Abstract
Objective
To describe the methods and baseline characteristics of a VA Cooperative Studies Program trial designed to compare the effectiveness of 1) alpha-tocopherol, a vitamin E antioxidant, 2) memantine (Namenda®), an NMDA antagonist, 3) their combination, and 4) placebo in delaying clinical progression in Alzheimer disease.
Design
Multi-center, randomized, placebo controlled trial
Setting
14 VA medical centers.
Patients
613 patients with a diagnosis of possible or probable Alzheimer's disease of mild-to-moderate severity defined as a Mini-Mental State Examination (MMSE) total score between 12 and 26 inclusive who were taking an acetylcholinesterase inhibitor.
Intervention
: 2,000 IU/day of alpha-tocopherol + placebo, 20 mg/day of memantine + placebo, 2,000 IU/day of alpha-tocopherol + 20 mg/day of memantine, or double placebo.
Main Outcome Measures
The primary outcome is the Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory (ADCS-ADL). Secondary outcome measures include the MMSE, the Alzheimer's Disease Assessment Scale - Cognitive portion, the Dependence Scale, the Neuropsychiatric Inventory, and the Caregiver Activity Survey.
Results
The majority of the enrolled patients were male (97%), white (86%) with a mean age of 79. The mean scores of the ADCS-ADL, and MMSE, at entry were 57, and 21, respectively.
Conclusion
This large multicenter trial will address the unanswered question of the long-term safety and effectiveness of alpha-tocopherol, memantine and their combination in patients with mild-to-moderate Alzheimer's disease taking an acetylcholinesterase inhibitor. The results are expected in early 2013.
Keywords: Alzheimer's disease, alpha-tocopherol, vitamin E, memantine, Namenda, acetylcholinesterase inhibitor, randomized clinical trial
Introduction
Cognitive impairment, functional decline, and behavioral symptoms that characterize Alzheimer's disease are associated with brain cholinergic loss,1, oxidative stress,2 and excessive glutamate activity.3,4 Current therapeutic strategies include efforts to 1) enhance cholinergic neuronal function with an acetlycholinesterase inhibitor, 2) promote neuroprotective effects with the administration of an antioxidant, and 3) block pathologic activity of excessive glutamate with a moderate-affinity NMDA antagonist. A combination of pharmacological therapies is potentially more effective than individual treatments alone. To test this hypothesis, this study examines the effectiveness of drug treatment with combinations of 1) an acetylcholinesterase inhibitor (AChEI), which facilitates central acetylcholine neurotransmission; 2) alpha-tocopherol (vitamin E), a fat soluble vitamin and antioxidant that has been shown to slow the rate of progression of moderately severe AD;5 and 3) memantine (Namenda©), a moderate-affinity NMDA antagonist that blocks excessive stimulation of NMDA receptors by glutamate6 and is FDA approved for the treatment of moderately severe Alzheimer's disease.
Methods
Overview of Study Design
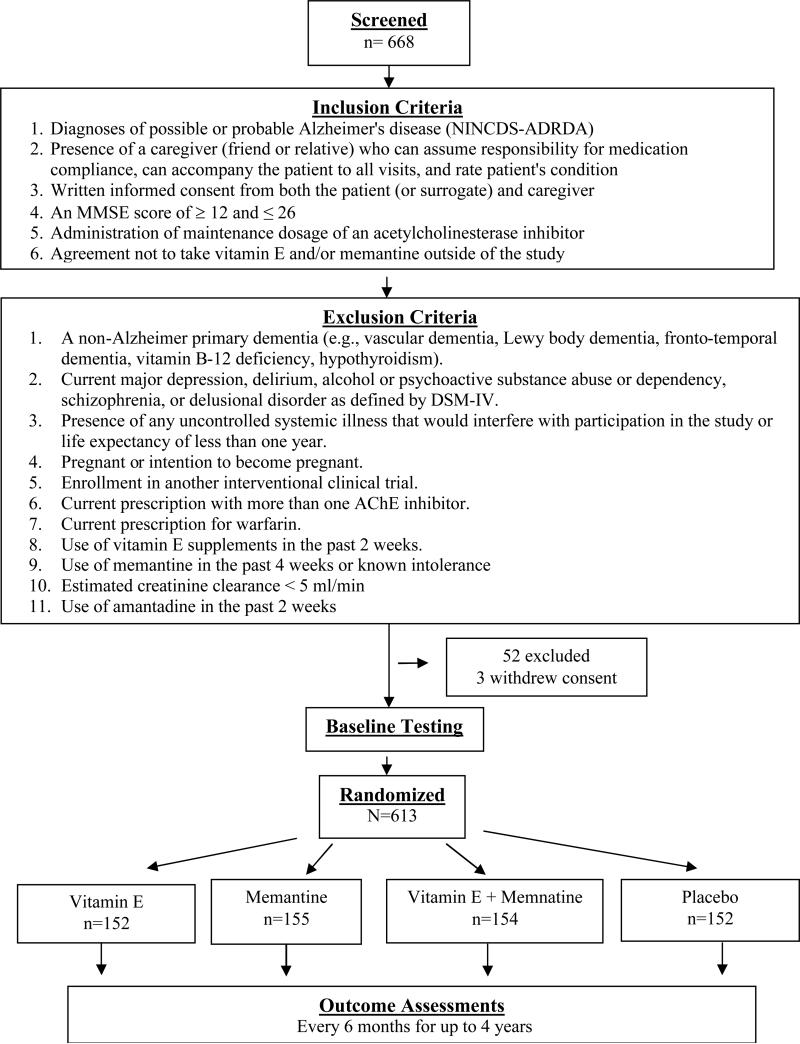
The Department of Veterans Affairs (VA) Cooperative Study Program (CSP) Trial of Vitamin E and Memantine in Alzheimer's disease (TEAM-AD) was designed as a double-blind, placebo-controlled, randomized clinical trial to assess the efficacy of 2,000 international units per day (IU/d) of alpha-tocopherol, 20 mg per day of memantine (Namenda®), and the combination in delaying clinical progression in patients with Alzheimer's disease who are currently taking an AChEI. The target population was Veterans with a diagnosis of possible or probable Alzheimer's disease7of mild-to-moderate severity defined as a Mini-Mental State Examination (MMSE) total score between 12 and 26 inclusive.8 Figure 1 displays an overall schematic of the study design.
Figure 1.
Flow of Participants in the Study
A total of fourteen VA medical centers participated in the trial (the organizational structure and study personnel for the trial are listed in the Appendix). The study protocol was approved by the institutional review boards at each participating site and the human rights committee at the West Haven CSP Coordinating Center. The trial is monitored for efficacy and safety by an independent Data Monitoring Committee (DMC) and is registered on www.clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT00235716). All participants or their surrogates gave written informed consent prior to study participation.
Study Hypotheses and Objectives
The primary study hypothesis is that compared with placebo, alpha-tocopherol and/or memantine will significantly delay clinical progression in mild-to-moderately demented patients with Alzheimer's disease who are currently taking an AChEI [donepezil (Aricept®), rivastigmine (Exelon®), or galantamine (Razadyne®)] and that combination treatment with alpha-tocopherol and memantine will add further incremental benefit. Secondary study hypotheses are that alpha-tocopherol, memantine and the combination will slow cognitive decline; slow functional decline; improve behavioral symptoms; and reduce caregiver burden, all relative to placebo. Randomization was performed centrally by the coordinating center and stratified by site using a random permuted block design of randomly varying sizes. The patient, caregivers, and all site investigators were blinded to the treatment assignment.
Treatment Regimens
Eligible patients who were currently taking an AChEI were randomly assigned to either 2,000 IU per day of alpha-tocopherol plus a matching placebo for memantine, 20 mg per day of memantine (Namenda®) plus a matching placebo for alpha-tocopherol, the combination of these two agents, or matching placebos for both memantine and alpha-tocopherol. Caregivers of study participants were requested to take on the responsibility of medication compliance for participants.
Alpha-tocopherol (or matching placebo) was given as an oral dose of 1000 IU twice a day. Dosage adjustments are allowed depending on participants’ tolerability of the regimen. The form of vitamin E used in this study is dl-alpha-tocopheryl acetate (“synthetic” vitamin E; DSM Nutritional Products, Inc.) formulated as hard-gelatin, liquid-filled capsules containing soybean oil (Arista Industries, Inc.). Encapsulation of the oils was completed by the VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center.
Memantine (or matching placebo) was titrated over four weeks to a maintenance dosage of 10 mg twice a day. . At the end of the titration period, participants were taking four 5-mg tablets daily, two in the morning and two in the evening. For an individual whose estimated creatinine clearance at entry or during follow-up was < 30 mL/min, site investigators were directed to reduce the dosage of memantine to 5 mg bid.
Outcome Measures
The primary endpoint of the study is the Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS/ADL) Inventory. 9 The ADCS/ADL Inventory is designed to assess functional abilities to perform activities of daily living in Alzheimer patients with a broad range of dementia severity. The total score ranges from 0 to 78 with higher scores indicating greater abilities.
Secondary outcome measures include 1) the MMSE,7 ( the most widely used instrument for the assessment of dementia severity and dementia progression; 2) the Alzheimer's Disease Assessment Scale - Cognitive Subscale (ADAS-cog),10,11 a widely used measure of cognitive function; 3) the Dependence Scale,12 an instrument used in patients with Alzheimer's disease to measure function; 4) the Neuropsychiatric Inventory (NPI),13 an assessment of psychological and behavioral problems in patients with dementia; and 5) the Caregiver Activity Survey (CAS),14 a measure of caregiver time and distress in caring for patients with dementia.
All serious adverse events (SAEs) and non-serious adverse events (AEs) were recorded. Caregivers and patients were queried in general about adverse experiences at each contact and specifically queried for patient falls, syncope, and congestive heart failure diagnoses due to concerns from previous studies that these events could be related to high dose alpha-tocopherol treatment.5,15 All randomized participants were followed in clinic every 6-months for a minimum of 6 months to a maximum of 4 years.
Analytical Plans
The original sample size for the study was 840 participants (155 per treatment group). This sample size was selected to provide 90% power to detect a 4-point mean treatment difference in the ADCS/ADL inventory by the end of an average of 2.5 years of follow-up with a type-I error of 0.0083 to control for 6 treatment comparisons and adjusted for 2.5% losses per 6 month follow-up. The original sample size used an estimated standard deviation of the ADCS/ADL of 12 units16 and the correlation of the repeated ADCS/ADL measurements within participants of 0.50. A re-estimation of sample size was conducted prior to the scheduled end of recruitment that included the observed variance and correlation of repeats of the primary outcome measure as well as the observed loss rate. To preserve the type-I error, the observed treatment effect was not used in the sample size re-estimation procedure. The outcome of the sample size re-estimation is presented in the Results section of this manuscript.
Interim analyses for treatment efficacy of the primary endpoint were planned for at least two time points using the methods of Haybittle and Peto17 but were designed to be flexible and allow the DMC to “look” on request. To protect the Type-I error, the three primary treatment comparisons of interest (the active treatment groups vs. the placebo group) were tested using a single 3 degrees of freedom test. If the joint test was significant at p<0.001, the individual treatment comparisons were examined by the DMC to consider recommending early termination of the trial, or eliminating one or more treatment arms.
The effect of treatment on the primary endpoint will be analyzed by a longitudinal repeated measures mixed effects model, adjusted for medical center as a random effect in the model and for the baseline ADCS/ADL Inventory score. Model building methods will be used to first determine the best mean structure of the outcome (e.g., time as linear or categorical, and treatment by time interactions) and then to determine the best fitting and most parsimonious covariance structure for the data. The sequentially rejective procedure of Hochberg will be used to determine statistical significance of all six possible treatment comparisons using an overall Type I error of 5% (two-sided).18 The same analytic approach will be used for the secondary outcomes except for the Dependence Scale, which will be analyzed by time to event (increase in dependence) model assuming a Weibull distribution to better account for interval censoring.19,20
Planned secondary analyses include an examination of baseline MMSE score on the treatment effect; an analysis of APO-E genotypes and their relationships with treatment effect and progression; the marginal effects of alpha-tocopherol and of memantine if there is no interaction among treatment arms in the four treatment group comparisons; and an examination of the relationship between serum alpha-tocopherol levels and/or serum memantine levels and outcomes.
Results
Between August 7, 2007 and March 31, 2012, 668 Veterans consented and were formally screened and 613 were randomized: 152 to Alpha-tocopherol alone, 155 to memantine alone, 154 to Alpha-tocopherol plus memantine, and 152 to placebo (Figure 1). The most common reasons for exclusion was a MMSE score out of the acceptable range of 12 to 26 (53% of those excluded).
In January 2011, the DMC was presented with the final sample size re-estimation that included an observed variance of 12.1 of the mean ADCS/ADL Inventory from the planned model, an observed correlation of 0.57 of the repeated ADCS/ADL measurements within participants, and an observed loss rate of 7.8% per 6 months follow-up. Based on these observations and an extension of the enrollment period of 1.5 years (to March, 2012) and the follow-up period of one year (to September, 2012), the sample size for the trial was re-estimated using the a protocol hypothesized slope of the treatment effect of 0.8 units per 6 months and original adjusted type-I error of 0.0083. The study extension increased the median follow-up from 2.5 years to approximately 3 years and thereby increased the estimated overall treatment effect from 4 units over 2.5 years to 4.8 units over 3 years. This increase in average treatment effect to 4.8 units increased the composite effect size (adjusted for attrition) from 37% to 45%. The re-estimated total sample size to achieve approximately 90% power was 600.
As expected in a sample of VA patients with mild-to-moderate AD, most Veterans were male (97%), white (86%), high school graduates or had some college education (78%) and slightly overweight (mean BMI = 27). Eleven percent of participants were Hispanic and 77% were white non-Hispanic. The mean age at enrollment was 79 years (SD = 7.1) with a range of 53 to 96. Notable positive clinical histories (>20%) include glaucoma or cataracts (36%), diabetes (27%), emotional problems (27%), musculoskeletal problems (27%), and heart disease (24%). The mean (SD) Charlson Risk Index score21 at entry was 2.5 (1.7) and the majority of participants (54%) had ≥ 2 comorbidity domains on the Kansas City Stroke Study Comorbidity Disease Index22,23 (Table 1).
Table 1.
Demographic and Clinical Characteristics of Randomized Participants
| Entry Characteristic | N=613 |
|---|---|
| Age in years, mean (SD), min–max | 78.8 (7.1), 53–96 |
| Male Sex, N (%) | 594 (97) |
| Race, N (%)* | |
| White | 530 (86) |
| Black or African American | 80 (13) |
| Other | 4 (1) |
| Hispanic ethnicity, N (%) | 66 (11) |
| Education, N (%) | |
| < High School Graduation | 137 (22) |
| High School Graduation | 207 (34) |
| Some College | 135 (22) |
| College Graduation or Advanced Degree | 134 (22) |
| Body Mass Index, mean (SD) | 26.7 (4.4) |
| Systolic Blood Pressure, mean (SD) | 134 (17) |
| Diastolic Blood Pressure, mean (SD) | 73 (11) |
| Laboratory Values, N, mean (SD) | |
| International normalized ratio (INR) | 590 1.0 (0.3) |
| HDL Cholesterol, mg/dL | 608 48 (15) |
| LDL Cholesterol, mg/dL | 605 96 (32) |
| Total Cholesterol, mg/dL | 609 169 (38) |
| Triglycerides, mg/dL | 608 131 (73) |
| Fasting Glucose, mg/dL | 610 109 (36) |
| Homocysteine, μmol/dL | 586 13.6 (4.9) |
| Thyroid-stimulating Hormone, μIU/mL | 608 2.1 (1.4) |
| Vitamin B12, pg/mL | 607 604 (322) |
| Creatinine Clearance, ml/mn | 612 62.8 (22.7) |
| Creatinine Clearance, N (%) < 30 ml/mn | 23 (3.8) |
| Medical History, N (%) | |
| Glaucoma or Cataract | 222 (36) |
| Diabetes | 167 (27) |
| Emotional Problems | 166 (27) |
| Musculoskeletal Problems | 166 (27) |
| Heart Disease** | 146 (24) |
| Sleep Disorder | 87 (14) |
| Cerebrovascular Disease | 61 (10) |
| Chronic Pain Syndrome | 53 (9) |
| Peripheral Vascular Disease | 50 (8) |
| Renal Disease | 28 (5) |
| Parkinson's Disease | 10 (2) |
| Smoker (current or past) | 387 (63) |
| Charlson Risk Index Score, Mean (SD) | 2.5 (1.7) |
| Comorbidity Disease Index, N (%) | |
| ≤1 Domain | 283 (46) |
| 2 Domains | 158 (26) |
| ≥3 Domains | 172 (28) |
Race and ethnicity were assessed in the study to able to demonstrate generalizability and to conduct possible subgroup analyses. Race and ethnicity were self identified by study participants. More than one race indicated by one participant.
Heart Disease includes a history of myocardial infarction, congestive heart failure, and/or angina.
A total of 612 patients (99.8%) were on an AChEI at baseline; one patient was not on an AChEI, which represented a protocol violation. Donepezil and galantamine were the most commonly prescribed AChEI at baseline;65% and 32%, respectively . The mean (SD) number of weeks from any AChEI initiation to randomization was 53 (66) with the overall percentage of participants who had been taking an AChEI > 12 weeks at 72% (Table 2).
Table 2.
Concomitant Medications Use at Entry
| Concomitant Medications, N (%) | |
|---|---|
| Acetylcholinesterase Inhibitors (AChEI) | |
| Donepezil (Aricept®) | 400 (65) |
| Galantamine (Razadyne®) | 194 (32) |
| Rivastigmine (Excelon®) | 18 (3) |
| Weeks from AChEI Initiation to Randomization, N (%) | |
| ≤ 12 weeks | 170 (28) |
| > 12 weeks | 442 (72) |
| Statins | 380 (62) |
| Aspirin | 375 (61) |
| Antiplatelets, Anticoagulants, or Thrombolytics | 43 (7) |
| Anticholinergics | 26 (4) |
| Tertiary Tricyclic Antidepressants | 18 (3) |
| Other Antidepressants | 213 (35) |
| Antipsychotics | 37 (6) |
| Sedatives/Hypnotics | 37 (6) |
| Skeletal Muscle Relaxants | 27 (4) |
| Vitamin C | 59 (10) |
| Other Antioxidants* | 122 (23) |
Other possible antioxidants included: Vitamin A, B6, B12, Folate, Zinc, Selenium, Lycopene, and Magnesium
The overall mean (SD) score for the ADCS/ADL Inventory was 56.8 (14.2), ranging from 8 to 78. The overall mean (SD) score for the MMSE was 21.0 (3.6), ranging from 12 to 26 (the range of eligibility for the study) (Table 3). The means (SD) for the ADAS-cog, NPI, and CAS were 18.8 (8.4), 12.5 (13.4), and 6.8 (10.9), respectively. The most frequent stages of Dependence were levels two (55%) and three (22%).
Table 3.
Primary and Secondary Outcome Assessments at Entry
| Assessments (range of scale), Mean (SD), Min-Max | |
|---|---|
| Alzheimer's Disease Cooperative Study/Activities of Daily Living (0-78) | 56.8 (14.2), 8-78 |
| Mini-Mental State Examination (0-30) | 21.0 (3.6), 12-26 |
| Alzheimer's Disease Assessment Scale - Cognitive portion (0-70) | 18.8 (8.4), 2.3-56 |
| Neuropsychiatric Inventory (0-144) | 12.5 (13.4), 0-95 |
| Caregiver Activity Survey (0-144 hours) | 6.8 (10.9), 0-144 |
| Dependence Scale, N (%) | |
| Level 0 | 22 (4) |
| Level 1 | 27 (4) |
| Level 2 | 335 (55) |
| Level 3 | 134 (22) |
| Level 4 | 29 (5) |
| Level 5 | 66 (11) |
Discussion
VA TEAM AD was designed to assess the efficacy of 2,000 international units per day of alpha-tocopherol, 20 mg per day of memantine, and the combination in delaying clinical progression in mild-to-moderately demented patients with Alzheimer's disease. With the exception of a study sample composed predominantly of men, participants in CSP #546 are typical of patients who have been enrolled in clinical trials of Alzheimer patients with mild-to- moderate disease severity. 24-29 The study will be one of the largest and longest treatment trials in Alzheimer patients with mild-to-moderate dementia.30 It will also be the first large-scale clinical trial to assess not only the effectiveness of alpha-tocopherol in Alzheimer patients with mild-to-moderate dementia but also the combination of alpha-tocopherol and memantine. In addition, the study will provide valuable information on reported safety issues of alpha-tocopherol31 that have resulted in decreased prescribing of alpha-tocopherol for patients with Alzheimer's disease.32
The length of follow-up for CSP #546 is considerably longer than the follow-up period in most AD clinical trials.24-29 In a Cochrane review of AChEI treatment in Alzheimer's disease, most of the Alzheimer's clinical trials (N=12) averaged between 24 and 26 weeks of follow-up with only two trials that were a year (52 weeks) or longer (104 weeks).33 For an alpha-tocopherol study of subjects with mild cognitive impairment 34 and the Sano et al. study in moderately severe AD patients,5 the trial duration was 3 and 2 years, respectively. In three recent memantine clinical trials in mild-to-moderate AD,35-37 the trial duration for each study was only 24 weeks. The knowledge gained from the relatively long-term follow-up in CSP #546 is an important component of the trial and will make the study results unique.
At the same time the long-term follow-up in an AD population has proved to be challenging. At the time of the last sample size re-estimation, the overall withdrawal rate was 31% and much greater (7.8% per 6-months) than anticipated during study design (2.5% per 6-months). On the other hand, the overall CSP #546 withdrawal rate at that time was comparable with withdrawal rates that were reported and analyzed for 15 recently published clinical trials in AD patients with mild-to-moderate dementia of shorter duration.30 Among these 15 studies, the average withdrawal rate was 26% with a range of 15% to 28%. Clinical trial duration was not a factor in predicting withdrawal rates nor was there a difference between placebo and active medication.
The CSP #546 Executive Committee closely monitored the study's withdrawal rate stressed the importance of retention with sites, and attempted to address issues when possible. Some of the successful techniques used by participating sites to improve retention were: assisting patients and caregivers in navigating the VA system; connecting patients and caregivers with VA social work services; scheduling study appointments in conjunction with other appointments at the medical center; and conducting study visits over the telephone or in a patient's home when possible.
The sample size re-estimation procedure used in the trial allowed for a reduction in the target sample size (840 to 600) based partly on a larger than expected correlation between repeated measures but primarily due to the increase in overall effect size by increasing the average follow-up time of participants..
The original target sample size of 840 could not be achieved due to a lower than expected number of eligible patients, greater than anticipated staff workload to enroll and follow patients, and higher refusal rates for study participation from patients and caregivers. The primary reason for excluding potential participants who signed the informed consent was a MMSE score out of the acceptable range of 12 to 26 (53%); however, site staff reported that the prominent reasons for exclusions prior to data collection were warfarin use, off label memantine use, overall caregiver burden, study pill burden, and concern about the safety of high dose alpha-tocopherol due to a published meta analysis of the possible risk of alpha-tocopherol31 and popular media coverage of the article.
During the course of the enrollment period the study's Executive Committee closely monitored recruitment and attempted to address issues when possible. For example, it was decided that warfarin use would not be removed as an exclusion criterion due to concern about a possible increased risk of bleeding in patients taking a combination of warfarin and high dose alpha-tocopherol. To encourage enforcement of VA guidelines that memantine should only be prescribed for patients with moderately severe disease (MMSE < 15), discussions were held with pharmacy managers within the participating VA medical centers. Prescriptions received outside of the VA, although relatively few, could not be addressed. To try and reduce caregiver burden and stress, the Committee encouraged site staff to connect caregivers to Alzheimer's disease support groups and to help them navigate the VA system for other needed medical care. Research coordinators were also encouraged to travel to patients’ homes for follow-up visits whenever possible and to conduct follow-up assessments over the telephone if needed. Following IRB approval caregivers were provided published materials on Alzheimer's disease and care giving and were given study newsletters with information about the study with tips on patient management and reduction in caregiver stress. To address concerns about the safety of high dose alpha-tocopherol, information was provided to study staff and potential participant and caregivers about the d Data Monitoring Committee monitoring procedures in the study, the possible benefits of alpha-tocopherol in Alzheimer's disease, and published information38 about the methodological limitations of the meta-analysis by Miller et al.31
Although representative of the gender ratio for the Veteran population, one limitation of the study is the small percentage of women. There is no reason, however, to expect that the effect of alpha-tocopherol or memantine will be significantly different in females compared to males based on the results of past controlled studies showing no gender effect on outcome.5,34,39,40 There is also no strong plausible mechanism to support the belief that these therapies would have a different effect in males compared to females. An additional limitation of the study may be the higher than anticipated loss rate if that loss rate turns out to be informative, which is not implausible since losses due to caregivers’ inability to manage patients, nursing home placement, and death could all be related to the functional and cognitive decline of the Alzheimer's disease study participants over time.
The VA TEAM-AD study is a large multicenter trial that will address the unanswered question of the long-term safety and effectiveness of alpha-tocopherol, memantine, and the combination in patients with mild-to-moderate Alzheimer's disease who are taking an acetylcholinesterase inhibitor. The results of the study are expected in early 2013.
Acknowledgements
VA TEAM-AD was funded by the Department of Veterans Affairs Cooperative Studies Program. Forest Research Institute, a Division of Forest Laboratories, Inc. donated the memantine and matching placebo tablets. DSM Nutritional Products, Inc., donated the dl-alpha-tocopheryl acetate oil and funding for the purchase of the soybean oil from Arista Industries, Inc. Department of Veterans Affairs Cooperative Studies Program as sponsor of the trial participated in the design and oversaw the conduct of the study but had no input into data collection, management, analysis, and interpretation of the data or preparation, review, or approval of the manuscript.
Appendix
The following persons participated in the VA TEAM AD Study: Planning Committee - S. Asthana, M. Dysken, P. Guarino, J. Hanlon, M. Kunik, P. Lavori, P. Peduzzi, E. Perry, M. Sano, G. Schellenberg, T. Sunderland, G. Vatasseryτ, J. Vertrees, L. Volicer; Executive Committee - M. Dysken (Chair), S. Asthana, P. Guarino, M. Llorente, S. Love, M. Pallaki, M. Sano, G. Schellenberg, G. Vatasseryτ, J. Vertrees; Data Monitoring Committee - K. Kieburtz (Chair), C. Kawas, E. Lonn (resigned), P. Rabins, J. Rochon, D. Sultzer, R. Thomas; VA Cooperative Studies Program Human Rights Committee, West Haven, CT - R. Marottoli (Chair), H. Allore, D. Beckwith, W. Farrell, R. Feldman, R. Mehta, J. Neiderman, E. Perry, S. Kasl, M. Zeman; VA Site Investigators and Coordinators - Ann Arbor, MI: R.S. Turner, J. Heidebrink, C. Bloehm, J. Lord, K. Belanger, N. Ricci, C. Nwankwo, C. Fletcher; Baltimore, MD: D. Loreck, L. Katzel, K. Anderson, G. Kavanagh, S. Carney, A. Loreck; Bay Pines, FL: S. Reddy, N. Purohit, R. Tamayo, K. Monnell, A. Cruz, S. Huda, S. Zachariah, W.C. McCarthy; Boston, MA: N. Kowall, B. Seltzer, M. Chopra, K. Kolbe; Charleston, SC: J. Mintzer, O. Brawman-Mintzer, A. Senseney, D. Courtney, M. Stuckey, S. Russell, J.A. Sweeney; Cleveland, OH: M. Pallaki, P. Chen, T. Hornick, T. Dolinar, L. Abood, A. Coulter, S. Truax, D. Davis; Dallas, TX: R. Bakshi, G. Trapp, L. Moody, N. Flye, D. Turner-Knight; Iowa City, IA: C. Turvey, C. Woodman, A. Ray, K. Ekstam Smith, N. Suiter; Madison, WI: S. Asthana, C. Gleason, S. Barczi, C. Carlsson, N. Lane, M. Wroblewski, Z. Zugin, J.J. Fruehling; Miami, FL: M. Llorente, F. Adan, J. Malphurs, S. Prieto, M. Horvath, D. Santiago, G. Athappilly, A. Cortes, A. Vazquez, R. Dreize, F. Ostovary, E. Palaois, M. Oliveira, J. Pino, L. Claude; Minneapolis, MN: J. McCarten, H. Fink, C. Erickson, L. Becker-Grandle; Salisbury, NC: K. Monnell, K. Phillips, D. Eknoyan, K. Gordon; San Juan, PR: A. Vidal-Cardona, L. Arroyo, A. Melendez, L. Santiago, B. Padilla; Seattle, WA: S. Craft, J. Breitner, S. Thielke, K. Enstrom, J. Tidwell, R. Bridgan, K. Bowton, D. Dahl; Study Chair's Office, VA Health Care System, Minneapolis, MN - M. Dysken (Study Chair), S. Love, J. Tomaska; Central Laboratory, VA Health Care System, Minneapolis, MN - G. Vatasseryτ, Y. Segal, H. Quach; VA Cooperative Studies Program Coordinating Center, VA Connecticut Healthcare System, West Haven, CT - P. Guarino (Director, Study Biostatistician), M. Antonelli, E. Jobes, C. Joncas, S. Joyner, K. Kirkwood, P. Peduzzi, M. Perry, E. Petrokaitis, J. Russo, J. Scholl, S. Yang, S. Zellner; VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, NM - M. Sather (Director), J. Vertrees (Study Clinical Research Pharmacist), S. Campbell, D. Conner, E. Copeland, A. Davis S. Jenkins, B. Matura; VA Cooperative Studies Program Site Monitoring, Auditing and Review Team, Albuquerque, NM - C. Haakenson, D. Krueger; VA Cooperative Studies Program Laboratory MAVERIC , VA Healthcare System, Boston, MA, - M. Brophy (Director), D. Humphries, D. Govan; VA Cooperative Studies Program DNA Bank Coordinating Center, VAMC Palo Alto, CA – J. Cockroft, S. Bobra, A. Baylosis, R. Dodson, I. Belitskaya-Levy; VA Office of Research and Development, Clinical Science R&D, Washington, DC - T. O'Leary (Director, Deputy CRADO), G. Huang (Deputy Director, Cooperative Studies Program)
τDeceased
Footnotes
SI's/Co-SI's
Judith L. Heidebrink, MD; VA Ann Arbor Healthcare System
David J. Loreck, MD; VA Maryland Healthcare System, Baltimore, MD
Leslie Katzel, MD; VA Maryland Healthcare System, Baltimore, MD
Karen Anderson, MD; VA Maryland Healthcare System; University of Maryland, Baltimore, MD
Angel R. Cruz, MD; Bay Pines VAMC
Shafe Huda, MD; Bay Pines VAMC
Sally B. Zachariah, MD; Bay Pines VAMC
Neil W. Kowall, MD; VA Boston Healthcare System
Mohit P. Chopra, MD; VA Boston Healthcare System
Jacobo E. Mintzer, MD; Ralph H. Johnson VAMC; Medical University of South Carolina, Charleston, SC
Donald Courtney, MD; Ralph H. Johnson VAMC, Charleston, SC
Peijun Chen, MD, MPH, PhD; Louis Stokes Cleveland VAMC
Rajbir S. Bakshi, MD; VA North Texas Healthcare System, Dallas, TX
George Trapp, MD, JD; VA North Texas Healthcare System, Dallas, TX
Carolyn L. Turvey, PhD; Iowa City VAMC; University of Iowa, Iowa City, IA
Catherine Woodman, MD, Iowa City VAMC; University of Iowa, Iowa City, IA
Julie Malphurs, PhD; VA Miami Healthcare System
Susana Prieto, MD; VA Miami Healthcare System
J. Riley McCarten, MD; VA Minneapolis Health Care System
Howard Fink, MD; VA Minneapolis Health Care System
Kimberly A. Monnell, MD; W.G. (Bill) Hefner VAMC, Salisbury, NC
Ana Vidal-Cardona, MD; VA Caribbean Healthcare System, San Juan, PR
Lillian M. Arroyo, MD; VA Caribbean Healthcare System, San Juan, PR
Suzanne Craft, PhD; VA Puget Sound Healthcare System, Seattle, WA
Stephen Thielke, MD; VA Puget Sound Healthcare System, Seattle, WA
Julie Tomaska, PhD; VA Minneapolis Health Care System
Contributor Information
Maurice W. Dysken, VA Minneapolis Health Care System; Dept of Psychiatry, University of Minnesota.
Peter D. Guarino, VA Connecticut Healthcare System, West Haven, CT.
Sanjay Asthana, William S. Middleton Memorial Veterans Hospital, Madison, WI.
Mary Sano, Bronx Veterans Medical Research Center.
Julia E. Vertrees, Cooperative Studies Program Clinical Research Pharmacy Coordinating Center (CSPCRPCC), Albuquerque, NM.
Gerard D. Schellenberg, University of Pennsylvania School of Medicine, Philadelphia, PA.
Susan. Love, VA Minneapolis Health Care System; Dept of Psychiatry, University of Minnesota.
Maria Llorente, Washington, DC VAMC.
Muralidhar Pallaki, Louis Stokes Cleveland VAMC.
References
- 1.Davies P. Studies on the neurochemistry of central cholinergic systems in Alzheimer's disease. In: Katzman R, Terry RD, Bick KL, editors. Alzheimer's disease: Senile dementia and related disorders. Raven Press; New York: 1978. pp. 453–459. [Google Scholar]
- 2.Markesbery WR. The roles of oxidative stress in Alzheimer disease. Arch Neurol. 1999;56:1449–1452. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 3.Cacabelos R, Takeda M, Winblad B. The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer's disease. Int J Geriatr Pscyhiatry. 1999;14:3–47. doi: 10.1002/(sici)1099-1166(199901)14:1<3::aid-gps897>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. NEJM. 1994;330:613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 5.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol or both as treatment for Alzheimer's disease. NEJM. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 6.Danysz W, Parsons C. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18:S23–S32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman DD, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force in Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Galasko D, Bennet D, Sano M, Ernesto C, Thomas R, Gundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11(2):S33–S39. [PubMed] [Google Scholar]
- 10.Mohs RC, Rosen WG, Davis KL. The Alzheimer's Disease Assessment Scale: An instrument for assessing treatment efficacy. Psychopharmacol Bull. 1983;19:448–450. [PubMed] [Google Scholar]
- 11.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 12.Stern Y, Albert SM, Sano M, Richards M, Miller L, Folstein M, Albert M, Bylsma FW, Lafleche G. Assessing patient dependence in Alzheimer's disease. J Gerontol. 1994;49:M216–M222. doi: 10.1093/geronj/49.5.m216. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi D, Gornbein J. The neuropsychiatric inventory: comprenhensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 14.Davis K, Marin D, Kane R, Patrick D, Peskind E, Raskind M, Puder K. The caregiver activity survey (CAS): development and validation of a new measure for caregivers of persons with Alzheimer's disease. Int J Geriatr. Psychiatry. 1997;11:978–988. doi: 10.1002/(sici)1099-1166(199710)12:10<978::aid-gps659>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Lonn E, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 16.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–26. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 17.Haybittle JL. Significance Testing in the Comparison of Survival Curves from Clinical Trials of Cancer Treatment. Eur J Cancer Clin Oncol. 1986;22:1279–1283. doi: 10.1016/0277-5379(86)90133-1. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 19.Kalbfleish JD, Prentice PL. The Statistical Analysis of Failure Time Data. John Wiley & Sons. New York, NY: 1980. [Google Scholar]
- 20.Odell PM, Anderson KM, D'Agostino RB. Maximum likelihood estimation for interval-censored data using a Weibull-based accelerated failure time model. Biometrics. 1992;48:951–9. [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40373:383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clinical Rehabilitation. 2002;16:420–8. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 23.Studenski S, Lai SM, Duncan PW, Rigler SK. The impact of self-reported cumulative comorbidity on stroke recovery. Ageing. 2004;33(2):195–8. doi: 10.1093/ageing/afh056. [DOI] [PubMed] [Google Scholar]
- 24.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 25.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, et al. Effect of dimebon on cognition, activities of daily living, behavior, and global function in patients with mild-to-moderate Alzheimer's disease: A randomized, double-blind, placebo-controlled study. The Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 27.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer's disease: A randomized trial. JAMA. 2011;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer's disease clinical trials. Alzheimer's Res Ther. 2010;2:34. doi: 10.1186/alzrt58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Int Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 32.Dysken MW, Kirk LN, Kuskowski M. Changes in vitamin E prescribing for Alzheimer patients. Am J Geriatr Psychiatry. 2009;17:621–4. doi: 10.1097/JGP.0b013e3181a31fcf. [DOI] [PubMed] [Google Scholar]
- 33.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database of Systematic Reviews. 2006;(1) doi: 10.1002/14651858.CD005593. Art. No.: CD005593. DOI: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. NEJM. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 35.Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14:704–15. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 36.Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT. Memantine MEM-MD-12 Study Group. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5:83–9. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 37.Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer's disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2008;13:97–107. doi: 10.3233/jad-2008-13110. [DOI] [PubMed] [Google Scholar]
- 38.Letters: Comments and Responses. Ann Int Med. 2005;143:150–160. [Google Scholar]
- 39.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer's disease. NEJM. 2003;348:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 40.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate-to-severe Alzheimer disease already receiving donepezil. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]