Abstract
In bilaterally symmetric organisms, the midline is a critical organizing center for the developing central nervous system. There is a striking conservation of the molecules and mechanisms that control axon path finding at the midline in vertebrate and invertebrate nervous systems. The majority of axons in the CNS cross the midline before projecting to their contralateral synaptic targets and this crossing decision is under exquisite spatial and temporal regulation. Growing commissural axons initially respond to attractive signals, while inhibiting responses to repulsive signals. Once across, repulsion dominates, allowing axons to leave and preventing them from re-entering the midline. Here we review recent advances in flies and mice that illuminate the molecular mechanisms underlying the establishment of precise connectivity at the midline.
Keywords: Growth Cone, Midline, Repulsion, Attraction, Slit, Robo, Netrin, DCC, Ephrin, Morphogen, Dscam, Spinal Cord
Introduction
It has been known for over a decade that guidance cues of the conserved Netrin and Slit protein families are secreted by midline cells and play central roles in controlling midline crossing. Commissural axons in flies and mice are initially attracted to the midline by Netrin acting through its deleted in colorectal carcinoma (DCC) receptor- Frazzled (Fra) in Drosophila- and are insensitive to the Slit repellant [1,2]. During midline crossing, commissural axons upregulate the surface expression of Robo receptors, thereby acquiring Slit responsiveness (Figure 1). Slit repulsion forces commissural axons to exit the midline and prevents them from abnormally crossing back to the other side of the CNS. Once across the midline, axons of CNS interneurons respond to additional signals (including Slits and morphogens of the Wnt and Sonic Hedgehog families) to turn anteriorly at a stereotyped lateral position relative to the midline and continue toward their synaptic targets.
Figure 1.
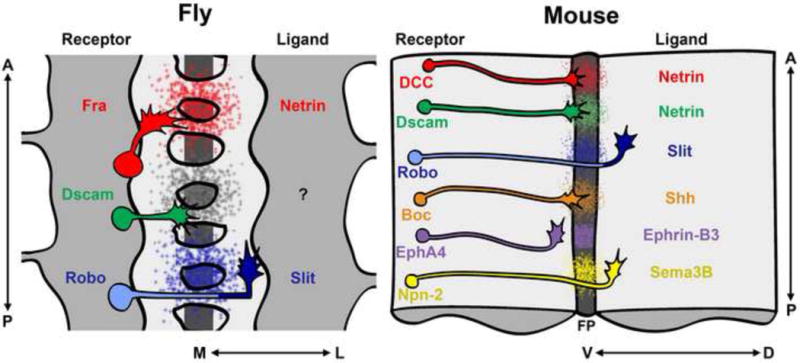
Ligands and receptors that mediate midline attraction and repulsion in fly and mouse. Diagrams show schematics of the Drosophila embryonic ventral nerve cord (left) and an open-book preparation of a mouse spinal cord (right). For both panels, anterior is up; the mediallateral axis of the fly CNS corresponds to the dorsal-ventral axis of the mouse spinal cord. In flies, Frazzled (Fra) and Dscam are the only known attractive midline receptors. Fra responds to a midline source of Netrin, while the attractive ligand for Dscam is unknown. Roundabout (Robo) receptors mediate midine repulsion in response to Slit in ipsilateral and post-crossing contralateral axons. Vertebrate homologs of Fra (DCC), Dscam, and Robo mediate similar guidance responses; however, in mice Dscam is an attractive Netrin receptor. In vertebrates, additional ligand-receptor systems influence midline crossing, including the Sonic hedgehog (Shh) morphogen, which promotes midline attraction via the Boc receptor, and the Ephrin-B3 (a transmembrane ephrin) and Sema3B (a secreted semaphorin) repellants, which signal through the EphA4 and Neuropilin-2 (Npn-2) receptors, respectively. For simplicity, schematics indicate guidance decisions of commissural axons and do not depict other components of Shh and Sema receptor complexes, but note that some repulsive ligand-receptor systems (for example Slit-Robo in fly and vertebrate, and EphrinB3-EphA4 in vertebrate) have been implicated in repelling both ipsilateral and post-crossing contralateral axons. FP, floor plate.
Here, we focus primarily on the vertebrate spinal cord and the Drosophila ventral nerve cord, considering each step of midline guidance sequentially and highlighting: 1) advances in understanding how morphogen signaling contributes to midline guidance, 2) the discovery of novel Netrin receptors that promote midline attraction, 3) new insights into mechanisms that regulate Slit responsiveness in mice and fly, 4) the role of Eph receptor repulsive signaling in regulating the establishment of midline circuits and 5) recent advances in delineating the mechanisms that guide post-crossing axons. Our discussion will emphasize events at the cell surface, as a full consideration of signaling mechanisms that act downstream of midline guidance receptors is beyond the scope of this review. We refer the reader to the following recent review of guidance receptor signaling [3].
Pre-crossing guidance I: Midline Attraction
Netrin and its DCC family receptors play a major role in axon attraction to the midline in worm, fly and all vertebrate systems that have been analyzed (Figure 1). Given Netrin’s ability to attract axons at long range, it has long been assumed to form a high ventral to low dorsal protein gradient; satisfyingly, in vivo visualization of such a gradient has recently been achieved in the vertebrate spinal cord [4]. However, whether all of Netrin function in commissural axon guidance depends on the formation of a diffusible gradient is an open question. Indeed, tethering endogenous Netrin at the fly midline can direct axon attraction and normal formation of axon commissures, although tethered Netrin is not sufficient to support long range Unc-5 receptor mediated repulsion [5]. Thus, in addition to acting as long-range diffusible chemoattractant, Netrin can also act as a contact dependent attractant.
Despite Netrin’s prominent role at the midline, genetic analysis in both flies and mice revealed that even in the total absence of Netrin, many commissural axons are still able to cross the midline- indicating that other midline attractants exist. A search for floor plate cues that could provide this function led to the exciting finding that the morphogen Sonic Hedgehog (Shh) acts as an attractant for commissural axons [6]. Shh isn’t the only morphogen to get into the act in directing midline guidance, indeed earlier studies had shown that the initial ventral growth of commissural axons is influenced by repulsive signaling by Bone Morphogenetic Proteins (BMPs) that are secreted from the roof plate [7]. How do morphogens that are defined by their ability to direct changes in gene expression in the nucleus steer growing axons- an event that clearly relies on local signaling to the growth cone cytoskeleton? In the case of Shh, identification of distinct receptors that mediate Shh’s chemoattractive effects, together with the demonstration that Shh-dependent guidance relies on non-canonical signaling through Src family kinases and does not require gene transcription has begun to answer this question [8,9]. In contrast, BMP-dependent axon guidance appears to rely on canonical BMP receptors, but it is likely that distinct downstream signaling pathways are engaged to repel axons ventrally toward the floor plate [10].
While Netrin mutant phenotypes in mice and fly suggested the existence of other attractants, experiments in cultured rat spinal cord explants also hinted that not all of Netrin’s influence on axon attraction is mediated by DCC. In the past year three groups, two working in vertebrate systems and the other in Drosophila, reported that the Down’s syndrome Cell Adhesion Molecule (DSCAM) is a high affinity Netrin-binding protein that can promote midline axon crossing (Figure 1) [11–13]. DSCAM is a type I transmembrane protein that has a similar extracellular domain composition to DCC and Robo proteins, including multiple immunoglobulin domains (Ig) and fibronectin type III repeats. In Drosophila, alternative splicing of the dscam locus generates an extraordinary number (~38016) of different DSCAM isoforms which mediate repulsive interactions during axon and dendrite morphogenesis through Ig-dependent isoform specific homophilic binding [14]. Although vertebrate DSCAM does not exhibit such diversity, it has also been implicated in neuronal arborization in mice and laminar targeting in the retina of chick [14].
To search for novel Netrin receptors, Ly and colleagues looked for Netrin-binding proteins that were expressed in commissural neurons and shared homology with DCC, while Liu et al., directly tested DSCAM as a candidate receptor since both Netrin and DSCAM had previously been shown to regulate p21-activated kinase activity. Inhibition of DSCAM using RNA interference in both mouse and chick results in a reduction in the ability of commissural axons to cross the midline [12,13]. Explant culture experiments show that DSCAM mediates Netrin-dependent turning of commissural axons in parallel to DCC: blocking DCC function alone partially inhibits Netrin turning responses, while blocking DCC and DSCAM together completely abolishes attractive turning [12,13]. Additional experiments in cultured Xenopus spinal neurons argue strongly that DSCAM can mediate Netrin-dependent attractive turning and that this effect requires its cytoplasmic domain [13]. How DSCAM activation leads to repulsion in the context of homophilic interactions and attraction in the context of heterophilic binding to Netrin is an important question for future studies.
Complementary genetic experiments in Drosophila also support a role for DSCAM in attracting axons to the midline; dscam mutations by themselves do not lead to major guidance defects, but they do strongly enhance the midline crossing defects observed in frazzled mutants, resulting in an almost complete absence of axon commissures [11]. Indeed the phenotype of dscam, frazzled double mutants is considerably stronger than the complete loss of Netrin, suggesting that at the fly midline dscam is likely to act in parallel to the Netrin-Fra pathway to promote attraction. This idea is further supported by the observation that over-expression of DSCAM leads to ectopic midline crossing, and that this effect does not depend on Netrin. Although the simplest interpretation of these results would argue that DSCAM does not act as a Netrin receptor at the fly midline, it is also possible that DSCAM contributes to both Netrin-dependent and Netrin-independent axon attraction; indeed, dose dependent genetic interactions between Netrin and dscam in the guidance of axons in the larval visual system support a role for DSCAM in Netrin-mediated guidance [11].
Pre-crossing guidance II: Preventing Premature Repulsion
Commissural axon projection is not achieved solely by promoting attraction toward the midline. Repulsive systems must be coordinately down-regulated to allow midline entry, and then quickly re-established to prevent lingering or re-crossing. An understanding of the synchronized regulation of attractive and repulsive signaling pathways is beginning to emerge, and it appears that distinct mechanisms are at work in two major branches of the animal kingdom.
The major midline repellant system in bilaterians is the Slit-Roundabout (Robo) pathway. Upon binding Slit, Robo receptors induce local rearrangement of the actin cytoskeleton resulting in growth cone repulsion [3]. The Slit-expressing midline thus represents a formidable barrier for Robo-expressing axons. How, then, are the majority of axons able to not only approach but cross this barrier?
The insect protein Commissureless (Comm) was first identified in a genetic screen for factors regulating midline crossing. As its name implies, in the absence of comm no axons cross the midline. Yet Comm does not mediate midline attraction per se; instead, Comm is a negative regulator of Slit-Robo repulsion and functions cell-autonomously in neurons to regulate intracellular sorting of Robo receptors. Transient activation of comm expression in pre-crossing commissural neurons ensures that newly-synthesized Robo proteins are trafficked not to the growth cone membrane, but to the late endocytic pathway, thus allowing axons to temporarily ignore the Slit-expressing midline barrier [15,16]. This mechanism allows temporally precise control of Slit responsiveness, as comm transcription is silenced shortly after midline crossing, allowing Robo trafficking to the growth cone to resume and thus restoring Slit sensitivity (Figure 2).
Figure 2.
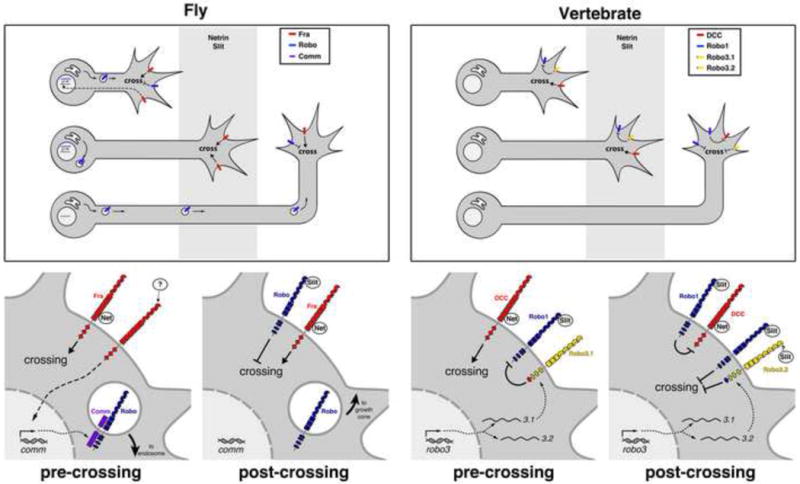
Pre-crossing and post-crossing regulation of attractive and repulsive guidance Top, schematics showing commissural axons before, during, and after crossing the CNS midline (grey). Bottom, enlarged schematics of pre- and post-crossing neurons indicating regulation and signaling output of axon guidance receptors. In pre-crossing fly commissural axons, Frazzled (Fra) mediates attractive signaling in response to midline-produced Netrin (Net). Fra also signals independently of Net to induce expression of commissureless (comm), a transmembrane sorting receptor that directs Robo-containing vesicles to the late endocytic pathway. After crossing, comm expression ceases and Robo is trafficked to the growth cone where it can mediate a Slit-dependent repulsive response and prevent re-crossing. Robo and Fra independently regulate crossing in fly axons. In vertebrates, the Fra homolog DCC signals Net-dependent attraction as axons approach the CNS ventral midline. Pre-crossing Slit sensitivity is antagonized by the Robo3.1 isoform of the Robo3/Rig-1 receptor. After crossing, Robo3.1 is replaced by Robo3.2, which functions alongside Robo1 (and Robo2, not shown) to prevent re-crossing. By analogy to Xenopus spinal axons, Robo1 could potentially act in post-crossing vertebrate commissural axons to silence the attractive output of DCC.
Notably, a recent report suggests that Comm’s Robo sorting activity cannot completely account for its in vivo function in promoting midline crossing. Gilestro (2008) replaced the endogenous robo gene with a mutant form insensitive to Comm sorting by homologous recombination (sorting-defective Robo: RoboSD); unexpectedly, freeing Robo from Comm-dependent sorting did not abolish commissure formation. Comm retained the ability to inhibit midline repulsion, however, as high-level misexpression of Comm produced a slit-like midline collapse phenotype despite simultaneous forced expression of RoboSD[17]. These provocative results suggest a second, sorting-independent mode of Robo regulation by Comm.
Recently, unexpected insight into the interplay between midline attraction and repulsion came from the observation that fra is necessary for comm expression in commissural neurons. Intriguingly, Fra activation of comm transcription is independent of the canonical Fra/DCC ligand Netrin. Thus, in addition to promoting midline attraction via classical Netrin-dependent signaling, Fra also functions in a Netrin-independent manner (presumably in response to some other, unidentified midline-derived cue) to activate comm expression and thereby down-regulate Slit-Robo repulsion (Figure 2) [18]. This example of cross-talk between attractive and repulsive midline signaling pathways gives a glimpse into the complex and delicate balance required for the precise control of commissural axon guidance.
Despite its absolute requirement for commissural axon guidance in Drosophila, Comm orthologs are undetectable in nematode or vertebrate genomes. Instead, other animal groups have invented alternative mechanisms for preventing premature midline repulsion. In vertebrates, the Robo family member Robo3/Rig-1 has taken on the Comm-like role of antagonizing repulsive Slit-Robo signaling to promote midline crossing. Like Comm, however, the function of Robo3 is more complex than initially thought.
Robo3/Rig-1 was initially described as an inhibitory component of the Slit-Robo pathway, expressed on pre-crossing commissural axons and promoting entry into the midline floor plate by decreasing their Slit sensitivity [19]. As Chen and colleagues recently discovered, however, this is only half the story. Robo3 exists in two functionally distinct isoforms generated by alternative splicing of the cytoplasmic tail [20]. One isoform, Robo3.1, is expressed on pre-crossing commissural axons as they approach and enter the floor plate; after crossing, Robo3.1 is turned off and Robo3.2 takes its place on post-crossing axons and functions as a repellant receptor to assist Robo1 and Robo2 in preventing re-crossing (Figure 2). Notably, the switch from Robo3.1 to Robo3.2 expression is not due to temporal regulation of transcription or splicing, suggesting functionally salient differences in translation or trafficking of the different isoforms [20]. Interestingly, in contrast to the spinal cord, in pre-cerebellar axons the failure of midline crossing that is observed in Robo3 mutants is not suppressed by removal of the other Robo receptors, suggesting that in certain contexts Robo3 can promote midline crossing independently of its ability to negatively regulate Slit responsiveness [21].
Although canonical repulsive pathways like Slit-Robo are essential for preventing recrossing by commissural axons, recent evidence indicates that repulsive signaling alone is insufficient to expel post-crossing axons from the midline. Stem Cell Factor (SCF) and its receptor Kit are both required for commissural axons to exit the floor plate [22]. In Steel (SCF) or Kit mutant mice, commissural axons extend toward and into the ventral floor plate normally, but then line up and linger at the contralateral edge. There is a switch in SCF sensitivity that corresponds with midline crossing and promotes floor plate exit by stimulating axon outgrowth. These results suggest that outgrowth-promoting pathways such as SCF-Kit cooperate with midline repellant pathways like Slit-Robo to ensure that the journey across the midline is one-way trip. Whether sensitivity to outgrowth-promoting factors is regulated by the same mechanisms that coordinate attractive and repulsive responses at the midline choice point will be an important issue to address.
Post-crossing guidance I: Midline Repulsion
Once across the midline, commissural axons increase the expression of Robo on their growth cones, thereby acquiring Slit sensitivity. How is the dramatic increase in Robo protein expression achieved? In Drosophila, comm expression is rapidly extinguished after midline crossing, which would presumably allow for the accumulation of Robo on the growth cone, but how comm expression is turned off is not known. In the spinal cord, essentially nothing is known about how this switch in Robo expression is achieved, nor is it known how the transition in the expression of Robo3 isoforms is controlled. Surprisingly the up-regulation of Robo in mice can even occur when commissural axons fail to cross the midline in Robo3 mutants [19]. Achieving a more complete understanding of the switch that controls the up-regulation of Robo expression is clearly a major challenge for the future. Genetic evidence in flies and mice strongly support the model that once on the growth cone Robo proteins expel commissural axons from the midline and prevent them from re-entering, and considerable progress has been made in understanding Robo signaling mechanisms [3]. In addition to its chemorepellent activity, experiments in cultured xenopus spinal neurons have shown that Robo can also directly antagonize and silence the output of the attractive Netrin receptor DCC, thereby coupling repulsion to the down-regulation of attraction and providing a possible molecular explanation for how post-crossing commissural axons lose sensitivity to Netrin [23]. It remains to be seen whether silencing occurs in vertebrates in vivo; however, the available genetic evidence in Drosophila suggests that if Robo does silence Fra/DCC, silencing is not strictly required to prevent axons from re-crossing the midline [24].
Although in Drosophila, Slit function appears to account for all midline repellent activity, this is clearly not the case in the vertebrate spinal cord, as semaphorins have also been implicated in repelling post-crossing axons [25]. More recently a role for ephrinB3 as a midline repellant in the spinal cord has emerged; mutations in either the ligand ephrinB3 or the receptor EphA4 cause abnormal midline crossing of corticospinal axons and interneuron axons in the central pattern generator (CPG), resulting in mice with a characteristic hopping gait phenotype[26]. These wiring defects are caused by a loss of ephrinB3/EphA4 forward signaling and can be attributed to a failure of axons to respond to midline ephrinB3. Incredibly, three independent groups have recently shown that mutations in a-chimaerin, a GTPase activating protein (GAP) for the Rac small GTPase, result in phenotypes almost identical to ephrinB3 −/− or EphA4 −/− mice, including the locomotory behavior phenotype [27–29]. Track tracing experiments in α-chimaerin mutants reveal that the corticospinal tract (CST) axons that control voluntary movements and axons of the CPG aberrantly cross the midline. The similarities of phenotypes and genetic interactions between ephrin B3 −/−, EphA4−/− and a-chimaerin mutants, together with the demonstration that a-chimaerin is a necessary mediator of ephrinB3/EphA4-induced growth cone collapse in cultured neurons, strongly argue that this GAP functions as an essential component in ephrinB3 forward signaling [27–29]. These observations are particularly exciting because they represent a clear example of a behavioral deficit associated with mis-regulation of midline guidance.
Post-crossing guidance II: Lateral Position
The influence of midline-derived cues on axon guidance does not end once commissural axons have reached the contralateral side of the CNS. After passing the midline choice point, commissural axons turn and extend along pathways running the length of the CNS at precise distances from the midline. The role of Shh and Wnt signals in regulating Anterior/Posterior guidance of post-crossing commissural axons has been reviewed recently [30], so here we briefly discuss the questions of how axons choose how far away from the midline to extend, and then, which specific pathway to join?
Nearly a decade ago, the Goodman and Dickson laboratories discovered that Robo receptors dictate longitudinal pathway choice in Drosophila, presumably in response to midline-produced Slit [1]. A few years later, Long and colleagues provided evidence that vertebrate Robo1 and Robo2 played a similar role in positioning post-crossing axons within the ventral and lateral funiculi of the mouse spinal cord [31]. However, these roles do not appear to be strictly analogous, as in mice loss of Robo1 or Robo2 redirected axons either farther from or closer to the midline, respectively, while in flies loss of robo2 or robo3 moved axons in one direction only: closer to the midline.
Recent work in other regions of the vertebrate CNS supports the idea that midline-derived cues influence longitudinal pathway formation. In chick and mouse hindbrain, descending longitudinal axons form a number of discrete tracts at specific distances from the midline. Ectopic induction of floor plate (in chick) or genetic ablation of floor plate (in mouse) altered the trajectory of these tracts, in the latter case resulting in both ectopic midline crossing and aberrant longitudinal guidance. Loss of Slit or its Robo1 and Robo2 receptors recapitulated guidance defects caused by loss of floor plate. However, unlike the previous studies in mouse spinal cord and fly embryonic CNS, no consistent shifts in hindbrain longitudinal tract position, either toward or away from the midline, resulted from the loss of Robo1 or Robo2 [32].
In the zebrafish posterior tuberculum, attractive and repulsive cues appear to cooperate in positioning the diencephalospinal longitudinal tract. Loss of astray/Robo2 causes dopaminergic (DA) axons to extend closer to the midline. Knocking down dcc or netrin1 function was sufficient to restore the normal positioning of DA axons, suggesting a balance between attractive Netrin-DCC signaling and repulsive Slit-Robo2 signaling in specifying the precise lateral position of this tract [33].
Conclusions
The past several years have seen significant progress in determining the molecular mechanisms that control midline guidance. In particular, the discovery of new pathways regulating midline attraction and midline exit and insights into how Slit responsiveness is regulated in invertebrate and vertebrate models continue to enrich our understanding of how attractive and repulsive signals are coordinated during midline crossing. Future insights into how the temporal transition in growth cone responses at the midline is achieved, how guidance receptors transmit their signals and how these signals are integrated within the growth cone will be instrumental in understanding how the best laid tracts of mice and flies seldom go awry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 2.Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and their receptors. Adv Exp Med Biol. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell M, Chance RK, Bashaw GJ. Axon Growth and Guidance: Receptor Regulation and Signal Transduction. Annu Rev Neurosci. 2009 doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 6.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 7.Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–141. doi: 10.1016/s0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- 8.Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- •9.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. In this paper, a comprehensive series of experiments demonstrate that the role of Shh in promoting commissural axon attraction to the floor plate is independent of its role in regulating gene expression through the Gli transcription factors. Instead, the authors show using a combination of in vitro co-culture experiments and in vivo genetic manipulations that Shh controls growth cone turning by regulating the asymmetric activation of Src family tyrosine kinases. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi K, Phan KD, Butler SJ. BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development. 2008;135:1119–1128. doi: 10.1242/dev.012989. [DOI] [PubMed] [Google Scholar]
- •11.Andrews GL, Tanglao S, Farmer WT, Morin S, Brotman S, Berberoglu MA, Price H, Fernandez GC, Mastick GS, Charron F, et al. Dscam guides embryonic axons by Netrin-dependent and -independent functions. Development. 2008 doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •12.Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, Wu JY. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci U S A. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••13.Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. These three studies report the discovery that in addition to its well-established role in regulating adhesive and repulsive interactions through selective homophilic binding, DSCAM can act as an attractive Netrin receptor. All three studies report high affinity binding data for DSCAM and Netrin and loss of function genetic experiments in all three papers support a role for DSCAM in promoting midline crossing. 12 and 13 provide in vitro evidence using co-culture experiments that DSCAM promotes attractive axon turning in response to Netrin, while 11 suggests that DSCAM can also promote midline crossing independently of Netrin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 16.Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- 17.Gilestro GF. Redundant mechanisms for regulation of midline crossing in Drosophila. PLoS One. 2008;3:e3798. doi: 10.1371/journal.pone.0003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••18.Yang L, Garbe DS, Bashaw GJ. A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance. Science. 2009 doi: 10.1126/science.1171320. This paper reports that in addition to mediating attraction in response to Netrin, the Drosophila Fra receptor functions independently of Netrin to regulate the mRNA expression of Commissureless, a key regulator of midline repulsion. Genetic evidence suggests that this dual function of Fra promotes midline crossing by coupling attraction to the down-regulation of Slit repulsion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- ••20.Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. 2008;58:325–332. doi: 10.1016/j.neuron.2008.02.016. In this study, the authors report the discovery that the divergent Robo receptor Rig1/Robo3 has dual functions in the control of midline crossing in the spinal cord. The authors demonstrate that the Robo3 locus is alternatively spliced to produce two isoforms that have distinct roles in pre versus post-crossing commissural axons. Robo 3.1 promotes crossing by preventing premature responses to Slit, while Robo 3.2 cooperates with the Robo 1 receptor to prevent post-crossing commissural axons from re-entering the midline. [DOI] [PubMed] [Google Scholar]
- 21.Di Meglio T, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A. Molecular mechanisms controlling midline crossing by precerebellar neurons. J Neurosci. 2008;28:6285–6294. doi: 10.1523/JNEUROSCI.0078-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •22.Gore BB, Wong KG, Tessier-Lavigne M. Stem cell factor functions as an outgrowth-promoting factor to enable axon exit from the midline intermediate target. Neuron. 2008;57:501–510. doi: 10.1016/j.neuron.2008.01.006. In this study a novel function for floor plate derived Stem Cell Factor (SCF) and its neuronal receptor Kit is reported. Loss of function genetic experiments demonstrate that in the absence of SCF or Kit commissural axons accumulate at the contralateral edge of the floor plate. In vitro, SCF is shown to selectively promote the outgrowth of post-crossing commissural axons. Thus, in addition to midline repellants, the regulation of midline exit of requires the outgrowth promoting function of SCF. [DOI] [PubMed] [Google Scholar]
- 23.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 24.Garbe DS, Bashaw GJ. Independent functions of Slit-Robo repulsion and Netrin-Frazzled attraction regulate axon crossing at the midline in Drosophila. J Neurosci. 2007;27:3584–3592. doi: 10.1523/JNEUROSCI.0301-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 26.Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- ••27.Wegmeyer H, Egea J, Rabe N, Gezelius H, Filosa A, Enjin A, Varoqueaux F, Deininger K, Schnütgen F, Brose N, et al. EphA4-dependent axon guidance is mediated by the RacGAP alpha2-chimaerin. Neuron. 2007;55:756–767. doi: 10.1016/j.neuron.2007.07.038. [DOI] [PubMed] [Google Scholar]
- ••28.Beg AA, Sommer JE, Martin JH, Scheiffele P. alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron. 2007;55:768–778. doi: 10.1016/j.neuron.2007.07.036. [DOI] [PubMed] [Google Scholar]
- ••29.Shi L, Fu WY, Hung KW, Porchetta C, Hall C, Fu AK, Ip NY. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc Natl Acad Sci U.S.A. 2007;104:16347–16352. doi: 10.1073/pnas.0706626104. These three studies describe the characterization of the Rac-specific GTPase activating protein alpha2-chimaerin and its role in EphA4 receptor forward signaling. Knock-out of alpha2-chimaerin leads to phenotypes at the anatomical and behavioral level that are indistinguishable from loss of either the ephrinB3 ligand or the EphA4 receptor. Specifically, alpha2-chimaerin is shown to be a key signaling molecule that regulates midline repulsion of axons of the corticospinal tract, as well as interneurons of the CPG. All three studies report biochemical and cell culture experiments that support the model that alpha2-chimaerin directly couples the EphA4 receptor to the regulation of Rac activity and is required for growth cone collapse. This is the clearest demonstration to date that an individual signaling molecule is required for all of the guidance activity of a specific receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoeckli ET. Longitudinal axon guidance. Curr Opin Neurobiol. 2006;16:35–39. doi: 10.1016/j.conb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 32.Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, Mastick GS. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development. 2008;135:3643–3653. doi: 10.1242/dev.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J. Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci. 2009;29:8914–8926. doi: 10.1523/JNEUROSCI.0568-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


