Abstract
Surfactant protein A (SP-A) is involved in lung innate immunity. Humans have two SP-A genes, SFTPA1 and SFTPA2, each with several variants. We examined the in vivo effects of treatment with specific SP-A variants on the alveolar macrophage (AM) proteome from SP-A knockout (KO) mice. KO mice received either SP-A1, SP-A2, or both. AM were collected and their proteomes examined with 2D-DIGE. We identified 90 proteins and categorized them as related to actin/cytoskeleton, oxidative stress, protease balance/chaperones, regulation of inflammation, and regulatory/developmental processes. SP-A1 and SP-A2 had different effects on the AM proteome and these effects differed between sexes. In males more changes occurred in the oxidative stress, protease/chaperones, and inflammation groups with SP-A2 treatment than with SP-A1. In females most SP-A1-induced changes were in the actin/cytoskeletal and oxidative stress groups. We conclude that after acute SP-A1 and SP-A2 treatment, sex-specific differences were observed in the AM proteomes from KO mice, and that these sex differences differ in response to SP-A1 and SP-A2. Females are more responsive to SP-A1, whereas the gene-specific differences in males were minimal. These observations not only demonstrate the therapeutic potential of exogenous SP-A, but also illustrate sex- and gene-specific differences in the response to it.
Keywords: surfactant, innate immunity, actin, oxidative stress, inflammation, autophagy
1. Introduction
As it fulfills its role in respiration, the lung must defend the largest mucosal surface in the body from pathogens and harmful substances in the environment. Much of the responsibility for defending the lung from these potential hazards falls to the alveolar macrophage (AM). Although the AM is the principal effector cell of innate immunity in the lung, its function is highly integrated with other cells in the distal lung. The type II alveolar epithelial cell, in addition to being the source of pulmonary surfactant, has also been shown to affect innate immunity by producing immune regulatory molecules and other substances that influence host defense function and the AM. Surfactant protein A (SP-A) is one of these molecules.
SP-A regulates multiple AM-mediated host defense functions including phagocytosis, chemotaxis, cytokine secretion, and reactive oxidant production, and has been shown to play a role in linking innate immunity to adaptive immunity. Numerous studies have shown that susceptibility to pneumonia, as well as other types of lung injury, is increased when SP-A is absent [1–9]. Animal studies have contributed to our understanding of SP-A function. However, the presence in humans of two distinct SP-A genes, SP-A1 and SP-A2, plus several variants for each gene, indicates that SP-A regulation and function is more complex than in animals. To aid in elucidation of the specific roles of the different human SP-A genes and variants our laboratory has developed stably transfected cell lines expressing SP-A variants and SP-A transgenic mice that express different SP-A variants. In a series of studies we have shown that the different variant SP-A molecules exhibit functional differences [10–20] and that AM from the SP-A humanized transgenic (hTG) mice have major differences in their AM proteomes [21].
In the present study we investigated the effects of a single in vivo dose of specific human SP-A variant or combinations of variants synthesized in vitro by stably transfected cell lines on the AM proteome of AM from mice lacking endogenous SP-A (SP-A knockout, KO) on the C57BL/6 genetic background. The AM from these mice were harvested by bronchoalveolar lavage, their intracellular proteomes examined with 2-dimensional difference gel electrophoresis, and the data analyzed by Ingenuity Pathways Analysis and by categorizing the identified proteins into functional groups.
2. Material and Methods
Animals
All mice were on the C57BL6/J genetic background and were 8–12 weeks of age. SP-A KO mice and humanized transgenic (hTG) SP-A2 mice that carried an SP-A2 variant were generated on the SP-A KO C57BL/6 background as previously described [22]. SP-A KO and hTG SP-A2 mice containing the 1A0 variant were propogated and raised in our breeding colony at the Penn State College of Medicine. All mice were maintained under pathogen-free conditions or in barrier facilities with free access to food and water. There was no evidence of respiratory pathogens in any of the strains or in sentinel animals housed in the same animal rooms. This study was approved by the Institutional Animal Care and Use Committee of the Penn State College of Medicine. Equal numbers of male and female animals were used. In all cases n=4, except KO males receiving SP-A1 + SP-A2 (5 μg of each) where n=3.
Treatment of mice with exogenous SP-A
For these experiments mice were anesthetized by injection with Ketamine (Ketaject, Phoenix Pharmaceuticals Inc., St. Joseph, MO) and Xylazine (XYLA-JECT, Phoenix Pharmaceuticals Inc., St. Joseph, MO). The experimental design is shown schematically in Figure 1. SP-A was purified from stably transfected CHO cells and isolated by mannose affinity chromatography as described previously [11]. SP-A1 preparations were made with the 6A2 variant and SP-A2 preparations with the 1A0 variant. Both of these variants are the ones occurring in the general population with the greatest frequency [23]. Exogenous SP-A preparations containing either SP-A1 (10 μg), SP-A2 (10 μg), SP-A1 + SP-A2 (5 μg of each), or SP-A1 + SP-A2 (10 μg of each) were prepared in 50 μl of sterile saline with 1 mM CaCl2. Control animals received 50 μl of saline and 1mM CaCl2 (vehicle) alone. Anesthetized mice were suspended by their maxillary incisors, the bolus containing SP-A or vehicle placed in the pharynx, and the nostrils briefly blocked, resulting in aspiration of the bolus. After recovery from anesthesia the mice were returned to their cages. In previous studies with a pneumonia model this route of delivery to the lungs has been very consistent and highly reproducible.
Figure 1.
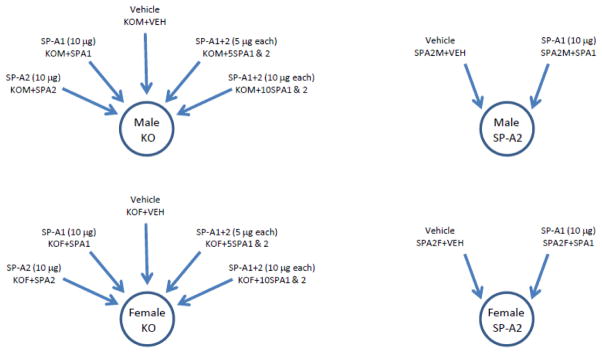
Schematic representation of experimental design. Male and female SP-A−/− mice (KO) received intrapharyngeal instillations of either: vehicle; SP-A1 (10μg); SP-A2 (10μg); SP-A1+SP-A2 (5μg each); or SP-A1+SP-A2 (10μg each). Male and female SP-A2 humanized transgenic mice received instillations of either: vehicle; or SP-A1 (10μg). Alveolar macrophages were then harvested 18 hours later by bronchoalveolar lavage and studied for protein expression.
Sample preparation
Eighteen hours after SP-A treatment the mice were euthanized and subjected to bronchoalveolar lavage (BAL) with phosphate-buffered saline (PBS), 1 mM EDTA to obtain alveolar macrophages. Cells were washed and counted. Cell aliquots of all BAL samples were subjected to cytospins and differential cell counts were performed to rule out the presence of any infectious or inflammatory lung conditions. In all cases BAL cells were >95% alveolar macrophages which were frozen for later analysis.
Preparation of samples for 2D-DIGE
Pellets of frozen macrophages were lyophilized until complete dryness and resuspended in 25 μL of standard cell lysis buffer (30 mM Tris HCl, 2 M thiourea, 7 M urea, 4% CHAPS, pH 8.5). The lysates underwent protein determinations using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA) and the concentration of protein was adjusted to 1 mg/ml for CyDye labeling.
CyDye labeling (minimal labeling) and electrophoresis for 2D-DIGE
These procedures have been described previously [24–26]. Information about the 2D-DIGE study is provided in a form that complies with the most recent version <http://www.psidev.info/miape/MIAPE_GE_1_4.pdf> of Minimum Information About a Proteomics Experiment – Gel Electrophoresis (MIAPE-GE) standards currently under development by the Human Proteome Organization Proteomics Standards Initiative (see Supplementary File 1). Briefly, 25 μg of each sample were labeled with either Cy3 or Cy5. An aliquot of the normalization pool was labeled with Cy2.
Gel imaging, image analysis, and statistics
Information about the acquisition and processing of data from the 2D-DIGE studies are provided in the form that complies with the most recent version of the guidelines established for Minimum Information about a Proteomics Experiment – Gel Informatics (MIAPE-GI) currently under development by the Human Proteome Organization Proteomics Standards Initiative <http://www.psidev.info/files/miape-gi-v1.pdf≥ (see Supplementary File 2). Briefly, gels were scanned using a Typhoon 9410 scanner and gel images were imported into the Progenesis SameSpots v4.0 program (Nonlinear Dynamics) for analysis. In cases where identified proteins had multiple isoforms, the normalized volumes of all isoforms of a given protein were added together and statistical analysis was performed on the totals using Microsoft Excel.
Protein identification by mass spectrometry
We have used this procedure in previous studies for other types of protein samples [25–27] and we recently published a detailed account including many modifications and refinements [24].
Peptides were analyzed by MALDI-ToF/ToF mass spectrometry (5800 Proteomic Analyzer Applied Biosystems, Foster City, CA) in the Mass Spectrometry Core at the Penn State University College of Medicine. The MS and MS/MS data were submitted to the MASCOT search engine using the NCBI non-redundant database and mouse taxonomy for identification. The search parameters included: trypsin digestion with a maximum of three missed cleavages; fixed modifications, carbamidomethylation; variable modifications, carbamylation, acetylation, deamidation, oxidation; peptide mass tolerance, 0.15 Da. MASCOT confidence interval scores of >95% combined with a ProteinPilot score of greater than 61 were considered as a positive protein identification. Out of all the gels comprising the study (each gel contained 2 experimental samples and an aliquot of the normalization pool) one was chosen as the reference gel and all other gel images were aligned with it. An image of the reference gel is shown in Figure 2 with all identified proteins circled and numbered. Protein names, accession numbers, and mass spectrometry information are given in Supplementary File 3.
Figure 2.
Reference gel of AM proteins and protein list. This figure shows an image of the reference gel with identified proteins circled and numbered. The legend contains the names of each identified protein. List of identified proteins in reference gel: 1) 14-3-3 protein beta/alpha; 2) 14-3-3 protein epsilon; 3) 14-3-3 protein gamma; 4) 14-3-3 protein zeta/delta; 5) 65-kDa macrophage protein (Plastin-2); 6) 6-phosphogluconolactonase; 7) Actin-related protein 3; 8) Actr2 protein; 9) Alpha-actinin-1; 10) Alpha-fetoprotein (Serum albumin); 11) Annexin A2; 12) Annexin A4; 13) Annexin A5; 14) ArsA arsenite transporter, ATP-binding, homolog 1; 15) Atp5b protein; 16) Calpain small subunit 1; 17) Calreticulin; 18) Capping protein (actin filament) muscle Z-line, alpha 2 (F-actin-capping protein subunit alpha-2); 19) Capping protein (actin filament) muscle Z-line, beta isoform a (F-actin-capping protein subunit beta); 20) Cathepsin D precursor; 21) Cathepsin S; 22) Chaperonin subunit 2 (beta); 23) Chia protein; 24) Chitinase 3-like 3 precursor; 25) Chloride intracellular channel 1 (Chloride intracellular channel protein 1); 26) Chloride intracellular channel 4 (mitochondrial); 27) CNDP dipeptidase 2 (Cytosolic non-specific dipeptidase); 28) Coatomer subunit epsilon; 29) Cytochrome b-c1 complex subunit 1, mitochondrial; 30) EF hand domain containing 2 (EF-hand domain-containing protein D2); 31) Endoplasmic reticulum resident protein 29; 32) Eno1 protein (Alpha-enolase); 33) Ezrin; 34) F-actin capping protein alpha-1 subunit (F-actin-capping protein subunit alpha-1); 35) Ferritin heavy chain 1 (Ferritin heavy chain); 36) Ferritin light chain 1; 37) Gamma-actin (Actin, cytoplasmic 2); 38) Gelsolin precursor; 39) Glucose-6-phosphate dehydrogenase X-linked; 40) Glucosidase 2 subunit beta; 41) Guanine deaminase; 42) Heat shock protein 1, beta; 43) Heat shock protein 5 precursor (78 kDa glucose-regulated protein); 44) Heat shock protein 65 (60 kDa heat shock protein, mitochondrial); 45) Heat shock protein 8 (Heat shock cognate 71 kDa protein); 46) Heat shock protein 90, beta (Grp94), member 1 (Endoplasmin); 47) Hematopoietic cell specific Lyn substrate 1 (Hematopoietic lineage cell-specific protein); 48) Heme-binding protein (Heme-binding protein 1); 49) Heterogeneous nuclear ribonucleoprotein K; 50) Heterogeneous nuclear ribonucleoproteins C1/C2; 51) High mobility group 1 protein; 52) Hnrpf protein (Heterogeneous nuclear ribonucleoprotein F); 53) Kappa-B motif-binding phosphoprotein; 54) Keratin type II; 55) Keratin, type I cytoskeletal 19; 56) Laminin receptor (40S ribosomal protein SA); 57) Major vault protein (MVP); 58) Malate dehydrogenase, cytoplasmic; 59) Microtubule-associated protein, RP/EB family, member 1; 60) Myosin light chain, regulatory B-like (Myosin regulatory light chain 12B); 61) N-acetyl-D-glucosamine kinase; 62) NSFL1 cofactor p47; 63) Nucleophosmin 1; 64) p50b (Lymphocyte-specific protein 1); 65) Peroxiredoxin-1; 66) Peroxiredoxin-2; 67) Platelet-activating factor acetylhydrolase IB subunit beta; 68) Prohibitin; 69) Prolyl 4-hydroxylase, beta polypeptide precursor; 70) Proteasome (prosome, macropain) 28 subunit, alpha (Proteasome activator complex subunit 1); 71) Proteasome alpha 1 subunit; 72) Protein CREG1; 73) Protein disulfide isomerase associated 6 (Protein disulfide-isomerase A6); 74) Protein disulfide-isomerase A3 precursor (Protein disulfide-isomerase A3); 75) Protein disulfide-isomerase A4; 76) Protein synthesis initiation factor 4A (Eukaryotic initiation factor 4A–I); 77) Purine nucleoside phosphorylase; 78) Put. beta-actin (aa 27–375) (Actin, cytoplasmic 2); 79) Rab GDP dissociation inhibitor beta; 80) Rho GDP dissociation inhibitor (GDI) alpha (Rho GDP-dissociation inhibitor 1); 81) Serine (or cysteine) proteinase inhibitor, clade B, member 1a (Leukocyte elastase inhibitor A); 82) Stress-70 protein, mitochondrial; 83) Translationally-controlled tumor protein; 84) Tropomodulin-3; 85) Tropomyosin 3, gamma (Tropomyosin alpha-3 chain); 86) Tubulin beta-4B chain; 87) Tubulin, beta 5; 88) Vacuolar adenosine triphosphatase subunit B; 89) Valosin-containing protein; 90) Vimentin.
3. Results
Identification and categorization of identified proteins
We used MALDI-ToF/ToF to identify 90 proteins with confidence intervals >95% and ProteinPilot scores of >61. Names of the proteins and their accession numbers and a reference gel are included as Supplementary Files 3 and 4 and Figure 2, respectively. Some of these identified proteins consisted of a single spot and others consisted of multiple spots representing isoforms of a specific protein. It should be noted that many of these proteins were also identified in our previous studies of the AM proteome [24,28], although several proteins resolved in the earlier studies were not present on these gels, and a number of additional proteins were identified. Normalized volumes for all identified proteins are given in Supplementary File 5.
We employed an approach similar to that used in previous publications and with other types of samples (BAL proteins, plasma proteins) [24,25,27,29] to assess the function of the 90 identified proteins and their biological relevance to AM function. A general overview of function was provided by analyzing the data with the Ingenuity Pathways Analysis (IPA) program. These analyses identified proteins involving several top canonical pathways: 1) remodeling of epithelial adherens junctions (p = 8.27E-10); 2) 14-3-3-mediated signaling (p = 7.26E-08); 3) RhoGDI signaling (p = 1.72E-06); 4) regulation of actin-based motility by Rho (p = 2.54E-06); and 5) epithelial adherens junction signaling (p = 5.26E-06). In addition, the Nrf2-mediated oxidative stress response (p = 1.89E-05) was also implicated by IPA. We also employed a manual curation approach, in which we tried to focus on findings from the literature that were particularly relevant to the lung and macrophages. We designed several broad groups that included a number of our identified proteins. The largest of these groups included proteins we referred to as “actin-related/cytoskeletal proteins” (ARC) which included 38 of the 90 proteins identified in our study (Supplementary File 4). Given the role of the AM as a mobile phagocyte, this was anticipated and the identification of a substantial subset of identified proteins involved in these processes indicates that SP-A plays a pivotal role in these macrophage functions.
We also assigned 26 proteins to a group involving the response to and regulation of oxidative stress (OX). Other major cellular processes implicated by our list of identified proteins included regulation of inflammation (ROI; 19 proteins), protease balance/chaperone function (PBCF; 25 proteins), and regulatory/developmental processes (RDP: 15 proteins). These functional groups represent important facets of AM biology and thereby constitute a valuable tool in assessing macrophage function in the response to treatment with different SP-A variants. AM reside in an oxygen-rich environment and may generate reactive oxidant species in their various host defense roles. They produce a number of proteins that play a role in the regulation of oxidative stress (OX). Many of the genes for these proteins possess antioxidant response elements (ARE) and are regulated by Nrf2. It is likely that the PBCF proteins are involved in the repair of damage to lung tissue and proteins potentially resulting from exposure to noxious material, pathogens, or other danger signals, as well as playing a role in autophagy. The ROI group is likely to be instrumental in regulating innate immune processes, since dysregulation of inflammation is known to play a major role in many pulmonary diseases. Finally, the profound differences between circulating blood monocytes and the AM [30–32] and between macrophages in different activation states [30, 33], indicate the presence of active regulatory mechanisms that regulate these differentiative processes. Proteins that may be involved in these processes that have known regulatory roles in other systems have been assigned to the regulatory/developmental processes (RDP) protein group. The functional groups to which each protein was assigned and the references responsible for that assignment are listed in Supplementary File 4; [34–76];[77–120]. Note that some proteins are included in more than one group.
Sex differences in the AM proteome of SP-A KO mice in response to rescue by SP-A variants
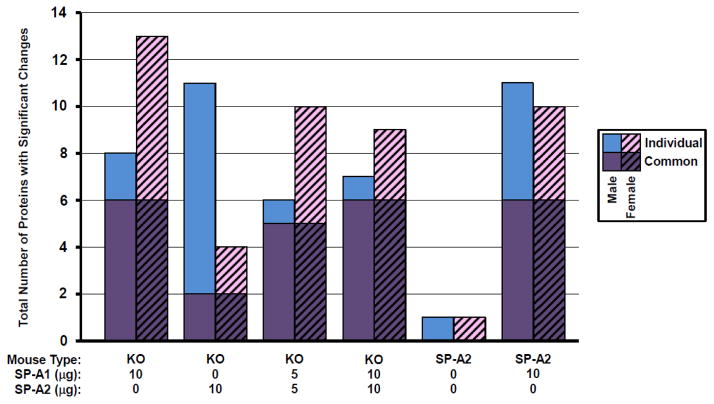
We first looked at the complete set of identified proteins in terms of their responses to rescue by various variants or combinations of variants. T-tests were performed to detect significant differences in AM from various treatment groups compared to AM from KO mice of the same sex (Figure 3). P-values and significant differences between groups of the same sex are listed in Supplementary File 5. The data for the 90 proteins analyzed and compared in this study between KO and the six different rescue groups revealed the following. Among all comparisons there were 44 significant changes from KO (out of a possible 540; i.e. 6 comparisons to KO/sex x 90 proteins) in males and 47 significant changes in females. Of these, 25 comparisons were significant in both males and females.
Figure 3.
Histogram summarizing all significant changes in both sexes. The total number of proteins with significant changes is graphed when each treatment group is compared to the KO + Vehicle group. Purple portions of bars are proteins with significant changes that are common to both males (solid) and females (hatched). Blue and pink portions of bars are additional proteins with significant changes that are unique to either males or females, respectively. As indicated in the key, male bars are blue solid and female bars are pink hatched.
When we examined the remainder of the significant changes which were unique to either males or females, an interesting pattern emerged. In females there were a greater number of unique changes in the SP-A1 rescue group (7 in females only; 2 in males only; 6 common to both males and females), whereas in males, the greatest concentration of unique changes was seen in the SP-A2 rescue group (9 in males only; 2 in females only; 2 common to both males and females)(Fig. 3). When mice received both SP-A1 and SP-A2 there were more unique changes in the females suggesting that the pattern was dominated by SP-A1, despite the inclusion of SP-A2 in the rescue treatment.
On the other hand, in the SP-A2 transgenics that were exposed to SP-A2 throughout their lives, after receiving vehicle there was only a single significant difference from KO in both sexes. When the SP-A2 transgenics received an SP-A1 rescue injection, they had just as many changes that were common to both sexes (n=6) as the KO mice had who received SP-A1 rescue (alone or in combination). Both sexes had nearly the same number of unique changes. This departure from the dimorphic pattern seen in the KO mice may reflect the presence of both SP-A1 (acutely) and SP-A2 (chronically).
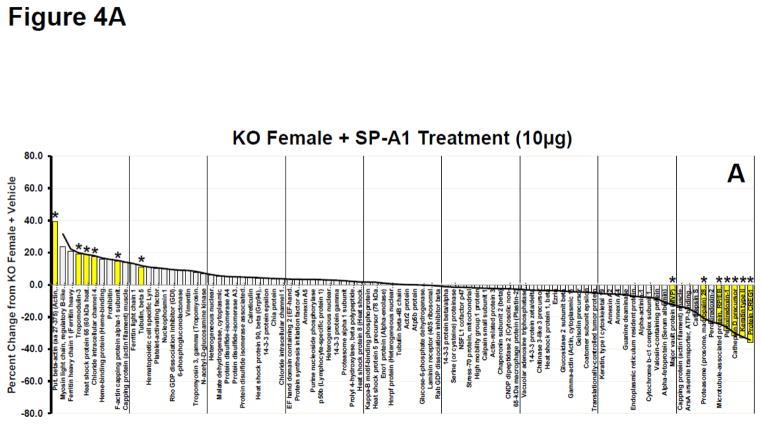
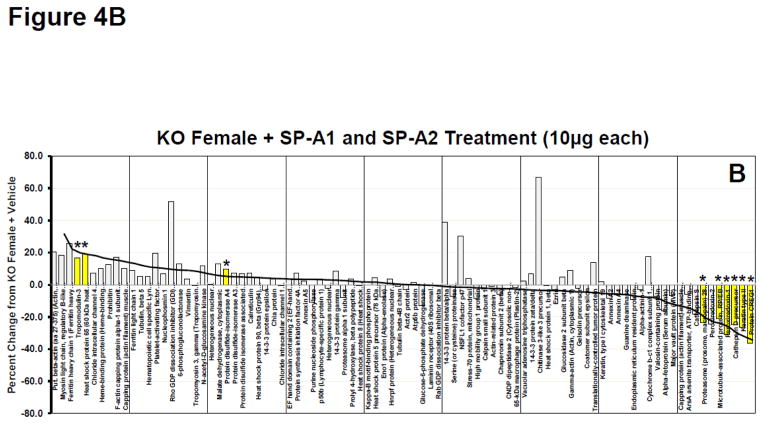
As a means of further assessing the overall response on the expression of the 90 identified proteins we constructed waterfall plots, plotting the percent change of the normalized volume of each protein in various rescue groups versus the levels expressed in AM from KO mice. Because the greatest number of significant changes from KO occurred in the female KO mice after being treated with SP-A1 we used this group as our index group and arranged the proteins from the highest percent change to the lowest (a waterfall plot)(Fig. 4A). The same protein order is also used in Panels B–D. Approximately half of the proteins had levels above those seen in KO (% change > 0) and half were expressed at lower levels than in KO (% change < 0). For reference purposes a trend line for the changes seen in the SP-A1-treated females is shown in all panels of these plots (Fig. 4A–D). This line approximates the value of the largest change for each bar and is used as a template to assess the degree of similarity of the other responses. In all panels significant changes vs KO values are indicated by the yellow bars with asterisks. The greatest similarity in protein expression pattern to the index group (% change from KO in SP-A1-treated KO females) was the plot from female KO mice rescued with 10 μg of SP-A1 and 10 μg of SP-A2, with 8 of 13 significant changes seen in the index group (Fig. 4B). There was a similar pattern and a similar number of significant differences in the female KO mice rescued with 5 μg of SP-A1 and 5 μg of SP-A2 (data not shown) although the magnitude of many of the larger differences was diminished slightly suggesting that the effects were dose-dependent. Mean normalized volumes, standard deviations, and p values for comparisons against KO for all proteins from all groups are listed in Supplementary File 5.
Figure 4.
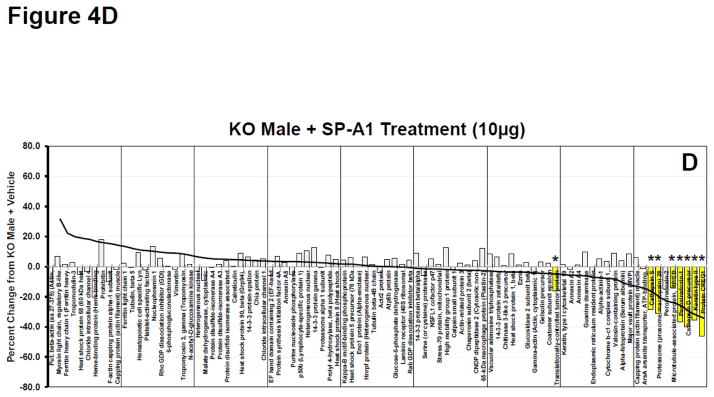
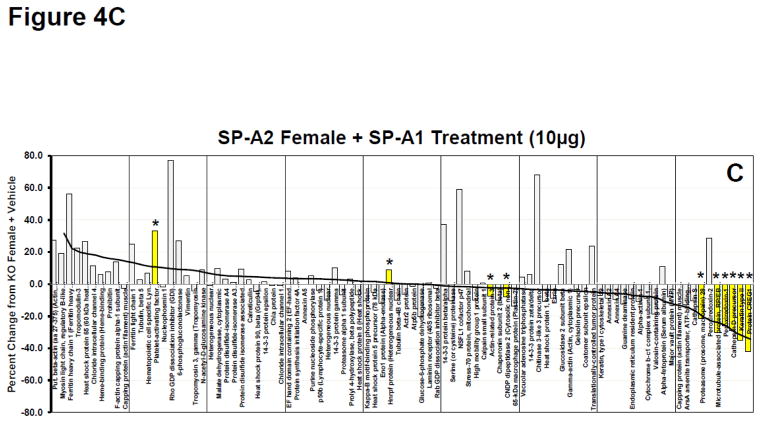
Waterfall plots of whole AM proteome. A. Waterfall plot showing the percent change for KO Female + SP-A1 treatment (10μg) compared to KO Female + Vehicle treatment for all proteins. This group is used as the index group and all plots (Panels AD) are presented with the proteins in this order. Proteins are arranged in order from greatest percent increase from KO+Vehicle to greatest percent decrease from KO+Vehicle as indicated by the trendline. Yellow bars with asterisks are proteins with significant changes (p<0.05) from KO + Vehicle. Names of proteins can be seen for each bar on the x-axis. B. Same as in 4A (with same protein order) for KO Female + SP-A1 and SP-A2 treatment (10 μg each). The trend line from 4A is superimposed on the histogram to facilitate comparison. C. Same as in 4A (with same protein order) for SP-A2 Female + SP-A1 treatment (10 μg). The trend line from 4A is superimposed on the histogram. D. Same as in 4A (with same protein order) for KO Male + SP-A1 treatment (10 μg). The trend line from 4A is superimposed on the histogram.
Also fairly similar was the pattern seen in the female SP-A2 transgenic mice rescued with SP-A1 (Fig. 4C), with 6 of the significant changes being in the same proteins as in the above mentioned groups (Fig. 4A–B). In both groups shown in Figures 4B and 4C, many of the same high and low expressing proteins were present as in Figure 4A, although there were a growing number of discordant responses (compare Figs. 4B and 4C to 4A). It is interesting to note that many of these discordant responses (i.e. increasing in Figs. 4B and 4C, but barely altered or slightly decreases in Fig. 4A, the SP-A1-treated females) are the same in the two groups shown in Figures 4B and 4C indicating their likely dependence on SP-A2, whether acutely available in the rescue group or chronically present in the SP-A2 transgenic mice.
We also examined the comparable plots of protein expression in the AM from male mice treated with SP-A1 (Fig. 4D). This plot was very different with the exception of 6 significantly changed proteins with the highest percent decreases (right side of graph). The persistence of this response subset (at the right end of the graph) in most male (data not shown) and female groups that received SP-A1, alone or in combination with SP-A2, suggests that these components of the response (right side of graph) are sex-independent. However, the remainder of the proteins differed quite markedly from the percent changes seen in the AM from female mice (compared to trend line). Many of the proteins showed markedly different percent changes as compared to KO AM from females (Figs. 4A–4C), perhaps indicating sex specificity in this part of the response pattern. Expression profiles from some of the other male groups (data not shown) resembled the male SP-A1 rescue group (Fig. 4D) and differed from the female responses.
Overall differences in significant changes in functional groups
We next examined the number of specific changes in the functional groups in each sex. The differences were demonstrated in bubble plots (Figs. 5–6) to provide an overview of the response patterns.
Figure 5.
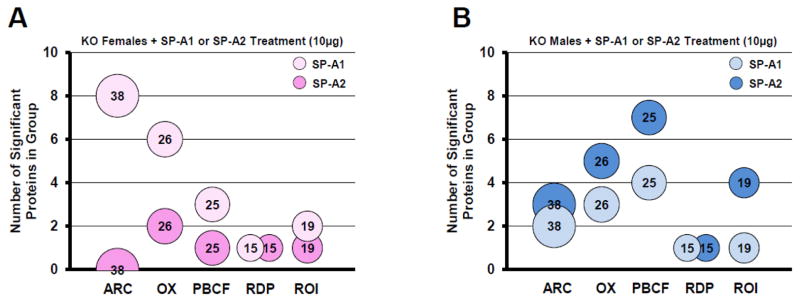
Changes in functional groups in each sex. Bubble charts depicting the number of significantly different proteins per functional group: ARC, actin-related/cytoskeletal; OX, oxidative stress; PBCF, protease balance/chaperone function; RDP, regulatory/developmental processes, and ROI, regulation of inflammation. Bubble size and the number within each bubble indicate the number of proteins in the overall group. The number of significant differences is indicated by y-axis values. Panel A shows groups from KO females treated with SP-A1 (10μg) (light pink bubbles) or treated with SP-A2 (10μg) (dark pink bubbles). Panel B shows groups from KO males treated with SP-A1 (10μg) (light blue bubbles) or treated with SP-A2 (10μg) (dark blue bubbles).
Figure 6.
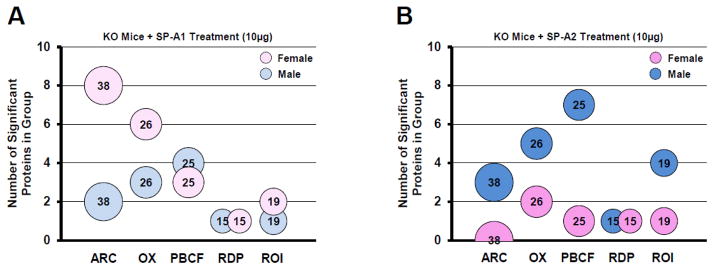
Changes in functional groups with each SP-A gene product. Bubble charts depicting the number of significantly different proteins per group: ARC, actin-related cytoskeletal; OX, oxidative stress; PBCF, protease balance/chaperone function; RDP, regulatory/developmental processes, and ROI, regulation of inflammation. Bubble size and the number within each bubble indicate the number of proteins in the overall group. The number of significant differences is indicated by the y-axis values. Panel A shows groups from KO mice treated with SP-A1 (10μg) for males (light blue) or females (light pink). Panel B shows groups from KO mice treated with SP-A2 (10μg) for males (dark blue) or females (dark pink).
i. as a function of SP-A genotype
Females had 20 significant differences in the SP-A1 group (light pink bubbles in Fig. 5A) and only 5 in the SP-A2 group (dark pink bubbles in Fig. 5A). Note that there are actually only 13 unique protein changes, but since several proteins are in multiple functional groups, in Figure 5A a total of 20 changes in SP-A1-treated females are observed when the changes in all functional groups are totaled. Fourteen of the 20 significant differences were in the SP-A1-treated ARC and OX groups (8 and 6 significant differences, respectively) (Fig. 5A).
Males showed a very different pattern. In the males the AM from SP-A1-treated KO mice had a total of 11 significant changes (8 are unique – see comment in previous paragraph) compared to KO + VEH among the 5 functional groups (light blue bubbles in Fig. 5B). By contrast, the SP-A2 rescue group had 20 (11 unique) significant differences from KO + VEH (dark blue bubbles in Fig. 5B). These changes were most different from the SP-A1 response in the functional groups related to PBCF (7 changes with SP-A2 and 4 with SP-A1) and in the ROI group (4 vs. 1). An increased number of changes with SP-A2 versus SP-A1 was also observed in the ARC and OX groups (Fig. 5A).
ii. as a function of sex
The differences shown in Figure 5 are replotted in Figure 6 to better compare sex differences. Figure 6A shows the SP-A1 responses for each sex, where the much higher number of significant differences in females versus males in the ARC and OX groups (light pink and blue bubbles in Fig. 6A) is readily evident. Slightly higher numbers of significant responses in males (n=4 in males; n=3 in females) were seen only in the PBCF group (Fig. 6A). In the chart summing up the SP-A2 responses (Fig. 6B) the pattern was much different than that of SP-A1 (Fig. 6A). There were more significant changes in males versus females in all functional groups except RDP in which both groups had a single significant change (dark pink and blue bubbles in Fig. 6B).
These data indicate that females are more responsive to SP-A1 (except RDP proteins), particularly with respect to actin metabolism and response to oxidative stress. It further shows that males are more responsive to SP-A2, with all functional groups, except RDP proteins, showing an increased response as compared to SP-A2-treated females, and with the greatest differences seen in the PBCF group (7 in males and 1 in females).
Sex specificity and specific differences in protein functional groups
We next used waterfall plots to study each functional group and further explore the sex differences.
i. Actin-related/cytoskeletal protein group
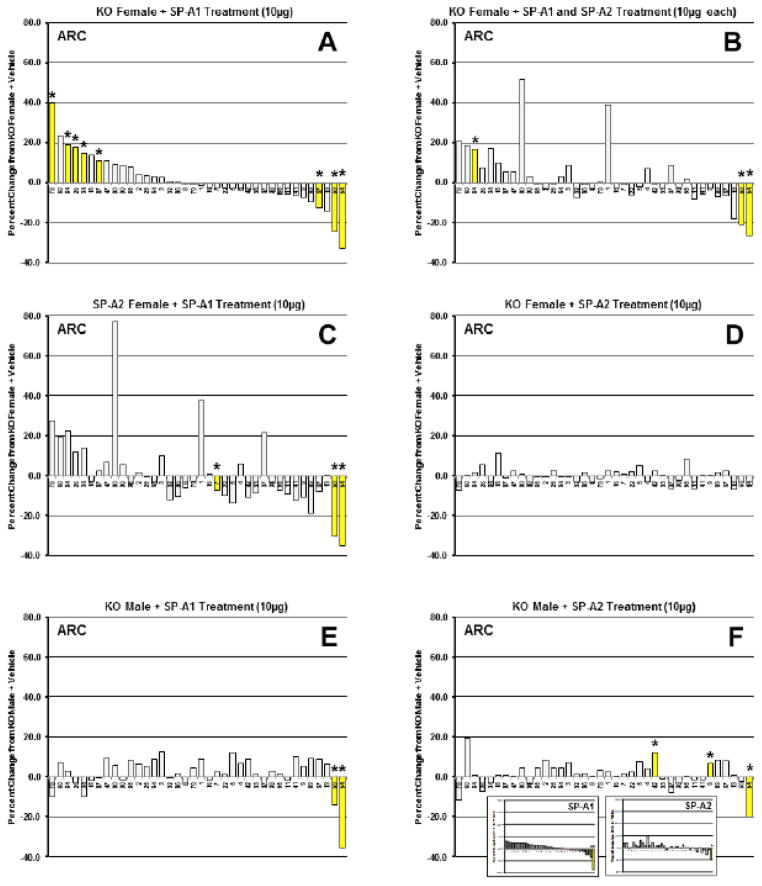
In the ARC functional group the greatest number of significant changes (8 of 38 proteins) was observed in SP-A1-treated KO females and the expression pattern is shown in Figure 7A. Bars where there were significant changes are in yellow with asterisks. Five of these changes were increases and 3 were decreases. Patterns very similar to that seen in the SP-A1-treated female, although with fewer significant changes, were obtained in the female KO mice treated with combinations of SP-A1 and SP-A2 (5 μg each)(not shown) and of SP-A1 and SP-A2 (10 μg each)(Fig. 7B). The magnitude of individual protein responses was slightly more pronounced in the group receiving 10 μg of each versus the 5 μg group, perhaps indicative of SP-A1 dose responsiveness. The profile of the female SP-A2 transgenic mice treated with SP-A1 (Fig. 7C) was also similar to the other female groups (particularly at either end of the plot) demonstrating the robust nature of the SP-A1 pattern. However, in terms of SP-A2 treatment, the KO females treated with SP-A2 had a very different pattern (Fig. 7D) from that seen with SP-A1, indicating the SP-A1-specific nature of the response in females. Of interest, the SP-A1-treated KO male group exhibited a pattern that bore little resemblance (Fig. 7E) to that seen in the females (Fig. 7A) indicating that with respect to this group of proteins, the SP-A1 response is sex-specific. Moreover, unlike the females, the response of the SP-A2 treated KO males (Fig. 7F) was similar (high on one end of the graph and low on the other end) to that of the SP-A1-treated males, showing that the male response in ARC proteins is not gene-specific. This is perhaps better appreciated in the inset in Figure 7F in which the changes in ARC proteins in SP-A1- and SP-A2-treated males have been rearranged in waterfall plots using the SP-A1-treated males as an index group.
Figure 7.
Waterfall plots for ARC proteins. Waterfall plots of percent change from KO + Vehicle for actin-related/cytoskeletal proteins (ARC). Panel A, KO female + SP-A1 treatment (10μg). This group is used as the index group, and all plots (Panels A-F, except inset in 7F) are presented with the proteins in this order (greatest percent increase from KO to greatest percent decrease from KO). Panel B, KO female + SP-A1 and SP-A2 treatment (10μg each); Panel C, SP-A2 female + SP-A1 treatment (10μg); Panel D, KO female + SP-A2 treatment (10μg); Panel E, KO male + SP-A1 treatment (10μg); Panel F, KO male + SP-A2 treatment (10μg). Inset: The inset in Panel F shows the expression patterns of the ARC proteins in males after SP-A1 or SP-A2 treatment (arranged by the SP-A1 treatment order) to facilitate comparison of these two treatments. Yellow bars with asterisks are proteins with significant changes (p<0.05) from KO + Vehicle. Numbers of proteins from the reference gel can be seen for each bar on the x-axis (see Fig. 2 and Supp. File 3 for protein names).
ii. Oxidative stress proteins
The oxidative stress (OX) group showed twice as many significant changes in the SP-A1-treated female as compared to the male (Supplementary File 6, panels A and B). In terms of male and female responses, in different treatment groups the OX protein group exhibited a similar overall trend to that seen with the ARC group. Six of 26 proteins in this group changed significantly in the SP-A1-treated KO females. The SP-A1-treated males (Supplementary File 6B) had a very different pattern from the SP-A1-treated females (Supp. File 6A), although both had significant decreases (right side of plot) in three proteins (keratin type II, #54; cathepsin D precursor, #20; peroxiredoxin 1, #65) that were also significantly altered in 9 or 10 out of the 12 experimental groups. Moreover, these female and male patterns were largely maintained when KO females (Supp. File 6C) and KO males (Supp. File 6D) were treated with the combination of SP-A1 and SP-A2 (10 μg each) as also shown for the ARC proteins (Fig. 7). In addition, when the female SP-A2 transgenic was treated with SP-A1 (Supp. File 6E), a similar pattern of protein expression as the other female treated groups emerged (Supp. File 6A, 6C). Similarly, the pattern of OX protein expression in KO males treated with SP-A1 + SP-A2 (10 μg) (Supp. File 6D) and the SP-A2 male mice treated with SP-A1 (Supp. File 6F) was similar to that seen in the SP-A1 treated males (Supp. File 6B). AM from KO females treated with SP-A2 exhibited a different pattern from that observed with SP-A1 treatment, although a couple of the proteins whose levels were decreased by treatment in nearly all other groups, were also decreased with SP-A2 treatment (Supp. File 6G). On the other hand, in SP-A2-treated males the pattern of protein expression in this group (OX) of proteins (Supp. File 6H) resembled that of males treated with SP-A1 (Supp. File 6B). This is best appreciated in the inset in Panel H where the proteins have been reordered in order of expression in SP-A1 treated KO males.
iii. Protease balance/chaperone function proteins
A very different situation was observed with the protease balance/chaperone function (PBCF) group. In this protein functional group there were many more significant changes in the male KO group treated with SP-A2 (Supp. File 7A) than in the females (Supp. File 7 B)(7 in the male, 1 in the female) so we chose to use the males as an index group and all panels in this figure have proteins in this order. Moreover, it is interesting to note that the pattern of expression for this protein group from male KO mice treated with SP-A1 (Supp. File 7 C) resembled that of the SP-A2-treated male mice (Supp. File 7 A), with a general correspondence between proteins increasing with treatment and those decreasing. This indicates a lack of specificity to SP-A1 or SP-A2 in the males in the PBCF protein group, much like that described above to the ARC (Fig. 7) and OX (Supp. File 6) protein groups. In the female mice the SP-A1 and SP-A2 responses seem to differ in the PBCF proteins (Supp. File 7 B, D) indicating an apparent gene specificity of the SP-A effects in females, as we saw previously for ARC (Fig. 7) and OX (Supp. File 6). The inset in Supplementary File 7D shows the waterfall plots for the female KO mice treated with either SP-A2 or SP-A1, reordered to facilitate their comparison. It should also be noted that the 3 proteins undergoing the greatest decreases after treatment (cathepsin D, #20; proteasome 28S subunit, #70; cathepsin S, #21) were similarly affected in nearly all treatment groups, and in many cases were significantly changed.
iv. other functional groups
The similarity in PBCF proteins between the male KO response to either SP-A1 or SP-A2 led us to re-examine this comparison in other functional groups. Supplementary File 8 shows the SP-A2 and SP-A1 responses of KO males for OX (Supp. File 8A, B), ROI (Supp. File 8C, D) and RDP (Supp. File 8E, F) proteins. The same general trend is observed, with both SP-A treatments eliciting similar patterns in the male. A similar comparison of females, using the protein order dictated by the female response to SP-A1 demonstrates much less similarity between SP-A1- and SP-A2-treated KO females (Supplementary File 9).
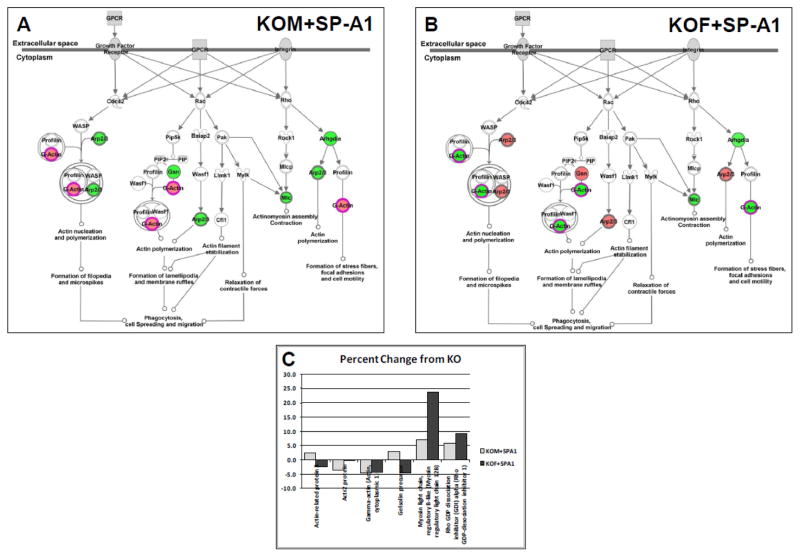
Actin-related pathways
Because of the large number of identified proteins in the actin-related/cytoskeletal (ARC) functional group and the prominence of an actin-related pathway in the analysis of our proteins by Ingenuity Pathways Analysis (IPA). We used IPA to compare responses in our study. In Figure 8 the canonical pathway for two treatment groups - “regulation of actin-based motility by Rho” - pathway is shown. The left panel (Fig. 8A) depicts the changes in KO males treated with SP-A1 and the right panel (Fig. 8B) shows KO females treated with SP-A1 (red=up-regulated; green=down-regulated). The response patterns (colors of spots) in the two groups are nearly the inverse of one another. All but two of the spots on the pathway are differentially regulated in males (Fig. 8A) and females (Fig. 8B). However, in one case, although the “upstream” spot is green in both sexes, the “downstream” molecules involved in “actin polymerization” and “formation of stress fibers, etc” are oppositely affected. Figure 8C shows the actual percent change from KO for each of the proteins involved in the pathways that are depicted. The data in the inset confirm the trends illustrated in the diagram and show the sex differences in the SP-A1 effect on AM and their likely physiological effects. The full complement of changes in these two treatment groups is shown in Figures 7A and 7E.
Figure 8.
Pathway diagram for ARC proteins after SP-A1 treatment. A diagram from Ingenuity Pathways Analysis is shown for some of the ARC proteins identified in this study. Red spots are increased relative to KO values and green spots are decreased. A. Changes in the pathways after KO males were treated with SP-A1. B. Changes in the pathways after KO females were treated with SP-A1. C. Histogram showing percent change from KO values (on y-axis) after SP-A1 treatment in males (light bars) and females (dark bars) for proteins depicted in the pathway diagram.
Proteomic changes and sex differences in SP-A2 transgenics at baseline levels
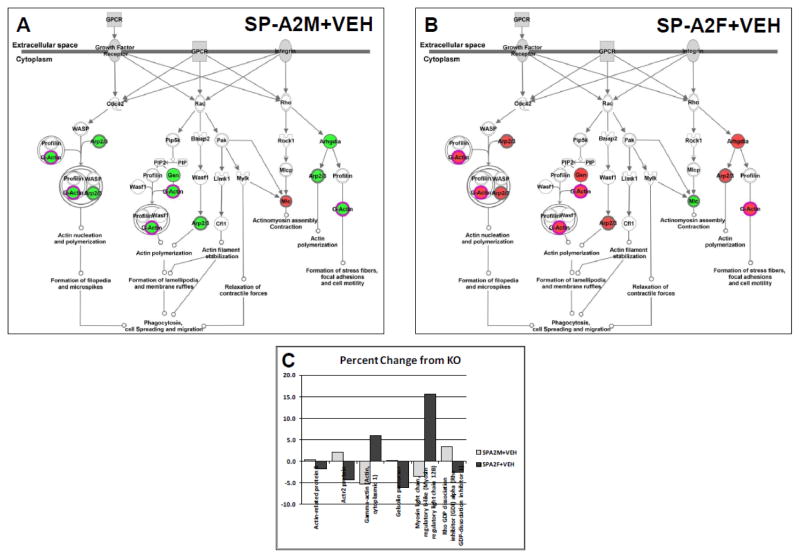
Most of the results described above involve the acute effects of a rescue with either SP-A1 or SP-A2. In most cases the rescue involved SP-A −/− mice, but some of the data described the SP-A1 rescue of SP-A2 transgenic mice. It is interesting to note that the chronic or constitutive presence of SP-A2 in the transgenic mice has a very different effect on the AM proteome than the acute/rescue exposure. With the entire set of 90 identified proteins there was only a single significant difference in SP-A2 transgenics compared to KO in both the males (coatomer subunit epsilon) and the females (peroxiredoxin 1), whereas there were many more changes in the KO mice rescued with SP-A2 as discussed above. However, marked albeit small, differences between the sexes in the relative levels of proteins in the ARC group were seen. In the males (Supp. File 10, Panel A) the majority of the ARC proteins (23 proteins) are higher than KO, whereas in the female (Supp. File 10, Panel B) the majority (29 out of 38) are expressed at levels lower than KO. The possible implications of these differences are seen in the IPA analysis of the canonical pathway “regulation of actin-based motility by Rho” (Fig. 9) where all but one spot are green in the male (Fig. 9A), and all but one spot are red in the female (Fig. 9B). The inset (Fig. 9C) shows histograms of all of the proteins represented in the pathway analysis and the marked differences between male and female are apparent.
Figure 9.
Pathway diagram for ARC proteins from SP-A2 transgenic mice. A diagram from Ingenuity Pathways Analysis is shown for some of the ARC proteins identified in this study. Red spots are increased relative to KO values and green spots are decreased. A. Changes in the pathways when KO males were compared with SP-A2 transgenic males. B. Changes in the pathways when KO females were compared with SP-A2 transgenic females. C. Histogram showing percent change from KO values (on y-axis) in SP-A2 transgenics in males (light bars) and females (dark bars) for proteins depicted in the pathway diagram.
4. Discussion
In a series of studies we have explored sex differences in lung innate immunity and the impact of oxidative stress on it [121–124] and have shown that some of these differences stem from changes in alveolar macrophage function [123]. In order to understand the basis for these sex differences we studied SP-A-dependent changes in AM gene expression, protein expression, and function [21,24,28]. We were able to demonstrate that the two human SP-A gene products, SP-A1 and SP-A2, have distinct roles [21,22,125].
In the present study we extend previous findings by conducting a rescue study in male and female SP-A −/− or SP-A2 hTG mice with human SP-A1, SP-A2, or mixtures of both gene products and studying the resultant changes in the AM proteome. Our results show different responses of each sex to SP-A1 or SP-A2 treatment as assessed by differential effects of these gene products on various AM functional protein groups in each sex.
The first indication of a sex difference was with a simple tabulation of significant changes with each treatment. More changes occurred in females than in males, with many more changes occurring in response to SP-A1 treatment than with SP-A2 treatment. The robust nature of the SP-A1 response in females became evident when we compared mice receiving the single dose of SP-A1 with other groups getting combinations of SP-A1 and SP-A2, or the administration of SP-A1 to SP-A2 transgenic mice and saw similar response patterns. Males, on the other hand, had similar numbers of changes with SP-A1 or SP-A2, although they appeared to be slightly more responsive to SP-A2 treatment than SP-A1 treatment. These observations indicate an apparent sex specificity of the responses to SP-A1 and SP-A2, with females displaying very different responses to the different gene products, and males exhibiting similar expression patterns after treatment with either gene product, but with a slightly higher degree of responsiveness to SP-A2.
We also found that when we studied the SP-A responses of different functional groups of proteins, some were preferentially affected in males and some in females. The females showed very dynamic responses and more significant changes in the ARC and OX proteins, while the males had more changes in the PBCF and ROI groups. Although many of these changes were modest, the fact that changes were often seen in multiple proteins affecting a given pathway or process raises the likelihood that biologically significant changes may be occurring in that activity. These response patterns consisted of both positive and negative changes with respect to the KO mice. The functional implications of these changes in the intact organism remain to be determined and could potentially be studied by combining the rescue protocol we used in this study with our models of infection and ozone-induced oxidant stress. However, we can speculate about what may be happening.
In our previous work we have shown that female mice have lower mortality rates than males in a Klebsiella pneumoniae model of infection [6, 123] and both sexes had more mortality in the absence of SP-A. Host defense by AM against bacterial infection of the lung probably depends on multiple cellular processes involving actin and other cytoskeletal elements, including chemotaxis, phagocytosis, internalization, and killing. We speculate that the SP-A-induced changes in protein expression correct some of the deficiencies seen in SP-A−/− mice vs wild-type animals, and that the more robust response in the females in processes involving ARC proteins may relate to their better clinical outcomes relative to the males [121, 123]. This may be due to a more dynamic actin metabolism resulting in more efficient host defense function in the females. If this is indeed the case we would expect SP-A1 to have a greater enhancement of survival than SP-A2 in female mice, but the benefit in males would be similar with SP-A1 and SP-A2. In addition, we have shown females to be more susceptible to ozone-induced oxidative injury than males [6]. We speculate that the robust response of OX proteins to SP-A1 treatment in the females would have a protective effect, whereas the much more limited response to SP-A2 would not. Here again, the similar response to both SP-A variants in the male may confer a similar benefit in the presence of oxidative stress.
There may be additional factors contributing to the negative impact of ozone exposure on infected female mice. These may relate to the enhanced regulation of PBCF and ROI proteins in the males compared to females, where these groups of proteins were less responsive in females, especially to SP-A2 treatment. We speculate that the SP-A2-induced changes in the expression of these protein groups could more effectively enhance the ability of male AM to eliminate ozone-damaged molecules and repair other cellular damage by processes like autophagy, and to resolve inflammation. These functions may be responsible for the fact that the males exhibit less of a decline in infection-induced mortality after ozone-induced oxidative stress than in the females [121, 123].
Based on the present findings, exogenous treatment with various SP-A variants may be useful to enhance different sex-specific processes of innate immunity in order to maintain lung structure and function. For example, SP-A1 treatment elicits a strong response in ARC proteins and via this (in females) may enhance the host defense response whereas in males SP-A1 treatment is almost as effective as SP-A2 for this group of proteins. To be maximally effective, this treatment may need to be administered prior to, or as soon as possible after the insults, and SP-A1 could be the treatment of choice. Moreover, in males the greatest impact of rescue therapy with SPA variants may be in dealing with the sequelae of infection and oxidative damage, as suggested by the higher SP-A2 response in PBCF and ROI protein groups. The data support the notion of a sex/genetic variant interaction in terms of SP-A1 and SP-A2 functional activity. We speculate that given the similar response of the male to either SP-A gene product that the relative quantities of the two gene products in humans may not be important, whereas in the female, where SP-A1 has a much greater effect than SP-A2 the ratio of the two may play an important role physiologically.
Not surprisingly, the acute effects on the KO AM proteome generated by SP-A rescue differed from those seen in transgenic mice that constitutively (or chronically) expressed SP-A2. The chronic effects were much more subtle with less pronounced changes, but there were clearly sex differences in the resultant phenotype. The “acute” effect may be important as a potential therapy, but the sex differences in the response indicate that effective therapeutics containing SP-A may differ depending on the sex of the recipient, as well as the indications for treatment. Also, the optimal time for treatment after a given insult may differ between males and females. In this study we have explored the effects of human SP-A1 and SP-A2 on male and female mouse AM, but have not investigated the mechanistic basis for these effects. It is not known, for example, whether the sex- and gene-specific differences we described would occur in human AM. Nor do we know whether treatment of mouse AM with mouse SP-A would elicit the same type of sex differences in the proteomic profile of the AM that we see after human SP-A treatment. Future studies are likely to address these issues. However, human SP-A (in terms of effect on phagocytic index) exhibited qualtitatively similar results with human or rat AM [16], as well as mouse AM (our unpublished data), indicating that rodent AM may be an appropriate model for human SP-A studies.
In this study we examined the AM from mice under baseline conditions. It would be of interest to compare the responses to infection, oxidative stress, or both in AM from male and female mice that either express SP-A1 or SP-A2 transgenes, or after rescue of SP-A −/− mice with exogenous SP-A1 or SP-A2. It is likely that this interaction between SP-A genotype and sex plays a role in the differential susceptibility and sex differences in the incidence and clinical course of some lung diseases.
Conclusion
In this study we have demonstrated: a) that alveolar macrophages from SP-A knockout mice have distinct responses to acute in vivo treatment with human SP-A1 and SP-A2; b) that there are sex differences in the nature of these responses; and c) that there may be differences between acute treatment and chronic exposure to SP-A1 and SP-A2. These results provide us with important clues about the role of SP-A1 and SP-A2 in the function of alveolar macrophages and perhaps in the pathogenesis of some lung diseases. Furthermore the sex differences observed, may provide insight into the biological basis for the differences observed between the sexes in the susceptibility to some pulmonary conditions.
Supplementary Material
Significance.
This study shows that changes occur in the alveolar macrophage proteome in response to a single in vivo treatment with exogenous SP-A1 and/or SP-A2. We demonstrate that SP-A1 and SP-A2 have different effects on the AM proteome and that sex differences exist in the response to each SP-A1 and SP-A2 gene product. This study illustrates the potential of exogenous SP-A1 and SP-A2 treatment for manipulation of macrophage function and indicates that the specific SP-A variant used for treatment may vary with sex and with the cellular functions being modified. The observed changes may contribute to sex differences in the incidence of some lung diseases.
Highlights.
Alveolar macrophages (AM) exhibit distinct responses to SP-A1 and SP-A2.
There are sex differences in the AM responses to SP-A1 and SP-A2.
AM from female mice exhibit a greater response to SP-A1 for selected protein groups.
AM from males have a greater response to SP-A2 for different protein groups.
The responsive protein groups for SP-A1 and SP-A2 differ between males and females.
Acknowledgments
This work was supported in part by NIH HL 34788 and R01 ES009882 from the National Heart, Lung and Blood Institute and the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goto H, Ledford JG, Mukherjee S, Noble PW, Williams KL, Wright JR. The role of surfactant protein A in bleomycin-induced acute lung injury. Am J Respir Crit Care Med. 2010;181:1336–1344. doi: 10.1164/rccm.200907-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gil HW, Oh MH, Woo KM, Lee EY, Oh MH, Hong SY. Relationship between pulmonary surfactant protein and lipid peroxidation in lung injury due to paraquat intoxication in rats. Korean J Intern Med. 2007;22:67–72. doi: 10.3904/kjim.2007.22.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madan T, Reid KB, Clark H, Singh M, Nayak A, Sarma PU, et al. Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis. Mol Immunol. 2010;47:1923–1930. doi: 10.1016/j.molimm.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Famuyide ME, Hasday JD, Carter HC, Chesko KL, He JR, Viscardi RM. Surfactant protein-A limits Ureaplasma-mediated lung inflammation in a murine pneumonia model. Pediatr Res. 2009;66:162–167. doi: 10.1203/PDR.0b013e3181aabd66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George CL, Goss KL, Meyerholz DK, Lamb FS, Snyder JM. Surfactant-associated protein A provides critical immunoprotection in neonatal mice. Infect Immun. 2008;76:380–390. doi: 10.1128/IAI.01043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikerov AN, Haque R, Gan X, Guo X, Phelps DS, Floros J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res. 2008;9:77. doi: 10.1186/1465-9921-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, et al. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol. 2007;220:72–82. doi: 10.1016/j.taap.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 9.LeVine AM, Bruno MD, Huelsman KM, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 10.Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol. 2000;278:L946–L954. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Umstead TM, Phelps DS, Al Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ Health Perspect. 2002;110:79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Wang G, Phelps DS, Al Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L546–L553. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Bates-Kenney SR, Tao JQ, Phelps DS, Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry. 2004;43:4227–4239. doi: 10.1021/bi036023i. [DOI] [PubMed] [Google Scholar]
- 14.Mikerov AN, Umstead TM, Gan X, Huang W, Guo X, Wang G, et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008;294:L121–L130. doi: 10.1152/ajplung.00288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005;288:L150–L158. doi: 10.1152/ajplung.00135.2004. [DOI] [PubMed] [Google Scholar]
- 16.Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, et al. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than Do SP-A1 variants. Infect Immun. 2007;75:1403–1412. doi: 10.1128/IAI.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Verdugo I, Wang G, Floros J, Casals C. Structural analysis and lipid-binding properties of recombinant human surfactant protein a derived from one or both genes. Biochemistry. 2002;41:14041–14053. doi: 10.1021/bi026540l. [DOI] [PubMed] [Google Scholar]
- 18.Oberley RE, Snyder JM. Recombinant human SP-A1 and SP-A2 proteins have different carbohydrate-binding characteristics. Am J Physiol Lung Cell Mol Physiol. 2003;284:L871–L881. doi: 10.1152/ajplung.00241.2002. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Myers C, Mikerov A, Floros J. Effect of cysteine 85 on biochemical properties and biological function of human surfactant protein A variants. Biochemistry. 2007;46:8425–8435. doi: 10.1021/bi7004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Taneva S, Keough KM, Floros J. Differential effects of human SP-A1 and SP-A2 variants on phospholipid monolayers containing surfactant protein B. Biochim Biophys Acta. 2007;1768:2060–2069. doi: 10.1016/j.bbamem.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelps DS, Umstead TM, Silveyra P, Hu S, Wang G, et al., editors. Differences in the alveolar macrophage proteome in transgenic mice expressing human SP-A1 and SP-A2. J Proteomic Genomic Res. 2013;1:2–26. doi: 10.14302/issn.2326-0793.jpgr-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, Guo X, DiAngelo S, Thomas NJ, Floros J. Humanized SFTPA1 and SFTPA2 transgenic mice reveal functional divergence of SP-A1 and SP-A2: formation of tubular myelin in vivo requires both gene products. J Biol Chem. 2010;285:11998–12010. doi: 10.1074/jbc.M109.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floros J, Hoover RR. Genetics of the hydrophilic surfactant proteins A and D. Biochim Biophys Acta. 1998;1408:312–322. doi: 10.1016/s0925-4439(98)00077-5. [DOI] [PubMed] [Google Scholar]
- 24.Phelps DS, Umstead TM, Quintero OA, Yengo CM, Floros J. In vivo rescue of alveolar macrophages from SP-A knockout mice with exogenous SP-A nearly restores a wild type intracellular proteome; actin involvement. Proteome Sci. 2011;9:67. doi: 10.1186/1477-5956-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umstead TM, Freeman WM, Chinchilli VM, Phelps DS. Age-related changes in the expression and oxidation of bronchoalveolar lavage proteins in the rat. Am J Physiol Lung Cell Mol Physiol. 2009;296:L14–L29. doi: 10.1152/ajplung.90366.2008. [DOI] [PubMed] [Google Scholar]
- 26.Umstead TM, Lu CJ, Freeman WM, Myers JL, Clark JB, Thomas NJ, et al. Dual-platform proteomics study of plasma biomarkers in pediatric patients undergoing cardiopulmonary bypass. Pediatr Res. 2010;67:641–649. doi: 10.1203/PDR.0b013e3181dceef5. [DOI] [PubMed] [Google Scholar]
- 27.Ali M, Umstead TM, Haque R, Mikerov AN, Freeman WM, Floros J, et al. Differences in the BAL proteome after Klebsiella pneumoniae infection in wild type and SP-A−/− mice. Proteome Sci. 2010;8:34. doi: 10.1186/1477-5956-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelps DS, Umstead TM, Floros J. Sex differences in the response of the alveolar macrophage proteome to treatment with exogenous surfactant protein-A. Proteome Sci. 2012;10:44. doi: 10.1186/1477-5956-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haque R, Umstead TM, Freeman WM, Floros J, Phelps DS. The impact of surfactant protein-A on ozone-induced changes in the mouse bronchoalveolar lavage proteome. Proteome Sci. 2009;7:12. doi: 10.1186/1477-5956-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiol. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Jin M, Opalek JM, Marsh CB, Wu HM. Proteome comparison of alveolar macrophages with monocytes reveals distinct protein characteristics. Am J Respir Cell Mol Biol. 2004;31:322–329. doi: 10.1165/rcmb.2004-0080OC. [DOI] [PubMed] [Google Scholar]
- 32.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L936–L946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bustos DM. The role of protein disorder in the 14-3-3 interaction network. Mol Biosyst. 2012;8:178–184. doi: 10.1039/c1mb05216k. [DOI] [PubMed] [Google Scholar]
- 35.Sluchanko NN, Gusev NB. 14-3-3 proteins and regulation of cytoskeleton. Biochemistry (Mosc) 2010;75:1528–1546. doi: 10.1134/s0006297910130031. [DOI] [PubMed] [Google Scholar]
- 36.Delanote V, Vandekerckhove J, Gettemans J. Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharmacol Sin. 2005;26:769–779. doi: 10.1111/j.1745-7254.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 37.Clarke JL, Mason PJ. Murine hexose-6-phosphate dehydrogenase: a bifunctional enzyme with broad substrate specificity and 6-phosphogluconolactonase activity. Arch Biochem Biophys. 2003;415:229–234. doi: 10.1016/s0003-9861(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 38.Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luikart S, Masri M, Wahl D, Hinkel T, Beck JM, Gyetko MR, et al. Urokinase is required for the formation of mactinin, an alpha-actinin fragment that promotes monocyte/macrophage maturation. Biochim Biophys Acta. 2002;1591:99–107. doi: 10.1016/s0167-4889(02)00255-0. [DOI] [PubMed] [Google Scholar]
- 41.Setiyono A, Budiyati AD, Purwantomo S, Anggelia MR, Fanany I, Wibowo GA, et al. Immunoregulatory effects of AFP domains on monocyte-derived dendritic cell function. BMC Immunol. 2011;12:4. doi: 10.1186/1471-2172-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morel E, Parton RG, Gruenberg J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev Cell. 2009;16:445–457. doi: 10.1016/j.devcel.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V. Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci. 2008;121:2177–2185. doi: 10.1242/jcs.028415. [DOI] [PubMed] [Google Scholar]
- 44.Laumonnier Y, Syrovets T, Burysek L, Simmet T. Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood. 2006;107:3342–3349. doi: 10.1182/blood-2005-07-2840. [DOI] [PubMed] [Google Scholar]
- 45.Madureira PA, Waisman DM. Annexin A2: the importance of being redox sensitive. Int J Mol Sci. 2013;14:3568–3594. doi: 10.3390/ijms14023568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeon YJ, Kim DH, Jung H, Chung SJ, Chi SW, Cho S, et al. Annexin A4 interacts with the NF-kappaB p50 subunit and modulates NF-kappaB transcriptional activity in a Ca2+-dependent manner. Cell Mol Life Sci. 2010;67:2271–2281. doi: 10.1007/s00018-010-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rand JH, Wu XX, Lin EY, Griffel A, Gialanella P, McKitrick JC. Annexin A5 binds to lipopolysaccharide and reduces its endotoxin activity. MBio. 2012;3 doi: 10.1128/mBio.00292-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Domeij H, Hua X, Su J, Backlund A, Yan Z, Frostegard AG, et al. Annexin A5 inhibits atherogenic and pro-inflammatory effects of lysophosphatidylcholine. Prostaglandins Other Lipid Mediat. 2013 doi: 10.1016/j.prostaglandins.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Kim HE, Lee SG. Induction of ATP synthase beta by H2O2 induces melanogenesis by activating PAH and cAMP/CREB/MITF signaling in melanoma cells. Int J Biochem Cell Biol. 2013;45:1217–1222. doi: 10.1016/j.biocel.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Potter DA, Tirnauer JS, Janssen R, Croall DE, Hughes CN, Fiacco KA, et al. Calpain regulates actin remodeling during cell spreading. J Cell Biol. 1998;141:647–662. doi: 10.1083/jcb.141.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 52.Bajor A, Tischer S, Figueiredo C, Wittmann M, Immenschuh S, Blasczyk R, et al. Modulatory role of calreticulin as chaperokine for dendritic cell-based immunotherapy. Clin exp Immunol. 2011;165:220–234. doi: 10.1111/j.1365-2249.2011.04423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 54.Cooper JA, Sept D. New insights into mechanism and regulation of actin capping protein. Int Rev Cell Mol Biol. 2008;267:183–206. doi: 10.1016/S1937-6448(08)00604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kostyukova AS. Capping complex formation at the slow-growing end of the actin filament. Biochemistry (Mosc) 2008;73:1467–1472. doi: 10.1134/s0006297908130075. [DOI] [PubMed] [Google Scholar]
- 56.Bewley MA, Marriott HM, Tulone C, Francis SE, Mitchell TJ, Read RC, et al. A cardinal role for cathepsin d in co-ordinating the host-mediated apoptosis of macrophages and killing of pneumococci. PLoS Pathog. 2011;7:e1001262. doi: 10.1371/journal.ppat.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitteringham NR, Abdullah A, Walsh J, Randle L, Jenkins RE, Sison R, et al. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteomics. 2010;73:1612–1631. doi: 10.1016/j.jprot.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirakawa H, Pierce RA, Bingol-Karakoc G, Karaaslan C, Weng M, Shi GP, et al. Cathepsin S deficiency confers protection from neonatal hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2007;176:778–785. doi: 10.1164/rccm.200704-519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lundin VF, Leroux MR, Stirling PC. Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci. 2010;35:288–297. doi: 10.1016/j.tibs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Brackley KI, Grantham J. Subunits of the chaperonin CCT interact with F-actin and influence cell shape and cytoskeletal assembly. Exp Cell Res. 2010;316:543–553. doi: 10.1016/j.yexcr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh H, Cousin MA, Ashley RH. Functional reconstitution of mammalian ‘chloride intracellular channels’ CLIC1, CLIC4 and CLIC5 reveals differential regulation by cytoskeletal actin. FEBS J. 2007;274:6306–6316. doi: 10.1111/j.1742-4658.2007.06145.x. [DOI] [PubMed] [Google Scholar]
- 63.Averaimo S, Milton RH, Duchen MR, Mazzanti M. Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett. 2010;584:2076–2084. doi: 10.1016/j.febslet.2010.02.073. [DOI] [PubMed] [Google Scholar]
- 64.Chuang JZ, Chou SY, Sung CH. Chloride intracellular channel 4 is critical for the epithelial morphogenesis of RPE cells and retinal attachment. Mol Biol Cell. 2010;21:3017–3028. doi: 10.1091/mbc.E09-10-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, et al. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006;79:1944–1955. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 66.Cuzzocrea S, Genovese T, Failla M, Vecchio G, Fruciano M, Mazzon E, et al. Protective effect of orally administered carnosine on bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1095–L1104. doi: 10.1152/ajplung.00283.2006. [DOI] [PubMed] [Google Scholar]
- 67.Zhang D, Richardson DR. Endoplasmic reticulum protein 29 (ERp29): An emerging role in cancer. Int J Biochem Cell Biol. 2011;43:33–36. doi: 10.1016/j.biocel.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 68.Keller A, Peltzer J, Carpentier G, Horvath I, Olah J, Duchesnay A, et al. Interactions of enolase isoforms with tubulin and microtubules during myogenesis. Biochim Biophys Acta. 2007;1770:919–926. doi: 10.1016/j.bbagen.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 69.Wygrecka M, Marsh LM, Morty RE, Henneke I, Guenther A, Lohmeyer J, et al. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;113:5588–5598. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- 70.Marion S, Hoffmann E, Holzer D, Le CC, Martin M, Sachse M, et al. Ezrin promotes actin assembly at the phagosome membrane and regulates phago-lysosomal fusion. Traffic. 2011;12:421–437. doi: 10.1111/j.1600-0854.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Tomso DJ, Chorley BN, Cho HY, Cheung VG, Kleeberger SR, et al. Identification of polymorphic antioxidant response elements in the human genome. Hum Mol Genet. 2007;16:1188–1200. doi: 10.1093/hmg/ddm066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 74.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez JR, Byrne B, Firestein BL. Phylogenetic analysis and molecular evolution of guanine deaminases: from guanine to dendrites. J Mol Evol. 2009;68:227–235. doi: 10.1007/s00239-009-9205-x. [DOI] [PubMed] [Google Scholar]
- 76.Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niture SK, Jaiswal AK. Hsp90 interaction with INrf2(Keap1) mediates stress-induced Nrf2 activation. J Biol Chem. 2010;285:36865–36875. doi: 10.1074/jbc.M110.175802. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Beck R, Dejeans N, Glorieux C, Creton M, Delaive E, Dieu M, et al. Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. PLoS One. 2012;7:e40795. doi: 10.1371/journal.pone.0040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wisniewska M, Karlberg T, Lehtio L, Johansson I, Kotenyova T, Moche M, et al. Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70–2, HSPA6/Hsp70B’, and HSPA5/BiP/GRP78. PLoS One. 2010;5:e8625. doi: 10.1371/journal.pone.0008625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Madore AM, Perron S, Turmel V, Laviolette M, Bissonnette EY, Laprise C. Alveolar macrophages in allergic asthma: an expression signature characterized by heat shock protein pathways. Hum Immunol. 2010;71:144–150. doi: 10.1016/j.humimm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas SG, Calaminus SD, Auger JM, Watson SP, Machesky LM. Studies on the actin-binding protein HS1 in platelets. BMC Cell Biol. 2007;8:46. doi: 10.1186/1471-2121-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 84.Gao JL, Guillabert A, Hu J, Le Y, Urizar E, Seligman E, et al. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J Immunol. 2007;178:1450–1456. doi: 10.4049/jimmunol.178.3.1450. [DOI] [PubMed] [Google Scholar]
- 85.Li H, Liu J. Identification of heterogeneous nuclear ribonucleoprotein K as a transactivator for human low density lipoprotein receptor gene transcription. J Biol Chem. 2010;285:17789–17797. doi: 10.1074/jbc.M109.082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hossain MN, Fuji M, Miki K, Endoh M, Ayusawa D. Downregulation of hnRNP C1/C2 by siRNA sensitizes HeLa cells to various stresses. Mol Cell Biochem. 2007;296:151–157. doi: 10.1007/s11010-006-9308-2. [DOI] [PubMed] [Google Scholar]
- 87.Tsan MF. Heat shock proteins and high mobility group box 1 protein lack cytokine function. J Leukoc Biol. 2011;89:847–853. doi: 10.1189/jlb.0810471. [DOI] [PubMed] [Google Scholar]
- 88.Goh ET, Pardo OE, Michael N, Niewiarowski A, Totty N, Volkova D, et al. Involvement of heterogeneous ribonucleoprotein F in the regulation of cell proliferation via the mammalian target of rapamycin/S6 kinase 2 pathway. J Biol Chem. 2010;285:17065–17076. doi: 10.1074/jbc.M109.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, et al. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traweek ST, Liu J, Battifora H. Keratin gene expression in non-epithelial tissues. Detection with polymerase chain reaction. Am J Pathol. 1993;142:1111–1118. [PMC free article] [PubMed] [Google Scholar]
- 91.Caulin C, Ware CF, Magin TM, Oshima RG. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol. 2000;149:17–22. doi: 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fradette J, Germain L, Seshaiah P, Coulombe PA. The type I keratin 19 possesses distinct and context-dependent assembly properties. J Biol Chem. 1998;273:35176–35184. doi: 10.1074/jbc.273.52.35176. [DOI] [PubMed] [Google Scholar]
- 93.Herrmann C, Golkaramnay E, Inman E, Rome L, Volknandt W. Recombinant major vault protein is targeted to neuritic tips of PC12 cells. J Cell Biol. 1999;144:1163–1172. doi: 10.1083/jcb.144.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–376. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 95.Chia WS, Chia DX, Rao F, Bar NS, Geifman SS. ATP binding to p97/VCP D1 domain regulates selective recruitment of adaptors to its proximal N-domain. PLoS One. 2012;7:e50490. doi: 10.1371/journal.pone.0050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hsu CY, Yung BY. Down-regulation of nucleophosmin/B23 during retinoic acid-induced differentiation of human promyelocytic leukemia HL-60 cells. Oncogene. 1998;16:915–923. doi: 10.1038/sj.onc.1201615. [DOI] [PubMed] [Google Scholar]
- 97.Watanabe N, Iwamura T, Shinoda T, Fujita T. Regulation of NFKB1 proteins by the candidate oncoprotein BCL-3: generation of NF-kappaB homodimers from the cytoplasmic pool of p50–p105 and nuclear translocation. EMBO J. 1997;16:3609–3620. doi: 10.1093/emboj/16.12.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Increased severity of bleomycin-induced skin fibrosis in mice with leukocyte-specific protein 1 deficiency. J Invest Dermatol. 2008;128:2767–2776. doi: 10.1038/jid.2008.164. [DOI] [PubMed] [Google Scholar]
- 99.Yanagisawa R, Warabi E, Inoue K, Yanagawa T, Koike E, Ichinose T, et al. Peroxiredoxin I null mice exhibits reduced acute lung inflammation following ozone exposure. J Biochem. 2012;152:595–601. doi: 10.1093/jb/mvs113. [DOI] [PubMed] [Google Scholar]
- 100.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J Clin Invest. 2010;120:3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mishra S, Ande SR, Nyomba BL. The role of prohibitin in cell signaling. FEBS J. 2010;277:3937–3946. doi: 10.1111/j.1742-4658.2010.07809.x. [DOI] [PubMed] [Google Scholar]
- 102.Natarajan R, Salloum FN, Fisher BJ, Smithson L, Almenara J, Fowler AA., III Prolyl hydroxylase inhibition attenuates post-ischemic cardiac injury via induction of endoplasmic reticulum stress genes. Vascul Pharmacol. 2009;51:110–118. doi: 10.1016/j.vph.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 103.Ito Y, Kondo E, Demachi-Okamura A, Akatsuka Y, Tsujimura K, Tanimoto M, et al. Three immunoproteasome-associated subunits cooperatively generate a cytotoxic T-lymphocyte epitope of Epstein-Barr virus LMP2A by overcoming specific structures resistant to epitope liberation. J Virol. 2006;80:883–890. doi: 10.1128/JVI.80.2.883-890.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hopitzan A, Himmelbauer H, Spevak W, Castanon MJ. The mouse Psma1 gene coding for the alpha-type C2 proteasome subunit: structural and functional analysis, mapping, and colocalization with Pde3b on mouse chromosome 7. Genomics. 2000;66:313–323. doi: 10.1006/geno.2000.6217. [DOI] [PubMed] [Google Scholar]
- 105.Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y, Tao J, Zhang J, Tian X, Liu S, Sun M, et al. Cellular repressor E1A-stimulated genes controls phenotypic switching of adventitial fibroblasts by blocking p38MAPK activation. Atherosclerosis. 2012;225:304–314. doi: 10.1016/j.atherosclerosis.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 107.Akama K, Horikoshi T, Sugiyama A, Nakahata S, Akitsu A, Niwa N, et al. Protein disulfide isomerase-P5, down-regulated in the final stage of boar epididymal sperm maturation, catalyzes disulfide formation to inhibit protein function in oxidative refolding of reduced denatured lysozyme. Biochim Biophys Acta. 2010;1804:1272–1284. doi: 10.1016/j.bbapap.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Laurindo FR, Pescatore LA, Fernandes DC. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med. 2012;52:1954–1969. doi: 10.1016/j.freeradbiomed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 109.Grune T, Reinheckel T, Li R, North JA, Davies KJ. Proteasome-dependent turnover of protein disulfide isomerase in oxidatively stressed cells. Arch Biochem Biophys. 2002;397:407–413. doi: 10.1006/abbi.2001.2719. [DOI] [PubMed] [Google Scholar]
- 110.Kozlov G, Maattanen P, Schrag JD, Hura GL, Gabrielli L, Cygler M, et al. Structure of the noncatalytic domains and global fold of the protein disulfide isomerase ERp72. Structure. 2009;17:651–659. doi: 10.1016/j.str.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 111.Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, et al. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol. 2011;12:235–245. doi: 10.1038/nrm3083. [DOI] [PubMed] [Google Scholar]
- 112.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu P, Bartz R, Zehmer JK, Ying YS, Zhu M, Serrero G, et al. Rab-regulated interaction of early endosomes with lipid droplets. Biochim Biophys Acta. 2007;1773:784–793. doi: 10.1016/j.bbamcr.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rivero F, Illenberger D, Somesh BP, Dislich H, Adam N, Meyer AK. Defects in cytokinesis, actin reorganization and the contractile vacuole in cells deficient in RhoGDI. EMBO J. 2002;21:4539–4549. doi: 10.1093/emboj/cdf449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benarafa C, Cooley J, Zeng W, Bird PI, Remold-O’Donnell E. Characterization of four murine homologs of the human ov-serpin monocyte neutrophil elastase inhibitor MNEI (SERPINB1) J Biol Chem. 2002;277:42028–42033. doi: 10.1074/jbc.M207080200. [DOI] [PubMed] [Google Scholar]
- 116.Morris JP, Thatje S, Hauton C. The use of stress-70 proteins in physiology: a re-appraisal. Mol Ecol. 2013;22:1494–1502. doi: 10.1111/mec.12216. [DOI] [PubMed] [Google Scholar]
- 117.Gnanasekar M, Dakshinamoorthy G, Ramaswamy K. Translationally controlled tumor protein is a novel heat shock protein with chaperone-like activity. Biochem Biophys Res Commun. 2009;386:333–337. doi: 10.1016/j.bbrc.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eng EW, Bettio A, Ibrahim J, Harrison RE. MTOC reorientation occurs during FcgammaR-mediated phagocytosis in macrophages. Mol Biol Cell. 2007;18:2389–2399. doi: 10.1091/mbc.E06-12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bodas M, Min T, Vij N. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLoS One. 2010;5:e15480. doi: 10.1371/journal.pone.0015480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 121.Mikerov AN, Hu S, Durrani F, Gan X, Wang G, Umstead TM, et al. Impact of sex and ozone exposure on the course of pneumonia in wild type and SP-A (−/−) mice. Microb Pathog. 2012;52:239–249. doi: 10.1016/j.micpath.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mikerov AN, Cooper TK, Wang G, Hu S, Umstead TM, Phelps DS, et al. Histopathologic evaluation of lung and extrapulmonary tissues show sex differences in Klebsiella pneumoniae - infected mice under different exposure conditions. Int J Physiol Pathophysiol Pharmacol. 2011;3:176–190. [PMC free article] [PubMed] [Google Scholar]
- 123.Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, et al. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9:24. doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Durrani F, Phelps DS, Weisz J, Silveyra P, Hu S, Mikerov AN, et al. Gonadal hormones and oxidative stress interaction differentially affects survival of male and female mice after lung Klebsiella Pneumoniae infection. Exp Lung Res. 2011;38:165–172. doi: 10.3109/01902148.2011.654045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Floros J, Wang G, Mikerov AN. Genetic complexity of the human innate host defense molecules, surfactant protein A1 (SP-A1) and SP-A2--impact on function. Crit Rev Eukaryot Gene Expr. 2009;19:125–137. doi: 10.1615/critreveukargeneexpr.v19.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.