Abstract
As the complexity of animal nervous systems has increased during evolution, developmental control of neuronal connectivity has become increasingly refined. How has functional diversification within related axon guidance molecules contributed to the evolution of nervous systems? To address this question, we explore the evolution of functional diversity within the Roundabout (Robo) family of axon guidance receptors. In Drosophila, Robo and Robo2 promote midline repulsion, while Robo2 and Robo3 specify the position of longitudinal axon pathways. The Robo family has expanded by gene duplication in insects; robo2 and robo3 exist as distinct genes only within dipterans, while other insects, like the flour beetle Tribolium castaneum, retain an ancestral robo2/3 gene. Both Robos from Tribolium can mediate midline repulsion in Drosophila, but unlike the fly Robos cannot be down-regulated by Commissureless. The overall architecture and arrangement of longitudinal pathways are remarkably conserved in Tribolium, despite it having only two Robos. Loss of TcSlit causes midline collapse of axons in the beetle, a phenotype recapitulated by simultaneous knockdown of both Robos. Single gene knockdowns reveal that beetle Robos have specialized axon guidance functions: TcRobo is dedicated to midline repulsion, while TcRobo2/3 also regulates longitudinal pathway formation. TcRobo2/3 knockdown reproduces aspects of both Drosophila robo2 and robo3 mutants, suggesting that TcRobo2/3 has two functions that in Drosophila are divided between Robo2 and Robo3. The ability of Tribolium to organize longitudinal axons into three discrete medial-lateral zones with only two Robo receptors demonstrates that beetle and fly achieve equivalent developmental outcomes using divergent genetic programs.
Keywords: Axon Guidance, Midline, Repulsion, Roundabout, Slit, Drosophila, Tribolium
Introduction
During development, neural circuits are formed as axons and dendrites extending from neuronal cell bodies follow a series of short- and long-range guidance cues toward their ultimate synaptic partners (Dickson, 2002; Tessier-Lavigne and Goodman, 1996). The concentration of neurites into a neuropile structure in modern animal nervous systems allows economical and efficient assembly and function of nerve pathways, and requires precise and coordinated control of multiple guidance decisions as axons and dendrites navigate an increasingly complex environment (Landgraf et al., 2003; Mauss et al., 2009; Zlatic et al., 2009). To facilitate this increase in complexity over evolutionary time, existing guidance pathways have been redeployed in diverse contexts, and novel activities have arisen as the repertoire of guidance molecules has expanded.
The midline repellant Slit and its cognate Roundabout (Robo) receptors constitute an ancient and widely conserved axon guidance pathway which regulates midline crossing in the nervous systems of bilaterally symmetric animals (Dickson and Gilestro, 2006; Evans and Bashaw, 2010a). In addition to this canonical midline repulsive role, Slit and its Robo receptors also control diverse axon guidance decisions in insects and vertebrates (Chen et al., 2008; Farmer et al., 2008; Jaworski et al., 2010; Kastenhuber et al., 2009; Long et al., 2004; Sabatier et al., 2004). In the Drosophila embryonic CNS, the three fly Robo receptors exhibit specialized guidance functions. Robo and Robo2 cooperate to promote Slit-dependent midline repulsion, while Robo2 and Robo3 direct the formation of longitudinal pathways within distinct regions of the neuropile (Evans and Bashaw, 2010b; Rajagopalan et al., 2000a; Rajagopalan et al., 2000b; Simpson et al., 2000a; Simpson et al., 2000b; Spitzweck et al., 2010). Alone among the Drosophila Robos, Robo2 can also promote midline crossing in addition to its conventional role in midline repulsion (Evans and Bashaw, 2010b; Simpson et al., 2000b; Spitzweck et al., 2010).
The divergent activities of the three Drosophila Robo receptors depend in part on differential expression patterns, and in part on functional distinctions between the receptors themselves (Evans and Bashaw, 2010b; Spitzweck et al., 2010). While robo and robo2 are both required for Slit-dependent midline repulsion, robo2 cannot functionally replace robo in this context. Similarly, neither robo nor robo3 can substitute for robo2 to promote the formation of axon pathways in the lateral regions of the neuropile. robo3’s role in longitudinal pathway formation in intermediate regions, however, does not depend on unique features of Robo3 as both robo and robo2 can substitute for robo3 in this context (Spitzweck et al., 2010). A complementary series of domain swapping studies from our lab and our colleagues in the Dickson lab recently demonstrated that Robo’s role in midline repulsion depends on unique sequences within its cytoplasmic region, while individual extracellular immunoglobulin-like (Ig) domains specify Robo2’s singular roles in lateral position and midline crossing (Evans and Bashaw, 2010b; Spitzweck et al., 2010).
How do related receptors acquire divergent axon guidance activities, and how has this contributed to the evolution of nervous systems? The study of conservation and divergence of developmental genetic mechanisms has provided enormous insight into the evolutionary history of animals, from establishing body axes to formation and patterning of the nervous system. Studies of neural differentiation and the initial formation of axon pathways in arthropods and related animals have provided a glimpse into the variety of neurodevelopmental mechanisms at work in this group (Duman-Scheel et al., 2007; Fischer and Scholtz, 2010; Linne and Stollewerk, 2011; Mayer and Whitington, 2009; Simanton et al., 2009; Stollewerk and Eriksson, 2010; Ungerer et al., 2011). Intriguingly, a recent analysis of axon pathway formation in onychophorans (worm-like relatives of arthropods with unsegmented bodies and unjointed legs) suggested that increasingly precise control of axon guidance may have been crucial to the evolution of the modern arthropod nervous system by facilitating intersegmental coordination between limb and body muscles (Mayer and Whitington, 2009; Whitington and Mayer, 2011). Illuminating the processes by which axon guidance mechanisms have changed over time may provide valuable insight into the evolution of this diverse and highly successful group of animals. While researchers have begun to investigate the function of conserved axon guidance genes in arthropods other than Drosophila (Clemons et al., 2011; Haugen et al., 2011; Linne and Stollewerk, 2011), a direct examination of the evolutionary diversification of axon guidance receptor function has not been attempted.
Here we describe the role of Slit/Robo signaling in midline repulsion and lateral positioning of longitudinal pathways in the beetle Tribolium castaneum. Unlike Drosophila, Tribolium retains an ancestral set of insect Robo receptors, allowing us to ask how Robo axon guidance functions have changed during evolution. We find that Slit/Robo-mediated midline repulsion is well conserved in Tribolium, but that Robo receptor control of longitudinal pathway formation differs from Drosophila. In Tribolium, a single ancestral Robo2/3 receptor controls distinct aspects of longitudinal pathway formation that in Drosophila are subdivided between Robo2 and Robo3. We also report that orthologs of Robo2/3 are not found outside insects, perhaps suggesting that expansion and functional diversification of the Robo family has contributed to the evolution of nervous system connectivity in insects and their relatives.
Materials and Methods
Tribolium culture and embryo collection
Tribolium castaneum were obtained from Carolina Biological (Burlington, NC) and cultured at 25°C on unbleached flour plus 5% dried yeast. For embryo collections, adults were incubated in flour for 24 hours, then removed using a 850 µm sieve and the embryos were either fixed immediately or allowed to develop for an additional 1–5 days at 25°C. Eggs were harvested using a 300 µm sieve and dechorionated with 50% bleach for 2 min, then rinsed thoroughly with water. Embryos were fixed in glass vials containing 10 ml of heptane and 10 ml of 8% formaldehyde for 30–45 min at room temperature with vigorous shaking, after which the fixative was removed and replaced with an equivalent volume of methanol. Embryos were devitellinized by 2–5 rounds of sonication for 5 seconds each using a Branson Sonifier 250 at 10% output. Devitellinized embryos and fragments (those that sank to the bottom of the methanol phase) were collected after each round of sonication. Embryos were stored at −20°C in 100% methanol.
Immunohistochemistry
Anti-TcFasII antibody was produced by GenScript (Piscataway NJ). Rabbits were immunized with a TcFasII-specific peptide (CKQRNSQDLSKSDRF) conjugated to KLH. Polyclonal antiserum was affinity-purified using the peptide antigen and used at 1:1000 dilution. Other antibodies used were: mouse MAb 1D4/anti-FasII (Developmental Studies Hybridoma Bank [DSHB]; 1:100), mouse anti-ßgal (DSHB; 1:150), FITC-conjugated goat anti-HRP (Jackson Immunoresearch; 1:50), Cy3 goat anti-rabbit (Jackson; 1:500), and Cy3 goat anti-mouse (Jackson; 1:1000). Antibody staining was performed as in Patel, 1994. Nerve cords were dissected and mounted in 70% glycerol/PBS. Confocal stacks were collected using a Leica Confocal TCS SL microscope and processed by NIH ImageJ and Adobe Photoshop software.
Phylogenetic analysis
Robo orthologs were identified by searching animal genome sequences by tblastn using Drosophila protein sequences. Intron-exon structures were manually annotated from the genomic sequence using the Drosophila sequences as references. Predicted Tribolium Slit and Robo sequences were confirmed by sequencing cDNA clones. Tribolium Robo sequences were deposited in the GenBank database. Accession numbers: JN634958 (TcRobo) and JN634959 (TcRobo2/3). Protein alignments of receptor ectodomains and resulting neighbor joining trees were generated using ClustalX. Trees were drawn using DrawTree from the PHYLIP software package.
Molecular biology
Total RNA was extracted from 0–6 day old Tribolium embryos using TRIzol (Invitrogen) and cDNA was synthesized using SuperScript (Invitrogen). Beetle genes were amplified by PCR using the following primers and cloned into TOPO vector (Invitrogen): TcSlit (5’-ATGCTAGCCTCCGGTGTCCCAGAGGATGC-3’ and 5’-GCCTCGAGTCAGTAGCATTTTTTCGTACACGC-3’), TcRobo (5’-TAGCTAGCCCGCGGATCACCGAGCACCCC-3’ and 5’-CGGGTACCTCACTTCCCGCACCTTTTCACCTGG-3’), TcRobo2/3 (5’-TAGCTAGCCCTCGAATCACCGAACACCCTGTAG-3’ and 5’-GCGGTACCTCACAACATATTGCCAGGCTCACTCC-3’). Drosophila and Tribolium Robo coding sequences (beginning with the first immunoglobulin [Ig1] domain) were subcloned into a pUAST vector including identical heterologous 5’UTR and signal sequences (derived from the Drosophila wingless gene) and an N-terminal 3×HA tag.
Double-stranded RNA (dsRNA) was synthesized with MEGAscript RNAi kit (Ambion) using ~800 bp PCR product templates. Forward and reverse PCR primers both included T7 promoter sequences at their 5’ ends; gene-specific sequences were as follows: TcSlit (5’-CATCTGGCTGATGAGCTGTTCAACGG-3’ and 5’-GTACACGCGCATTTGCGCACGATGTC-3’), TcRobo (5’-CAAGGCCCTTATTCCAACCAAGTGCC-3’ and 5’-TCACTTCCCGCACCTTTTCACCTGGC-3’, TcRobo2/3 (5’-CCGTAAAAGTGGCAGCAATGACACGC-3’ and 5’-TCACAACATATTGCCAGGCTCACTCC-3’).
Genetics
The following Drosophila stocks were used: (1) w1118, (2) slit2/CyO,wg-lacZ, (3) slitE158/CyO,wg-lacZ, roboGA285/CyO,wg-lacZ, (4) robo2x33/CyO,wg-lacZ, (5) robo2x123/CyO,wg-lacZ, (6) robo31/CyO,wg-lacZ, (7) roboGA285,robo2x33/CyO,wg-lacZ, (8) roboGA285,robo2x123/CyO,wg-lacZ, (9) elav-GAL4, (10) roboGA285/CyO,wg-lacZ; elav-GAL4, (11) eg-GAL4, UAS-TauMycGFP/TM3,tub-GAL80, (12) roboGA285,UAS-TauMycGFP/CyO,tub-GAL80; eg-GAL4, (13) UAS-Comm/CyO,tub-GAL80; elav-GAL4. Transgenic flies were generated by BestGene Inc (Chino Hills, CA) using ΦC31-directed site-specific integration into the same landing site for all constructs (at cytological position 86FB) to ensure comparable expression levels between lines. All crosses were carried out at 25°C.
Parental RNAi
Female Tribolium pupae were collected just before eclosion and glued by the posterior abdomen to a glass microscope slide using rubber cement. Approximately 0.1–0.2 ul of dsRNA (0.25–1.0 ug/ul concentration) was injected laterally into the ventral abdomen, and slides were placed upside down in flour until adult beetles eclosed. Injected females were mated to untreated males and egg collections began one week after mating.
Results
Expansion of the Robo family by gene duplication in insects
In Drosophila, three Robo receptors (Robo, Robo2, and Robo3) each control a unique set of axon guidance decisions in the embryonic CNS (Rajagopalan et al., 2000a; Rajagopalan et al., 2000b; Simpson et al., 2000a; Simpson et al., 2000b). This functional diversity depends in part on differential expression patterns and in part on structural differences between the three receptors (Evans and Bashaw, 2010b; Spitzweck et al., 2010). The close proximity and genomic organization of robo2 and robo3 suggest that these two genes are the product of a recent gene duplication, and raises the possibility that their divergent axon guidance functions are a recent evolutionary development. To gain insight into the evolutionary history of the Robo family, we examined the distribution of Robo orthologs in the sequenced genomes of insects and other animal groups.
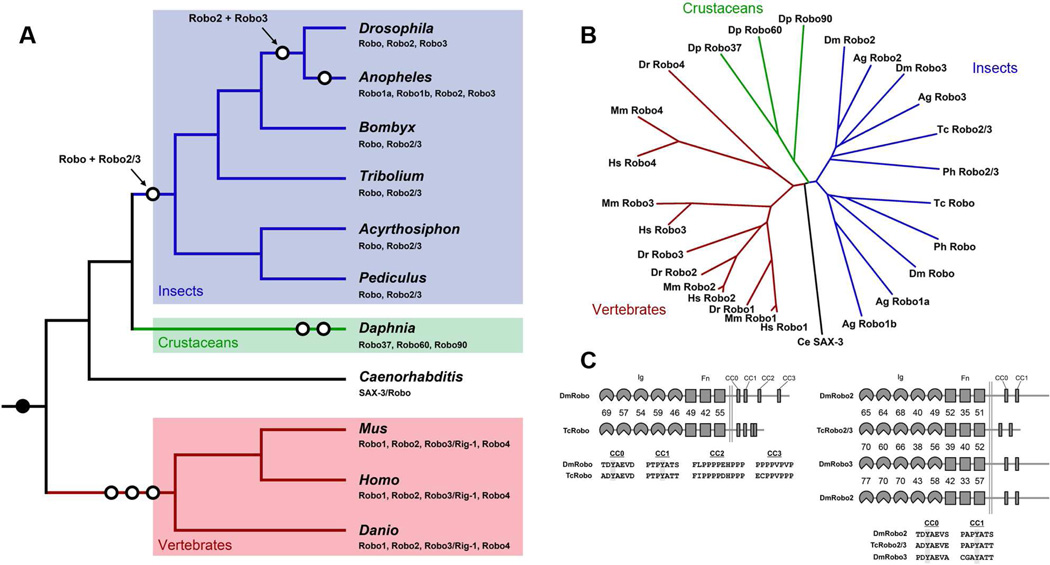
Orthologs of all three D. melanogaster Robos can be found in each of the twelve sequenced Drosophila genomes (Clark et al., 2007), and in the mosquitoes Anopheles gambiae (Holt et al., 2002) and Aedes aegypti (Nene et al., 2007). In addition, a fourth Robo gene in mosquitoes appears to be the result of a duplication of robo in this group. Thus each of the three Drosophila Robos are conserved throughout the Diptera. In contrast, the genomes of representatives from four other insect orders (Lepidoptera: Bombyx mori (International Silkworm Genomics Consortium, 2008); Coleoptera: Tribolium castaneum (Richards et al., 2008); Hemiptera: Acyrthosiphon pisum (International Aphid Genomics Consortium, 2010); Phthiraptera: Pediculus humanus (Kirkness et al., 2010)) each encode only two Robo genes: one an ortholog of Drosophila Robo and the other equally similar to Drosophila Robo2 and Robo3 (Figure 1). We refer to this ancestral Robo2/Robo3 receptor as Robo2/3. Thus the gene duplication event that produced robo2 and robo3 occurred in a common ancestor of flies and mosquitoes, while the split between robo and robo2/3 was a more ancient event (Figure 1A).
Figure 1. Expansion of the Robo family by gene duplication.
A. Schematic phylogeny showing the evolutionary relationship between selected animals, and the complement of Robo receptors present in each animal’s genome. Predicted gene duplication events are represented by open circles. Filled black circle represents the appearance of the ancestral Robo receptor. The Robo family has expanded via gene duplication in insects (blue), crustaceans (green), and vertebrates (red). Daphnia Robos are given numerical designation based on the genomic contig in which they are located. Branch lengths are not to scale.
B. Phylogenetic analysis of Robo family members using extracellular (Ig+Fn) protein sequences. Insect (blue), crustacean (green), and vertebrate (red) receptors cluster together, reflecting their independent derivation from a single common ancestral receptor. Note also that Robo2 and Robo3 orthologs in fly (Dm) and mosquito (Ag) are more similar to each other than to Robo2/3 from beetle (Tc) and louse (Ph). A single Robo receptor (SAX-3) has been retained in nematodes. Abbreviations: Dm, Drosophila melanogaster; Ag, Anopheles gambiae; Tc, Tribolium castaneum; Ph, Pediculus humanus; Dp, Daphnia pulex; Ce, Caenorhabditis elegans; Hs, Homo sapiens; Mm, Mus musculus; Dr, Danio rerio.
C. Schematic comparison of Drosophila and Tribolium Robo receptors showing percent sequence identity between individual protein domains, and highlighting the conserved cytoplasmic (CC) motifs. Lengths of cytodomains are roughly to scale. TcRobo retains all four CC motifs despite its much smaller cytodomain. Note that Drosophila Robo2 and Robo3 are more similar to each other than to Tribolium Robo2/3.
A single Robo receptor has been described in the nematode Caenorhabditis elegans (known as SAX-3) (Zallen et al., 1998), raising the possibility that Robo and Robo2/3 may exist as distinct genes only within arthropods. We searched available non-insect arthropod genomes for Robo receptor orthologs. The genome of the branchiopod crustacean Daphnia pulex (Colbourne et al., 2011; Colbourne et al., 2005) does not encode distinct orthologs of Robo and Robo2/3; the three Daphnia Robo receptors are more similar to each other than to any insect or vertebrate Robos, indicating that they are also the products of independent gene duplication (Figure 1B). Similarly, the preliminary assembly of the Ixodes scapularis (deer tick) genome (http://iscapularis.vectorbase.org) encodes four predicted Robo-like sequences, also apparently reflecting robo gene duplication in this lineage (not shown). These results suggest that the ancestral arthropod, like nematodes, possessed a single Robo receptor that was duplicated independently in three major arthropod groups (insects, crustaceans, and chelicerates). Within the insects, robo2 and robo3 exist as separate genes only in dipterans.
When were the distinct axon guidance functions associated with the three Drosophila Robos acquired? What role did robo gene duplication and functional diversification play in the evolution of the nervous system in insects and other animal groups? Do divergent guidance receptor complements promote similar or distinct guidance outcomes in related animals? To address these questions, we set out to examine the functions of Robo receptors in the beetle Tribolium castaneum, an experimentally tractable insect that retains an ancestral set of Robo receptors.
Tribolium Robos can mediate midline repulsion in Drosophila
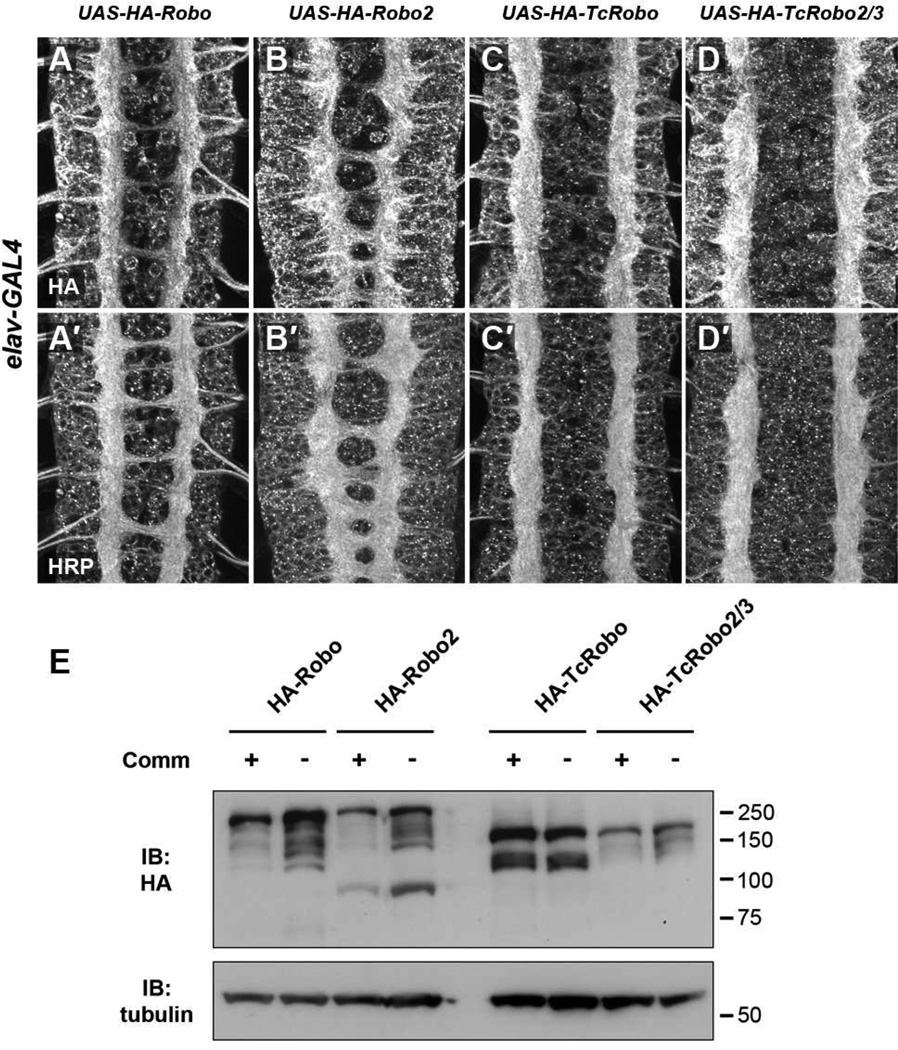
Our first step was to ask whether one or both of the two Tribolium Robos could mediate midline repulsion in response to Slit. To this end, we cloned both Tribolium Robos via RT-PCR from beetle embryos and introduced them into Drosophila using the GAL4/UAS system (Brand and Perrimon, 1993). We used ΦC31-directed site-specific integration to insert all of our UAS transgenes into the same genomic landing site to ensure comparable mRNA expression levels between lines. Using an N-terminal epitope tag, we confirmed that both beetle receptors could be stably expressed in Drosophila embryonic neurons, and exhibited axonal localization patterns similar to those observed for Drosophila Robos under equivalent ectopic expression conditions (Figure 2A–D). In an otherwise wild type background, misexpression of TcRobo or TcRobo2/3 either pan-neurally (with elav-GAL4; Figure 2) or in a subset of commissural neurons (EW neurons, with eg-GAL4; Figure S3) in the embryonic CNS prevented commissural axons from crossing the midline, indicating that both Tribolium Robos can trigger midline repulsion in the Drosophila embryonic CNS. This phenotype was similar to that caused by high level misexpression of Drosophila Robo or Robo2, although the Tribolium Robos appeared much more potent at mediating midline repulsion (Figure 2A′–D′). Importantly, this appeared to be a specific effect on axon guidance, as we observed no apparent defects in neuronal survival or axon outgrowth in these embryos.
Figure 2. Tribolium Robos are potent midline repellant receptors in Drosophila.
A–D. Stage 16–17 Drosophila embryos carrying elav-GAL4 and the indicated UAS-Robo transgenes. In this and all subsequent figures, all UAS-Robo transgenes have identical 5’UTR, signal sequence and 3×HA N-terminal tags, and are inserted into the same genomic location (at cytological position 86FB). Anti-HA staining shows that Robo receptors from fly and beetle localize to axons when expressed pan-neurally in the Drosophila embryonic CNS (A–D). Tribolium Robos are much more effective at preventing axons from crossing the midline than Drosophila Robos, producing a commissureless phenotype as revealed by anti-HRP staining (A′–D′).
E. Western blot of total lysates from 0–24 hour embryos carrying elav-GAL4 and the indicated UAS-Robo transgenes, with or without UAS-Comm. Lysates were subjected to SDS-PAGE and immunoblotted with anti-HA to detect the levels of HA-tagged receptors, and anti-tubulin as a loading control. Levels of HA-tagged Drosophila Robo and Robo2 are strongly reduced in the presence of exogenous Comm, while the levels of HA-tagged TcRobo and TcRobo2/3 were similar in the presence or absence of exogenous Comm.
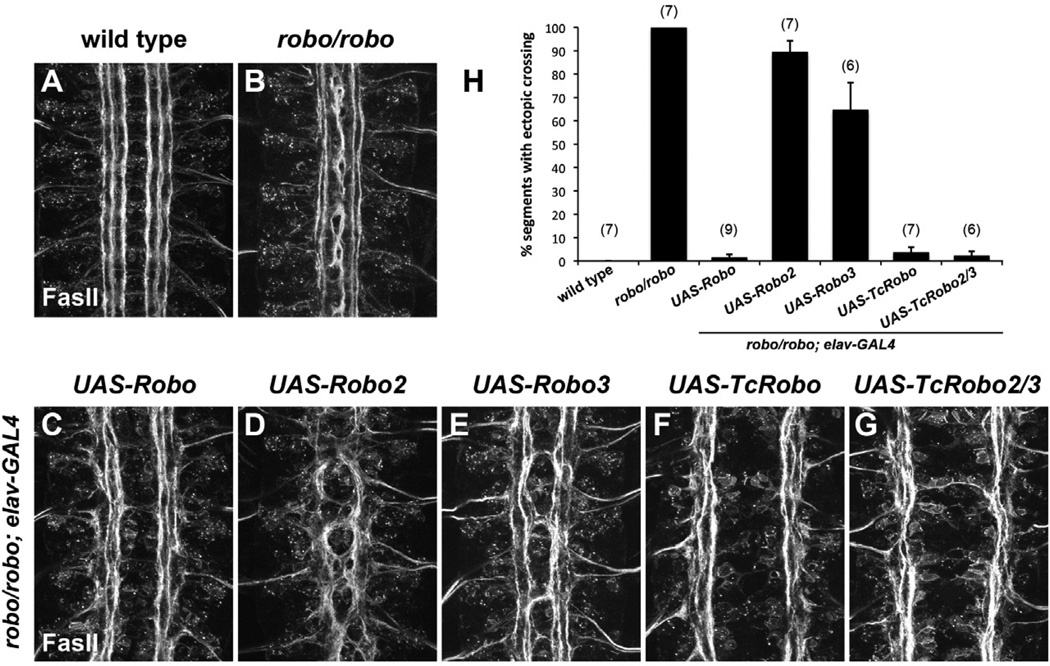
To test whether one or both beetle Robos could substitute for fly Robo in the context of midline repulsion, we performed a similar set of experiments in a robo mutant background. Both TcRobo and TcRobo2/3 were able to prevent FasII axons from crossing the midline in the absence of robo (Figure 3). Indeed, loss of robo had little effect on the commissureless phenotype caused by pan-neural misexpression of TcRobo or TcRobo2/3, as nearly all segments were commissureless in these embryos. Similarly, both TcRobo and TcRobo2/3 were able to prevent commissural EW axons from crossing the midline in nearly 100% of segments in a robo mutant background (Figure S4). This set of experiments confirmed that the Tribolium Robos are able to respond to Drosophila Slit and activate downstream signaling components in Drosophila neurons in the absence of any contribution from endogenous Robo.
Figure 3. Tribolium Robos can mediate midline repulsion in Drosophila in the absence of robo.
A–G. Stage 16–17 Drosophila embryos stained with mAb 1D4 (anti-FasII).
A. Wild type embryo. FasII-positive axon pathways do not cross the midline.
B. In robo mutant embryos, axons from the medial FasII pathways cross the midline in every segment.
C–G. robo mutant embryos carrying elav-GAL4 and indicated UAS-Robo transgenes.
C. Pan-neural expression of Drosophila Robo fully rescues the robo loss of function phenotype: FasII-positive axons no longer cross the midline.
D,E. Expression of Drosophila Robo2 (D) or Robo3 (E) does not prevent ectopic midline crossing in a robo mutant background.
F,G. Expression of Tribolium Robo (D) or Tribolium Robo2/3 (E) is sufficient to prevent ectopic midline crossing in the absence of endogenous robo.
H. Quantification of ectopic midline crossing (average percent segments with ectopic FasII crossing per embryo) in the genetic backgrounds shown in A–G. Error bars represent s.e.m. Number of embryos scored for each genotype is indicated in parentheses.
Tribolium Robos are insensitive to Drosophila Comm
In the course of characterizing our UAS-TcRobo and UAS-TcRobo2/3 transgenic lines, we were struck by the potency of these receptors in mediating midline repulsion in Drosophila embryos. Both TcRobo and TcRobo2/3 transgenics consistently displayed stronger midline repulsion activity than any of the transgenic lines we analyzed for the three Drosophila Robos. We reasoned that the Tribolium Robos might not be subject to the same regulatory mechanisms as the Drosophila Robos when expressed in fly neurons. Commissureless (Comm) is a Drosophila protein that directs endosomal sorting and degradation of Robo in pre-crossing fly commissural neurons, thus preventing premature Slit sensitivity while axons are crossing the midline (Keleman et al., 2002; Keleman et al., 2005). To test whether Comm could downregulate TcRobo or TcRobo2/3 in the fly embryonic CNS, we expressed HA-tagged forms of fly and beetle Robos in Drosophila embryonic neurons using elav-GAL4, in the presence or absence of UAS-Comm (Figure 2E). While misexpression of Comm strongly reduced the expression levels of both Drosophila Robo and Robo2, reflecting Comm-dependent trafficking and endosomal degradation, the levels of TcRobo and TcRobo2/3 were not affected by the presence of Comm (Figure 2E). These results suggest that Drosophila Comm is unable to negatively regulate Tribolium Robos in fly neurons. Notably, we have been unable to detect orthologs of any of the three Drosophila Comm genes in the Tribolium genome, perhaps indicating that the Drosophila system of Comm-dependent Robo regulation is not conserved in Tribolium. Having determined that Tribolium Robos can function as midline repellant receptors in the Drosophila CNS, we next set out to examine the roles of TcRobo and TcRobo2/3 in axon guidance in the beetle embryonic CNS.
Conservation of CNS architecture in flies and beetles
As axon guidance in the beetle embryonic CNS has not previously been described, we first compared the pattern of axon tracts in wild type Tribolium and Drosophila embryos. To examine axon guidance in Tribolium, we used an antibody against horseradish peroxidase (HRP), which recognizes a pan-neural epitope in insects (Haase et al., 2001; Snow et al., 1987). In hopes of developing a more informative marker for quantifying midline crossing phenotypes and examining the formation of longitudinal pathways, we also generated a polyclonal antibody against the Tribolium ortholog of Fasciclin II (TcFasII), a neural cell adhesion molecule which labels individual longitudinal axon tracts in the Drosophila and grasshopper embryonic CNS (Bastiani et al., 1987; Grenningloh et al., 1991).
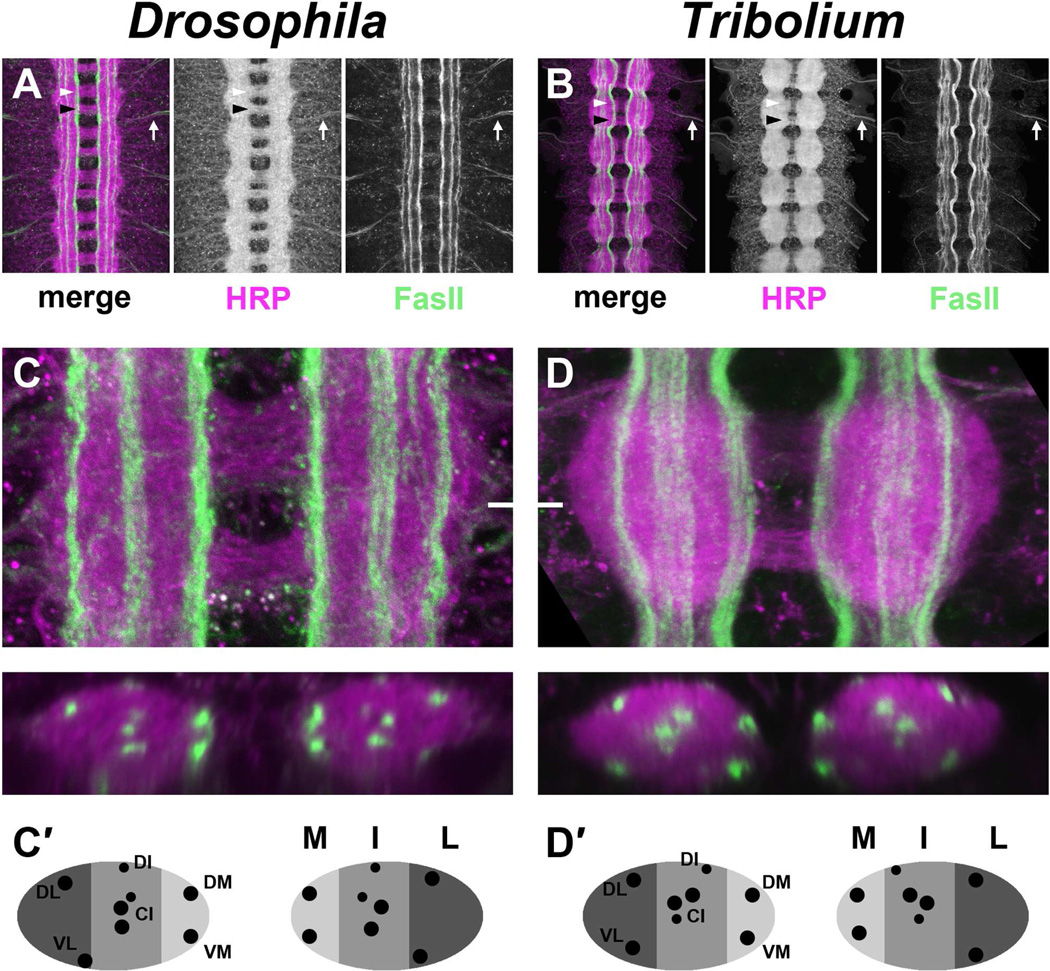
As in Drosophila and grasshopper, anti-HRP staining of Tribolium embryos revealed a ladder-like arrangement of segmental ganglia in the ventral nerve cord, with two commissures per segment and segmental (SN) and intersegmental (ISN) nerve roots exiting the CNS (Figure 4A,B). Both anti-HRP and anti-TcFasII antibodies labeled motor axons as they extend into the periphery and innervate body wall and limb muscles. A number of distinct FasII-positive longitudinal pathways were visible in the beetle embryonic CNS, localized (as in the fly) into discrete medial (M), intermediate (I), and lateral (L) regions (Figure 4C,D). Optical cross-sections of fly and beetle embryonic nerve cords revealed that corresponding individual FasII pathways can be identified at similar dorsoventral and mediolateral locations in late-stage embryos of both species (Figure 4C′,D′). While some commissural segments of axons are FasII-positive at earlier stages, as in Drosophila, in older beetle embryos no FasII-positive axon segments can be found extending across the midline. Thus in Tribolium, as in Drosophila, FasII is an informative marker for assaying midline repulsion, longitudinal pathway formation, and motor axon guidance. The high degree of similarity in number and position of longitudinal axon pathways is particularly remarkable given that three Robo receptors are required to establish this pattern in Drosophila, while Tribolium has only two Robos. How do Tribolium Slit and its two Robo receptors contribute to midline repulsion and longitudinal pathway formation in the beetle embryonic CNS?
Figure 4. Conservation of CNS architecture in Drosophila and Tribolium.
A–B. Dissected ventral nerve cords from a stage 16–17 Drosophila embryo (A) and 4–5 day old Tribolium embryo (B) stained with antibodies against HRP (magenta; detects all axons in the CNS) and the longitudinal pathway marker FasciclinII (FasII; green). Both insects exhibit a ladder-like arrangement of axon pathways in the CNS consisting of bilateral longitudinal connectives and two commissures per segment. Anterior and posterior commissures in a single segment are indicated by white and black arrowheads, respectively. Individual FasII-positive axon pathways are visible in both species, and they do not cross the midline. Peripheral nerves are labeled by both anti-HRP and anti-FasII in each species as they exit the CNS (arrows).
C–D. Single abdominal segments of Drosophila (C) and Tribolium (D) embryos stained as in A–B. Maximum confocal projections through the entire neuropile are shown above; optical cross sections taken at the level of the white hash mark are shown below.
(C′,D′). Schematic representation of cross sections shown in C,D denoting medial (M, light gray), intermediate (I, medium gray) and lateral (L, dark gray) regions of the neuropile and showing relative positions of individual longitudinal pathways (black dots).
C–C′. FasII-positive pathways form in three distinct zones within the Drosophila neuropile. At least eight distinct FasII pathways can be seen in cross section: the dorsal medial (DM) and ventral medial (VM) pathways form within the medial (M) zone; three central intermediate (CI) and one dorsal intermediate (DI) pathway form within the intermediate (I) zone; the dorsal lateral (DL) and ventral lateral (VL) pathways form within the lateral (L) zone. Pathway nomenclature is after Landgraf et al 2003.
D–D′. Similar arrangement of axon pathways in the Tribolium embryonic CNS. Distinct FasII-positive pathways are visible at medial, intermediate, and lateral positions. The same number of individual pathways can be seen in cross section at equivalent dorsoventral and mediolateral locations to those in Drosophila. In this and all subsequent figures, Tribolium embryos are shown scaled to 75% original size for easier comparison with the smaller Drosophila embryos.
Slit-Robo signaling regulates midline crossing in Tribolium
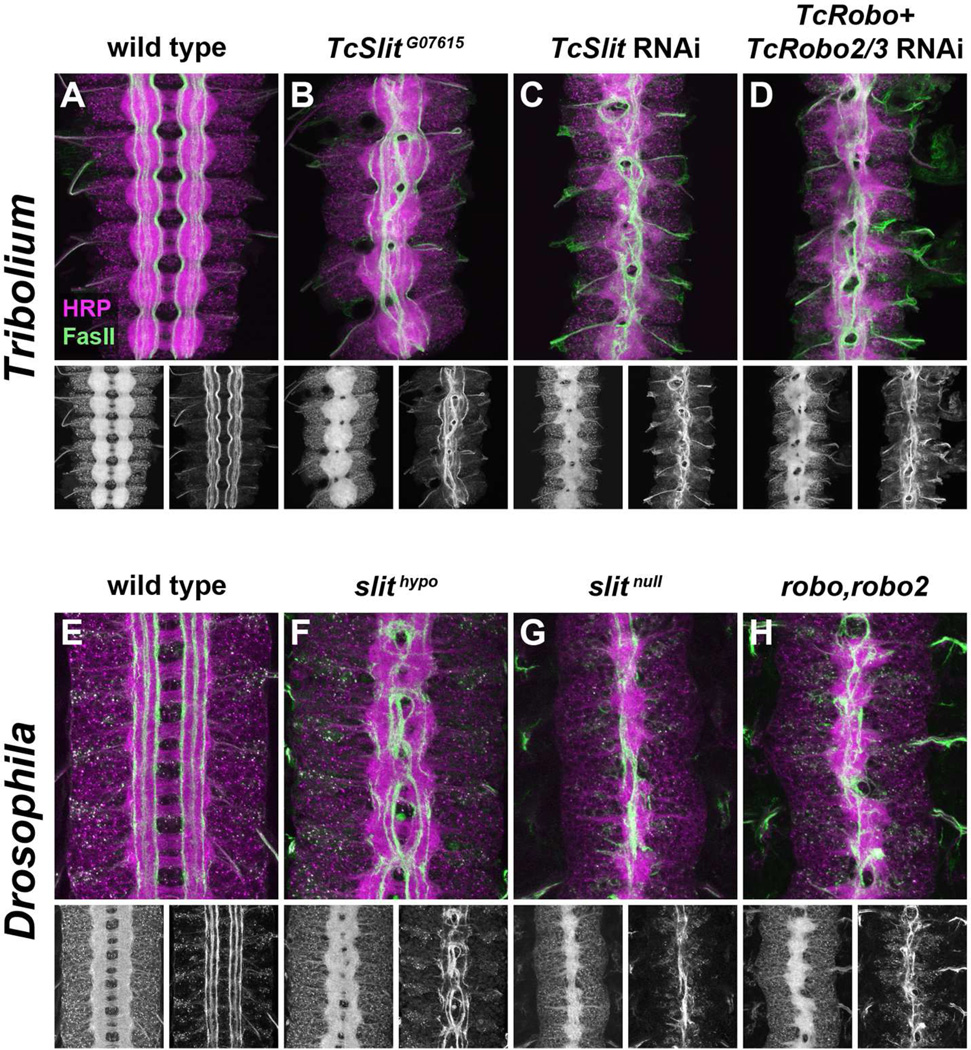
In Drosophila, Slit is produced at the midline of the embryonic CNS and prevents Robo-expressing axons from crossing the midline (Kidd et al., 1999). Both robo and robo2 contribute to slit-dependent midline repulsion in flies, and robo,robo2 double mutants phenocopy the midline collapse phenotype of slit mutants (Rajagopalan et al., 2000a; Simpson et al., 2000b). To investigate the contributions of Slit and Robos to midline repulsion in Tribolium, we used parental RNAi (Bucher et al., 2002) to knock down these genes during embryogenesis and assayed axon guidance in the embryonic CNS using anti-HRP and anti-TcFasII antibodies.
RNAi-mediated knockdown of TcSlit produced a severe midline collapse phenotype reflecting a loss of midline repulsion similar to Drosophila slit mutants: distinct longitudinal tracts do not form properly, and the normally well separated bilateral connectives fuse as axons enter the midline and do not leave (Figure 5C,G). The TcSlit knockdown phenotype appears slightly less severe than in Drosophila slit null mutants, as midline gaps between the connectives can still be seen, encircled by FasII-positive axons as they cross and re-cross the midline. This difference likely reflects an incomplete knockout of TcSlit function under RNAi conditions.
Figure 5. Slit/Robo signaling mediates midline repulsion in Tribolium.
A–D. 4–5 day old Tribolium embryos stained with anti-HRP (magenta) and anti-TcFasII (green). Small panels below show individual HRP (left) and FasII (right) channels.
A. Wild type Tribolium embryo. The ladder-like axon scaffold is formed by segmentally repeated pairs of commissures and bilateral longitudinal connectives. Distinct FasII-positive pathways are visible, and they do not cross the midline.
B. In TcSlitG07615 embryos, commissures are fused and FasII-positive axons linger at the midline, similar to the phenotype seen in Drosophila hypomorphic slit mutants (F). Some longitudinal pathways still form relatively normally in these embryos.
C. Embryos from mothers injected with dsRNA targeting TcSlit display a strong midline collapse phenotype. The width of the axon scaffold is reduced and longitudinal pathways do not form properly, as many FasII-positive axons enter the midline and fail to leave.
D. Embryos in which TcRobo and TcRobo2/3 are targeted in combination phenocopy the TcSlit knockdown phenotype.
E–H. Stage 16–17 Drosophila embryos stained with anti-HRP (magenta) and mAb 1D4 (anti-FasII; green).
E. In wild type Drosophila embryos, the longitudinal connectives are well separated and connected by two commissures per segment. Three distinct FasII-positive pathways can be seen on either side of the midline; they do not cross the midline.
F. In hypomorphic slit mutants, the connectives are closer together, commissures thicken and begin to fuse, and FasII-positive axons cross the midline in every segment.
G. In slit null mutants, all axons collapse at the midline, reflecting a complete absence of midline repulsion.
H. Double mutant robo,robo2 embryos display a slit-like midline collapse phenotype.
To complement our RNAi-based knockdown approach, we searched among the collection of recessive lethal piggyBac transposon insertions generated by the GEKU consortium for potential loss-of-function alleles of TcSlit, TcRobo, or TcRobo2/3 (Trauner et al., 2009). While no insertions mapped to the TcRobo or TcRobo2/3 loci, one line (designated G07615) was reported to carry an insertion within the sixth intron of the TcSlit gene. We found that embryos from heterozygous G07615 parents exhibited strong ectopic crossing phenotypes similar to, but less severe than, the TcSlit RNAi phenotype (Figure 5B). A similar range of phenotypes can be seen when comparing hypomorphic and null alleles of Drosophila slit (Figure 5F,G) (Kidd et al., 1999). We conclude that the G07615 transposon insertion is a hypomorphic allele of TcSlit, and thus refer to this allele as TcSlitG07615.
In Drosophila, robo and robo2 single mutants both exhibit ectopic midline crossing, but simultaneous removal of both genes is necessary to produce a slit-like midline collapse phenotype (Figure 5H) (Rajagopalan et al., 2000a; Simpson et al., 2000b). Similarly, individual knockdown of TcRobo or TcRobo2/3 did not reproduce the TcSlit midline collapse phenotype (see below), suggesting that neither of these receptors is solely responsible for mediating Slit-dependent midline repulsion. We therefore asked whether simultaneous knockdown of both TcRobo and TcRobo2/3 would recapitulate the TcSlit phenotype. Indeed, embryos from mothers injected with a mix of TcRobo and TcRobo2/3 dsRNAs displayed strong midline collapse phenotypes virtually identical to those caused by TcSlit RNAi (Figure 5D). Thus in Tribolium, as in Drosophila, two Robo receptors cooperate to mediate Slit-dependent midline repulsion in the embryonic CNS.
Tribolium Robo receptors have specialized roles in axon guidance
Drosophila Robo receptors perform specialized roles in axon guidance: while both Robo and Robo2 contribute to midline repulsion, Robo2 (but not Robo) cooperates with Robo3 to specify the lateral position of longitudinal axon pathways in the embryonic CNS (Evans and Bashaw, 2010b; Rajagopalan et al., 2000a; Rajagopalan et al., 2000b; Simpson et al., 2000a; Simpson et al., 2000b; Spitzweck et al., 2010). Notably, while Robo2 can substitute for Robo3 to promote formation of axon pathways in the intermediate region of the neuropile, Robo3 cannot functionally replace Robo2 to promote formation of lateral pathways (Spitzweck et al., 2010). Thus the divergent functions of Robo2 and Robo3 cannot be accounted for solely by differences in expression, and must also reflect intrinsic functional differences in the receptors themselves. How do these different activities relate to those of the ancestral Robo2/3 receptor? To determine whether Tribolium Robos exhibit similar functional specialization, we examined the effects of knocking down TcRobo or TcRobo2/3 individually on axon guidance in the Tribolium embryonic CNS.
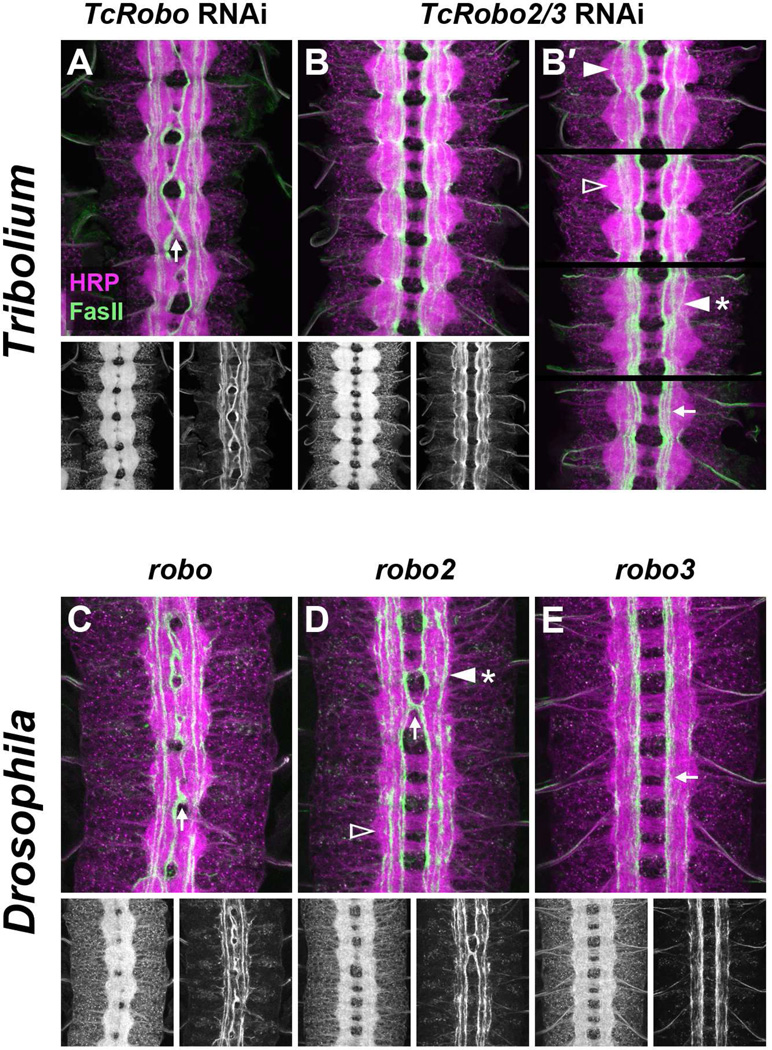
TcRobo knockdown produced an ectopic midline crossing phenotype very similar to Drosophila robo mutants: thickened commissures and reduced connectives were revealed by anti-HRP staining, and medial FasII-positive pathways ectopically crossed the CNS midline in over 90% of examined segments (Figure 6A,C; Table 1). Notably, as in Drosophila robo mutants, intermediate and lateral FasII-positive axon pathways appeared unaffected in TcRobo knockdown embryos, indicating that TcRobo, like Drosophila robo, is dedicated to midline repulsion and does not contribute to longitudinal pathway formation.
Figure 6. Tribolium Robo receptors exhibit specialized axon guidance functions.
Tribolium (A,B) and Drosophila (C–E) embryos prepared as in Figure 5.
A. TcRobo knockdown embryo. Commissures are thickened and FasII axons from medial pathways ectopically cross the midline (arrow). Intermediate and lateral longitudinal pathways are formed relatively normally.
B. TcRobo2/3 knockdown embryo. The axon scaffold has an overall normal appearance, and no FasII-positive axons cross the midline. Longitudinal pathways are disorganized and often fail to form at their normally stereotyped positions.
B′. Partial confocal projections from four different TcRobo2/3 knockdown embryos displaying longitudinal pathway defects: missing lateral pathways (empty arrowhead); FasII-positive axons wandering between longitudinal pathways (arrowhead with asterisk); intermediate and lateral pathways compressed into medial zone (arrow). In a minority of segments, intermediate and lateral pathways form relatively normally (solid arrowhead). Projections were made from 8–15 0.2 µm confocal sections and include all dorsal pathways in their entirety while excluding the majority of ventral pathways.
C. robo mutant embryo. As in TcRobo knockdown embryos (A), thickened commissures and ectopic midline crossing of FasII axons can be seen (arrow).
D. robo2 mutant embryo. Although the axon scaffold appears relatively normal, medial FasII axons can occasionally be seen crossing the midline (arrow). In addition, lateral FasII pathways are often absent (empty arrowhead) or fuse with intermediate pathways (arrowhead with asterisk).
E. robo3 mutant embryo. The axon scaffold appears normal, and no ectopic crossing is detectable. Intermediate FasII pathways shift towards the midline and fuse with medial pathways (arrow).
Table 1.
Quantification of Tribolium Robo knockdown phenotypes.
| genetic background |
FasII-positive axons at midlinea |
medial shift of intermediate pathwayb |
dorsal lateral pathway defectsb | |
|---|---|---|---|---|
| medial shiftc | missing | |||
| wild type | 0.0 (n=48/8) | 0.0 (n=93/8) | 0.0 (n=95/8) | 0.0 (n=95/8) |
| TcRobo RNAi | 91.9 (n=74/11) | 1.5 (n=131/11) | 0.8 (n=119/11) | 0.0 (n=119/11) |
| TcRobo2/3 RNAi | 0.0 (n=51/10) | 83.3 (n=102/10) | 11.0 (n=100/10) | 69.0 (n=100/10) |
4–5 day old embryos were stained with anti-HRP and anti-TcFasII and abdominal segments were scored for defects in midline repulsion and longitudinal pathway formation. Percentage of segments or hemisegments displaying each phenotype are shown.
n = number of segments/number of embryos scored.
n = number of hemisegments/number of embryos scored.
hemisegments were scored as “medial shift” if axons could be seen transitioning from lateral to intermediate pathways within the hemisegment.
In contrast, the overall organization of the axon scaffold appeared normal in anti-HRP stained TcRobo2/3 knockdown embryos, and we did not observe any instances of ectopic midline crossing of FasII-positive axons. Instead, anti-TcFasII staining revealed defects in longitudinal pathway formation in these embryos (Figure 6B). The normally distinct FasII pathways were disorganized in TcRobo2/3 knockdown embryos, in some cases forming in inappropriate positions or crossing between zones to fuse with other pathways (Figure 6B′; Table 1).
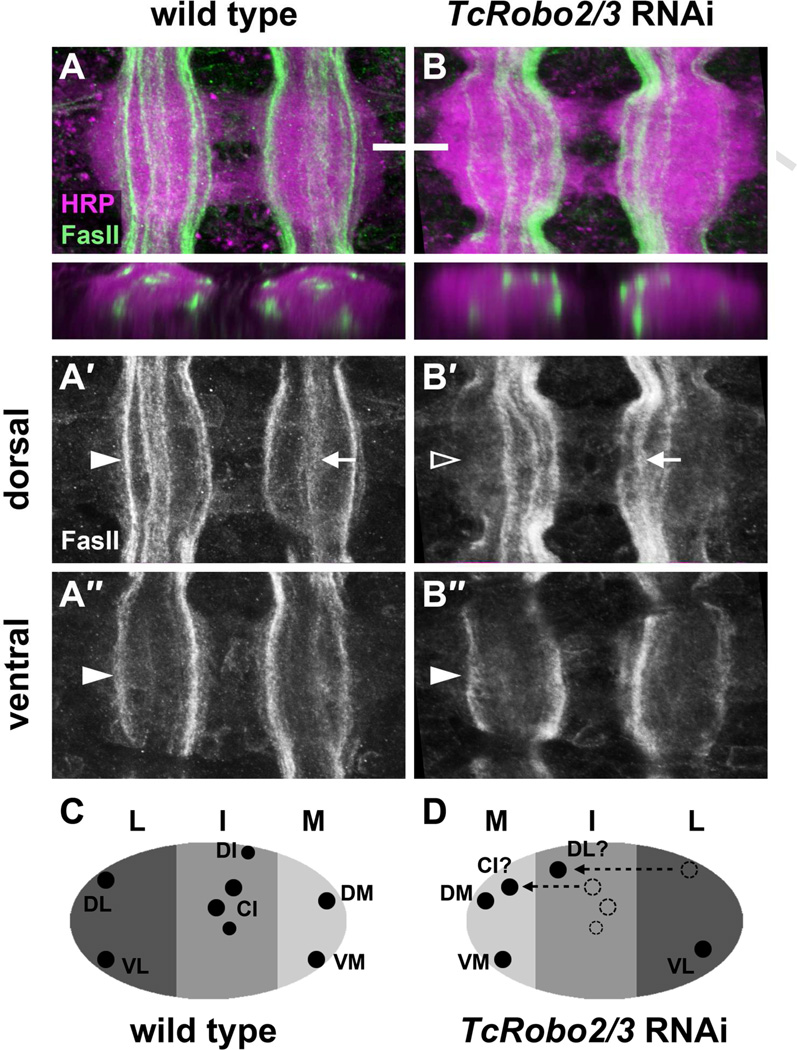
To more thoroughly characterize this phenotype, we used higher magnification imaging and optical cross sections to examine the formation of individual longitudinal pathways (Figure 7). In wild type embryos, two major FasII-positive pathways form in the lateral region of the neuropile: one dorsal and one ventral (Figure 7A). In TcRobo2/3 knockdown embryos, the dorsal lateral pathway was missing in nearly 70% of hemisegments. In a further 11% of hemisegments, this pathway could be seen shifting medially to join one of the intermediate pathways (Figure 7B; Table 1). Neither of these phenotypes were observed in wild type or TcRobo knockdown embryos. The ventral lateral pathway was unaffected by the loss of TcRobo2/3 (Figure 7B″).
Figure 7. TcRobo2/3 controls multiple aspects of longitudinal pathway formation.
A,B. High-magnification views of single abdominal segments from wild type (A) and TcRobo2/3 knockdown (B) embryos. Maximum confocal projections through the entire neuropile are shown above; optical cross-sections taken at the level of the white hash mark are shown below.
A′,B′. Partial confocal projections showing only the dorsal-most FasII pathways.
A″,B″. Partial confocal projections showing only the ventral-most FasII pathways.
In wild type embryos, a major FasII pathway forms in the dorsal lateral region of the neuropile (solid arrowhead in A′). In TcRobo2/3 knockdown embryos, this pathway is missing in the majority of segments (empty arrowhead in B′). The ventral lateral FasII pathway appears unaffected by the loss of TcRobo2/3 (solid arrowhead in A″, B″). In addition, the 3–4 FasII pathways that normally form in the intermediate region (arrow in A, A′) are shifted medially in TcRobo2/3 knockdown embryos (arrow in B, B′).
C,D. Schematic comparison of longitudinal pathways in wild type (C) and TcRobo2/3 knockdown (D) embryos. Loss of TcRobo2/3 results in the absence (dashed circles) or medial shifting (dashed arrows) of both lateral and intermediate pathways. In the absence of more restricted molecular markers, the identity of ectopic pathways forming within the medial zone cannot be determined unless axons can be detected shifting from more lateral pathways. Medial (DM and VM) and ventral lateral (VL) pathways appear unaffected by loss of TcRobo2/3. See Figure 4 legend for longitudinal pathway nomenclature.
Knockdown of TcRobo2/3 also altered the position of intermediate FasII pathways. In wild type embryos, a group of 3–4 FasII-positive pathways form within the intermediate region of the neuropile, well separated from the two major medial pathways (Figure 7A). In TcRobo2/3 knockdown embryos, these intermediate pathways formed closer to the midline, where they mingled with and were often indistinguishable from the normally medial pathways (Figure 7B). Loss of TcRobo2/3 in beetle embryos therefore results in axon guidance defects that combine aspects of Drosophila robo2 and robo3 mutant phenotypes: loss of lateral axon pathways and medial shifting of intermediate pathways are characteristic of robo2 and robo3 mutants, respectively (Figure 6D,E). It is unclear whether the robo2 loss of function phenotype is specific to the dorsal lateral pathway in Drosophila, as the ventral lateral pathway is less robust in flies than in beetles and we were sometimes unable to detect it even in wild type Drosophila embryos (not shown).
Discussion
Modern animals sense and respond to their environment in highly diverse and sophisticated ways. As the complexity of animal nervous systems has increased, developmental control of neuronal connectivity has become increasingly refined. How have axon guidance molecule repertoires changed over evolutionary time? How has functional diversity been acquired within related groups of guidance molecules, and how has this contributed to the evolution of nervous system connectivity?
Here we describe Slit/Robo-mediated axon guidance in the beetle Tribolium castaneum and report on the evolution of the Roundabout (Robo) family of axon guidance receptors in insects. We conclude that the functional diversity exhibited by Drosophila Robo receptors was present early in insect evolution, and that the combined role of Robo2 and Robo3 in specifying lateral position of longitudinal pathways in Drosophila is an ancestral function performed by a single Robo2/3 receptor in other insects. Robo2/3 orthologs are not found outside insects, suggesting a role for expansion and functional diversification of the Robo family in the evolution of nervous system connectivity.
Slit/Robo-mediated axon guidance in Tribolium
Much of our current knowledge of how the Slit/Robo pathway controls axon guidance decisions has come from studies in Drosophila, C. elegans, and mouse. To gain broader insight into Slit/Robo signaling in insects, we now extend this analysis to a second experimentally tractable insect, the flour beetle Tribolium castaneum. To our knowledge, this work represents the first investigation of axon guidance in the Tribolium embryonic CNS. While there is a high degree of conservation in Slit/Robo-mediated guidance between these two insects, we also note some important differences. First and most obvious, Tribolium has only two Robo receptors (TcRobo and TcRobo2/3), while Drosophila has three (Robo, Robo2, and Robo3). Despite this divergent complement of axon guidance receptors, the architecture of the embryonic CNS in Drosophila and Tribolium, including the number and position of individual axon pathways, is remarkably similar.
Secondly, our results also suggest that the regulation of Slit/Robo signaling differs between Drosophila and Tribolium. In flies, Commissureless (Comm) is essential for preventing premature response to Slit by preventing Robo receptor molecules from reaching the growth cone surface in pre-crossing commissural axons (Keleman et al., 2002; Keleman et al., 2005). Tribolium Robos appear insensitive to regulation by Comm when expressed in Drosophila neurons, and the Tribolium genome does not appear to encode a Comm ortholog. How, then, is Slit responsiveness suppressed in pre-crossing commissural axons in Tribolium? A close examination of Robo receptor expression (at both mRNA and protein levels) and localization in different classes of Tribolium neurons may provide insight into the regulation of Slit/Robo signaling in beetles. Novel negative regulators of Slit/Robo signaling in Tribolium could be uncovered by forward genetic screens to identify genes required for midline crossing, similar to the screen that identified Drosophila comm (Seeger et al., 1993).
Functional specialization of Tribolium Robos
Our findings demonstrate that at least two of the axon guidance functions performed by Drosophila Robo receptors—midline repulsion and lateral position—are shared by Robos in the beetle Tribolium. As in Drosophila, TcRobo (the ortholog of Drosophila Robo) is dedicated to midline repulsion, and does not appear to play a role in lateral positioning of longitudinal axon tracts. Similarly, TcRobo2/3 (orthologous to the ancestor of Drosophila Robo2 and Robo3) controls longitudinal pathway formation and also contributes to midline repulsion. Unlike in Drosophila robo2 mutants, however, we could not detect ectopic midline crossing in TcRobo2/3 knockdown embryos. While this may indicate that our RNAi-based approach only achieves a partial reduction of TcRobo2/3 function, the much more severe midline collapse phenotype seen in TcRobo+TcRobo2/3 double knockdowns compared to TcRobo single knockdowns suggests that we are indeed strongly reducing or eliminating TcRobo2/3’s midline repulsive activity (even at one half the dose of the single knockdown). Indeed, we did not observe an increase in severity of TcRobo2/3’s lateral positioning phenotype, or an appearance of ectopic midline crossing, when we increased the concentration of TcRobo2/3 dsRNA nearly ten-fold (to 5ug/ul; not shown). We therefore interpret the lack of ectopic midline crossing in TcRobo2/3 single knockdowns as evidence that TcRobo2/3’s role in midline repulsion is largely or completely redundant with that of TcRobo.
Our finding that TcRobo2/3 performs a dual role in positioning both intermediate and lateral pathways indicates that this activity predates the divergence of Robo2 and Robo3, and that these two receptors in flies now subdivide a role that is performed by a single ancestral receptor in other insects. Thus Robo receptor control of lateral position of longitudinal pathways is likely to be widespread among insects. As Robo2/3 appears to be unique to insects, when did this activity arise? We can envision two possibilities: 1) lateral positioning activity was present in the ancestral Robo receptor, and retained by Robo2/3 after the gene duplication event that separated it from Robo; 2) Robo2/3 gained lateral positioning activity after it separated from Robo. Examining the roles of Robo and Robo2/3 in axon guidance in additional insects, or related animals that lack a disinct Robo2/3 receptor (e.g. crustaceans) may distinguish between these possibilities.
The third, and least well understood, function of Robos in axon guidance in the Drosophila embryonic CNS is Robo2’s role in promoting midline crossing. Commissural axons fail to cross the midline when robo2 and the attractive ligand netrin or its receptor frazzled/DCC (fra) are removed in combination, and pan-neural misexpression of Robo2 can cause ectopic midline crossing (Evans and Bashaw, 2010b; Simpson et al., 2000b; Spitzweck et al., 2010). This activity is restricted to Robo2, as neither Robo nor Robo3 produces the same effect in gain of function assays, and neither robo nor robo3 can rescue the pro-crossing function of robo2 in fra,robo2robo1 or fra,robo2robo3 mutants (Evans and Bashaw, 2010b; Spitzweck et al., 2010). We did not detect any defects in commissure formation after TcRobo2/3 knockdown in Tribolium embryos, and pan-neural misexpression of TcRobo2/3 did not produce ectopic midline crossing in Drosophila embryos. Two direct ways to test whether TcRobo2/3 shares Drosophila Robo2’s pro-midline crossing activity would be to examine the effect of simultaneously knocking down TcFra and TcRobo2/3 on commissure formation in the Tribolium embryonic CNS, or by replacing Drosophila robo2 with TcRobo2/3 and asking whether it can promote commissure formation in a fra mutant background.
Conservation of neural architecture in insects with divergent sets of axon guidance receptors
The coordinated targeting of axons and dendrites to specific regions within the three-dimensional network of neurites in the CNS is necessary for the proper assembly of motor and sensory circuits. In the Drosophila embryonic ventral nerve cord, the Slit/Robo and Semaphorin (Sema)/Plexin signaling pathways regulate mediolateral and dorsoventral positioning of neurites, respectively (Wu et al., 2011; Zlatic et al., 2003; Zlatic et al., 2009). Robo2 is required for the formation of longitudinal axon pathways in the lateral region of the neuropile, while Robo3 directs axons to join pathways in intermediate regions (Rajagopalan et al., 2000b; Simpson et al., 2000a).
Do the same mechanisms regulate longitudinal pathway choice in other animals? We generated an antibody against the Tribolium ortholog of FasII (TcFasII) in order to determine the number and location of FasII-positive axon pathways in the beetle embryonic CNS. Surprisingly, we found that both the number and position of FasII-positive longitudinal pathways are conserved in the fly and beetle embryonic CNS, despite the fact that only two Robo receptors exist in Tribolium. Thus, in these two insect species, divergent genetic programs lead to equivalent developmental outcomes. Further, Drosophila’s system of three Robo receptors specifying three medial-lateral zones in the neuropile is not typical of insects, as most insects (like Tribolium) have only two Robos.
Gene replacement experiments in Drosophila demonstrate that Robo2 and Robo3 are not functionally equivalent (Spitzweck et al., 2010), yet a single Robo2/3 receptor performs both roles in Tribolium. How is this achieved? Our data suggest that the ancestor of Robo2 and Robo3 was able to specify both intermediate and lateral pathway formation. Subsequent to the gene duplication that produced robo2 and robo3 in dipterans, Robo3 apparently lost the ability to promote lateral pathway formation. In contrast, Robo2 retains the ability to specify both intermediate and lateral pathways, but its expression was lost in neurons whose axons join the intermediate pathways. This model makes a number of testable predictions: TcRobo2/3 should be expressed on axons found in the intermediate and lateral zones of the axon scaffold in Tribolium embryos, similar to Robo3 expression in Drosophila; TcRobo2/3 should be able to substitute for both robo3 and robo2 to promote intermediate and lateral pathway formation in Drosophila; and Drosophila robo2, but not robo3, should be able to substitute for TcRobo2/3 to promote lateral pathway formation in Tribolium.
One possibility is that TcRobo2/3 expression in the neurons that make up the intermediate and lateral pathways may be differentially regulated, such that TcRobo2/3 is expressed at different levels or at different times in these two neuronal classes, and that these expression differences might be important for the sequential formation of distinct axon pathways. We note that embryogenesis in Tribolium takes around six times as long as Drosophila at 25C, perhaps allowing temporal regulation of gene expression to play a more important role in specifying distinct guidance outcomes than in the rapidly developing fly embryo. Direct examination of the expression patterns of both beetle Robos will provide insight into how the regulation of Robo expression differs between Drosophila and Tribolium, and may also explain why the lateral position of dorsal and ventral lateral pathways exhibit a differential requirement for TcRobo2/3 activity.
Expansion of axon guidance molecule families by gene duplication
Our phylogenetic analysis indicates that the Robo family has expanded by gene duplication independently in a number of animal groups, including vertebrates, crustaceans, chelicerates and insects. In contrast, nematodes have retained a single Robo receptor. Notably, expansion of the Robo family by gene duplication does not appear to correlate with whole genome duplication events in insects, and does not appear to be common among insect axon guidance genes. We found only a single ortholog of Slit in each of the analyzed insect and nematode genomes; similarly, the number of Plexin (two) and Frazzled/DCC (one) receptors is the same in all insects and C. elegans.
The pattern of distribution of Robo orthologs together with sequence comparisons therefore suggest that a single Robo receptor was present in the last common ancestor of protostomes and deuterostomes. This ancestral Robo was almost certainly involved in Slit-dependent midline repulsion, but it is unclear whether it would have possessed any of the other known Robo axon guidance functions. An intriguing possibility is that additional activities were acquired by Robo paralogs subsequent to gene duplication in the lineages leading to vertebrates and insects, and that this functional diversification may have contributed to increases in nervous system complexity in these groups.
Robo receptors and the evolution of the arthropod nervous system
What molecular mechanisms have facilitated increases in nervous system complexity over evolutionary time? The duplication and functional diversification of axon guidance pathway components is one possible mechanism providing increasingly precise control of axon guidance. This appears to be the case with the Robo family of axon guidance receptors, whose members exhibit functional diversity in a number of animal groups with complex nervous systems. Importantly, one key aspect of nervous system patterning and circuit formation in insects, specifying mediolateral targeting of neurites in the CNS neuropile, is controlled by Robo orthologs that appear to be restricted to insects (Robo2/3 and its descendants, Robo2 and Robo3). Correspondingly, insect nervous systems display a level of precision in axon guidance that is not present in close relatives outside the arthropods (Whitington and Mayer, 2011). Elucidating the axon guidance roles of Robo receptors in additional insects and other animals (for example, crustaceans and onychophorans) should provide further insight into the origins of functional diversity in this important family of axon guidance molecules, and the role they have played in the evolution of animal nervous systems.
Supplementary Material
Highlights.
-
>
The Robo receptor family has expanded by gene duplication in insects.
-
>
Slit and Robos have highly conserved roles in midline repulsion in flies and beetles.
-
>
Insects build similar nervous systems using different numbers of Robos receptors.
-
>
TcRobo2/3 combines lateral positioning functions of DmRobo2 and DmRobo3.
-
>
Flies and beetles employ distinct mechanisms to regulate Robo receptor activity.
Acknowledgements
We thank G. Bucher and the GEKU consortium for TcSlitG07615 beetles and members of the Bashaw laboratory for helpful discussions and comments on the manuscript. This work was supported by grants from the National Institutes of Health (R01-NS046333 and R01-NS054739 to G.J.B. and F32-NS060357 to T.A.E.) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bastiani MJ, Harrelson AL, Snow PM, Goodman CS. Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell. 1987;48:745–755. doi: 10.1016/0092-8674(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bucher G, Scholten J, Klingler M. Parental RNAi in Tribolium (Coleoptera) Curr Biol. 2002;12:R85–R86. doi: 10.1016/s0960-9822(02)00666-8. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. 2008;58:325–332. doi: 10.1016/j.neuron.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, Iyer VN, Pollard DA, Sackton TB, Larracuente AM, Singh ND, Abad JP, Abt DN, Adryan B, Aguade M, Akashi H, Anderson WW, Aquadro CF, Ardell DH, Arguello R, Artieri CG, Barbash DA, Barker D, Barsanti P, Batterham P, Batzoglou S, Begun D, Bhutkar A, Blanco E, Bosak SA, Bradley RK, Brand AD, Brent MR, Brooks AN, Brown RH, Butlin RK, Caggese C, Calvi BR, Bernardo de Carvalho A, Caspi A, Castrezana S, Celniker SE, Chang JL, Chapple C, Chatterji S, Chinwalla A, Civetta A, Clifton SW, Comeron JM, Costello JC, Coyne JA, Daub J, David RG, Delcher AL, Delehaunty K, Do CB, Ebling H, Edwards K, Eickbush T, Evans JD, Filipski A, Findeiss S, Freyhult E, Fulton L, Fulton R, Garcia AC, Gardiner A, Garfield DA, Garvin BE, Gibson G, Gilbert D, Gnerre S, Godfrey J, Good R, Gotea V, Gravely B, Greenberg AJ, Griffiths-Jones S, Gross S, Guigo R, Gustafson EA, Haerty W, Hahn MW, Halligan DL, Halpern AL, Halter GM, Han MV, Heger A, Hillier L, Hinrichs AS, Holmes I, Hoskins RA, Hubisz MJ, Hultmark D, Huntley MA, Jaffe DB, Jagadeeshan S, Jeck WR, Johnson J, Jones CD, Jordan WC, Karpen GH, Kataoka E, Keightley PD, Kheradpour P, Kirkness EF, Koerich LB, Kristiansen K, Kudrna D, Kulathinal RJ, Kumar S, Kwok R, Lander E, Langley CH, Lapoint R, Lazzaro BP, Lee SJ, Levesque L, Li R, Lin CF, Lin MF, Lindblad-Toh K, Llopart A, Long M, Low L, Lozovsky E, Lu J, Luo M, Machado CA, Makalowski W, Marzo M, Matsuda M, Matzkin L, McAllister B, McBride CS, McKernan B, McKernan K, Mendez-Lago M, Minx P, Mollenhauer MU, Montooth K, Mount SM, Mu X, Myers E, Negre B, Newfeld S, Nielsen R, Noor MA, O'Grady P, Pachter L, Papaceit M, Parisi MJ, Parisi M, Parts L, Pedersen JS, Pesole G, Phillippy AM, Ponting CP, Pop M, Porcelli D, Powell JR, Prohaska S, Pruitt K, Puig M, Quesneville H, Ram KR, Rand D, Rasmussen MD, Reed LK, Reenan R, Reily A, Remington KA, Rieger TT, Ritchie MG, Robin C, Rogers YH, Rohde C, Rozas J, Rubenfield MJ, Ruiz A, Russo S, Salzberg SL, Sanchez-Gracia A, Saranga DJ, Sato H, Schaeffer SW, Schatz MC, Schlenke T, Schwartz R, Segarra C, Singh RS, Sirot L, Sirota M, Sisneros NB, Smith CD, Smith TF, Spieth J, Stage DE, Stark A, Stephan W, Strausberg RL, Strempel S, Sturgill D, Sutton G, Sutton GG, Tao W, Teichmann S, Tobari YN, Tomimura Y, Tsolas JM, Valente VL, Venter E, Venter JC, Vicario S, Vieira FG, Vilella AJ, Villasante A, Walenz B, Wang J, Wasserman M, Watts T, Wilson D, Wilson RK, Wing RA, Wolfner MF, Wong A, Wong GK, Wu CI, Wu G, Yamamoto D, Yang HP, Yang SP, Yorke JA, Yoshida K, Zdobnov E, Zhang P, Zhang Y, Zimin AV, Baldwin J, Abdouelleil A, Abdulkadir J, Abebe A, Abera B, Abreu J, Acer SC, Aftuck L, Alexander A, An P, Anderson E, Anderson S, Arachi H, Azer M, Bachantsang P, Barry A, Bayul T, Berlin A, Bessette D, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Bourzgui I, Brown A, Cahill P, Channer S, Cheshatsang Y, Chuda L, Citroen M, Collymore A, Cooke P, Costello M, D'Aco K, Daza R, De Haan G, DeGray S, DeMaso C, Dhargay N, Dooley K, Dooley E, Doricent M, Dorje P, Dorjee K, Dupes A, Elong R, Falk J, Farina A, Faro S, Ferguson D, Fisher S, Foley CD, Franke A, Friedrich D, Gadbois L, Gearin G, Gearin CR, Giannoukos G, Goode T, Graham J, Grandbois E, Grewal S, Gyaltsen K, Hafez N, Hagos B, Hall J, Henson C, Hollinger A, Honan T, Huard MD, Hughes L, Hurhula B, Husby ME, Kamat A, Kanga B, Kashin S, Khazanovich D, Kisner P, Lance K, Lara M, Lee W, Lennon N, Letendre F, LeVine R, Lipovsky A, Liu X, Liu J, Liu S, Lokyitsang T, Lokyitsang Y, Lubonja R, Lui A, MacDonald P, Magnisalis V, Maru K, Matthews C, McCusker W, McDonough S, Mehta T, Meldrim J, Meneus L, Mihai O, Mihalev A, Mihova T, Mittelman R, Mlenga V, Montmayeur A, Mulrain L, Navidi A, Naylor J, Negash T, Nguyen T, Nguyen N, Nicol R, Norbu C, Norbu N, Novod N, O'Neill B, Osman S, Markiewicz E, Oyono OL, Patti C, Phunkhang P, Pierre F, Priest M, Raghuraman S, Rege F, Reyes R, Rise C, Rogov P, Ross K, Ryan E, Settipalli S, Shea T, Sherpa N, Shi L, Shih D, Sparrow T, Spaulding J, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Strader C, Tesfaye S, Thomson T, Thoulutsang Y, Thoulutsang D, Topham K, Topping I, Tsamla T, Vassiliev H, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Young G, Yu Q, Zembek L, Zhong D, Zimmer A, Zwirko Z, Alvarez P, Brockman W, Butler J, Chin C, Grabherr M, Kleber M, Mauceli E, MacCallum I. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- Clemons A, Haugen M, Le C, Mori A, Tomchaney M, Severson DW, Duman-Scheel M. siRNA-mediated gene targeting in Aedes aegypti embryos reveals that frazzled regulates vector mosquito CNS development. PLoS One. 2011;6:e16730. doi: 10.1371/journal.pone.0016730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, Frohlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak JR, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Koonin EV, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne JK, Singan VR, Gilbert DG. wFleaBase: the Daphnia genome database. BMC Bioinformatics. 2005;6:45. doi: 10.1186/1471-2105-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- Duman-Scheel M, Clark SM, Grunow ET, Hasley AO, Hill BL, Simanton WL. Delayed onset of midline netrin expression in Artemia franciscana coincides with commissural axon growth and provides evidence for homology of midline cells in distantly related arthropods. Evol Dev. 2007;9:131–140. doi: 10.1111/j.1525-142X.2007.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ. Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol. 2010a;20:79–85. doi: 10.1016/j.conb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ. Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr Biol. 2010b;20:567–572. doi: 10.1016/j.cub.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, Mastick GS. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development. 2008;135:3643–3653. doi: 10.1242/dev.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AHL, Scholtz G. Axogenesis in the stomatopod crustacean Gonodactylaceus falcatus (Malacostraca) Invertebrate Biology. 2010;129:59–76. [Google Scholar]
- Grenningloh G, Rehm EJ, Goodman CS. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell. 1991;67:45–57. doi: 10.1016/0092-8674(91)90571-f. [DOI] [PubMed] [Google Scholar]
- Haase A, Stern M, Wachtler K, Bicker G. A tissue-specific marker of Ecdysozoa. Dev Genes Evol. 2001;211:428–433. doi: 10.1007/s004270100173. [DOI] [PubMed] [Google Scholar]
- Haugen M, Flannery E, Tomchaney M, Mori A, Behura SK, Severson DW, Duman-Scheel M. Semaphorin-1a is required for Aedes aegypti embryonic nerve cord development. PLoS One. 2011;6:e21694. doi: 10.1371/journal.pone.0021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O'Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- International Aphid Genomics Consortium. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010;8:e1000313. doi: 10.1371/journal.pbio.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Silkworm Genomics Consortium. The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem Mol Biol. 2008;38:1036–1045. doi: 10.1016/j.ibmb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci. 2010;30:9445–9453. doi: 10.1523/JNEUROSCI.6290-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J. Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci. 2009;29:8914–8926. doi: 10.1523/JNEUROSCI.0568-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kirkness EF, Haas BJ, Sun W, Braig HR, Perotti MA, Clark JM, Lee SH, Robertson HM, Kennedy RC, Elhaik E, Gerlach D, Kriventseva EV, Elsik CG, Graur D, Hill CA, Veenstra JA, Walenz B, Tubio JM, Ribeiro JM, Rozas J, Johnston JS, Reese JT, Popadic A, Tojo M, Raoult D, Reed DL, Tomoyasu Y, Kraus E, Mittapalli O, Margam VM, Li HM, Meyer JM, Johnson RM, Romero-Severson J, Vanzee JP, Alvarez-Ponce D, Vieira FG, Aguade M, Guirao-Rico S, Anzola JM, Yoon KS, Strycharz JP, Unger MF, Christley S, Lobo NF, Seufferheld MJ, Wang N, Dasch GA, Struchiner CJ, Madey G, Hannick LI, Bidwell S, Joardar V, Caler E, Shao R, Barker SC, Cameron S, Bruggner RV, Regier A, Johnson J, Viswanathan L, Utterback TR, Sutton GG, Lawson D, Waterhouse RM, Venter JC, Strausberg RL, Berenbaum MR, Collins FH, Zdobnov EM, Pittendrigh BR. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc Natl Acad Sci U S A. 2010;107:12168–12173. doi: 10.1073/pnas.1003379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Sanchez-Soriano N, Technau GM, Urban J, Prokop A. Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev Biol. 2003;260:207–225. doi: 10.1016/s0012-1606(03)00215-x. [DOI] [PubMed] [Google Scholar]
- Linne V, Stollewerk A. Conserved and novel functions for Netrin in the formation of the axonal scaffold and glial sheath cells in spiders. Dev Biol. 2011;353:134–146. doi: 10.1016/j.ydbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Mauss A, Tripodi M, Evers JF, Landgraf M. Midline signalling systems direct the formation of a neural map by dendritic targeting in the Drosophila motor system. PLoS Biol. 2009;7:e1000200. doi: 10.1371/journal.pbio.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer G, Whitington PM. Neural development in Onychophora (velvet worms) suggests a step-wise evolution of segmentation in the nervous system of Panarthropoda. Dev Biol. 2009;335:263–275. doi: 10.1016/j.ydbio.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000a;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000b;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Bucher G, Friedrich M, Grimmelikhuijzen CJ, Klingler M, Lorenzen M, Roth S, Schroder R, Tautz D, Zdobnov EM, Muzny D, Attaway T, Bell S, Buhay CJ, Chandrabose MN, Chavez D, Clerk-Blankenburg KP, Cree A, Dao M, Davis C, Chacko J, Dinh H, Dugan-Rocha S, Fowler G, Garner TT, Garnes J, Gnirke A, Hawes A, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Jackson L, Kovar C, Kowis A, Lee S, Lewis LR, Margolis J, Morgan M, Nazareth LV, Nguyen N, Okwuonu G, Parker D, Ruiz SJ, Santibanez J, Savard J, Scherer SE, Schneider B, Sodergren E, Vattahil S, Villasana D, White CS, Wright R, Park Y, Lord J, Oppert B, Brown S, Wang L, Weinstock G, Liu Y, Worley K, Elsik CG, Reese JT, Elhaik E, Landan G, Graur D, Arensburger P, Atkinson P, Beidler J, Demuth JP, Drury DW, Du YZ, Fujiwara H, Maselli V, Osanai M, Robertson HM, Tu Z, Wang JJ, Wang S, Song H, Zhang L, Werner D, Stanke M, Morgenstern B, Solovyev V, Kosarev P, Brown G, Chen HC, Ermolaeva O, Hlavina W, Kapustin Y, Kiryutin B, Kitts P, Maglott D, Pruitt K, Sapojnikov V, Souvorov A, Mackey AJ, Waterhouse RM, Wyder S, Kriventseva EV, Kadowaki T, Bork P, Aranda M, Bao R, Beermann A, Berns N, Bolognesi R, Bonneton F, Bopp D, Butts T, Chaumot A, Denell RE, Ferrier DE, Gordon CM, Jindra M, Lan Q, Lattorff HM, Laudet V, von Levetsow C, Liu Z, Lutz R, Lynch JA, da Fonseca RN, Posnien N, Reuter R, Schinko JB, Schmitt C, Schoppmeier M, Shippy TD, Simonnet F, Marques- Souza H, Tomoyasu Y, Trauner J, Van der Zee M, Vervoort M, Wittkopp N, Wimmer EA, Yang X, Jones AK, Sattelle DB, Ebert PR, Nelson D, Scott JG, Muthukrishnan S, Kramer KJ, Arakane Y, Zhu Q, Hogenkamp D, Dixit R, Jiang H, Zou Z, Marshall J, Elpidina E, Vinokurov K, Oppert C, Evans J, Lu Z, Zhao P, Sumathipala N, Altincicek B, Vilcinskas A, Williams M, Hultmark D, Hetru C, Hauser F, Cazzamali G, Williamson M, Li B, Tanaka Y, Predel R, Neupert S, Schachtner J, Verleyen P, Raible F, Walden KK, Angeli S, Foret S, Schuetz S, Maleszka R, Miller SC, Grossmann D. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–955. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Simanton W, Clark S, Clemons A, Jacowski C, Farrell-VanZomeren A, Beach P, Browne WE, Duman-Scheel M. Conservation of arthropod midline netrin accumulation revealed with a cross-reactive antibody provides evidence for midline cell homology. Evol Dev. 2009;11:260–268. doi: 10.1111/j.1525-142X.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell. 2000a;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000b;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Snow PM, Patel NH, Harrelson AL, Goodman CS. Neural-specific carbohydrate moiety shared by many surface glycoproteins in Drosophila and grasshopper embryos. J Neurosci. 1987;7:4137–4144. doi: 10.1523/JNEUROSCI.07-12-04137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzweck B, Brankatschk M, Dickson BJ. Distinct protein domains and expression patterns confer divergent axon guidance functions for Drosophila Robo receptors. Cell. 2010;140:409–420. doi: 10.1016/j.cell.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Stollewerk A, Eriksson BJ. Expression patterns of neural genes in Euperipatoides kanangrensis suggest divergent evolution of onychophoran and euarthropod neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22576–22581. doi: 10.1073/pnas.1008822108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Trauner J, Schinko J, Lorenzen MD, Shippy TD, Wimmer EA, Beeman RW, Klingler M, Bucher G, Brown SJ. Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC Biol. 2009;7:73. doi: 10.1186/1741-7007-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerer P, Wolff C, Geppert M. Axogenesis in the central and peripheral nervous system of the amphipod crustacean Orchestia cavimana. Integrative Zoology. 2011;6:28–44. doi: 10.1111/j.1749-4877.2010.00227.x. [DOI] [PubMed] [Google Scholar]
- Whitington PM, Mayer G. The origins of the arthropod nervous system: insights from the Onychophora. Arthropod Struct Dev. 2011;40:193–209. doi: 10.1016/j.asd.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Wu Z, Sweeney LB, Ayoob JC, Chak K, Andreone BJ, Ohyama T, Kerr R, Luo L, Zlatic M, Kolodkin AL. A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS. Neuron. 2011;70:281–298. doi: 10.1016/j.neuron.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors: atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37:41–51. doi: 10.1016/s0896-6273(02)01131-5. [DOI] [PubMed] [Google Scholar]
- Zlatic M, Li F, Strigini M, Grueber W, Bate M. Positional cues in the Drosophila nerve cord: semaphorins pattern the dorso-ventral axis. PLoS Biol. 2009;7:e1000135. doi: 10.1371/journal.pbio.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.