Summary
Transcription factors establish neural diversity and wiring specificity; however, how they orchestrate changes in cell morphology remains poorly understood. The Drosophila Roundabout (Robo) receptors regulate connectivity in the central nervous system, but how their precise expression domains are established is unknown. Here we show that the homeodomain transcription factor Hb9 acts upstream of Robo2 and Robo3 to regulate axon guidance in the Drosophila embryo. In ventrally-projecting motor neurons, hb9 is required for robo2 expression, and restoring Robo2 activity in hb9 mutants rescues motor axon defects. Hb9 requires its conserved repressor domain and functions in parallel with Nkx6 to regulate robo2. Moreover, hb9 can regulate the medio-lateral position of axons through robo2 and robo3, and restoring robo3 expression in hb9 mutants rescues the lateral position defects of a subset of neurons. Together, these data identify Robo2 and Robo3 as key effectors of Hb9 in regulating nervous system development.
Keywords: Axon Guidance, Lateral Position, Robo receptors, Hb9, Transcription factors
Introduction
Combinations of transcription factors specify the tremendous diversity of cell types in the nervous system (Dasen, 2009; Hobert, 2011; Shirasaki and Pfaff, 2002). Many studies have identified requirements for transcription factors in regulating specific events in circuit formation as neurons migrate, form dendritic and axonal extensions, and select their final synaptic targets (reviewed in Polleux et al., 2007; Zarin et al., 2013). In most cases the downstream effectors through which transcription factors control changes in neuronal morphology and connectivity remain unknown, although several functional relationships have been demonstrated (van den Berghe et al., 2013; Jinushi-Nakao et al., 2007; Labrador et al., 2005; Luria et al., 2008; Marcos-Mondéjar et al., 2012; Nóbrega-Pereira et al., 2008; Wilson et al., 2008).
Conserved homeodomain transcription factors regulate motor neuron development across phyla. Studies in vertebrates and invertebrates have shown that motor neurons that project to common target areas often express common sets of transcription factors, which act instructively to direct motor axon guidance (Kania and Jessell, 2003; Kania et al., 2000; Landgraf et al., 1999; Thor and Thomas, 1997). In mouse and chick, Nkx6.1/Nkx6.2 and MNR2/Hb9 are required for the specification of spinal cord motor neurons, and for axon pathfinding and muscle targeting in specific motor nerves (Arber et al., 1999; De Marco Garcia and Jessell, 2008; Sander et al., 2000; Thaler et al., 1999; Vallstedt et al., 2001). In Drosophila, Nkx6 and Hb9 are expressed in embryonic motor neurons that project to ventral or lateral body wall muscles, and although they are not individually required for specification, they are essential for the pathfinding of ventrally-projecting motor axons (Broihier and Skeath, 2002; Broihier et al., 2004; Odden et al., 2002). Axons that project to dorsal muscles express the homeodomain transcription factor Even-skipped (Eve), which regulates guidance in part through the Netrin receptor Unc5 (Fujioka et al., 2003; Labrador et al., 2005; Landgraf et al., 1999). Eve exhibits cross-repressive interactions with hb9 and nkx6, which function in parallel to repress eve and promote islet and lim3 expression (Broihier and Skeath, 2002; Broihier et al., 2004). Hb9 and Nkx6 act as repressors to regulate transcription factors in the spinal cord (Lee et al., 2008; Muhr et al., 2001; William et al., 2003); however, guidance receptors that act downstream of Hb9 and Nkx6 have not been characterized. Interestingly, in both flies and vertebrates, Hb9 and Nkx6 are also expressed in a subset of interneurons, and knockdown experiments in Drosophila have suggested a role for hb9 in regulating midline crossing (Broihier et al., 2004; Odden et al., 2002; Sander et al., 2000; Vallstedt et al., 2001; Wilson et al., 2005).
Robo receptors regulate midline crossing and lateral position within the developing central nervous systems of invertebrates and vertebrates (Jaworski et al., 2010; Kastenhuber et al., 2009; Kidd et al., 1998; Long et al., 2004; Rajagopalan et al., 2000a, 2000b; Sabatier et al., 2004; Simpson et al., 2000a, 2000b). Two recent studies in mice have also identified a role for Robos in regulating motor axon guidance in specific motor neuron populations (Bravo-Ambrosio et al., 2012; Jaworski and Tessier-Lavigne, 2012). The three Drosophila Robo receptors have diversified in their expression patterns and functions. Robo, hereafter referred to as Robo1, is broadly expressed in the ventral nerve cord and prevents inappropriate midline crossing by signaling repulsion in response to midline-derived Slit (Kidd et al., 1998, 1999). Robo2 is initially expressed in many ipsilateral pioneers, and also contributes to Slit-mediated repulsion (Rajagopalan et al., 2000a; Simpson et al., 2000a). Subsequently, robo2 expression is more restricted, and it is required to specify the medio-lateral position of axons (Rajagopalan et al., 2000b; Simpson et al., 2000b). Robo3 is expressed in a subset of CNS neurons, and also regulates lateral position (Rajagopalan et al., 2000b; Simpson et al., 2000b).
Characterization of the expression domains of the Drosophila Robos revealed an intriguing pattern, in which Robo1 is expressed on axons throughout the width of the CNS, Robo3 is found on axons in intermediate and lateral zones, and Robo2 is enriched on the most lateral axons (Rajagopalan et al., 2000b; Simpson et al., 2000b). These patterns are transcriptional in origin, as replacing any robo gene with the coding sequence of another Robo receptor results in a protein distribution that matches the endogenous expression of the replaced gene (Spitzweck et al., 2010) (C.S., T. Evans and G.J.B., unpublished). A phenotypic analysis of these gene-swap alleles revealed the importance of transcriptional regulation for the diversification of robo gene function (Spitzweck et al., 2010). Robo2 and robo3’s roles in regulating lateral position are largely dependent on their expression patterns, although unique structures within the Robo2 receptor are also important for its function in lateral position (Evans and Bashaw, 2010; Spitzweck et al., 2010). In the peripheral nervous system, the atonal transcription factor regulates robo3 in chordotonal sensory neurons, directing the position of their axon terminals (Zlatic et al., 2003). In the CNS, the transcription factors lola and midline contribute to the induction of robo1 (Crowner et al., 2002; Liu et al., 2009). However, how the expression patterns of robo2 and robo3 are established to direct axons to specific medial-lateral zones within the CNS remains unknown.
This study identifies a functional relationship between Hb9 and the Robo2 and Robo3 receptors in multiple contexts. We show that Hb9 acts through Robo2 to regulate motor axon guidance, and can direct the medio-lateral position of axons in the nerve cord through its effects on robo2 and robo3. Furthermore, hb9 interacts genetically with nkx6 and requires its conserved repressor domain to regulate robo2. Together, these data establish a link between transcriptional regulators and cell surface guidance receptors, providing an example of how upstream factors act through specific guidance receptors to direct circuit formation.
Results
Robo2 is required in neurons for motor axon pathfinding
Hb9 regulates motor axon pathfinding across species, but its downstream effectors remain unknown. In Drosophila, hb9 is required for the formation of the ISNb nerve, which innervates a group of ventral muscles (Broihier and Skeath, 2002). In our hands, approximately 20% of hemisegments in hb9 mutant embryos lack innervation at the muscle 6/7 cleft, while these defects are rarely observed in wild type animals or hb9 heterozygotes (Figure 1). To identify potential targets of hb9, we examined the expression patterns of axon guidance genes by in situ hybridization. We found that during the stages when motor axons navigate the muscle field, robo2 mRNA is enriched in ventrally-projecting motor neurons (Figure S1).
Figure 1. Robo2 and hb9 mutants have similar motor axon guidance defects, and hb9 is required for robo2 expression in the RP motor neurons.
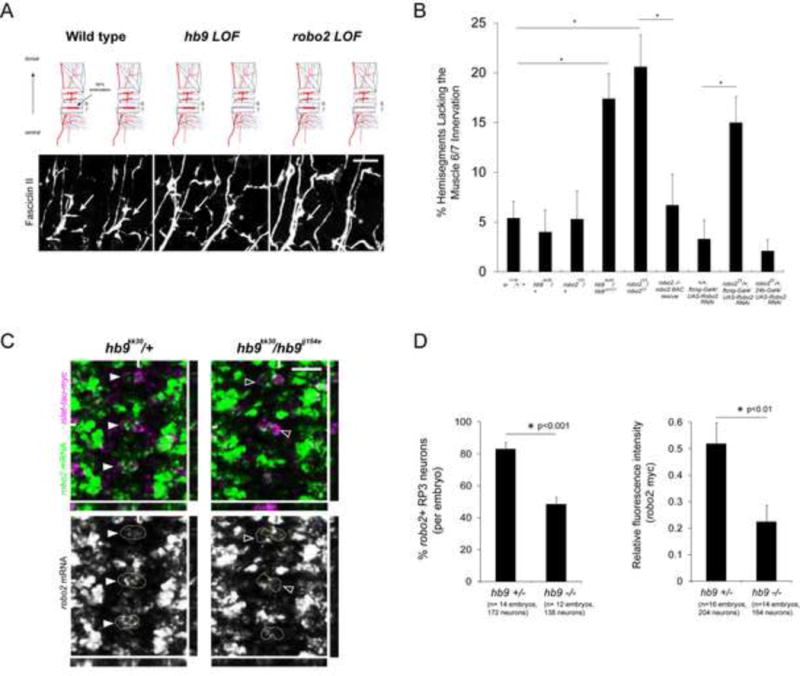
A: Stage 17 embryos stained for Fasciclin II (FasII). Anterior is left. Arrows point to the muscle 6/7 innervation, which is often absent in hb9 or robo2 mutants (asterisks). B: The percentage of hemisegments lacking the 6/7 innervation is shown; asterisks indicate a significant difference (Student’s t-test, p<0.01). Error bars = s.e.m. C: Fluorescent in situ for robo2 mRNA in Stage 16 embryos. Anterior is up. The RP3 motor neurons are labeled by the islet-tau-myc transgene, and circled in the single-channel images. Most RP3 neurons express robo2 in hb9 heterozygotes (filled arrowheads), whereas many RP3 neurons do not express robo2 in hb9 mutants (empty arrowheads). YZ and XZ cross-sections are shown; hash marks indicate the planes of the sections. D, Left: RP3 neurons were scored as positive or negative for robo2. Hb9 mutants have significantly fewer robo2+ RP3 neurons than heterozygous siblings (Student’s t-test, p<0.001). Error bars = s.e.m. D, Right: The mean gray value of the robo2 mRNA signal in RP3 neurons was normalized to the mean gray value of the myc signal. The average relative fluorescence intensity of robo2 mRNA is significantly lower in hb9 mutants than in hb9 heterozygotes (Student’s t-test, p<0.01). Error bars = s.e.m. Numbers of embryos and neurons analyzed are shown in parentheses. Scale bars represent 10 μm. Robo2 −/− robo2 BAC rescue denotes robo2123, 22K18robo2BAC/ robo233. Hb9 +/− denotes hb9kk30, isl-taumyc/TM3. Hb9 −/− denotes hb9kk30, isl-taumyc/hb9jj154e. See also Figure S1.
To determine whether robo2 regulates motor axon guidance, we examined robo2 mutant embryos for innervation defe cts. In 20% of hemisegments in robo2 mutants, the axon that normally innervates the muscle 6/7 cleft is either absent or stalled at the main ISNb trunk (Figure 1). This phenotype is similar to that of hb9 mutants, and is observed using multiple robo2 alleles (Figure 1 and data not shown). Robo2 heterozygotes and robo2/+; hb9/+ double heterozygotes do not have significant defects (Figure 1 and data not shown). Robo2 mutants have no defects in axons forming the ISN, SNa, SNc, TN, or ISNd nerves. Importantly, restoring one copy of an 83.9 kb BAC transgene that contains the robo2 locus and its flanking genomic sequence fully rescues the 6/7 innervation defects of robo2 mutants (Figure 1B).
Robo2 is expressed in ventral muscles and in motor neurons (Figure S1). To determine if robo2 acts in neurons to regulate motor axon pathfinding, we expressed a UAS-Robo2RNAi transgene using ftzng-Gal4, which drives expression in many motor neurons and their precursors (Thor et al., 1999). Expressing UAS-Robo2RNAi with ftzng-Gal4 in an otherwise wild type background produces no effect, but causes significant 6/7 innervation defects when expressed in robo2 heterozygotes (Figure 1B). Conversely, expressing UAS-Robo2 RNAi in robo2 heterozygotes using the pan-muscle driver 24bgal4 has no effect (Figure 1B). Together, these data suggest that robo2 is required neuronally to regulate ISNb pathfinding.
Hb9 is required for robo2 expression in the RP motor neurons
To test if hb9 regulates robo2 in ventrally-projecting motor neurons, we examined robo2’s expression pattern in hb9 mutants. In Stage 16 wild type or hb9 heterozygote embryos, robo2 mRNA is readily detected in the RP motor neurons (Figure S1 and Figure 1C). In particular, robo2 transcript is enriched in RP3, the neuron that innervates the muscle 6/7 cleft (Figure 1C). In hb9 mutants, robo2 mRNA is significantly decreased in the RP motor neurons (Figure 1D). An average of 83% of RP3 neurons in hb9kk30/+ heterozygous embryos, but only 49% of RP3 neurons in hb9kk30/hb9jj154e mutants express detectable robo2 at Stage 16 (p<0.001, Student’s t-test) (Figure 1D). This difference is observed as early as Stage 14, when robo2 mRNA begins to accumulate in RP3, and is detected using multiple hb9 alleles (Figures 1, 3 and data not shown). Interestingly, hb9 mutants show no change in the expression of robo1, which is broadly expressed in many motor neurons including the RPs (data not shown). To quantify the fluorescent robo2 mRNA signal in RP3 neurons, we measured pixel intensity and normalized the mRNA signal to the myc signal from islet-tau-myc. The average relative fluorescence intensity of robo2 mRNA in hb9 heterozygotes is more than twice the average value measured in hb9 mutants (p<0.01, Student’s t-test) (Figure 1D). We conclude that hb9 is an essential regulator of robo2 in the RP motor neurons.
Figure 3. Hb9’s Eh domain is required for its activity in motor axon guidance and for robo2 regulation.
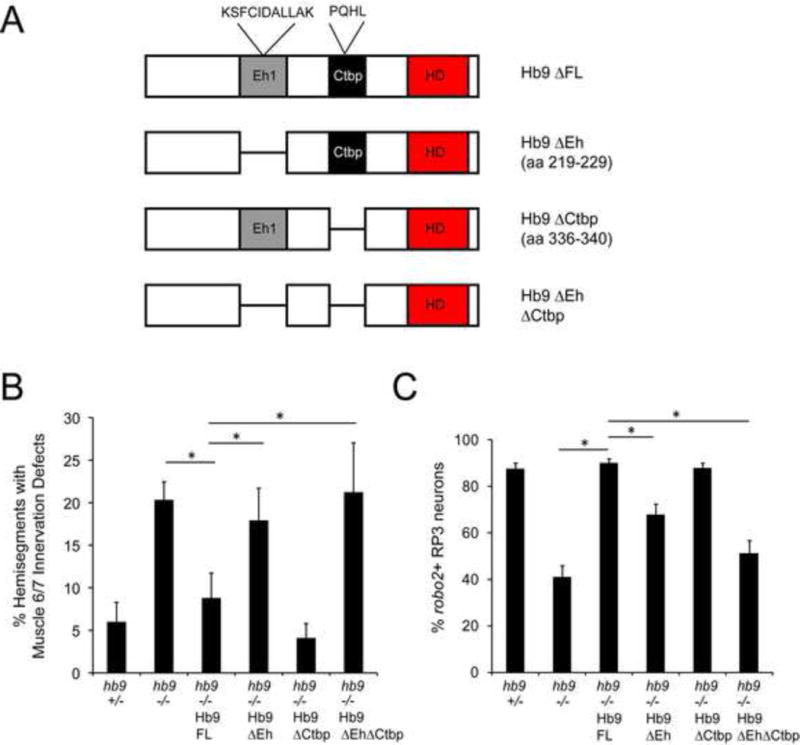
A: Schematic of the Hb9 variants analyzed for their ability to rescue hb9 mutants. B: Muscle 6/7 innervation was quantified in late Stage 17 embryos; asterisks indicate a significant difference (Student’s t-test, p<0.01). Hb9 transgenes lacking the Eh domain failed to rescue motor axon guidance defects in hb9 mutants. C: The percentage of robo2+ RP3 neurons per embryo is shown; asterisks indicate a significant difference (Student’s t-test, p<0.01). Hb9’s Eh domain is required for rescue of robo2 expression. Error bars = s.e.m. Hb9 +/− denotes hb9gal4/TM3. Hb9 −/− denotes hb9gal4/hb9kk30. Hb9 −/− Hb9 (variant) denotes UAS-Hb9 (variant)/+; hb9gal4/hb9kk30.
Robo2’s activity in motor axon guidance depends on unique features of its cytodomain
Robo2 has multiple activities in the embryonic CNS, some of which cannot be substituted for by the other Robo receptors (Evans and Bashaw, 2010; Spitzweck et al., 2010). To determine if Robo2’s activity in motor axon guidance is a unique property of Robo2, we examined knock-in alleles in which the coding sequences of Robo1, Robo2, or Robo3 are knocked into the robo2 locus, hereafter referred to as robo2X, where X represents the inserted coding sequence (Spitzweck et al., 2010). Embryos homozygous for the robo2robo2 allele have no significant defects in motor axon pathfinding, whereas embryos homozygous for either robo2robo1 or robo2robo3 have as many RP3 innervation defects as robo2 mutants (Figure 2B). To define the protein domains required for Robo2’s activity in motor axon guidance, we examined knock-in alleles encoding either of two chimeric receptors: Robo2-1 (Robo2’s ectodomain and Robo1’s cytodomain) or Robo1-2 (Robo1’s ectodomain and Robo2’s cytodomain) (Spitzweck et al., 2010) (Figure 2A). We found that robo2robo2-1 embryos have as strong a motor axon phenotype as robo2 mutants, while robo2robo1-2 embryos are phenotypically normal (Figure 2B). Together, these results suggest that neither Robo1 nor Robo3 can substitute for Robo2 in motor axon guidance, and that this Robo2-specific activity maps to its cytodomain.
Figure 2. Restoring Robo2 activity in hb9 mutants rescues motor axon guidance defects.
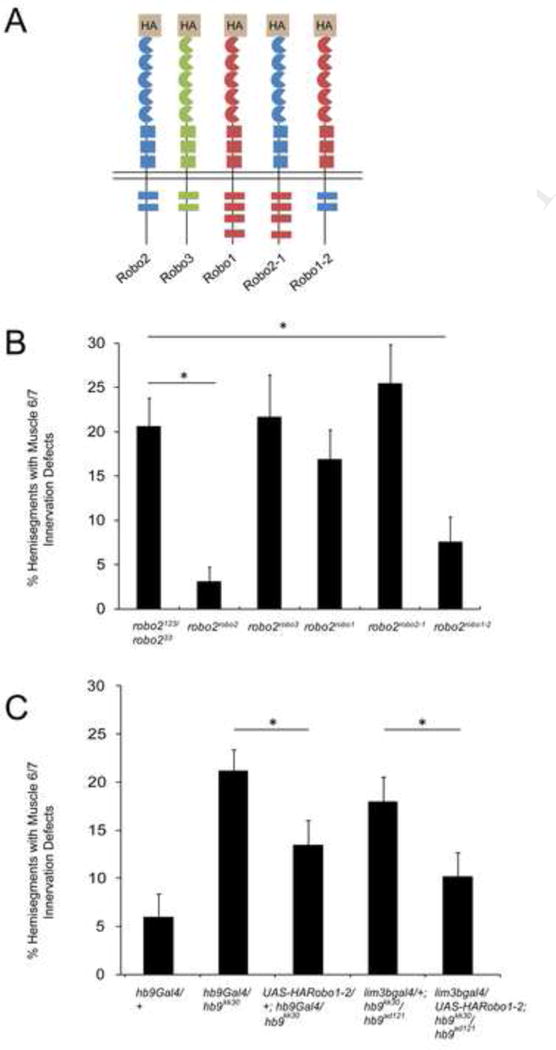
A: Schematic of the Robo receptors analyzed for their ability to replace endogenous Robo2. B: Embryos homozygous for knock-in alleles in which the coding sequences of Robo2, Robo3, Robo1, Robo2-1, or Robo1-2 are inserted in the robo2 locus were analyzed for motor axon guidance defects. Only Robo2 and Robo1-2 can restore muscle 6/7 innervation. Asterisks indicate a significant difference (Student’s t-test, p<0.01). Error bars = s.e.m. B: Hb9 mutant embryos over-expressing UAS-HARobo1-2 have fewer defects than mutants lacking the transgene (Student’s t-test, p<0.05). All hb9 mutants were scored blind to genotype. Error bars = s.e.m. See also Figure S2.
Restoring Robo2 activity in hb9 mutants rescues motor axon guidance defects
To determine if Robo2 acts as an effector of Hb9 during motor axon guidance, we tested whether over-expressing robo2 in hb9 mutants rescues their muscle 6/7 innervation defects. However, over-expressing a UAS-Robo2 transgene using hb9gal4 in otherwise wild type embryos produces severe motor axon defects, affecting RP3 innervation in more than 50% of hemisegments (Figure S2). We therefore sought to identify a variant of the Robo2 receptor that retains its endogenous activity in ISNb pathfinding, but does not generate defects when over-expressed. As our results with the knock-in alleles indicate a requirement for Robo2’s cytodomain in motor axon guidance (Figure 2B), we tested whether over-expression of a chimeric receptor that contains the ectodomain of Robo1 and the cytodomain of Robo2 (Robo1-2) results in motor axon guidance defects. We found that over-expression of UAS-Robo1-2 with hb9gal4 does not result in 6/7 innervation defects, whereas expressing the reciprocal chimera (Robo2-1) produces significant errors in motor axon pathfinding (Figure S2).
We could now test if expressing a receptor that is functional in robo2’s endogenous context (Robo1-2) rescues motor axon guidance in hb9 mutants. We used the hb9gal4 enhancer trap to perform this experiment (Broihier and Skeath, 2002), as we have found that when placed over a null hb9 allele, this allelic combination results in nearly undetectable levels of hb9 protein, and has as strong a motor axon phenotype as the null itself (Figure 2C and data not shown). Over-expressing UAS-Robo1-2 in hb9 mutants using hb9gal4 significantly rescues RP3 innervation defects (22% of hemisegments to 13%, p=0.03, Student’s t-test) (Figure 2C). A similar result is observed using the lim3bgal4 driver (Certel and Thor, 2004) and a different hb9 allelic combination (18% to 10%, p =0.04, Student’s t-test) (Figure 2C). The incomplete rescue may be a consequence of the timing or expression levels caused by Gal4-driven expression. Alternatively, robo2 may be one of multiple downstream targets of hb9, and restoring Robo2 activity might not be sufficient to fully rescue hb9 mutants. Nevertheless, together with the loss of function phenotypes and the requirement for hb9 in promoting robo2 expression, these results strongly suggest that Robo2 acts as a downstream effector of Hb9 during motor axon guidance.
Hb9 requires its conserved repressor domain and functions in parallel with Nkx6 to regulate robo2
Vertebrate Hb9 acts as a repressor to regulate gene expression when over-expressed in the spinal cord, but the requirement for Hb9’s repressor activity for axon guidance has not been studied (Lee et al., 2008; William et al., 2003). Two conserved putative repressor domains are found in Drosophila Hb9: an Engrailed homology (Eh) domain similar to sequences that interact with the Groucho co-repressor (Broihier and Skeath, 2002; Smith and Jaynes, 1996), and a domain similar to sequences that interact with the C-terminal binding protein (CtBP) co-repressor (William et al., 2003). To test the contribution of these domains to Hb9 function, we generated Hb9 transgenes in which either or both domains were deleted, and compared their ability to rescue hb9 mutants relative to full length Hb9 (Figure 3). All transgenes are inserted in the same genomic location and are expressed at similar levels (data not shown). We found that whereas a full-length Hb9 transgene (Hb9 FL) fully rescues both muscle 6/7 innervation defects and robo2 expression in hb9 mutants, the Eh domain deletion (Hb9ΔEh) does not rescue motor axon pathfinding, and only weakly rescues robo2 expression (Figure 3). Conversely, the CtBP-binding domain deletion (Hb9ΔCtBP) fully rescues both guidance and robo2 expression (Figure 3). The double deletion (Hb9ΔEhΔCtBP) is not significantly different from Hb9ΔEh in either assay (Figure 3). These results suggest that Hb9 indirectly activates robo2, perhaps by repressing a direct regulator of robo2, likely through a Groucho-dependent mechanism.
The embryonic expression patterns of hb9 and the homeodomain transcription factor nkx6 largely overlap, and genetic analyses suggest that Hb9 and Nkx6 act in parallel to regulate motor axon guidance and multiple transcription factors (Broihier et al., 2004). We hypothesized that robo2 might be a shared downstream target of hb9 and nkx6. Indeed, nkx6 mutants have a significant decrease in robo2 expression in the RP motor neurons (81% robo2+ RP3 neurons in nkx6 heterozygotes versus 51.4% robo2+ RP3 neurons in nkx6 mutants, p<0.001, Student’s t-test) (Figure 4A and B). To determine if hb9 and nkx6 function in parallel to regulate robo2, we examined robo2 expression in hb9, nkx6 double mutants and observed a decrease relative to either single mutant (data not shown). However, we were not able to quantify robo2 expression in the double mutants, as many cells are not labeled by hb9gal4 or islet-tau-myc. Therefore, we looked for an alternative background to address whether nkx6 regulates robo2 in parallel with hb9. Removing one copy of nkx6 in hb9 mutants strongly enhances the motor axon phenotype (from 21.6% of hemisegments with 6/7 innervation defects in hb9/hb9 embryos to 45% in hb9, nkx6/hb9,+ embryos, p<0.001, Student’s t-test) without producing the changes in markers observed in hb9, nkx6 double mutants (Figure 4C and D). In this background robo2 expression is significantly decreased relative to hb9 mutants (from 41% robo2+ RP3 neurons in hb9/hb9 embryos to 19% in hb9, nkx6/hb9,+ embryos, p<0.001, Student’s t-test) suggesting that nkx6 promotes robo2 expression independently of hb9 (Figure 4B). Nkx6 single mutants have a severe ISNb phenotype in which most ventrally-projecting motor axons fail to exit the nerve cord (Broihier et al., 2004), implying that Nkx6 regulates downstream targets other than robo2. Nevertheless, our data argue that Hb9 and Nkx6 are essential regulators of robo2 in the RP motor neurons and that they act in parallel to regulate ISNb guidance and achieve normal levels of robo2 expression, thus demonstrating how a combination of transcription factors regulates axon guidance by impinging on a common downstream target.
Figure 4. Hb9 and Nkx6 function in parallel to regulate motor axon guidance and robo2.
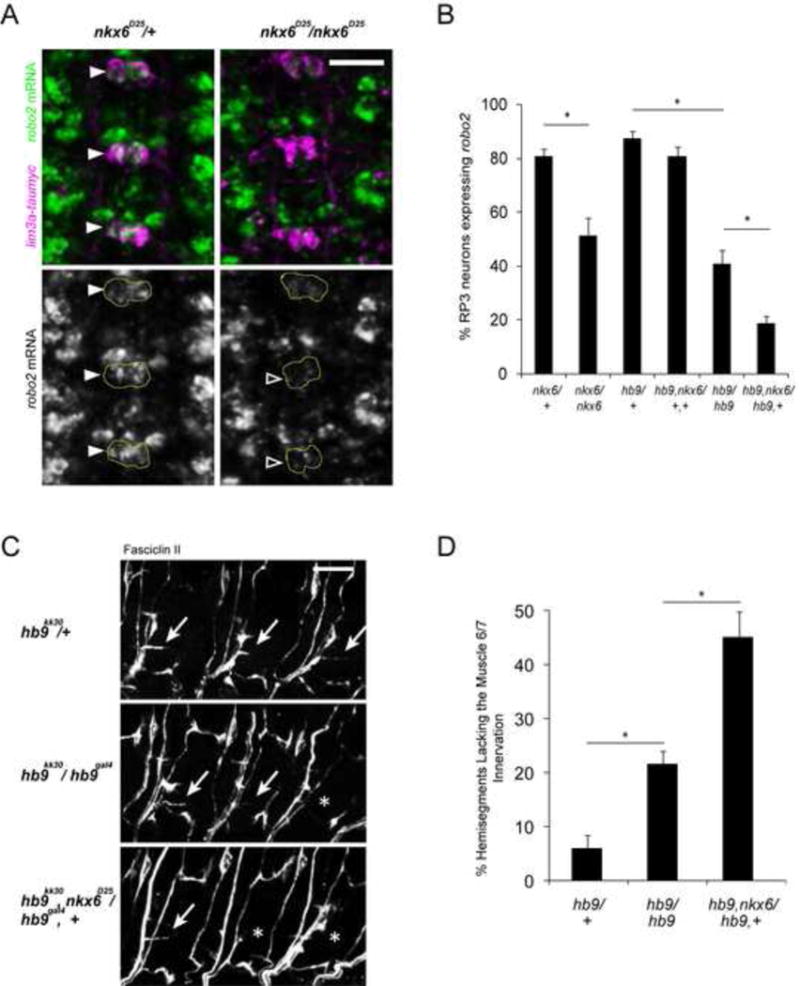
A: Fluorescent in situ for robo2 mRNA (green) in Stage 16 embryos. Anterior is up. The RP motor neurons are labeled by the lim3a-taumyc transgene (magenta). Filled arrowheads point to robo2+ RP3 neurons; empty arrowheads indicate robo2- neurons. B: Nkx6 mutants have fewer robo2+ RP3 neurons than nkx6 heterozygotes (p<0.001, Student’s t-test). Removing one copy of nkx6 enhances the loss of robo2 in hb9 mutants (p<0.001, Student’s t-test). Error bars = s.e.m. C: Stage 17 embryos stained for FasII. Anterior is left. The arrows point to the muscle 6/7 innervation, while asterisks indicate its absence. D: The percentage of hemisegments lacking the 6/7 innervation was quantified; asterisks indicate a significant difference (p<0.001, Student’s t-test). Loss of nkx6 dominantly enhances the 6/7 innervation defects of hb9 mutants. Error bars = s.e.m. Scale bars represent 10 μm. Nxk6/+ denotes nkx6D25/TM6B. Nkx6/nkx6 denotes nkx6D25/nkx6D25. Hb9/+ denotes hb9kk30/TM3. Hb9, nxk6/+,+ denotes hb9gal4, nkx6D25/TM3. Hb9/hb9 denotes hb9gal4/hb9kk30. Hb9, nkx6/hb9,+ denotes hb9gal4, nkx6D25/ hb9kk30. See also Figure S3.
Hb9 regulates lateral position in a subset of neurons
Robo2 regulates midline crossing and lateral position within the embryonic CNS (Rajagopalan et al., 2000a, 2000b; Simpson et al., 2000a, 2000b). As hb9 is expressed in many neurons other than the RP motor neurons, we asked if it acts through robo2 to regulate axon guidance in other contexts. The enhancer trap hb9gal4 is expressed in all neurons that endogenously express hb9 (Broihier and Skeath, 2002), labeling three parallel axon tracts on either side of the midline (Figure 5A). These align with, but are distinct from, Fasciclin II (FasII)-expressing axons, which form three bundles at specific medio-lateral positions (Figure 5A). Hb9 mutants do not have defects in the organization of FasII axons (Figure 5A and data not shown). However, in hb9 mutants, the two outer hb9gal4+ bundles are often disrupted and the inner pathway appears thicker (Figure 5A). The lateral-most hb9gal4+ pathway is missing or discontinuous in approximately 30% of hemisegments, and the intermediate pathway is missing in close to 50% of hemisegments (Figure 5B). These defects are fully rescued by expression of a UAS-Hb9 transgene (Figure 5). No changes in the number of hb9gal4+ neurons are observed (data not shown). To determine if nxk6 also regulates the trajectory of hb9gal4+ axons, we examined the organization of these pathways in embryos with reduced nkx6 activity. Nkx6 mutants have no significant defects in the lateral position of hb9gal4+ axons (Figure S3). However, hb9 mutants heterozygous for nkx6 have a significantly stronger disruption of the outer-most hb9gal4+ pathway relative to hb9 mutants (Figure S3), suggesting that nkx6 also regulates lateral position, although its requirement is only revealed in the absence of hb9.
Figure 5. The lateral position of hb9Gal4-expressing axons is disrupted in the absence of hb9, robo2, or robo3.
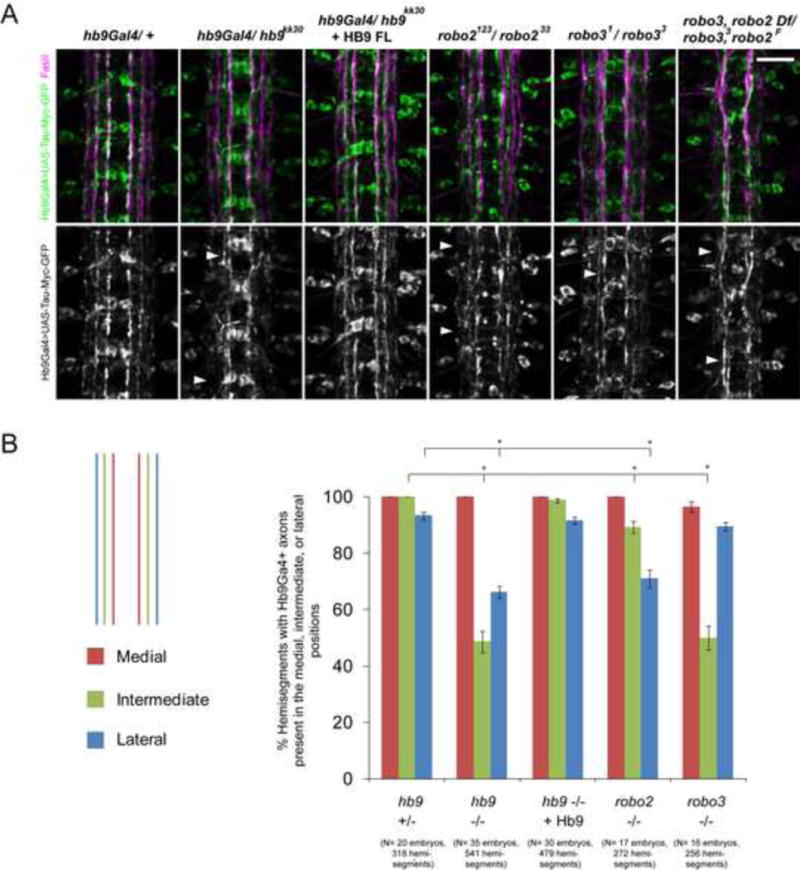
A: Stage 17 embryos, anterior is up. FasII staining is shown in magenta. Hb9Gal4> UAS-TauMycGFP (green) labels axons that form three bundles on each side of the midline in hb9 heterozygotes. In hb9 mutants, the outer hb9Gal4+ pathways are disrupted or shifted medially (arrowheads). Robo2 and robo3 mutants partially phenocopy these defects (arrowheads). B: The percentage of hemisegments containing hb9Gal4+ axons in the medial, intermediate, or lateral positions is shown. Asterisks indicate a significant difference (Student’s t-test, p<0.001). Error bars = s.e.m. Numbers of embryos and hemisegments scored are shown in parentheses. Scale bars represent 10 μm. Hb9 +/− denotes hb9gal4/TM6B. Hb9 −/− denotes hb9gal4/hb9kk30. Hb9 −/− + HB9 denotes UAS-Hb9/+; hb9gal4/hb9kk30. Robo2 −/− denotes robo2123/robo233; hb9gal4/+. Robo3 −/− denotes robo31 / robo33; hb9gal4/+. Robo3, robo2 Df/ robo33, robo2F denotes Df(2L)ED108/ robo2F, robo33; hb9gal4/+. See also Figure S4.
Robo2 and robo3 are major regulators of lateral position in the developing CNS (Evans and Bashaw, 2010; Rajagopalan et al., 2000b; Simpson et al., 2000b; Spitzweck et al., 2010). Their expression patterns mirror their requirements: robo2 is expressed on axons that select a lateral trajectory, and is required for the formation of lateral pathways, while robo3 is expressed in both lateral and intermediate zones and is required for the formation of intermediate pathways (Rajagopalan et al., 2000b; Simpson et al., 2000b). Gene-swap experiments underscored the importance of the transcriptional regulation of robo2 and robo3 for their function in lateral position (Spitzweck et al., 2010), but upstream regulators within the CNS remain unknown. To determine if hb9 regulates medio-lateral position through robo2 or robo3, we first asked whether robo2 or robo3 regulate the position of axons labeled by hb9gal4. In robo2 mutants, the outer hb9gal4+ pathway is missing in approximately 30% of hemisegments (Figure 5B). The intermediate pathway is mildly affected, while the medial pathway appears intact (Figure 5). In robo3 mutants, the intermediate hb9gal4+ pathway is absent or strongly shifted in close to 50% of hemisegments, the outer pathway is not disrupted, and the medial pathway is intact (Figure 5). Robo2, robo3 double mutants have a stronger phenotype in which the outer two hb9gal4+ pathways are disrupted in a majority of hemisegments (Figure 5). However, the dramatic decrease in the width of the nerve cord in robo2, robo3 double mutants made it difficult to quantify the presence of lateral pathways. We conclude that loss of robo2 and robo3 reproduces the lateral position defects observed in hb9 mutants.
Hb9 can regulate lateral position by inducing robo2
To test whether hb9 regulates lateral position through robo2 or robo3, we searched for hb9-expressing neurons that also express robo2 or robo3 and project to intermediate or lateral zones. Several hb9+ cells co-express robo2, including a cluster of neurons found immediately anterior and slightly dorsal to dMP2 (Figure S4). We scored robo2 expression in these cells and observed a decrease in the percentage expressing robo2 mRNA in hb9 mutants compared to heterozygotes (52% to 24%, p<0.0001, Student’s t-test, Figure S4). However, we were not able to achieve the resolution necessary to determine whether these neurons contribute to lateral pathways. It is likely that most of these cells are interneurons, as few motor neuron cell bodies reside in this area of the nerve cord (Landgraf et al., 1997). Together with the similarity in the lateral position defects of hb9 and robo2 mutants, as well as the observation that Robo2 is an effector of hb9 in motor neurons, these data suggest that hb9 may endogenously regulate the medio-lateral position of a subset of interneurons via its effect on robo2.
To study the consequences of manipulating hb9 levels on lateral position in a defined group of neurons, we used the apterous-Gal4 driver, which labels ipsilateral interneurons that normally do not express hb9, and express little robo2 and robo3 (Figure 6 and data not shown). In wild type embryos, the apterous (ap) axons form a fascicle that projects along the medial FasII bundle on either side of the midline (Figure 6B). Over-expressing Robo2 or Robo3 in the ap neurons causes their axons to shift laterally away from the midline (Evans and Bashaw, 2010; Rajagopalan et al., 2000b; Simpson et al., 2000b). We found that over-expressing Hb9 produces a very similar phenotype, in which ap axons are shifted in more than 75% of hemisegments, now aligning with the intermediate or lateral FasII tracts (Figure 7B). To determine if this phenotype is due to the induction of robo2 or robo3, we examined the effect of hb9 over-expression on robo2 and robo3 mRNA levels. Over-expression of Hb9 in ap neurons does not result in robo3 induction (data not shown). In contrast, we observed significant upregulation of robo2 (Figure 6A). In control embryos, robo2 mRNA is detected in less than 20% of ventral ap cells, whereas more than 60% of ventral ap neurons express robo2 when Hb9 is present (p<0.001, Student’s t-test) (Figure 6A). Interestingly, we do not observe robo2 induction in the dorsal ap neurons (data not shown) which express a different transcription factor profile than their ventral counterparts (Allan et al., 2005; Baumgardt et al., 2007).
Figure 6. Hb9 gain of function in ap neurons induces robo2 expression and a robo2-dependent lateral shift.
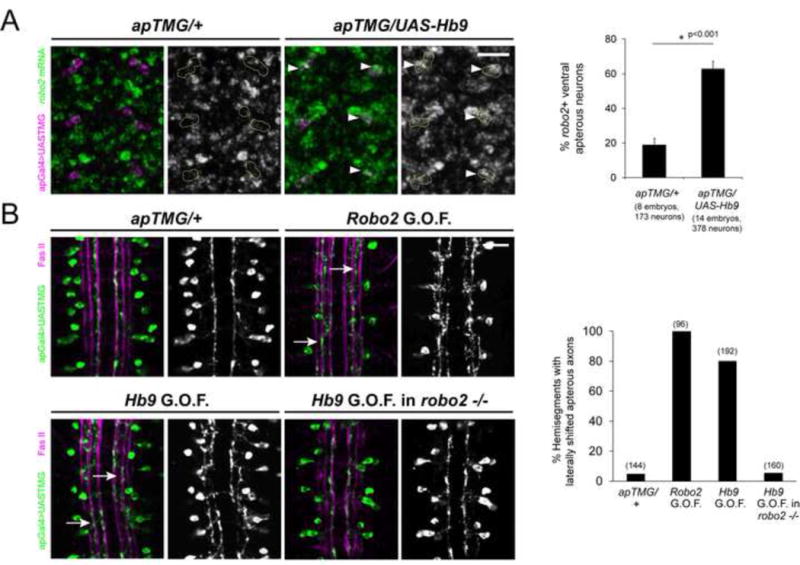
A, Left: Fluorescent in situ for robo2 mRNA (green) in Stage 15 embryos. Anterior is up. The ventral ap neurons are labeled in magenta and circled in the single channel images. Wild-type embryos express little robo2 in the ap neurons, whereas many ventral ap neurons express robo2 when Hb9 is present (arrowheads). A, Right: The percentage of ventral ap neurons expressing robo2 is shown. Hb9 gain of function results in a significant increase compared to controls (p<0.001, Student’s t-test). Error bars = s.e.m. B, Left: Stage 17 embryos stained for FasII (magenta) and GFP (green), which labels the ap axons. Over-expression of robo2 or hb9 in ap neurons shifts their axons laterally (arrows). Hb9 over-expression in robo2 mutants does not induce a lateral shift phenotype. B, Right: The percentage of hemisegments in which ap axons project along the intermediate or lateral FasII tracts is shown. Numbers of hemisegments scored are indicated in parentheses. Scale bars represent 10 μm. apTMG/+ denotes apGal4,UAS-TauMycGFP/CyO. Robo2 G.O.F. denotes UAS-HARobo2.T1/apGal4, UAS-TauMycGFP. Hb9 G.O.F denotes UAS-Hb9/apGal4,UAS-TauMycGFP. Hb9 G.O.F. in robo2 −/− denotes robo2123,UAS-Hb9/robo233, apGal4; UAS-TauMycGFP/+.
Figure 7. Robo3 acts downstream of Hb9 to direct the lateral position of MP1 axons.
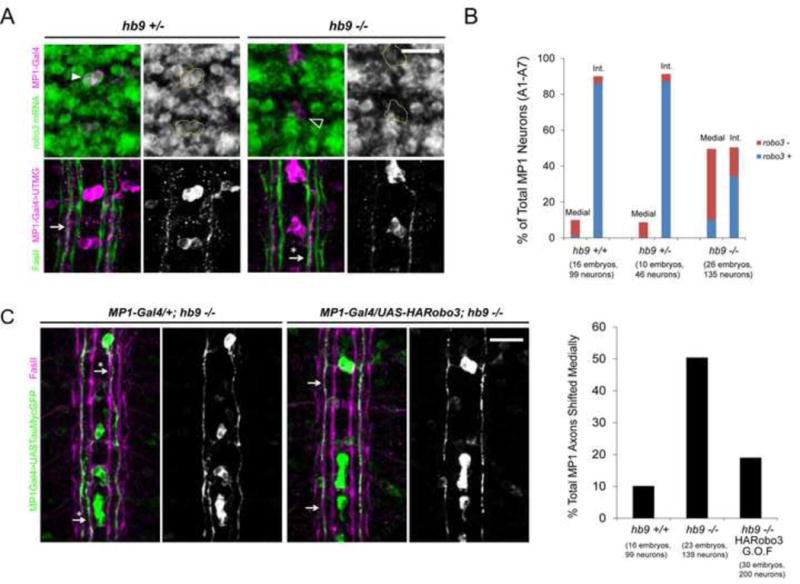
A, Top: Fluorescent in situ for robo3 mRNA (green) in Stage 16 embryos. Anterior is up. MP1 neurons are labeled by C544-Gal4 in magenta and circled in the single-channel images. Many MP1 neurons do not express robo3 in hb9 mutants (empty arrowhead). A, Bottom: MP1 axons project along the intermediate FasII bundle in hb9 heterozygotes (arrow), but are often shifted to the medial pathway in hb9 mutants (arrow with an asterisk). B: MP1 neurons were scored as robo3+ or robo3− and as projecting along the medial or intermediate (Int.) FasII tract. A significant correlation was detected between robo3 expression and lateral position in both hb9 +/− and hb9−/− embryos (Fisher’s exact test, p<0.001). C: Over-expressing robo3 rescues the medial shift phenotype of MP1 axons in hb9 mutants (p<0.001, Fisher’s Exact Test). Arrows point to MP1 axons in the correct position; arrows with asterisks point to medially shifted axons. All mutants were scored blind to genotype. Scale bars represent 10 μm. Hb9 +/+ denotes C544-Gal4/+; UAS-TauMycGFP/+. Hb9 +/− denotes C544-Gal4/+; hb9ad121, UAS-TauMycGFP/TM3. Hb9 −/− denotes C544-Gal4/+; hb9ad121, UAS-TauMycGFP/hb9kk30. Hb9 −/− HARobo3 G.O.F. denotes C544-Gal4/UAS-HARobo3.T15; hb9ad121, UAS-TauMycGFP/hb9kk30. See also Figure S5.
To determine if the lateral shift phenotype caused by Hb9 over-expression in ap neurons is due to the induction of robo2, we over-expressed Hb9 in robo2 mutants. Strikingly, removing both copies of robo2 results in a full suppression of Hb9’s gain of function phenotype, and ap axons appear wild type (Figure 6B). Together, these data indicate that ectopic expression of Hb9 is sufficient to induce robo2, and that Hb9-driven changes in robo2 expression can dramatically affect the medio-lateral position of axons.
Hb9 endogenously regulates lateral position through robo3
The requirement for hb9 in regulating the position of intermediate hb9gal4+ axons suggests it may also regulate robo3, which is expressed on axons that project to intermediate regions of the nerve cord and is essential for the formation of intermediate axonal pathways (Rajagopalan et al., 2000b; Simpson et al., 2000b). The peptidergic midline neuron MP1 expresses both hb9 and robo3 and is one of the pioneers for the intermediate FasII pathway (Broihier and Skeath, 2002; Hidalgo and Brand, 1997; Simpson et al., 2000a). We used the C544-Gal4 driver (Wheeler et al., 2006) to identify MP1 neurons and score robo3 expression and the position of the MP1 axon. The mosaic expression of C544-Gal4 allowed us to score the axonal trajectory of individual cells. Whereas almost all MP1 neurons express high levels of robo3 mRNA and project along the intermediate FasII bundle in hb9 heterozygous embryos, in hb9 mutants 56 % of MP1 neurons do not express robo3 and 47% of MP1 axons project along the medial FasII tract (Figure 7A and B). A strong correlation between robo3 expression and the lateral position of a cell’s axon is detected in both hb9 heterozygotes and mutants, suggesting that the loss of robo3 is responsible for the medial shift phenotype (p<0.0001, Fisher’s exact test) (Figure 7B). MP1 neurons also express nkx6; however, we detected no significant change in robo3 expression or in the MP1 axonal projection in nkx6 mutants (Figure S3).
To determine if restoring Robo3 rescues the lateral position of MP1 axons in hb9 mutants, we used C544-Gal4 to over-express a UAS-HARobo3 transgene. Robo3 over-expression produces no effect on the lateral position of MP1 axons in hb9 heterozygous embryos (data not shown), but results in a robust rescue of the lateral position defects of hb9 mutants (50.4% of MP1 axons shifted medially in hb9 mutants versus 19% in hb9 mutants over-expressing Robo3, p<0.0001, Fisher’s exact test) (Figure 7C). We conclude that in at least one defined group of neurons, hb9 acts through robo3 to direct the selection of an intermediate pathway.
Interestingly, all of the Hb9 deletion variants fully rescue the lateral position defects of the intermediate hb9gal4+ axons in hb9 mutants (Figure S5). Moreover, they all rescue robo3 expression in MP1 neurons, and while variants lacking the Eh domain are slightly weaker than Hb9 FL in this assay, these differences are not statistically significant (Figure S5). While we cannot rule out that Hb9 acts as a repressor to regulate robo3, the observation that its Engrailed homology domain is not required for robo3 regulation suggests the intriguing possibility that Hb9 may regulate robo2 and robo3 via distinct mechanisms.
Discussion
We have demonstrated a functional relationship between Hb9 and the Robo2 and Robo3 receptors in multiple contexts in the Drosophila embryo. In the RP motor neurons, hb9 is required for robo2 expression, and genetic rescue experiments indicate that robo2 acts downstream of hb9. Hb9 requires its conserved repressor domain and acts in parallel with Nkx6 to regulate robo2 and motor axon guidance. Moreover, hb9 contributes to the endogenous expression patterns of robo2 and robo3 and the lateral position of a subset of axons in the CNS, and can redirect axons laterally when over-expressed via upregulation of robo2. Finally, restoring Robo3 rescues the medial shift of MP1 axons in hb9 mutants, indicating that hb9 acts through robo3 to regulate medio-lateral position in a defined subset of neurons.
Robo2 is a downstream effector of Hb9 during motor axon guidance
Hb9 and nkx6 are required for the expression of robo2 in motor neurons, and rescue experiments suggest that the loss of robo2 contributes to the phenotype of hb9 mutants. However, nkx6 mutants and hb9 mutants heterozygous for nkx6 have a stronger ISNb phenotype than robo2 mutants, implying the existence of additional downstream targets. One candidate is the cell adhesion molecule Fasciclin III, which is normally expressed in the RP motor neurons and appears reduced in nkx6 mutant embryos (Broihier et al., 2004). Identifying the constellation of effectors that function downstream of Hb9 and Nkx6 will be key to understanding how transcription factors expressed in specific neurons work together to drive the expression of the cell surface receptors that regulate axon guidance and target selection.
We have identified a new activity for Drosophila Robo2 in regulating motor axon guidance. While Robo1 can replace Robo2’s repulsive activity at the midline (Spitzweck et al., 2010), Robo2’s function in motor axon guidance is not shared by either Robo1 or Robo3. Moreover, Robo2’s anti-repulsive activity at the midline and its ability to shift axons laterally when over-expressed both map to Robo2’s ectodomain, whereas we have found that Robo2’s activity in motor axon guidance maps to its cytodomain (Evans and Bashaw, 2010; Spitzweck et al., 2010). The signaling outputs of Robo2’s cytodomain remain unknown, as it lacks the conserved motifs within Robo1 that engage downstream signaling partners (Bashaw et al., 2000; Fan et al., 2003; Yang and Bashaw, 2006). How does Robo2 function during motor axon guidance? In mice, Robo receptors are expressed in spinal motor neurons and prevent the defasciculation of a subset of motor axons (Jaworski and Tessier-Lavigne, 2012). Does Drosophila Robo2 regulate motor axon fasciculation? The levels of adhesion between ISNb axons and other nerves must be precisely controlled during the different stages of motor axon growth and target selection, and several regulators of adhesion are required for ISNb guidance (Fambrough and Goodman, 1996; Huang et al., 2007; Winberg et al., 1998). Furthermore, whereas Slit can be detected on ventral muscles, it is not visibly enriched in a pattern that suggests directionality in guiding motor axons (Kramer et al., 2001), making it difficult to envision how Robo2-mediated repulsive or attractive signaling might contribute to ISNb pathfinding. Future work will determine how Robo2’s cytodomain mediates motor axon guidance, whether this activity is Slit-dependent, and whether Robo2 signals attraction, repulsion, or modulates adhesion in Drosophila motor axons.
Hb9 regulates lateral position through robo2 and robo3
Elegant gene swap experiments revealed the importance of transcriptional regulation in establishing the different expression patterns and functions of the Drosophila Robo receptors (Spitzweck et al., 2010). By analyzing a previously uncharacterized subset of axon pathways, we have uncovered a requirement for Hb9 in regulating lateral position in the CNS. While Hb9 can act instructively to direct lateral position when over-expressed, its endogenous expression in a subset of medially-projecting neurons suggests that its ability to shift axons laterally is context-dependent. A complex picture emerges in which multiple factors act in different groups of neurons to regulate robo2 and robo3. In a subset of interneurons, hb9 is endogenously required for lateral position through the upregulation of robo3 and likely robo2. In other neurons, such as those that form the outer FasII tracts, the expression patterns of robo2 and robo3 rely on additional upstream factors. What might be the significance of a regulatory network in which multiple sets of transcription factors direct lateral position in different groups of neurons? One possibility is that hb9-expressing neurons may share specific functional properties, such as the expression of particular neurotransmitters or ion channels. Alternatively, hb9 may regulate other aspects of connectivity. Indeed, Robo receptors mediate dendritic targeting in the Drosophila CNS (Furrer et al., 2003), raising the exciting possibility that hb9 regulates both axonal and dendritic guidance through its effects on guidance receptor expression.
How does Hb9 regulate robo2 and robo3?
What is the mechanism by which Hb9 regulates the expression of robo2, robo3, and its other downstream effectors? We have found that Hb9 requires its conserved putative repressor domain and acts in parallel with Nkx6 to regulate robo2 and motor axon guidance. It has previously been shown that hb9 and nkx6 function in parallel to regulate several transcription factors (Broihier and Skeath, 2002; Broihier et al., 2004). Hb9, nkx6 double mutants show decreased expression of islet and lim3, and upregulation of eve and the Nkx2 ortholog vnd (Broihier et al., 2004). Are Hb9 and Nkx6 regulating robo2 or robo3 through any of their previously identified targets? Hb9 and nkx6 single mutants show no change in islet, lim3, or vnd expression (Broihier and Skeath, 2002; Broihier et al., 2004), arguing that hb9 and nkx6 do not act solely through these factors to regulate robo2 or robo3. Eve expression is unaffected in nkx6 mutants (Broihier et al., 2004), and while it is ectopically expressed in two neurons per hemisegment in hb9 mutants (Broihier and Skeath, 2002), these do not correspond to RP3 or MP1, the identifiable cells in which we can detect changes in robo2 and robo3 (data not shown). Therefore, our data do not support the hypothesis that Hb9 and Nkx6 regulate robo2 or robo3 primarily through their previously identified targets islet, lim3, vnd or eve.
Gain of function experiments in vertebrates suggest that Hb9 and Nkx6 act as repressors to regulate gene expression in the spinal cord (Lee et al., 2008; Muhr et al., 2001; William et al., 2003). Our finding that Hb9’s Engrailed homology domain is required for motor axon pathfinding and robo2 regulation suggests that Hb9 acts as a repressor in this context as well, most likely through a previously unidentified intermediate target. On the other hand, the Eh domain is not required for Hb9’s ability to regulate robo3 or lateral position in hb9gal4+ neurons that project to intermediate zones of the CNS. The finding that Hb9ΔEh retains significant activity in rescuing lateral position and robo3 expression indicates that Hb9 may regulate robo2 and robo3 via distinct mechanisms, perhaps involving different transcriptional co-factors or intermediate targets. In support of this hypothesis, hb9 over-expression in the apterous neurons can induce robo2, but not robo3. These data raise the intriguing possibility that Hb9’s ability to regulate robo2 and robo3 via different mechanisms contributed to the diversification of their expression patterns in the CNS.
Determining how Hb9 and Nkx6 regulate their effectors will be key to achieving a complete understanding of how these conserved transcription factors control changes in cell morphology and axon pathfinding during development. Of note, Hb9 mutant mice exhibit defects in a subset of motor nerves, including the phrenic and intercostal nerves, which are also affected in Robo mutants (Arber et al., 1999; Jaworski and Tessier-Lavigne, 2012; Thaler et al., 1999). It will be of great interest to determine if despite the vast divergence in the evolution of nervous system development between invertebrates and vertebrates, Hb9 or Nkx6 have retained a role for regulating Robo receptors across species.
Experimental Procedures
Molecular Biology
Hb9 constructs with an N-terminal Myc tag were cloned into a pUAST vector containing 10xUAS and an attB site for ΦC31-mediated targeted insertion. Hb9ΔEh (lacking amino acids 219-229) and Hb9ΔCtbp (lacking amino acids 336–340) were generated by serial overlap extension PCR. Transgenes were inserted at cytological site 51C by Best Gene (Chino Hills, CA, USA). The 22K18-robo2 BAC was obtained from BACPAC Resources (Children’s Hospital, Oakland) and inserted at 51C by Rainbow Transgenics (Carmarillo, CA, USA).
Fluorescent in situ hybridization and quantification
Fluorescent mRNA in situ hybridization was performed as described (Labrador et al., 2005). Fluorescence quantification was performed using ImageJ as described (Yang et al., 2009); see the supplemental experimental procedures.
Immunostaining and imaging
Embryo fixation and staining were performed as described (Kidd et al., 1998). Images were acquired with Volocity using a spinning disk confocal (Perkin Elmer) using a Nikon 40x objective with a Hamamatsu C10600-10B CCD camera and Yokogawa CSU-10 scanner head. Images were processed using ImageJ.
Supplementary Material
Highlights.
Hb9 acts through Robo2 to regulate motor axon pathfinding
Robo2’s novel activity in motor axon guidance maps to its cytoplasmic domain
Hb9 regulates medio-lateral position in longitudinal axons through robo2 and robo3
Hb9 requires its conserved repressor domain to regulate robo2, but not robo3
Acknowledgments
We thank members of the Bashaw lab for thoughtful feedback during the development of this manuscript. In particular, we thank Tim Evans, Melissa Hernandez, Alexandra Neuhaus-Follini and Mike O’Donnell for intellectual and experimental contributions. We thank Dr. Barry Dickson, Dr. Lawrence Zipursky, Dr. Stephen Crews, and Dr. James Skeath for fly stocks and antibodies. C.S. was supported by an NSF pre-doctoral training grant. This work was supported by National Institutes of Health Grants NS-046333, NS054739 and March of Dimes grant #1-FY12-445 to G.J.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan DW, Park D, St Pierre SE, Taghert PH, Thor S. Regulators acting in combinatorial codes also act independently in single differentiating neurons. Neuron. 2005;45:689–700. doi: 10.1016/j.neuron.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Baumgardt M, Miguel-Aliaga I, Karlsson D, Ekman H, Thor S. Specification of neuronal identities by feedforward combinatorial coding. PLoS Biol. 2007;5:e37. doi: 10.1371/journal.pbio.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe V, Stappers E, Vandesande B, Dimidschstein J, Kroes R, Francis A, Conidi A, Lesage F, Dries R, Cazzola S, et al. Directed migration of cortical interneurons depends on the cell-autonomous action of Sip1. Neuron. 2013;77:70–82. doi: 10.1016/j.neuron.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Bravo-Ambrosio A, Mastick G, Kaprielian Z. Motor axon exit from the mammalian spinal cord is controlled by the homeodomain protein Nkx2.9 via Robo-Slit signaling. Development. 2012;139:1435–1446. doi: 10.1242/dev.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broihier HT, Skeath JB. Drosophila homeodomain protein dHb9 directs neuronal fate via crossrepressive and cell-nonautonomous mechanisms. Neuron. 2002;35:39–50. doi: 10.1016/s0896-6273(02)00743-2. [DOI] [PubMed] [Google Scholar]
- Broihier HT, Kuzin A, Zhu Y, Odenwald W, Skeath JB. Drosophila homeodomain protein Nkx6 coordinates motoneuron subtype identity and axonogenesis. Development. 2004;131:5233–5242. doi: 10.1242/dev.01394. [DOI] [PubMed] [Google Scholar]
- Certel SJ, Thor S. Specification of Drosophila motoneuron identity by the combinatorial action of POU and LIM-HD factors. Development. 2004;131:5429–5439. doi: 10.1242/dev.01418. [DOI] [PubMed] [Google Scholar]
- Crowner D, Madden K, Goeke S, Giniger E. Lola regulates midline crossing of CNS axons in Drosophila. Development. 2002;129:1317–1325. doi: 10.1242/dev.129.6.1317. [DOI] [PubMed] [Google Scholar]
- Dasen JS. Transcriptional networks in the early development of sensory-motor circuits. Elsevier Inc; 2009. [DOI] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ. Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr Biol. 2010;20:567–572. doi: 10.1016/j.cub.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D, Goodman CS. The Drosophila beaten path gene encodes a novel secreted protein that regulates defasciculation at motor axon choice points. Cell. 1996;87:1049–1058. doi: 10.1016/s0092-8674(00)81799-7. [DOI] [PubMed] [Google Scholar]
- Fan X, Labrador JP, Hing H, Bashaw GJ. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40:113–127. doi: 10.1016/s0896-6273(03)00591-9. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Lear BC, Landgraf M, Yusibova GL, Zhou J, Riley KM, Patel NH, Jaynes JB. Even-skipped, acting as a repressor, regulates axonal projections in Drosophila. Development. 2003;130:5385–5400. doi: 10.1242/dev.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer MP, Kim S, Wolf B, Chiba A. Robo and Frazzled/DCC mediate dendritic guidance at the CNS midline. Nature. 2003;6:223–230. doi: 10.1038/nn1017. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Brand AH. Targeted neuronal ablation : the role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development. 1997;124:3253–3262. doi: 10.1242/dev.124.17.3253. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol. 2011;27:681–696. doi: 10.1146/annurev-cellbio-092910-154226. [DOI] [PubMed] [Google Scholar]
- Huang Z, Yazdani U, Thompson-peer KL, Kolodkin AL, Terman JR. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development. 2007;134:2337–2347. doi: 10.1242/dev.004242. [DOI] [PubMed] [Google Scholar]
- Jaworski A, Tessier-Lavigne M. Autocrine/juxtaparacrine regulation of axon fasciculation by Slit-Robo signaling. Nat Neurosci. 2012;15:367–369. doi: 10.1038/nn.3037. [DOI] [PubMed] [Google Scholar]
- Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci. 2010;30:9445–9453. doi: 10.1523/JNEUROSCI.6290-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi-Nakao S, Arvind R, Amikura R, Kinameri E, Liu AW, Moore AW. Knot/Collier and Cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102:161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J. Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci. 2009;29:8914–8926. doi: 10.1523/JNEUROSCI.0568-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kramer SG, Kidd T, Simpson JH, Goodman CS. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–740. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- Labrador JP, O’keefe D, Yoshikawa S, McKinnon RD, Thomas JB, Bashaw GJ. The homeobox transcription factor even-skipped regulates netrin-receptor expression to control dorsal motor-axon projections in Drosophila. Curr Biol. 2005;15:1413–1419. doi: 10.1016/j.cub.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Landgraf M, Bossing T, Technau GM, Bate M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J Neurosci. 1997;17:9642–9655. doi: 10.1523/JNEUROSCI.17-24-09642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Roy S, Prokop A, VijayRaghavan K, Bate M. even-skipped determines the dorsal growth of motor axons in Drosophila. Neuron. 1999;22:43–52. doi: 10.1016/s0896-6273(00)80677-7. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee B, Joshi K, Pfaff SL, Lee JW, Lee SK. A regulatory network to segregate the identity of neuronal subtypes. Dev Cell. 2008;14:877–889. doi: 10.1016/j.devcel.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QX, Hiramoto M, Ueda H, Gojobori T, Hiromi Y, Hirose S. Midline governs axon pathfinding by coordinating expression of two major guidance systems. Genes Dev. 2009;23:1165–1170. doi: 10.1101/gad.1774209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Luria V, Krawchuk D, Jessell TM, Laufer E, Kania A. Specification of Motor Axon Trajectory by Ephrin-B: EphB Signaling: Symmetrical Control of Axonal Patterning in the Developing Limb. Neuron. 2008;60:1039–1053. doi: 10.1016/j.neuron.2008.11.011. [DOI] [PubMed] [Google Scholar]
- De Marco Garcia NV, Jessell TM. Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity. Neuron. 2008;57:217–231. doi: 10.1016/j.neuron.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos-Mondéjar P, Peregrín S, Li JY, Carlsson L, Tole S, López-Bendito G. The lhx2 transcription factor controls thalamocortical axonal guidance by specific regulation of robo1 and robo2 receptors. J Neurosci. 2012;32:4372–4385. doi: 10.1523/JNEUROSCI.5851-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr J, Andersson E, Persson M, Jessell TM, Ericson J. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell. 2001;104:861–873. doi: 10.1016/s0092-8674(01)00283-5. [DOI] [PubMed] [Google Scholar]
- Nóbrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson S, Marín O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odden JP, Holbrook S, Doe CQ. Drosophila HB9 is expressed in a subset of motoneurons and interneurons, where it regulates gene expression and axon pathfinding. J Neurosci. 2002;22:9143–9149. doi: 10.1523/JNEUROSCI.22-21-09143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Ince-Dunn G, Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev Neurosci. 2007;8:331–340. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000a;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000b;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EYHP, Tessier-Lavigne M. The divergent Robo family protein Rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Sakai N, Insolera R, Sillitoe RV, Shi SH, Kaprielian Z. Axon sorting within the spinal cord marginal zone via Robo-mediated inhibition of N-cadherin controls spinocerebellar tract formation. J Neurosci. 2012;32:15377–15387. doi: 10.1523/JNEUROSCI.2225-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Paydar S, Ericson J, Briscoe J, Berber E, German M, Jessell TM, Rubenstein JLR. Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 2000;14:2134–2139. doi: 10.1101/gad.820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000a;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell. 2000b;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Smith ST, Jaynes JB. A conserved region of engrailed, shared among all en-, gsc-, Nk1-, Nk2-and msh-class homeoproteins, mediates active transcriptional repression in vivo. Development. 1996;122:3141–3150. doi: 10.1242/dev.122.10.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzweck B, Brankatschk M, Dickson BJ. Distinct protein domains and expression patterns confer divergent axon guidance functions for Drosophila Robo receptors. Cell. 2010;140:409–420. doi: 10.1016/j.cell.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Thor S, Thomas JB. The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron. 1997;18:397–409. doi: 10.1016/s0896-6273(00)81241-6. [DOI] [PubMed] [Google Scholar]
- Thor S, Andersson SGE, Tomlinson A, Thomas JB. A LIM-homeodomain combinatorial code for motor- neuron pathway selection. Nature. 1999;397:76–80. doi: 10.1038/16275. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron. 2001;31:743–755. doi: 10.1016/s0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Wheeler SR, Kearney JB, Guardiola AR, Crews ST. Single-cell mapping of neural and glial gene expression in the developing Drosophila CNS midline cells. Dev Biol. 2006;294:509–524. doi: 10.1016/j.ydbio.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William CM, Tanabe Y, Jessell TM. Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development. 2003;130:1523–1536. doi: 10.1242/dev.00358. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt Ja, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SI, Shafer B, Lee KJ, Dodd J. A molecular program for contralateral trajectory: Rig-1 control by LIM homeodomain transcription factors. Neuron. 2008;59:413–424. doi: 10.1016/j.neuron.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Yang L, Bashaw GJ. Son of sevenless directly links the Robo receptor to rac activation to control axon repulsion at the midline. Neuron. 2006;52:595–607. doi: 10.1016/j.neuron.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Yang L, Garbe DS, Bashaw GJ. A frazzled/DCC-dependent transcriptional switch regulates midline axon guidance. Science. 2009;324:944–947. doi: 10.1126/science.1171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarin AA, Asadzadeh J, Labrador JP. Transcriptional regulation of guidance at the midline and in motor circuits. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors: atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37:41–51. doi: 10.1016/s0896-6273(02)01131-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


