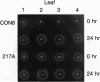
Abstract
Tobacco plants were transformed with a cDNA clone of chymotrypsin/trypsin-specific potato proteinase inhibitor II (PI2) under the control of a constitutive promoter. Although considerable levels of transgene expression could be demonstrated, the growth of Spodoptera exigua larvae fed with detached leaves of PI2-expressing plants was not affected. Analysis of the composition of tryptic gut activity demonstrated that only 18% of the proteinase activity of insects reared on these transgenic plants was sensitive to inhibition by PI2, whereas 78% was sensitive in insects reared on control plants. Larvae had compensated for this loss of tryptic activity by a 2.5-fold induction of new activity that was insensitive to inhibition by PI2. PI2-insensitive proteolytic activity was also induced in response to endogenous proteinase inhibitors of tobacco; therefore, induction of such proteinase activity may represent the mechanism by which insects that feed on plants overcome plant proteinase inhibitor defense.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson A. H., Heath R. L., Simpson R. J., Clarke A. E., Anderson M. A. Proteinase inhibitors in Nicotiana alata stigmas are derived from a precursor protein which is processed into five homologous inhibitors. Plant Cell. 1993 Feb;5(2):203–213. doi: 10.1105/tpc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. L., Begué-Cantón M. L., Blakeley R. L., Brubacher L. J., Feder J., Gunter C. R., Kézdy F. J., Killheffer J. V., Jr, Marshall T. H., Miller C. G. The determination of the concentration of hydrolytic enzyme solutions: alpha-chymotrypsin, trypsin, papain, elastase, subtilisin, and acetylcholinesterase. J Am Chem Soc. 1966 Dec 20;88(24):5890–5913. doi: 10.1021/ja00976a034. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- GREEN N. M. Competition among trypsin inhibitors. J Biol Chem. 1953 Dec;205(2):535–551. [PubMed] [Google Scholar]
- GREEN N. M. Kinetics of the reaction between trypsin and the pancreatic trypsin inhibitor. Biochem J. 1957 Jul;66(3):407–415. doi: 10.1042/bj0660407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M. Use of trypsin inhibitors to prevent autoactivation in the assay of enterokinase. Clin Chim Acta. 1983 Nov 15;134(3):363–367. doi: 10.1016/0009-8981(83)90375-3. [DOI] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Holm H., Jørgensen A., Hanssen L. E. Raw soy and purified proteinase inhibitors induce the appearance of inhibitor-resistant trypsin and chymotrypsin activities in Wistar rat duodenal juice. J Nutr. 1991 Apr;121(4):532–538. doi: 10.1093/jn/121.4.532. [DOI] [PubMed] [Google Scholar]
- Holm H., Krogdahl A., Hanssen L. E. High and low inhibitor soybean meals affect human duodenal proteinase activity differently: in vitro comparison of proteinase inhibition. J Nutr. 1988 Apr;118(4):521–525. doi: 10.1093/jn/118.4.521. [DOI] [PubMed] [Google Scholar]
- Iwai K., Fushiki T., Fukuoka S. Pancreatic enzyme secretion mediated by novel peptide: monitor peptide hypothesis. Pancreas. 1988;3(6):720–728. doi: 10.1097/00006676-198812000-00013. [DOI] [PubMed] [Google Scholar]
- Johnson R., Narvaez J., An G., Ryan C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9871–9875. doi: 10.1073/pnas.86.24.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M. A., Bakker P. L., Stiekema W. J. Quantitative determination of serine proteinase inhibitor activity using a radial diffusion assay. Anal Biochem. 1993 Jul;212(1):79–84. doi: 10.1006/abio.1993.1294. [DOI] [PubMed] [Google Scholar]
- Keeling D. J., Malcolm R. C., Laing S. M., Ife R. J., Leach C. A. SK&F 96067 is a reversible, lumenally acting inhibitor of the gastric (H+ + K+)-ATPase. Biochem Pharmacol. 1991 Jun 21;42(1):123–130. doi: 10.1016/0006-2952(91)90690-7. [DOI] [PubMed] [Google Scholar]
- McGurl B., Pearce G., Orozco-Cardenas M., Ryan C. A. Structure, expression, and antisense inhibition of the systemin precursor gene. Science. 1992 Mar 20;255(5051):1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- Pearce G., Johnson S., Ryan C. A. Purification and characterization from tobacco (Nicotiana tabacum) leaves of six small, wound-inducible, proteinase isoinhibitors of the potato inhibitor II family. Plant Physiol. 1993 Jun;102(2):639–644. doi: 10.1104/pp.102.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. P., White M., Marcus S., Wigler M. Concerted action of RAS and G proteins in the sexual response pathways of Schizosaccharomyces pombe. Mol Cell Biol. 1994 Jan;14(1):50–58. doi: 10.1128/mcb.14.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Salm T., Bosch D., Honée G., Feng L., Munsterman E., Bakker P., Stiekema W. J., Visser B. Insect resistance of transgenic plants that express modified Bacillus thuringiensis cryIA(b) and cryIC genes: a resistance management strategy. Plant Mol Biol. 1994 Oct;26(1):51–59. doi: 10.1007/BF00039519. [DOI] [PubMed] [Google Scholar]