic GC treatment (Table 1). Taken together, these data demonstrate chronic GC treatment to decreases NK cell cytolytic activity and at the same time prime NK cells for enhanced proinflammatory cytokine production upon stimulation. Secondly, these data demonstrate this effect at the cellular level in that the percentages of cells positive for cytokines or lytic molecules were essentially unchanged. Rather a shift in the MFI at the individual cellular level was observed. As such, chronic GC treatment imparts an apparent dichotomous phenotype on NK cells, which results in reduced NKCA and an enhanced capacity for proinflammatory cytokine production.
3.3. Chronic GC treatment induced histone post translational modifications at proinflammatory gene regulatory regions
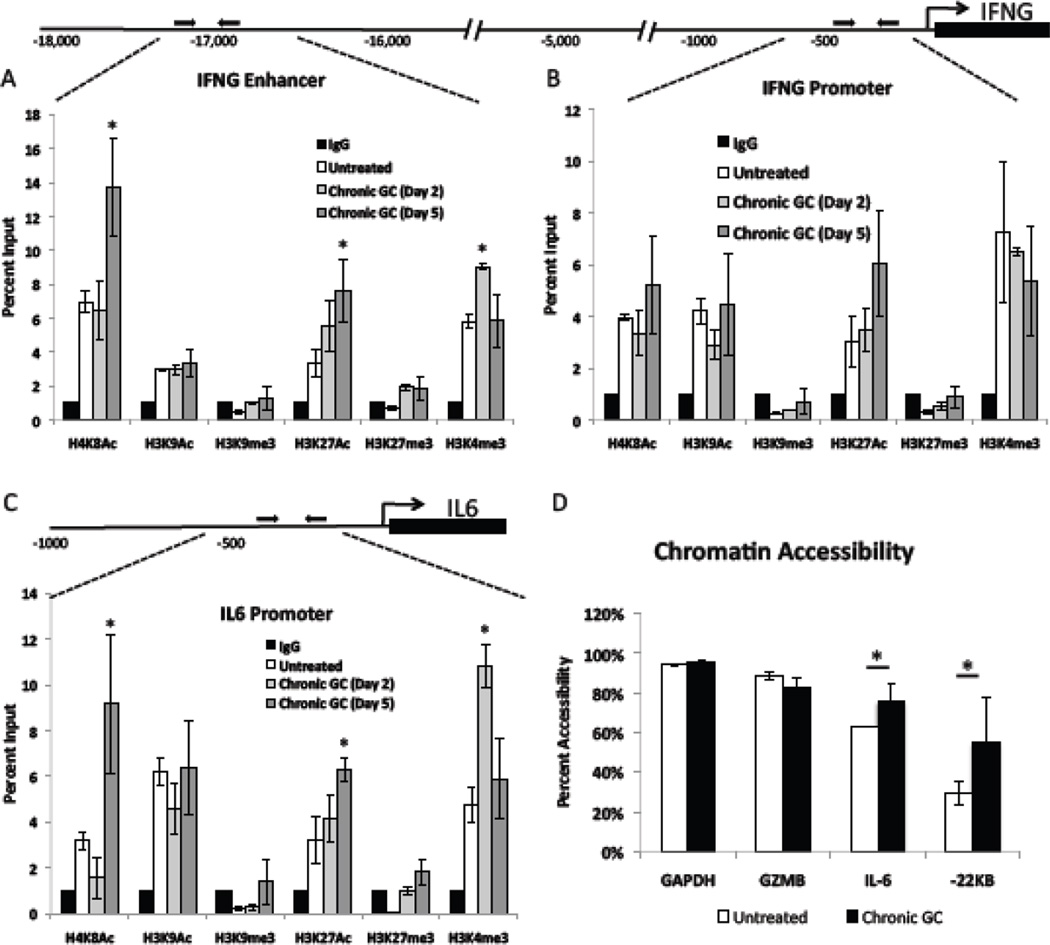
Ligand activated glucocorticoid receptor (GR) has been shown to interact with histone modifying enzymes and to influence histone tail post translational modifications at genomic regulatory regions [50; 52; 53]. Histone post translational modifications at genomic regulatory regions can not only affect active transcription rates but can also serve to prime genes for future transcription. Enhanced transcription after stimulation was observed after 5 days of chronic GC treatment (Fig. 1C). Hence, six histone post translational modifications at the promoter and −22KB enhancer region of the IFNG locus, and the IL6 promoter were assessed in chronic GC treated and untreated cells. See Fig. 2. By chromatin immunoprecipitation, each of the three regulatory regions of untreated NK cells exhibited moderate H4K8 acetylation (Ac), H3K27Ac, and H3K4 trimethylation (me3); low but detectable H3K9Ac and H3K27me3; and undetectable/below background (IgG) H3K9me3 levels. In cells treated with GC for 5 days, the IFNG enhancer, which is critical for IFNG transcription [54; 55], exhibited a significant increase in H4K8Ac as well as H3K27Ac (Fig. 2A). This pattern of acetylation for H4K8 and H3K27 corresponded to the enhanced IFN gamma production and secretion following chronic GC treatment and IL-12 stimulation (Fig. 1A and C). No GC treatment effects were observed at the proximal promoter of IFNG (Fig. 2B) or the TNF promoter (data are not shown). Like the IFNG enhancer, the IL6 proximal promoter exhibited a significant increase in H4K8Ac as well as H3K27Ac (Fig. 2C) after 5 day of GC treatment. This increase corresponded to the two fold increase in IL-6 production following chronic GC treatment and IL-2 stimulation (Fig. 1B). These data indicate that proinflammatory gene regulatory regions exhibit increased acetylation following chronic GC treatment and suggest histone post translational modifications to contribute to the enhanced proinflammatory cytokine production following cellular stimulation. For both the IFNG enhancer and the IL6 promoter an increase in H3K4me3 at day 2 of GC treatment (grey bars) but not at day 5 of GC treatment was noted.
Fig. 2.
Histone post translational modifications at NK proinflammatory gene regulatory regions. Schematic diagrams and percent input calculations of posttranslational histone modifications at the (A) IFNG enhancer, (B) IFNG promoter, and (C) the IL6 promoter after 2 days (grey bars) and 5 days (dark grey bars) of chronic GC treatment, compared to untreated cells (white bars) and to the IgG (black bars) negative control. Untreated cell values are the average for each modification at day 2 and day 5. No difference in these levels over time was observed. (D) Percent change in sensitivity of indicated genomic regions to nuclease digestion as determined by the Bio-Rad EpiQ chromatin accessibility kit.
Accessibility is calculated as the ability of nuclease to digest indicated regions setting GAPDH accessibility as 100% in untreated (white bars) and 5 day chronic GC treated cells (black bars). Data represent the average of three independent experiments performed in duplicate n=6 ± SEM *; p<0.05.
3.4. Chronic GC induced effects on chromatin accessibility
Acetylation increases the accessibility of gene regulatory regions and enhances transcription of genes upon cellular activation [56; 57; 58]. Chromatin from 5 day chronic GC treated and untreated cells was subjected to nuclease digestion. qRT-PCR was used to quantify the degree to which each regulatory region was resistant to digestion compared to GAPDH, a constitutively open and transcribed gene. Chronic GC treatment increased the accessibility of the IL6 promoter by 18% and increased the accessibility of the IFNG enhancer by 37% (Fig. 2D). As no increase in transcription was observed prior to cytokine stimulation (Fig. 1A) it is likely that this increased accessibility is the result of the increased acetylation at proinflammatory cytokine gene regulatory regions (Fig. 2A and C) which primes NK cells for enhanced cytokine production when stimulated. Analysis of the GZMB promoter yielded no significant difference in accessibility (Fig. 2D). Although granzyme B transcripts were diminished, chronic GC treatment did not appear to alter chromatin accessibility, suggesting that GZMB is affected by GC in a manner different from cytokine regulatory regions.
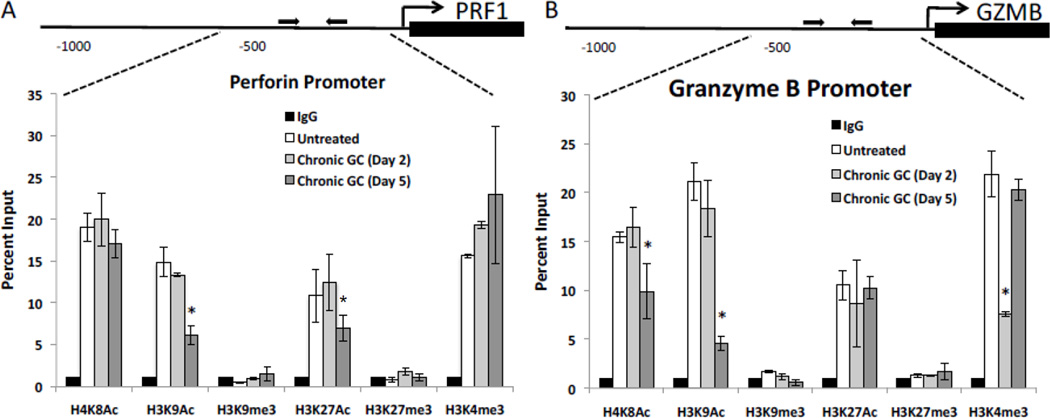
3.5. Chronic GC treatment induced histone post translational modifications at PRF1 and GZMB regulatory regions
In contrast to the cytokine loci, regulatory regions for the granule constituents of untreated NK cells exhibited high levels of H4K8Ac, H3K9Ac, H3K27Ac, and H3K4me3 while H3K9me3 and H3K27me3 were at or below background (IgG) levels. These histone post translational modifications are indicative of genes undergoing active transcription (high H3K9Ac and H3K27Ac) with no heterochromatin marks (H3K9me3 and H3K27me3) [56; 57; 58]. However, with 5 days of chronic GC treatment (dark grey bars) the perforin (PRF1) promoter exhibited significantly reduced H3K9Ac and H3K27Ac, corresponding to reduced perforin transcripts (Fig. 3B and Fig. 1F). Likewise, the proximal promoter of GZMB exhibited significant reductions in H3K9Ac and H4K8Ac (Fig. 3B) after 5 days of GC treatment, corresponding to reduced transcripts for granzyme B (Fig. 1E). These data suggest chronic GC treatment to reduce NKCA at least in part through reduced acetylation of histones associated with the promoter regions of these two granule constituents. For the GZMB promoter a decrease in H3K4me3 at day 2 of GC treatment (grey bars) was noted.
Fig. 3.
Histone post translational modifications at NK cells granule constituent regulatory regions. Schematic diagrams and percent input calculations of posttranslational histone modifications at the (A) proximal promoter of the PRF1 gene and the (B) proximal promoter of the GZMB gene after 2 days (grey bars) and 5 days (dark grey bars) of chronic GC treatment, compared to untreated cells (white bars) and to the IgG (black bars) negative control. Untreated cell values are the average for each modification at day 2 and day 5. No difference in these levels over time was observed. Data represents the average of three independent experiments performed in duplicate n=6 ± SEM *; p<0.05.
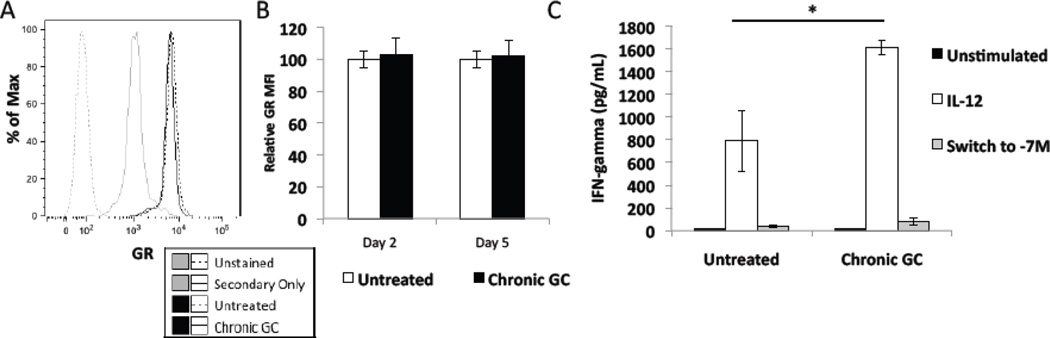
3.6.Effect of chronic GC treatment on GR Cellular Levels and GR Sensitivity to GC
It is possible that continued GC exposure may desensitize NK cells to the effect of the hormone [59; 60] by reducing cellular levels of GR or by reducing the sensitivity of GR to GC. No difference in GR levels, as judged by MFI at either day 2 or day 5, was found between chronic GC treated and untreated cells. A representative histogram is shown Fig. 4A and quantification of GR MFI in Fig. 4B, with untreated cells set to 100%. To assess the functional activity of GR in NK cells treated for 5 days with GC, NK cells were exposed to a high concentration of GC (10−7M) during 4 hours of stimulation with IL-12 (Switch to −7M). Fig. 4C demonstrates that 10−7M GC equally reduced IFN gamma production in both chronic GC treated cells and in untreated cells. These data demonstrate no effect of chronic GC treatment on GR levels or sensitivity to GC.
Fig. 4.
Effect of chronic GC treatment on the glucocorticoid receptor (GR). (A) Representative histogram of GR protein levels in untreated and chronic GC treated (5 days) NK92 cells assessed by flow cytometric analysis. (B) Quantification of MFI from flow cytometric analysis of GR mean fluorescent intensity levels (MFI) of cells harvested after 2 days and 5 days of GC treatment. MFI of untreated cells was set to 100%. (C) IFN-gamma production by untreated (black bars) and chronic GC (5 days) (white bars) treated NK92 cells that then received an additional 4 hour treatment with a high concentration of GC (10-7M) (switch to −7M) and IL-12 (40 pg). Data represent the average of three independent experiments n=3 ± SEM *; p<0.05.
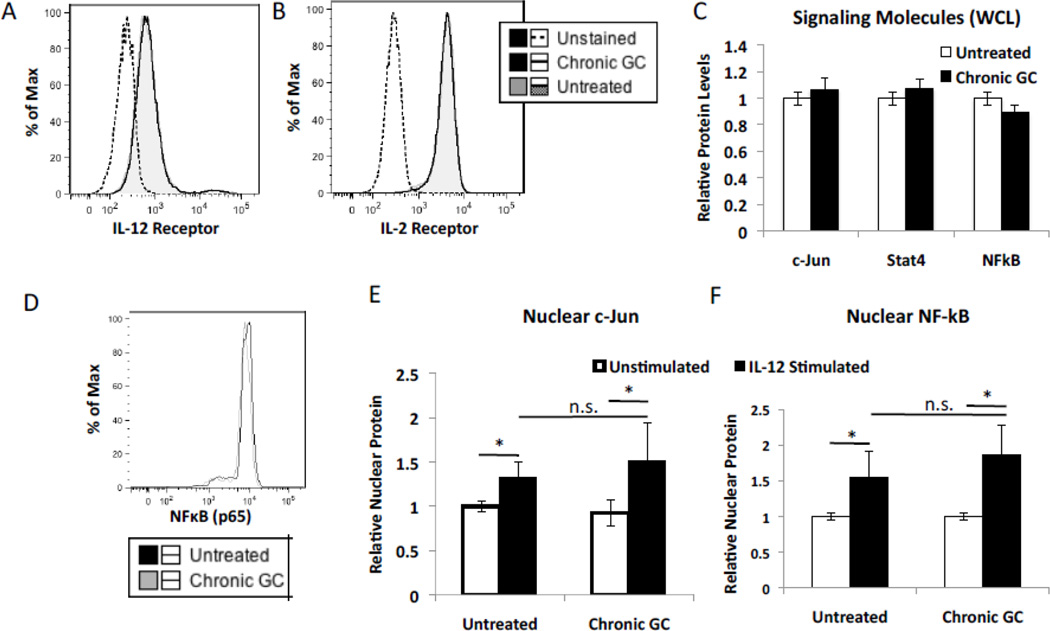
3.7 Effect of chronic GC treatment on cytokine receptor levels and signal transduction pathways
It is possible that chronic GC treatment may affect levels of cytokine receptors or alternatively the capacity of NK cells to complete signal transduction. Chronic GC treatment had no impact on the levels of the IL-12 receptor beta chain or the IL-2 receptor alpha chain, implying that chronic GC did not alter surface receptor density of these two cytokine receptors (Fig. 5A and B). Downstream of GR signaling is the activation and subsequent nuclear localization of the canonical proinflammatory signaling transcription factors NFκBp65 and AP-1 (c-Jun) as well as STAT4. Western blot analysis and ImageJ quantification demonstrated 5 days of chronic GC treatment to have no impact on the total cellular levels of these three transcription factors (Fig. 5C). Likewise, 5 days of chronic GC treatment did not alter the total cellular level of NFκBp65 (Fig. 5D). Quantification of NFκBp65 and AP-1 (c-Jun) in cell nuclear fractions isolated from NK cells treated for 5 days with GC or not (Untreated) demonstrated no difference in nuclear localization after IL-12 stimulation. (Fig. 5E–F). These data demonstrate NK cells exposed to chronic GC treatment to have no apparent signal transduction defect nor any apparent deficit in cytokine surface receptor levels.
Fig. 5.
Effect of chronic GC (5 day) treatment on NK cell receptor expression and signaling pathways. Flow cytometric analysis of (A) IL-12 and (B) IL-2 receptor surface expression by NK92 cells treated with GC for 5 days or not (untreated). (C) ImageJ quantification of whole cell lysates probed by western blot analysis for c-Jun, Stat4, and NF-kB (p65) levels in chronic GC treated and untreated cells. (D) Flow cytometric analysis of NF-kB (p65) in 5 day chronic GC treated and untreated cells. ImageJ quantification of nuclear (E) c-Jun and (F) NF-kB (p65) in chronic GC treated and untreated cells prior to and after IL-12 stimulation (40 pg). In all cases, relative protein levels in GC treated cells were compared to untreated cells set to 1. n=3 *; p<0.05.
4. Discussion
This investigation evaluated the effects of chronic low concentration GC exposure on the human NK cell line, NK92. This exposure to GC, termed chronic GC treatment, reduced histone acetylation of the proximal promoter regions for two NK granule constituents, perforin and granzyme B. The reduction in histone acetylation corresponded with reduced mRNA and protein level for each and was associated with reduced NK cell cytolytic activity (NKCA). In contrast, chronic GC treatment increased histone acetylation at the −22 kb enhancer region of IFNG and the proximal promoter region of IL6, as well as chromatin accessibility for each of these gene regulatory regions, priming the regions for transcription upon cellular stimulation. Increases in histone acetylation were associated with quantitatively increased mRNA and protein levels for these cytokines. These epigenetic and immunologic effects were demonstrated at the individual cellular level, in that chronic GC treatment affected levels of perforin, granzyme B, and IFN-gamma within individual cells, with little or no change in the percentage of cells positive for each of these NK cell effector proteins.
Conceptually, histone tail proteins possess highly basic amino-terminal tail domains located outside of the core nucleosome particle, which are accessible to multiple post-translational covalent modifications, especially acetylation [61]. Lysine acetylation neutralizes histone proteins, whereas deacetylation restores the positive charge of lysines. Following acetylation, nucleosome mobility on the DNA is enhanced, increasing accessibility of the promoter for transcription machinery [62]. Thus, acetylation increases and deacetylation decreases transcription factor access to regulatory regions. With 5 days of GC treatment, a quantitative reduction in the acetylation of H3K9 at the PRF1 and GZMB proximal promoters was noted. H3K9Ac is typically found at active promoters and enhancers and is highly correlated with transcription [63]. In the case of the PRF1 promoter a reduction in H3K27Ac and for the GZMB promoter H4K8Ac were also observed. These histone post translational modifications are typically found at active gene regulatory regions [56]. Reductions in histone acetylation at these gene regulatory regions would reduce gene transcription. As such, these reductions in histone acetylation likely contribute to the reduced production of transcripts for both of these effector proteins. In contrast, GC treatment increased acetylation of H4K8 and H3K27 for both the IFNG enhancer and the IL6 promoter. Increased histone acetylation at these gene regulatory regions would be expected to increase production of transcripts for both of these proteins upon cellular stimulation. For untreated cells, H3K4me3 with no H3K9me3 or H3K27me3 was noted. H3K4me3 typically marks transcription start sites and is associated with transcription potential at gene regulatory regions [64; 65]. H3K9me3 marks constitutive heterochromatin and H3K27me3 marks silenced genes [63; 66; 67; 68] indicating these gene regulatory regions to be located within euchromatin, either actively transcribed (PRF1 and GZMB) or with the potential for transcription (IFNG and IL6).
By use of this in vitro culture system it was possible to evaluate the effect of GC on NK cell histone post translational modifications over time. After 2 days of chronic GC treatment no significant change in the acetylation status of either the PRF1 or GZMB promoter region was noted. Only a slight numerical reduction in H3K9Ac was detected for both promoter regions at day 2. Significant reductions in H3K9Ac and H3K27AC were only identified by day 5 of chronic GC treatment. The reduced acetylation status of these residues corresponded with maximal reductions in perforin and granzyme B levels as well as maximal reductions in NK cell cytolytic activity. It is worth noting that after 2 and 5 days of chronic GC treatment, transcripts for PRF1 and GZMB were reduced, with detected reductions in perforin at days 4 and 5 and for granzyme B only at day 5. These data suggest that the transient reduction in transcript levels at day 2 were not sufficient to effect NKCA. The effect of GC on NKCA was only manifest after 5 days of continuous culture with GC when maximal reductions in acetylation at both promoters was observed. It is worth noting that a significant reduction in H3K4me3 was observed at day 2 of GC treatment for the GZMB promoter. H3K4me3 is associated with an open chromatin structure and transcriptional activation [69; 70]. It is possible that a reduction in this histone post translational modification contributed to reduced transcript levels on that day. The unexpected observation was the acetylation status of the enhancer region of IFNG and the promoter region of IL6. Both were increased with 5 days of chronic GC treatment. These epigenetic modifications coincided with increased transcription and protein levels of these proinflammatory cytokines when NK cells were stimulated, suggesting that increased acetylation status primed these promoters regions for increased transcription upon cellular stimulation. Interestingly, H3K4me3 was increased with GC treatment for both the enhancer region of IFNG and the promoter region of IL6, suggesting that these regulatory regions were open and primed for transcription by day 2. It is possible that this histone post translational modification may pioneer the changes in acetylation noted by day 5. The effect of GC on PRF1 and GZMB regulatory regions directly affected function, while the effect on proinflammatory cytokine gene regions was indirect resulting in a primed or poised condition, apparent only after cellular stimulation. These data demonstrate that GC can dichotomously regulate NK cell effector function epigenetically through two apparently independent mechanisms. These mechanisms do not appear to relate to a direct influence of chronic GC on; the cellular levels of GR, cytokine receptor density, GR sensitivity or signal transduction pathways. Chronic GC did not alter steady state GR or cytokine receptor protein levels. Although ligand activated GR can directly interact with proinflammatory transcription factors like AP-1 (Fos-Jun heterodimers) and NF-κB (p65-p50heterodimers) [71; 72; 73], chronic GC treatment did not alter their steady state levels. Nor did chronic GCs treatment affect lymphocyte responsiveness via the JAK-STAT signaling pathway and or via NF-kB or AP-1. Taken together, the effect of chronic GC treatment on NK cell function is through epigenetic modification of chromatin accessibility, both suppression of NKC A and priming of proinflammatory cytokine loci.
These observations may be of particular relevance to understanding stressful conditions in which GC levels are influenced by the experiences of chronic and or immediate stress. For example in humans, prior adverse experiences, which dysregulate GC have been shown to “prime” inflammatory responses, promoting excessive cytokine production [74; 75; 76; 77; 78; 79]. Likewise, in experimental animals stress has been shown to potentiate neuro-inflammation [20; 21; 22; 80; 81] increasing proinflammatory cytokine production in response to LPS challenge [82; 83]. In those experimental studies, blockade of the GC receptor with the GC receptor antagonist RU486 abolished the stress-induced inflammatory response, while exogenous GC administration, mimicked the effect of chronic stress. It is important to note that chronic stress did not by itself tonically increase proinflammatory cytokine levels, rather the effect of prior stress shifted the animal’s response toward a preferential proinflammatory phenotype [20: 21; 22]. It has been suggested that the proinflammatory effects of GC are limited to the brain [83]. However, GC have been shown to potentiate the peripheral (liver) proinflammatory response to a challenge of LPS, if GCs are administered prior to challenge. The observations reported herein may also provide insight into various human conditions in which an apparent dichotomous phenotype is demonstrable. For example, psychological stress has been associated with reduced peripheral blood NKCA and with increased serum or produced levels of proinflammatory cytokines [4; 19; 85; 86; 87]. In those cases psychological stress was typically associated with hypothalamic-pituitary-adrenal (HPA) axis activation and GC dysregulation. Of particular note is the condition of individuals with major depression, which is a disorder that is accompanied by not just immune dysregulation but also HPA axis activation [43; 88; 89; 90; 91; 92; 93]. Several systematic reviews and meta-analyses have found that major depression is reliably associated with a reduction in NKCA with an increase in circulating levels of proinflammatory cytokines [94; 95; 96]. However, no direct relationship was found between NKCA and proinflammatory cytokines [93]. The observations of this study, support the possibility that both immune suppression and immune enhancement can exist within a single cell population and that it may be essential to stimulate the cell population to uncover such a dichotomous phenotype.
There are limitations to this investigation. The work has been accomplished in a single human NK cell line, although this work does follow our previous investigative approach with this particular cell line [50]. Also, we have explored only a finite number of proinflammatory cytokines and granule constituents, but these are hallmark molecules for each. Further, we have investigated only 6 different histone post translational modifications, but the evaluation of those has identified unique differences relevant to transcription status. Finally, analysis of the observed effects was accomplished only in the presence of GC. The effect of GC removal on the observed effects was not evaluated.
In conclusion, a low concentration of GC reduced NKCA as well as the histone acetylation of the promoter regions of PRF1 and GZMB, which corresponded with reduced levels of perforin and granzyme B. In contrast, low GC concentration increased enhancer/promoter histone acetylation and accessibility for IFNG and IL6. These GC induced epigenetic effects resulted in a “preparative” or “priming” effect on the enhancer/promoter in that increased mRNA and protein levels were only detected upon stimulation (cellular activation) of the GC treated cells. Taken together, treatment with a low concentration GC decreased NK cell cytolytic activity and at the same time primed NK cells for enhanced proinflammatory cytokine production. This apparent dichotomous NK phenotype corresponded with histone post translational modifications at the promoter/enhancer gene regions of these immune effector molecules.
Highlights.
Glucocorticoids reduce NK cell cytotoxicity while at the same time prime NK cells for proinflammatory cytokine production.
Reduced NK cell lytic activity is associated with reduced histone promoter acetylation for perforin and granzyme B.
Increased proinflammatory cytokine production is associated with increased histone acetylation of regulatory gene regions.
Acknowledgements
The study was supported in part by the National Cancer Institute R01-CA-134736. The authors gratefully acknowledge the expertise of Patricia Simms, Flow Cytometry Facility, Loyola University Chicago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicting interests.
References
- 1.Lannan EA, Galliher-Beckley AJ, Scoltock AB, Cidlowski JA. Proinflammatory actions of glucocorticoids: glucocorticoids and TNFalpha coregulate gene expression in vitro and in vivo. Endocrinology. 2012;153:3701–3712. doi: 10.1210/en.2012-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda TB, Morris SA, Hager GL. Complex genomic interactions in the dynamic regulation of transcription by the glucocorticoid receptor. Mol Cell Endocrinol. 2013;380:16–24. doi: 10.1016/j.mce.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revollo JR, Oakley RH, Lu NZ, Kadmiel M, Gandhavadi M, Cidlowski JA. HES1 is a master regulator of glucocorticoid receptor-dependent gene expression. Sci Signal. 2013;6 doi: 10.1126/scisignal.2004389. ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biondi M. Effects of stress on immune functions: An overview. In: Ader R, Cohen N, editors. Psychoneuroimmunology. New York: Academic Press; 2001. pp. 189–226. [Google Scholar]
- 5.Holbrook NJ, Cox WI, Horner HC. Direct suppression of natural killer activity in human peripheral blood leukocyte cultures by glucocorticoids and its modulation by interferon. Cancer Res. 1983;43:4019–4025. [PubMed] [Google Scholar]
- 6.Arima M, Plitt J, Stellato C, Bickel C, Motojima S, Makino S, Fukuda T, Schleimer RP. Expression of interleukin-16 by human epithelial cells. Inhibition by dexamethasone. Am J Respir Cell Mol Biol. 1999;21:684–692. doi: 10.1165/ajrcmb.21.6.3671. [DOI] [PubMed] [Google Scholar]
- 7.Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996;108:374–381. [PubMed] [Google Scholar]
- 8.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Snyers L, De Wit L, Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci U S A. 1990;87:2838–2842. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strickland RW, Wahl LM, Finbloom DS. Corticosteroids enhance the binding of recombinant interferon-gamma to cultured human monocytes. J Immunol. 1986;137:1577–1580. [PubMed] [Google Scholar]
- 11.Yeager MP, Pioli PA, Wardwell K, Beach ML, Martel P, Lee HK, Rassias AJ, Guyre PM. In vivo exposure to high or low cortisol has biphasic effects on inflammatory response pathways of human monocytes. Anesth Analg. 2008;107:1726–1734. doi: 10.1213/ane.0b013e3181875fb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiecolt-Glaser JK, Glaser R, Shuttleworth EC, Dyer CS, Ogrocki P, Speicher CE. Chronic stress and immunity in family caregivers of Alzheimer's disease victims. Psychosom Med. 1987;49:523–535. doi: 10.1097/00006842-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Barber A, Illner H, Shires GT. Bacterial translocation in burn injury. Semin Nephrol. 1993;13:416–419. [PubMed] [Google Scholar]
- 14.Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 15.Lim HY, Muller N, Herold MJ, van den Brandt J, Reichardt HM. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology. 2007;122:47–53. doi: 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren MK, Vogel SN. Opposing effects of glucocorticoids on interferon-gamma-induced murine macrophage Fc receptor and Ia antigen expression. J Immunol. 1985;134:2462–2469. [PubMed] [Google Scholar]
- 17.Bosch JA, Berntson GG, Cacioppo JT, Marucha PT. Differential mobilization of functionally distinct natural killer subsets during acute psychologic stress. Psychosom Med. 2005;67:366–375. doi: 10.1097/01.psy.0000160469.00312.8e. [DOI] [PubMed] [Google Scholar]
- 18.Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witek-Janusek L, Gabram S, Mathews HL. Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology. 2007;32:22–35. doi: 10.1016/j.psyneuen.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Frank MG, Watkins LR, Maier SF. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun 25 Suppl. 2011;1:S21–S28. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun. 2011;25:1408–1415. doi: 10.1016/j.bbi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapolsky RM, Glucocorticoids stress. and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 24.Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grayson J, Dooley NJ, Koski IR, Blaese RM. Immunoglobulin production induced in vitro by glucocorticoid hormones: T cell-dependent stimulation of immunoglobulin production without B cell proliferation in cultures of human peripheral blood lymphocytes. J Clin Invest. 1981;68:1539–1547. doi: 10.1172/JCI110408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1987;5:621–669. doi: 10.1146/annurev.iy.05.040187.003201. [DOI] [PubMed] [Google Scholar]
- 27.Wiegers GJ, Knoflach M, Bock G, Niederegger H, Dietrich H, Falus A, Boyd R, Wick G. CD4(+)CD8(+)TCR(low) thymocytes express low levels of glucocorticoid receptors while being sensitive to glucocorticoid-induced apoptosis. Eur J Immunol. 2001;31:2293–2301. doi: 10.1002/1521-4141(200108)31:8<2293::aid-immu2293>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 28.Gutsol AA, Sokhonevich NA, Seledtsov VI, Litvinova LS. Dexamethasone effects on activation and proliferation of immune memory T cells. Bull Exp Biol Med. 2013;155:474–476. doi: 10.1007/s10517-013-2182-5. [DOI] [PubMed] [Google Scholar]
- 29.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 30.Wiegers GJ, Reul JM. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol Sci. 1998;19:317–321. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- 31.Guyre CA, Gomes D, Smith KA, Kaplan JM, Perricone MA. Development of an in vivo antibody-mediated killing (IVAK) model, a flow cytometric method to rapidly evaluate therapeutic antibodies. J Immunol Methods. 2008;333:51–60. doi: 10.1016/j.jim.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 33.Dhabhar FS. Enhancing versus Suppressive Effects of Stress on Immune Function: Implications for Immunoprotection versus Immunopathology. Allergy Asthma Clin Immunol. 2008;4:2–11. doi: 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhabhar FS, McEwen BS. Psychoneuroimmunology. Burlington, MA: Elsevier Academic Press; 2007. Bi-directional effects of stress on immune function: Possible explanations for salubrious as well as harmful effects; pp. 723–760. [Google Scholar]
- 36.Eastman HB, Fawcett TW, Udelsman R, Holbrook NJ. Effects of perturbations of the hypothalamic-pituitary-adrenal axis on the acute phase response: altered C/EBP and acute phase response gene expression in lipopolysaccharide-treated rats. Shock. 1996;6:286–292. doi: 10.1097/00024382-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsusue S, Walser M. Healing of intestinal anastomoses in adrenalectomized rats given corticosterone. Am J Physiol. 1992;263:R164–R168. doi: 10.1152/ajpregu.1992.263.1.R164. [DOI] [PubMed] [Google Scholar]
- 39.Lekander M, Axen J, Knutsson U, Hoglund COlgart, Werner S, Wikstrom AC, Stierna P. Cytokine inhibition after glucocorticoid exposure in healthy men with low versus high basal cortisol levels. Neuroimmunomodulation. 2009;16:245–250. doi: 10.1159/000212385. [DOI] [PubMed] [Google Scholar]
- 40.Yeager MP, Rassias AJ, Pioli PA, Beach ML, Wardwell K, Collins JE, Lee HK, Guyre PM. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit Care Med. 2009;37:2727–2732. doi: 10.1097/ccm.0b013e3181a592b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole SW. Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 44.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller AH. Inflammation versus glucocorticoids as purveyors of pathology during stress: have we reached the tipping point? Biol Psychiatry. 2008;64:263–265. doi: 10.1016/j.biopsych.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ 11 Suppl. 2004;1:S45–S55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 47.Chan HW, Miller JS, Moore MB, Lutz CT. Epigenetic control of highly homologous killer Ig-like receptor gene alleles. J Immunol. 2005;175:5966–5974. doi: 10.4049/jimmunol.175.9.5966. [DOI] [PubMed] [Google Scholar]
- 48.Cippitelli M, Sica A, Viggiano V, Ye J, Ghosh P, Birrer MJ, Young HA. Negative transcriptional regulation of the interferon-gamma promoter by glucocorticoids and dominant negative mutants of c-Jun. J Biol Chem. 1995;270:12548–1256. doi: 10.1074/jbc.270.21.12548. [DOI] [PubMed] [Google Scholar]
- 49.Santourlidis S, Trompeter HI, Weinhold S, Eisermann B, Meyer KL, Wernet P, Uhrberg M. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J Immunol. 2002;169:4253–4261. doi: 10.4049/jimmunol.169.8.4253. [DOI] [PubMed] [Google Scholar]
- 50.Krukowski K, Eddy J, Kosik KL, Konley T, Janusek LW, Mathews HL. Glucocorticoid dysregulation of natural killer cell function through epigenetic modification. Brain Behav Immun. 2011;25:239–249. doi: 10.1016/j.bbi.2010.07.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bush KA, Krukowski K, Eddy JL, Janusek LW, Mathews HL. Glucocorticoid receptor mediated suppression of natural killer cell activity: identification of associated deacetylase and corepressor molecules. Cell Immunol. 2012;275:80–89. doi: 10.1016/j.cellimm.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burkhart BA, Ivey ML, Archer TK. Long-term low level glucocorticoid exposure induces persistent repression in chromatin. Mol Cell Endocrinol. 2009;298:66–75. doi: 10.1016/j.mce.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shnyreva M, Weaver WM, Blanchette M, Taylor SL, Tompa M, Fitzpatrick DR, Wilson CB. Evolutionarily conserved sequence elements that positively regulate IFN-gamma expression in T cells. Proc Natl Acad Sci U S A. 2004;101:12622–12627. doi: 10.1073/pnas.0400849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatton RD, Harrington LE, Luther RJ, Wakefield T, Janowski KM, Oliver JR, Lallone RL, Murphy KM, Weaver CT. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity. 2006;25:717–729. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hezroni H, Sailaja BS, Meshorer E. Pluripotency-related, valproic acid (VPA)- induced genome-wide histone H3 lysine 9 (H3K9) acetylation patterns in embryonic stem cells. J Biol Chem. 2011;286:35977–35988. doi: 10.1074/jbc.M111.266254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hezroni H, Tzchori I, Davidi A, Mattout A, Biran A, Nissim-Rafinia M, Westphal H, Meshorer E. H3K9 histone acetylation predicts pluripotency and reprogramming capacity of ES cells. Nucleus. 2011;2:300–309. doi: 10.4161/nucl.2.4.16767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest. 2008;134:394–401. doi: 10.1378/chest.08-0440. [DOI] [PubMed] [Google Scholar]
- 60.Ramamoorthy S, Cidlowski JA. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr Dev. 2013;24:41–56. doi: 10.1159/000342502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spencer VA, Davie JR. Role of covalent modifications of histones in regulating gene expression. Gene. 1999;240:1–12. doi: 10.1016/s0378-1119(99)00405-9. [DOI] [PubMed] [Google Scholar]
- 62.Ura K, Wolffe AP. Reconstruction of transcriptionally active and silent chromatin. Methods Enzymol. 1996;274:257–271. doi: 10.1016/s0076-6879(96)74022-3. [DOI] [PubMed] [Google Scholar]
- 63.Northrup DL, Zhao K. Application of ChIP-Seq and related techniques to the study of immune function. Immunity. 2011;34:830–842. doi: 10.1016/j.immuni.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izzo A, Schneider R. Chatting histone modifications in mammals. Brief Funct Genomics. 2010;9:429–443. doi: 10.1093/bfgp/elq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliver SS, Denu JM. Dynamic interplay between histone H3 modifications and protein interpreters: emerging evidence for a "histone language". Chembiochem. 2011;12:299–307. doi: 10.1002/cbic.201000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato S, Yokoyama A, Fujiki R. Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem Sci. 2011;36:272–281. doi: 10.1016/j.tibs.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Cuddapah S, Barski A, Zhao K. Epigenomics of T cell activation, differentiation, and memory. Curr Opin Immunol. 2010;22:341–347. doi: 10.1016/j.coi.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem. 2011;80:473–499. doi: 10.1146/annurev-biochem-061809-175347. [DOI] [PubMed] [Google Scholar]
- 69.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 70.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 71.Barnes PJ, Adcock IM. Transcription factors and asthma. Eur Respir J. 1998;12:221–234. doi: 10.1183/09031936.98.12010221. [DOI] [PubMed] [Google Scholar]
- 72.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993;14:459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- 74.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 75.Dantzer R, Wollman E, Vitkovic L, Yirmiya R. Cytokines and depression: fortuitous or causative association. Mol Psychiatry. 1999;4:328–332. doi: 10.1038/sj.mp.4000572. [DOI] [PubMed] [Google Scholar]
- 76.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 77.Johnson JD, O'Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF. Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology. 2002;27:353–365. doi: 10.1016/s0306-4530(01)00057-9. [DOI] [PubMed] [Google Scholar]
- 78.Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- 79.Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord. 2001;63:85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- 80.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Johnson JD, O'Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–R432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- 82.Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, Cano J. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging. 2011;32:85–102. doi: 10.1016/j.neurobiolaging.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 83.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 85.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 86.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J, stress Chronic, stressors daily. and circulating inflammatory markers. Health Psychol. 2012;31:264–268. doi: 10.1037/a0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 89.Kronfol Z, House JD, Silva J, Jr., Greden J, Carroll BJ. Depression, urinary free cortisol excretion and lymphocyte function. Br J Psychiatry. 1986;148:70–73. doi: 10.1192/bjp.148.1.70. [DOI] [PubMed] [Google Scholar]
- 90.Maes M, Bosmans E, Suy E, Minner B, Raus J. A further exploration of the relationships between immune parameters and the HPA-axis activity in depressed patients. Psychol Med. 1991;21:313–320. doi: 10.1017/s0033291700020419. [DOI] [PubMed] [Google Scholar]
- 91.Miller AH, Asnis GM, Lackner C, Halbreich U, Norin AJ. Depression, natural killer cell activity, and cortisol secretion. Biol Psychiatry. 1991;29:878–886. doi: 10.1016/0006-3223(91)90054-p. [DOI] [PubMed] [Google Scholar]
- 92.Miller GE, Cohen S, Herbert TB. Pathways linking major depression and immunity in ambulatory female patients. Psychosom Med. 1999;61:850–860. doi: 10.1097/00006842-199911000-00021. [DOI] [PubMed] [Google Scholar]
- 93.Pike JL, Irwin MR. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain Behav Immun. 2006;20:169–174. doi: 10.1016/j.bbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 94.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 95.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 96.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]